Abstract
Adults with psychopathy have a high propensity for substance abuse, generally starting from a young age. This investigation tested hypotheses about differences in the neural responses associated with drug craving among high-risk young offenders with histories of abuse of stimulants and other drugs as a function of psychopathic traits. Fifty-four male adolescents (44 with a history of stimulant abuse and 10 controls) incarcerated at a maximum-security facility (M age = 17.08 years) completed a drug-cue exposure task while brain hemodynamic activity was monitored using functional magnetic resonance imaging (fMRI) with a mobile MRI scanner stationed at the facility. Psychopathic traits were assessed using the Hare Psychopathy Checklist: Youth Version (PCL:YV). In the stimulant abuser group, drug cues elicited activity in classic reward circuitry. Consistent with studies of adult psychopathic traits and substance abuse, there was a negative association between PCL-YV scores and hemodynamic response related to drug craving in the amygdala and ACC in youth with a history of stimulant abuse. However, there were considerably more negative associations between the PCL:YV and hemodynamic response among youth than adults and this was primarily due to callous-unemotional traits rather than interpersonal or behavioral traits. The implications for how personality traits modulate motivations for drug-seeking behavior among adolescent offenders are discussed.
Keywords: Delinquency, Psychopathic traits, Drug abuse, Craving, Cocaine, Brain imaging
Psychopathic Traits Modulate Brain Drug Craving Response in Substance Abusing Young Offenders
Substance use disorders and crime are two issues with significant financial burden to society. The latest figures from the National Institute of Drug Abuse estimate the annual cost of alcohol and illicit drug abuse in the United States (U.S.) is approximately $417 billion. The annual cost of crime in the U.S. has been estimated to exceed $3.2 trillion (Anderson, 2012; Kiehl & Hoffman, 2011) – a sum that rivals the cost of all health care in the U.S. Individuals with psychopathic personality disorder have a high likelihood of being chronic lifetime offenders (Piquero, Farrington, Fontaine, Vincent, & Coid, 2012), have a high propensity towards drug and alcohol abuse (Hemphill, Hart, & Hare, 1994), and are at the highest risk of engaging in persistent violence (e.g., Leistico, Salekin, DeCoster, & Rogers, 2008), which is significantly amplified when paired with substance abuse (Steadman et al., 2000).
Arguably, if we want to prevent psychopathy and extreme antisocial behavior in adults, we need to look at earlier identification and effective interventions for youth because these traits start at a young age (Robins, 1978). Similarly, effective treatment or prevention of substance use during adolescence may greatly decrease the likelihood of addiction in adulthood for which early initiation is a significant predictor (Grant & Dawson, 1998). Early initiation of substance use is particularly prominent among youth with psychopathic traits (Mailloux, Forth, & Kroner, 1997). As such, effective substance abuse prevention or interventions designed for youth, particularly those at increased risk for psychopathy in adulthood, may have a lifetime impact on the youth and on communities. Neuroimaging evidence suggests substance abusing adults with psychopathy have significantly different neurological responses associated with drug craving than other offenders with substance abuse histories, which may be a result of differences in their motivations for drug abuse (Cope et al., 2014). The current study sought to examine whether this is also the case with youth by investigating the underlying neurobiology of drug craving among a sample of serious substance abusing high-risk offenders in a secure correctional facility.
Addiction and the Brain
Drug addiction is defined by both a compulsion to seek and take drugs and a loss of control in the ability to limit one’s intake (Koob & Volkow, 2010). The compulsion aspect of addiction is commonly known as drug craving or an intense desire or urge or use drugs. One popular method for studying drug craving is the cue-exposure paradigm (Wilson, Sayette, & Fiez, 2004). Recently, researchers have paired the drug cue-exposure paradigm with functional magnetic resonance (fMRI) neuroimaging technology to examine the neural basis of cue-elicited craving. Neuroimaging studies using the cue-exposure paradigm have been performed across multiple drugs of abuse using a variety of cue-modalities (Wilson et al., 2004). Researchers have identified an association between increased brain activation in sensory, motor, and cognitive-emotional processing areas during these cue-exposure tasks with eventual relapse (Kosten et al., 2006).
Volkow, Fowler, Wang, and Goldstein (2002) hypothesized that addiction may be perpetuated by a disruption to the frontal cortical circuits that regulate motivation and self-control, as well as a disruption in memory circuits that promote the salience of drug stimuli. Evidence for their hypothesis comes from neuroimaging drug studies with animals and cue-exposure studies with adults with substance abuse, which indicated there were neuropsychological reactions or hyperactivity in a network including the orbitofrontal cortex (OFC), dorsal striatum (caudate and putamen), prefrontal cortex, amygdala, hippocampus, and insula (Koob & Volkow, 2010). Koob and Volkow (2010) also noted that lack of behavioral control over drug use has been associated with disruption in the anterior and posterior cingulate gyri (ACC and PCC), dorsolateral prefrontal cortex, and inferior frontal areas. Research with adults has demonstrated many of these regions that are also known dopaminergic targets (e.g., the ACC, striatum, amygdala and frontal areas like the OFC) are activated by exposure to cocaine cues and by the self-administration of cocaine among individuals addicted to cocaine (Breiter et al., 1997; Childress et al., 1999; Grant et al., 1996; Risinger et al., 2005), making cocaine an appealing option for testing drug craving in cue-exposure tasks.
Psychopathic Traits and Substance Abuse in Adults
Individuals with psychopathy have a high predilection towards substance abuse. Psychopathy is a personality disorder that is traditionally thought of as comprising two interrelated factors; Factor 1 comprises emotional deficits (e.g., callousness, lack of remorse) and an arrogant and deceitful interpersonal style, and Factor 2 comprises antisocial behavioral or social deviance features such as impulsivity and stimulation-seeking (Cleckley, 1976; Hare, 2003). Clinical assessments of psychopathy most commonly use the Hare Psychopathy Checklist-Revised (PCL-R; Hare, 2003) to identify these traits. Studies using a version of the PCL-R with offenders have indicated that, relative to non-psychopathic offenders, psychopathic offenders are more likely to have a diagnosis of alcohol or substance abuse, more likely to be polysubstance abusers, and are more likely to abuse stimulants and cocaine (Smith & Newman, 1990). Early studies of psychopathy and substance abuse indicated that the degree of substance abuse and dependence were more strongly associated with the impulsive and social deviance traits (Factor 2) of psychopathy than the affective and interpersonal traits (Hemphill et al., 1994; Rutherford, Alterman, Cacciola, & McKay, 1997). More recently, however, Walsh, Allen, and Kossen (2007). colleagues found that the affective and arrogant personality traits (Factor 1) of psychopathy were positively correlated with abuse of certain drugs, particularly cocaine.
Adult offenders with psychopathy have significant functional brain abnormalities, relative to non-psychopathic offenders (e.g. Kiehl, 2006; Philippi et al., 2015), many of which overlap with regions implicated in drug addiction but in an inverse manner. For example, individuals with psychopathy show reduced activation in the bilateral amygdala, rostral ACC, and PCC during emotional processing tasks (see Kiehl, 2006), areas that tend to be hyperactive among individuals who abuse substances when exposed to drug cues. Adults with psychopathy also have shown reduced activation during aversive conditioning in regions that are also related to a difficulty in inhibiting responses to a perceived reward and attention (e.g., Kiehl, 2006; Veit et al., 2002).
A recent fMRI drug-cue exposure study of adult offenders with poly-substance abuse histories indicated psychopathic traits modulated the drug craving response (Cope et al., 2014). Psychopathic traits were negatively correlated with the neurobiological craving response in limbic and paralimbic areas, namely, the ACC, PCC, hippocampus, amygdala, caudate, pallidum, and areas of the prefrontal cortex. These negative associations were most strongly related to the behavioral features of psychopathy (Factor 2), whereas Factor 1 showed positive associations to drug cues in the pallidum, insula, and areas of the cerebellum. The negative correlation between brain activation to drug cues and Factor 2 may seem surprising in light of the strong positive correlations between Factor 2 and actual drug use. However, the authors explained that this was likely due to an overall tendency for individuals with psychopathy to not experience drug craving or withdrawal and their general deficiencies in regions related to reward processing. The authors suggested individuals with psychopathic traits may differ from other substance abusers in their motivation for abusing drugs, and potentially for relapse.
Shifting Focus to Adolescents
Research investigating whether findings in adults extend to adolescents must consider some important differences between these developmental periods, particularly with respect to psychopathic traits. We expect adolescents to be more prone to impulsive and reward-seeking behavior than adults as their brain structure and functioning is still undergoing development, particularly in areas of the prefrontal cortex (e.g., Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Somerville, Jones, & Casey, 2010) that are known to mature slower. Development of the prefrontal cortex may be differentially impacted (even slower) among both substance users and those with striking behavioral issues characteristic of psychopathy. Many young people with impulsivity and serious conduct problems do not go on to be psychopathic adults as they mature; however, youth exhibiting significant and impairing patterns of both conduct problems and callous-unemotional (CU) traits seem to be at highest risk for having psychopathic personality disorder as adults (Frick, 2006). Youth with CU traits have a number of classic psychopathy correlates, including aggression, attachment difficulties, deficits in affective processing, and attentional deficits (see Vincent, Kimonis, & Clark, 2015, for a review). We refer to these youth as having CU or as being ‘high in psychopathic traits’ in light of the issues with assessing psychopathic personality at young ages (Seagrave & Grisso, 2002; Vincent, 2006).
Despite developmental changes that occur with brain maturation, neuroimaging studies have documented many abnormalities for youth with CU or high psychopathic traits that are similar to those found in adults (Waller, Murray, Dotterer, & Hyde, 2015). This appears to be the case in relation to emotional processing, where they have low activation in the amygdala in response to affective stimuli (Marsh et al., 2008) and in response to other people’s pain (Decety, Skelly, & Kiehl, 2013; Marsh et al., 2013). The reduced amygdala response appears to be most strongly related to the callous-unemotional traits (e.g., Viding et al., 2012). There is also evidence among youth scoring high on measures of psychopathic traits of disruption in reward-related processing. They have been reported to have reduced reactivity in the caudate and OFC (Finger et al., 2011), and generally lowered responsiveness in areas of the dorsal striatum, ventral striatum, and amygdala (Cohn et al., 2015) during reward outcomes. However, as noted by Blair (2015), this reduced reactivity or sensitivity to rewards has also been identified in youth with serious behavioral disorders and substance abuse (Crowley et al., 2010), so it is unclear whether there is something unique about youth with both disruptive behavior problems and CU traits. At least one study demonstrated CU traits were unrelated to reduced activity in the caudate and other areas in response to environmental rewards (White et al., 2014). A challenge in the interpretation of the inconsistent results between some studies is their use of different self-report measures to assess psychopathic traits among youth. These measures do not correlate highly with clinical ratings of psychopathic traits (i.e., the PCL:YV), particularly for the affective and interpersonal dimensions (Cauffman, Kimonis, Dmitrieva, & Monahan, 2009; Fink, Tant, Tremba, & Kiehl, 2012).
Current Study
Youth scoring high on assessments of psychopathic traits, like their adult counterparts, appear to be at increased risk for drug use disorders (Roussey & Toupin, 2000), have tried significantly more drugs, have more severe drug use, and have a younger onset of drug use (Mailloux et al., 1997). To date, neurological studies regarding the association between psychopathic traits and craving response among youth have not been reported. Although the evidence is mixed, there is support for the notion that adults with psychopathy and youths with high psychopathic traits are vulnerable to reduced reactivity in areas of their reward systems. This may affect their response to drugs of abuse. For example, the neurological deficits in areas responsible for affective processing may make them less likely to demonstrate significant increases in neural activation in areas of the reward system associated with a preoccupation and compulsion to seek drugs. On the other hand, the functional neurocognitive abnormalities related to their difficulties inhibiting reward-related responses in the face of punishment (Budhani & Blair, 2005) may compel youth with psychopathic traits to have difficulty limiting their intake of substances even when this is associated with extreme consequences. If this were the case, it could affect the efficacy of traditional approaches to substance abuse treatment with this group. Whether deficiencies that have been identified in reward and punishment processing are unique to youth with CU traits and conduct disorder or are simply a function of their disruptive behavior disorder and possibly substance abuse remain unclear.
The current study used fMRI and an incarcerated sample of substance abusing young offenders with histories of cocaine or other stimulant abuse to investigate their neural craving response. We utilized a similar drug-cue exposure task as Cope et al. (2014). In order to obtain a sample of serious substance abusers, a history of stimulant abuse (cocaine, methamphetamine, or prescription stimulants ingested nasally) and naming a stimulant as the primary drug of choice was a requirement for inclusion. Stimulant abuse was also required, at a minimum, because: a) stimulant abuse history increases the likelihood of obtaining a higher prevalence of psychopathic traits due to their relatively greater proclivity towards cocaine abuse (Smith & Newman, 1990; Walsh et al., 2007), and b) stimulants and cocaine elicit a strong craving response (Carter & Tiffany, 1999). There were two primary hypotheses. First, consistent with Cope et al. (2014), we expected psychopathic traits to negatively modulate the neural response to drug cues, particularly in areas of the limbic system associated with emotion, emotional memory, and reward (e.g., amygdala, ACC, hippocampus). Second, in contrast to Cope et al. (2014), we expected the negative association to be due primarily to the Factor 1 traits of psychopathy, rather than the behavioral features. There are a few prominent reasons for this hypothesis. First, the population studied is adolescents where CU traits appear to have a strong effect for predicting who is more likely to have stable psychopathic traits (see Frick, 2006, and Vincent, Kimonis, & Clark, 2015, for a review). Second, the impulsive and irresponsible behavioral traits associated with psychopathy in adults may have a higher prevalence in adolescents as a function of their stage of brain development, and impulsivity and irresponsibility are likely to be even more prevalent among youth who are incarcerated and are substance abusers. In other words, the characteristics of the high-risk population under study may make Factor 1 traits more discriminating than the behavioral features. Third, there is some evidence from an item response theory comparison of youth and adults that the CU traits are the most discriminating feature of a psychopathy syndrome in adolescents, whereas behavioral features were less discriminating and interpersonal features were discriminating but less common than one sees in adults (Vincent, 2002). The positive associations with the craving response reported for Factor 1 by Cope et al. (2014) were primarily due to the interpersonal features of psychopathy in their adult sample, which may only be found among youth at very high levels of a latent trait.
Method
Participants
Participants were 54 male adolescent offenders, aged 14 to 19 years, from a maximum-security juvenile correctional facility. Participants were selected from a larger dataset (Southwest Advanced Neuroimaging Cohort-Youth; SWANC-Y; NIMH R01 MH071896: K.A.K., PI) of male and female offenders from the facility. Forty-four of these participants were serious substance abusers: They had to (a) meet criteria for lifetime abuse of methamphetamine or cocaine on the substance use disorders module of the Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, Rao & Ryan, 1996), (b) report cocaine or methamphetamine as their primary drug of choice, and (c) report regular use (i.e., three times a week for a period of three months or more) of at least one stimulant. These participants could have histories of abuse of other drugs as well, but stimulants had to be the primary. We will herein refer to this group as the “stimulant abuser group”. The other 10 participants were non-stimulant abusers and served as a control group. To be eligible for the control group, the participants could not have reported regular use of any substance other than alcohol or marijuana. Four of the controls met criteria for marijuana abuse and six met criteria for alcohol abuse on the K-SADS-PL. The purpose of the control group was to examine the specificity of the task, which should not have engaged the craving circuits for individuals who had not abused hard drugs due to the nature of the drug-cue pictures.
All participants were English speaking and had adequate English reading comprehension as measured by the Wide Range Achievement Test version 3 (WRAT-3; Wilkinson, 1993). Youth were excluded from participation in this study if they were under the age of 18 and wards of the state (because parental consent would be challenging to obtain), or had an estimated full-scale IQ less than 70 (as measured by the short form of the WAIS), metal in their bodies, past or current central nervous system disease, or a history of psychosis. A history of abuse or dependence on alcohol or drugs other than stimulants was not grounds for exclusion in the stimulant abuser group. Four participants were excluded due to motion recorded during scanning or poor image quality. The final sample consisted of 40 stimulant abusers (M age = 17.08 years, SD = 1.29) and 10 controls (M = 17.10, SD = 1.29). Table 1 illustrates that the demographic characteristics did not differ significantly between these groups (see Table 1).
Table 1.
Sample characteristics
| Substance Abusers (n = 40) |
Controls (n = 10) |
Test | |
|---|---|---|---|
| Demographic | |||
| Age at enrollment | 17.08(1.27) | 17.10(1.29) | t(48) = −.06, p = .96 |
| Last grade completed | 9.03(1.36) | 9.89(1.61) | t(44) = −1.64, p = .11 |
| Intelligence Quotient | 94.22(11.43) | 97.4(17.76) | t(44) = −.68, p = .50 |
| Left handed | 7.5% | 10% | X2(1) = 0.07, p = .79 |
| Race | |||
| White | 90% | 100% | X2(1) = 1.09, p = .30 |
| American Indian | 10% | 0 | |
| Ethnicity - Hispanic | 87.5% | 80.0% | X2(1) = 0.37, p = .54 |
| Psychopathy (PCL:YV) | |||
| Total score | 24.26(6.02) | 15.28(5.98) | t(48) = 4.23, p < .01 |
| Interpersonal/Affective Factor 1 | 6.91(3.69) | 4.70(2.83) | t(48) = 1.76, p = .08 |
| Interpersonal | 2.53(2.28) | 1.30(1.49) | |
| Affective | 4.38(1.82) | 3.40(1.65) | |
| Behavioral Factor 2 | 14.94(2.43) | 9.13(4.13) | t(48) = 5.82, p < .01 |
| Lifestyle | 6.90(1.73) | 3.40(1.78) | |
| Antisocial | 8.04(1.24) | 5.73(2.63) | |
| Substance Use History | |||
| Age regular use (any drug) | 11.15(2.44) | 13.00 (2.83)a | |
| Age regular use (stimulants) | 14.54(1.85) | – | |
| Days since last use | 138.80(129.55) | – | |
| % Regular use marijuanab | 97.5% | 20% | X2(1) = 32.55, p < .01 |
| % Regular use alcohol-high thresholdb- | 76.9% | 10% | X2(1) = 15.34, p < .01 |
| % Regular use opiatesb | 45.0% | 0% | X2(1) = 7.03, p < .01 |
Note.
–Average is based on the two subjects that regularly used marijuana only.
– Refers to regular use at any point in the past.
Because incarcerated adolescents are a vulnerable population, extra care was taken to minimize the potential for coercive influences that might reduce their ability to provide voluntary consent to participate. Members of the research team recruited participants using facility-wide group announcements, noting that participation was completely voluntary and agreeing or disagreeing to participate would not affect their facility status or release. Researchers contacted guardians to provide written informed consent for their youth’s participation (after receiving the youth’s written assent) for youths under age 18. Participants aged 18 or older provided their own written informed consent. Participants were financially compensated at a rate comparable to the average institutional pay rate in the facility.
Measures
Hare Psychopathy Checklist-Youth Version (PCL:YV; Forth et al., 2003)
The PCL:YV is a downward extension of the adult Hare Psychopathy Checklist-Revised (Hare, 1991, 2003), and is used to provide a dimensional assessment of the prototypical psychopath among adolescents aged 12 to 19 years. The PCL:YV is an expert symptom rating scale that comprises 20 items scored on a three-point scale (0 = item does not apply; 1 = item applies somewhat; 2 = item definitely applies) based on each symptom’s pervasiveness, severity, and chronicity. The PCL scales have traditionally been considered to have two factors: Factor 1 contains interpersonal and affective symptoms, and Factor 2 contains lifestyle and antisocial behavioral symptoms (Forth et al., 2003; Hare, 2003). Recent confirmatory factor analytic (CFA) studies suggest the PCL:YV has more than one valid test structure, both a three-factor model (Interpersonal, Affective, and Lifestyle behavioral) and a four-factor model (Interpersonal, Affective, Lifestyle, and Antisocial) whereby the Interpersonal and Affective Factors are highly correlated and the Lifestyle and Antisocial Factors are highly correlated (Neumann, Kosson, Forth, & Hare, 2006). This study focused on the traditional two-factor model to reduce the number of tests due to a relatively small sample and to be consistent with Cope et al. (2014). Factor 1 and Factor 2 scores were highly correlated in this sample (r = .56, p < .001), which is common in offender samples, including young offenders (see Forth et al., 2003).
PCL:YV assessments were completed following a 60 to 90 minute semi-structured interview and a review of collateral information, which included psychosocial histories and juvenile arrest and institutional records. Interviewers completed a rigorous training process, which included a two-day training workshop, supervision, and on-going booster training involving rating videos. Inter-rater reliability (IRR) for total scores calculated on 35 double-rated cases was excellent (ICC2 = .95) and consistent with IRR reported in the manual (r = .90–.96; Forth et al., 2003). There is no set cut-off on the PCL:YV to identify high scorers. However, 27.5% of the stimulant abuser sample and none of the controls would qualify as high scorers on the PCL:YV if we used the traditional Total score cut-off of 30 from the adult PCL (Hare, 2003).
Substance use
Two variables related to substance use were examined as covariates:
Length of abstinence. Just prior to fMRI scanning, participants were asked about their most recent drug use. We calculated the number of days between their scan and last drug use (excluding alcohol), which averaged 4.62 months in the substance abuse sample (see Table 1). This is a relatively long period of abstinence due to use of an institutional sample, which rarely used drugs while in the institution. Length of abstinence was included as a control variable because it is negatively related to neurobiological response (Volkow et al., 1996).
Age of onset of regular use of any drug. Age of onset of regular use (i.e., three or more times per week) of any drug was included as a measure of substance abuse severity. Interviewers used a modified version of the Addiction Severity Index (ASI; McLellan et al., 1992) to assess youth’s substance use history. The ASI is a brief interview that asks details about the duration, frequency, and amount of use of multiple types of drugs. This included questions about their earliest age of regular use for several types of drugs: heroin, cocaine, methamphetamine, cannabis, hallucinogens, and inhalants. The earliest age of regular use of any drug (excluding alcohol) was included as a covariate.
Stimuli and Task
All participants were required to have at least one-week abstinence from all substances prior to completing the fMRI. Two types of pictures (32 drug-related and 32 neutral) were selected from the popular media. Drug-related pictures depicted drugs or drug paraphernalia related to cocaine, heroin, and/or methamphetamine (e.g., white powder with a razor blade, a hand holding a syringe, a pipe). Neutral pictures depicted non-drug objects and scenes (e.g., white fluffy clouds, folded hands, a pen). Participants were instructed that they would see a series of pictures presented one at a time, each for 6 seconds. For each picture, the instructions were to determine if anything in the picture “gave them a craving feeling or desire to use drugs”. Then they were instructed to rate their intensity of drug craving (in the form of a growing red bar) on a scale from 1 (no craving) to 5 (extreme craving) based on their immediate level of desire, not how they think they should feel or would hope to feel. After the rating screen, a black screen with a white fixation cross was presented for 4 seconds. Twenty null fixation trials the same duration as picture trials (i.e., picture + rating + fixation = 14 seconds) were interspersed randomly. Each participant completed two runs of 52 trials (16 drug cues, 16 neutral, and 20 null fixation stimuli per run). Research has demonstrated this drug cue task elicits robust engagement of brain regions involved in craving and addiction in a sample of incarcerated adults regardless of whether their drug of choice was cocaine, methamphetamine, or heroin (Cope et al., 2014).
MRI Data Acquisition and Statistical Analysis
Participants were scanned on the Mind Research Network 1.5T Siemens Avanto mobile MRI, stationed at the correctional facility, using an EPI gradient-echo pulse sequence (TR=2000ms, TE=39ms, flip angle=75°, FOV24 × 24 cm, 64 × 64 matrix, 3.8 × 3.8mm in-plane resolution, 4mm slice thickness with 1mm gap, 27 slices).
Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm). The ArtRepair Toolbox (Mazaika, Hoeft, Glover, & Reiss, 2009) was used to detect and remove severe image artifacts. ArtRepair-detected artifactual images were replaced and a regressor to remove the effects of any offending images in the statistical analyses. Following ArtRepair each run was motion-corrected using INRIAlign, an algorithm that is unbiased by local signal changes (Freire, Roche, & Mangin, 2002). The realignment parameters (three translations and three rotations) and second-order movement parameters were entered as covariates in the statistical models below in order to covary any residual variance due to head movement. Realigned images were spatially normalized to the Montreal Neurological Institute (MNI) template and smoothed with an 8mm full-width at half-maximum (FWHM) Gaussian smoothing kernel. Low frequency noise was removed using a high pass filter (cutoff: 1/128s). Talairach daemon was used to assist with anatomical labeling after conversion from MNI space. All anatomical images and coordinates are reported in MNI space.
Drug-related pictures, neutral pictures, the rating period, and null fixation trials were modeled separately. Participants’ craving ratings to each drug-related and neutral picture also were included as parametric modulators. Analyses examined the hemodynamic activation present when comparing the drug-cue and neutral-cue picture conditions. The T-map of main effects was produced from one-sample t-tests in SPM12 to detect differences in the drug-related versus neutral pictures. Main effect analyses were thresholded at the p < .05 FWE correction for multiple comparisons with an extent threshold of 10 voxels.
We performed analyses with the PCL and covariates using anatomical regions of interest (ROIs) where we had a priori hypotheses. These ROIs were selected based on a combination of the previously cited craving and psychopathy literatures and the ROIs used by Cope et al (2014). We defined 25 ROIs: the ACC, PCC, medial OFC, and left and right regions of the lateral OFC, insula, hippocampus, parahippocampal gyrus, amygdala, precuneus, thalamus, and areas of the basal ganglia and striatum (nucleus accumbens, caudate, putamen, and pallidum). These regions were consistent with the 20 ROIs used by Cope et al. (2014) with a few exceptions. We added the parahippocampal gyrus and precuneus due to previously identified associations with psychopathy (Kiehl, 2006; Motzkin, Newman, Kiehl, & Koenigs, 2011) and split the lateral OFC into left and right in the event the Cope et al. (2014) study did not find any effects here possibly because their region was too large. Linear regressions were performed in SPM12 on the peak beta values from the ROIs’ association with the T-map of main effects (neural correlates of drug craving) to examine associations with hemodynamic activity to craving and PCL:YV Total and Factor scores. Peak betas were used rather than average betas because the fMRI data had already been spatially smoothed.
In addition to these primary regression analyses with PCL:YV Total score, Factor 1, and Factor 2 (25 regressions per predictor, one for each ROI, correcting for the number of tests within each dependent variable by setting a threshold of p < .002, using a Bonferroni correction), supplementary analyses were performed to evaluate the robustness of the effect of the different PCL:YV scores on the hemodynamic response to drug-related stimuli after including covariates (i.e., age onset of substance use, length of abstinence, scan age, and IQ). Peak beta values from the ROIs were imported from SPM12 into SPSS to examine their correlation with each of the potential covariates. Variables with a significant correlation (p < .050) within a particular ROI were included as covariates in supplementary regressions for that ROI. In order to assess potential effects for the rest of the brain, we report post hoc whole-brain analyses using a threshold p < .005, uncorrected, for significance with an extent threshold of 10 voxels.
Results
Table 1 provides the means and standard deviations on the PCL:YV and select substance use variables from the ASI for both the stimulant abuser group and the controls. The substance abusers had significantly higher PCL:YV scores than controls with the exception of Factor 1 (see Table 1). The stimulant abuser group had a significant history of drug use, with the average age of onset for regular use of any drug at 11.15 years (SD = 2.44 years) and regular use of any stimulant at age 14.5 years (see Table 1). The majority of these youths had regularly used (i.e., three or more times a week) marijuana or alcohol at a high threshold (five or more drinks at a time) at some point in the past, and almost half had regularly used heroin or another opiate. This group had significant drug use histories, with 70% meeting lifetime criteria for cocaine or other stimulant dependence on the K-SADS and almost 40% having lifetime opiate dependence. In contrast, only two of the controls had used marijuana regularly in the past with an average age of onset of 13 years (SD = 2.83). One had a history of consuming a high threshold of alcohol regularly (five drinks or more, three or more times a week).
Correlations Between PCL And Potential Covariates
Among the stimulant abuse group (n = 40), the PCL:YV Total, r(38)= −.39, p = .012, Factor 1, r(38) = −.36, p = .022, and Factor 2 scores, r(38) = −.40, p = .011, were significantly negatively correlated with the age of onset of regular drug use. The PCL:YV Total, r(38)= −.34, p = .034, and Factor 2, r(38) = −.36, p = .024, scores also were negatively correlated with length of abstinence from all drugs, but Factor 1 was not, r(38)= −.24, p = .132. The PCL:YV total and factor scores were not correlated with demographic variables, such as the subjects’ age at time of assessment, race or ethnicity, or IQ at p-values of less than .05.
Behavioral Craving Ratings
The stimulant abuse group (n = 40) rated the drug cue pictures (M = 2.31, SD = 0.99) as eliciting significantly more craving (defined for subjects as the immediate desire for drugs) than neutral pictures (M = 1.51, SD = 0.41) indicating the drug-related pictures produced the desired response, t(39) = 5.39, p < .001, d = .85. PCL:YV Total scores were positively correlated with self-reported craving, r(38) = .35, p = .015, and this could be attributed to both Factor 1, r(38) = .32, p = .021, and Factor 2, r(38) = .39, p = .007. Surprisingly, Factor 2 scores also were related to higher reported craving on neutral pictures, r(38) = .28, p = .040, but Total and Factor 1 scores were not.
Brain Imaging and Main Effect of Drug Cues
Consistent with previous literature, the comparison of hemodynamic activity associated with viewing drug-related pictures relative to neutral pictures set at a threshold p < .05, FWE corrected, showed positive engagement of the ACC, bilateral precuneus, right thalamus, left PCC, left parietal lobule, left cingulate gyrus, areas of the frontal lobe (right inferior and left medial frontal gyrus), and regions in the occipital lobe. Figure 1 illustrates the positive associations for the serious substance abuse group at p < .005, uncorrected, to best display the results. There was significant negative response in the areas of the bilateral insula, precentral gyri, bilateral postcentral gyri, and the mid/superior temporal gyri.
Figure 1. Main effect of viewing drug-related pictures vs. neutral pictures for stimulant abusers (n = 40). p < 0.005, uncorrected.
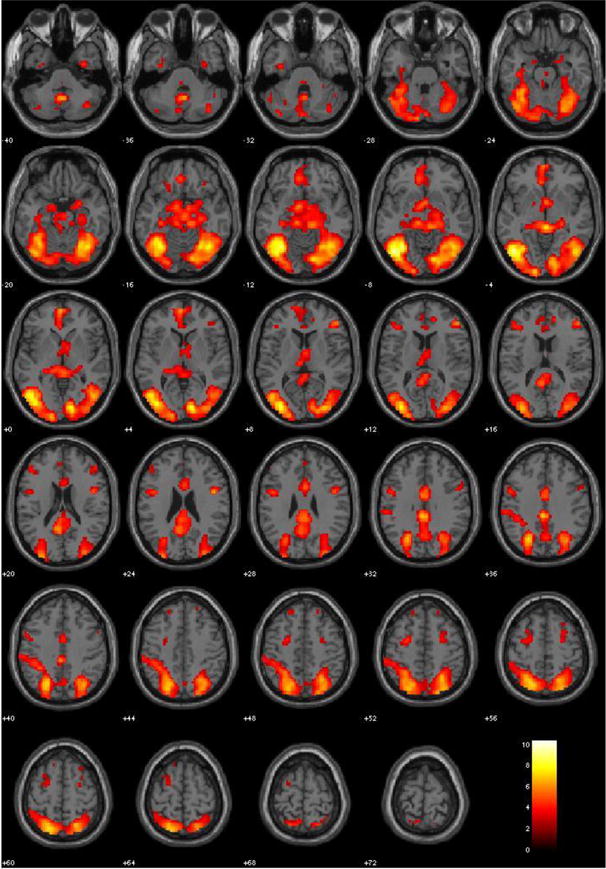
Note. These regions are significant in the whole brain at p < 0.005, uncorrected with a 10 voxel extent. Numeric values indicate the MNI z-coordinate of the slice, and the color bar represents t-values. Warm colors represent positive activation.
As a manipulation check, we conducted the same analyses for the 10 controls. Controls did not show a statistically significant hemodynamic response to drug-related pictures relative to neutral pictures (p < .05, FWE corrected); however, the effect sizes were large (r’s ranging from 0.76 to 0.94) in regions that are not traditionally associated with craving. The controls showed positive engagement of areas of the bilateral parahippocampus and bilateral precuneus (regions of interest due to reported associations with psychopathy rather than craving), left superior parietal lobule, the mid/inferior temporal gyri, and areas of the occipital lobe. They showed negative response in some areas traditionally associated with drug craving (left thalmus, bilateral caudate, left ACC, bilateral insula) in addition to the bilateral superior temporal gyri, bilateral cingulate gyri, and the mid/superior temporal gyri. Thus, positive activation in multiple craving regions appeared to be specific to the stimulant abuser group and remaining analyses were conducted with only this group.
Brain Imaging and Potential Covariates
With respect to the potential covariates with neural activity associated with drug craving in the ROIs, current age was negatively correlated with activity in the left precuneus, r(38) = −.27, p = .050, and left thalamus, r(38) = −.28, p = .040. Age of onset was positively correlated with activity in the ACC, r(38) = .30, p = .032, left caudate, r(38) = .31, p = .026, left insula, r(38) = .34, p = .017, and medial OFC, r(38) = .30, p = .031. Length of abstinence was positively correlated with activity in the left nucleus accumbens, r(38) = .33, p = .020, left amygdala, r(38) = .28, p = .040, left hippocampus, r(38) = .33, p = .018, and the left precuneus, r(38) = .30, p = .032. IQ was not significantly correlated with any region. To take a conservative approach, we included covariates in the relevant supplementary analyses if they were significant at p < .05 before correction.
Brain Imaging and Modulating Effects of Psychopathy
PCL:YV Total scores
Regression analyses with the ROIs indicated significant negative associations between PCL:YV Total scores and activity to drug-related cues in many areas at p < .002 (Bonferroni correction threshold set for p < .05 after correcting for 25 tests; see Table 2). The areas included the ACC, left amygdala, right caudate, left hippocampus, left and right insula, left and right pallidum, and left putamen. There were no significant positive hemodynamic associations with Total PCL-YV scores. Figure 2 illustrates the negative associations in most of the ROIs provided in Table 2 at p < .005 to best display the results.
Table 2.
Regression Results: Negative Associations Between PCL-R Total Scores And Hemodynamic Activity For Viewing Drug-Related Pictures vs. Neutral Pictures in Peak Voxels Within AAL Regions of Interest
| ROI | x | y | z | hemi | t-value | β [CI] | r | p-value |
|---|---|---|---|---|---|---|---|---|
| Anterior Cingulate | 0 | 18 | 27 | R & L | −3.89 | −.016 [−.025, −.008] | .53 | < .0002 |
| Amygdala | −27 | −6 | −12 | L | −4.38 | −.015 [−.021, −.008] | .56 | < .0001 |
| Caudate | 12 | 3 | 21 | R | −3.87 | −.016 [−.025, −.008] | .53 | .0002 |
| Hippocampus | −30 | −6 | −15 | L | −4.01 | −.012 [−.018, −.006] | .55 | .0001 |
| Insula | 42 | 12 | −9 | R | −4.12 | −.017 [−.026, −.009] | .56 | < .0001 |
| Insula | −42 | 9 | 3 | L | −3.48 | −.018 [−.028, −.007] | .49 | .0006 |
| Pallidum | 15 | 6 | −3 | R | −3.33 | −.011 [−.017, −.004] | .48 | < .001 |
| Pallidum | −21 | 3 | −3 | L | −3.62 | −.012 [−.018, −.005] | .51 | .0004 |
| Putamen | −24 | 6 | −3 | L | −4.04 | −.014 [−.021, −.007] | .43 | .0001 |
Note. Hemi = hemisphere of the ROI. β [CI] = the unstandardized peak beta and its confidence interval. r = Pearson’s correlation as a measure of effect size, calculated by sqrt (t2/t2 + df). P-values represent the uncorrected p-value. All areas are significant at p < .05 following a Bonferroni correction.
Figure 2. Negative associations between PCL:YV Total (upper) and PCL:YV Factor 1 (lower) scores and hemodynamic activity for viewing drug-related pictures vs. neutral pictures for stimulant abusers (n = 40). p < 0.005, uncorrected.
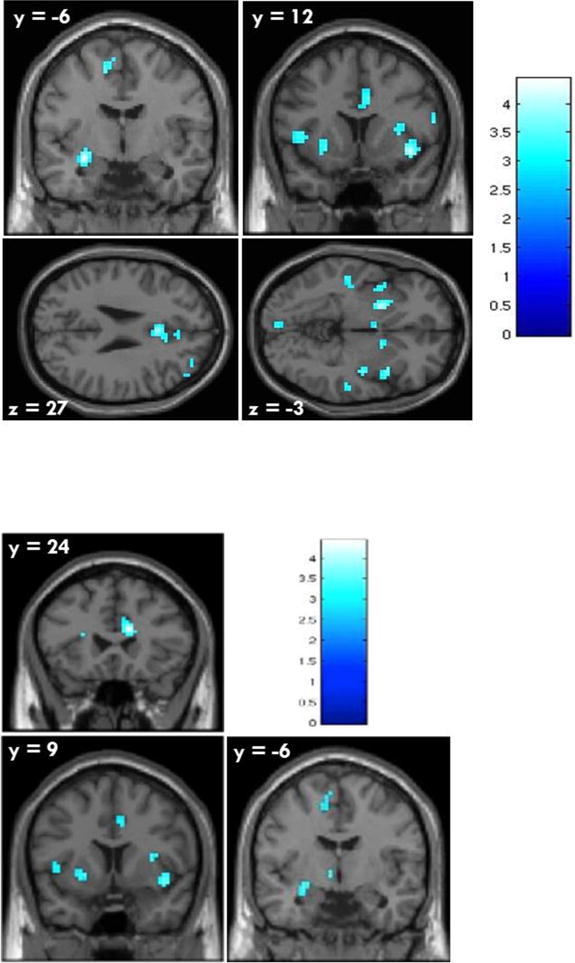
Note. Numeric values indicate the MNI coordinate of the slice, and the color bar represents t-values. Cold colors represent negative association.
Supplemental analyses were conducted so that each significant covariate (current age, age onset, or length of abstinence) was added to models for the respective ROIs when comparing drug-related pictures to neutral pictures, with PCL:YV Total score as the main predictor. PCL:YV Total scores were still significantly associated with the ACC and left insula after including age of onset, and were still significantly related to the left amygdala and left hippocampus after including length of abstinence. Inclusion of covariates in the model with other ROIs did not change the non-significant associations with the PCL after correction.
Factor 1 scores
Regressions with the ROIs indicated there were significant negative associations between PCL Factor 1 scores and hemodynamic activity in areas of the left amygdala, ACC, right caudate, left and right insula, left and right hippocampus, left pallidum, and left putamen at p < .002 (see Table 3). Factor 1 was not significantly positively associated with activity in any region. Figure 2 illustrates the negative associations in most of the ROIs provided for Factor 1 in Table 3 at p < .005. Supplementary analyses for Factor 1 controlling for covariates led to a non-significant association between PCL:YV Factor 1 and the right insula, t(38) = −2.32, p = .026, d = .37, left insula, t(38) = −2.39, p = .022, d = .38, and left caudate, t(38) = −2.04, p =.048, d = .32, after the addition of age of onset, following correction. Addition of length of abstinence did not change the negative association with the left amygdala but did render the association with the left hippocampus non-significant following correction, t(38)= −2.83, p = .008, d = 45.
Table 3.
Regression Results: Negative associations between PCL:YV Factor scores and hemodynamic activity for viewing drug-related pictures vs. neutral pictures at peak voxels within AAL ROIs
| ROI | x | y | z | hemi | β [CI] | t-value | r | p-value |
|---|---|---|---|---|---|---|---|---|
| PCL:YV Factor 1 | ||||||||
| Anterior Cingulate | 12 | 24 | 24 | R & L | −.012 [−.019, −.006] | −3.74 | .27 | .0003 |
| Amygdala | −27 | −6 | −12 | L | −.021 [−.032 – −.009] | −3.56 | .25 | .0005 |
| Caudate | 12 | 3 | 21 | R | −.026 [−.040 – −.011] | −3.66 | .26 | .0004 |
| Hippocampus | 21 | −9 | −18 | R | −.019 [−.032, −.006] | −3.01 | .19 | .0023 |
| Hippocampus | −27 | −9 | −12 | L | −.019 [−.031, −.007] | −3.13 | .21 | .0017 |
| Insula | 42 | 9 | −9 | R | −.026 [−.040, −.012] | −3.69 | .26 | .0003 |
| Insula | −45 | 9 | 3 | L | −.029 [−.049 – −.009] | −2.98 | .19 | .0025 |
| Pallidum | −21 | 3 | −3 | L | −.017 [−.028 – −.006] | −3.13 | .20 | .0016 |
| Putamen | −24 | 6 | −3 | L | −.020 [−.032 – −.008] | −3.36 | .18 | .0009 |
| PCL:YV Factor 2 | ||||||||
| Amygdala | −27 | −6 | −12 | L | −.031 [−.049 – −.013] | −3.52 | .22 | < .0006 |
| Hippocampus | −30 | −6 | −15 | L | −.028 [−.043 – −.012] | −3.53 | .25 | .0005 |
| Putamen | −24 | 6 | −3 | L | −.030 [−.049 – −.012] | −3.31 | .25 | .001 |
Note. Hemi = hemisphere of the ROI. β [CI] = the unstandardized peak beta and its confidence interval. r = Pearson’s correlation as a measure of effect size, calculated by sqrt (t2/t2 + df). P-values represent the uncorrected p-value. All areas are significant at p < .05 following a Bonferroni correction.
Factor 2 scores
Regressions indicated there were significant negative associations between PCL:YV Factor 2 scores and hemodynamic activity in the left amygdala, left hippocampus, and left putamen (see Table 3). Supplementary analyses for Factor 2 controlling for length of abstinence led to a non-significant association with the left hippocampus, t(38) = −2.90, p = .006, d = .46, but did not affect the left amygdala following correction, t(38) = −2.99, p = .002, d = .47. Inclusion of covariates did not affect other associations with PCL:YV Factor 2.
Exploratory analyses
We conducted whole brain analyses with the PCL:YV for exploratory purposes. Significant negative associations were found between Total scores and hemodynamic activity to drug-related cues in areas of the amygdala, ACC, insula, precentral gyrus, parahippocampal gyrus, caudate, temporal gyrus, PCC, cuneus, and cerebellum (p < .005, uncorrected, see Table S1 in online supplemental materials). Negative associations with Factor 1 were similar to the ROI analyses already reported, with the inclusion of many areas of the cerebellum.
We conducted additional analyses to examine the associations between traits measured by the PCL:YV and hemodynamic activity related to drug cues using the PCL:YV four-factor model. Investigation of these more homogenous groupings of traits was important for exploring patterns; however, the results should be interpreted with caution because the larger number of tests may inflate Type I error. Splitting Factor 1 into two factors indicated the negative associations were explained by callous-unemotional traits; whereas interpersonal traits had only positive associations and these were only present in two ROIs (posterior cingulate, precuneus; see Table S2 in online supplementary materials). Splitting Factor 2 into two factors indicated the negative associations were strongly due to the antisocial traits; whereas the lifestyle traits had mainly positive associations with activity (see Table S2). Positive associations with the lifestyle traits were in the same regions as the interpersonal traits (e.g., PCC, precunus), but also in some areas that showed negative associations with the Affective and/or Antisocial Factors (e.g., lateral OFC, medial OFC, hippocampus, and caudate). This Lifestyle Factor was also the only factor to be significantly related to youth’s craving ratings; however, it was positively related to craving ratings for both the drug cue, r(38) = .42, p < .010, and the neutral pictures, r(38) = .34, p < .010.
The different pattern of findings for the Affective and Interpersonal factors is somewhat surprising because these PCL:YV factor scores were highly correlated in this sample, r(38) = .63, p < .010. Conversely, the Affective and Antisocial Factors had a strong correlation in this sample of serious substance abusers, r(38) = .52, p < .010, which may explain the greater consistency in the associations with hemodynamic activity for these factors. The discrepancies between the Antisocial and Lifestyle Factors were less surprising because these factors had a weaker correlation in this sample, r(38) = .31, p = < .050.
Discussion
This study investigated whether psychopathic traits moderated the brain’s response to drug cues among high-risk young offenders in a correctional facility with histories of significant substance abuse, which included cocaine and other stimulants. Consistent with the hypotheses, psychopathic traits were negatively associated with engagement of many areas of the limbic and paralimbic systems in response to drug cues, including the ACC, left amygdala, bilateral hippocampus, bilateral insula, and areas of the striatum. Also, consistent with the hypotheses, most of the negative associations were accounted for by Factor 1. This differed from findings reported with adults where Factor 1 as a whole had positive associations with some classic craving regions (e.g., insula; Cope et al., 2014). It is noteworthy that both the Cope et al. (2014) study and our adolescent study reported primarily negative associations between affective traits and hemodynamic activity in response to drug cues, but positive associations with the interpersonal traits. It seems the CU traits overshadowed the interpersonal traits in the adolescent sample, resulting in an overall negative association with Factor 1 whereas the opposite was true with adults.
Counter to the hypotheses, the behavioral features of psychopathy among these adolescents also showed negative association with activation in craving regions, namely, in the left hippocampus, left putamen, and left amygdala. However, effects in the left amygdala washed out after accounting for length of abstinence. Previous findings indicate that Factor 2 had consistently more negative associations in craving regions with adults (Cope et al., 2014) than among this group of adolescents. Exploratory analyses with the adolescent sample using the four-factor model suggested that the antisocial traits had strong negative associations with activity in many craving regions (e.g., ACC, amygdala, hippocampus, insula, OFC), but this may have been overshadowed by the many positive associations with the impulsive lifestyle traits in similar regions (e.g., hippocampus, OFC) when combining the two clusters of traits into Factor 2. This pattern differs from that reported in adults where the combined Factor 2 and both clusters comprising Factor 2 had far more negative associations with neural activity during the craving task. Although the consistencies and inconsistencies between adolescents and adults with respect to findings from the four-factor model are interesting, any interpretation should be done with extreme caution due to the relatively small sample of adolescents here and the potential for inflation of Type I error.
In general, the findings indicated youth with both substance abuse and high scores on the PCL:YV did not have a strong neural craving response, but surprisingly, self-reported experiencing significant craving to drug cues. Exploratory analyses with the four-factor model suggested the high craving ratings were related to only the lifestyle traits of psychopathy. It is unclear why lifestyle traits were related to higher self-reported craving. Especially for this task, for which giving higher ratings required individuals to let the craving bar grow before pressing a button to stop it. One would expect impulsive individuals to report lower craving if their impulsivity was interfering with the task. Of note, research with adults has demonstrated that self-reported craving during these tasks is not associated with relapse but activation in some brain regions is associated with relapse (Kosten et al., 2006). Thus, studies of relapse in youth with serious substance abuse are an important next step.
Previous research with normative adolescents indicates behavioral disinhibition is a strong risk factor for substance abuse (Iacono, Carlson, Taylor, Elkins, & McGue, 1999). Clearly, behavioral disinhibition is also characteristic of psychopathy. The current study of incarcerated youth with serious substance abuse histories indicates that youth with these behavioral features in addition to CU may have different craving reactions as well as utilization of another pathway to substance abuse.
Neural Responses
The amygdala is thought to be responsible for emotional memory and placing emotional significance on associations between relevant stimuli. This area is strongly related to the anticipation and preoccupation with using drugs in animal studies (Koob & Volkow, 2010) and in humans (Franklin et al., 2007), and tends to be hyperactive among adults with stimulant abuse histories in response to drug cues (Childress et al., 1999; Grant et al., 1996). Similarly, the hippocampus is thought to be part of this motivational action system (Koob & Volkow, 2010). The amygdala is under-reactive in psychopaths during moral decision-making (Harenski, Harenski, Shane, & Kiehl, 2010), emotional processing tasks (see Kiehl, 2006 for a review), and exposure to drug cues (Cope et al., 2014). Amygdala dysfunction is thought to be integral to the development of psychopathy (Blair, 2008) and is under-reactive to negative emotional stimuli (see Waller et al., 2015 for a review) and under responsive in reward processing (Cohn et al., 2015; Finger et al., 2011). The hippocampus also is under-reactive in antisocial youth, particularly in response to rewards (Rubia et al., 2009). The negative association between activation in these areas during the craving task and the PCL:YV may be due to those with CU traits not having as strong a compulsion to seek drugs as other substance abusers or may be a result of biochemical differences. Surprisingly, the amygdala and hippocampus were not engaged among the sample of adolescents with substance abuse as a whole in response to drug cues, which is inconsistent with other fMRI studies of addiction including Cope et al.’s (2014) study. Thus, there is a need for a more rigorous examination of developmental differences involved in the craving response in general as well as in diverse sub-groups.
Consistent with adults, adolescent psychopathic traits showed negative association in the ACC; however, in the youth sample this was strongest for Factor 1 traits as opposed to Factor 2. The anterior cingulate is thought to be responsible for cognitive control, conflict monitoring, and avoidance learning (Carter et al., 1999; Kiehl, Liddle, & Hopfinger, 2000). Addiction researchers have postulated that the ACC and OFC underlie addiction because the ACC is responsible for inhibitory control of emotional responses and the OFC is response for signal reward prediction (Volkow et al., 2002). Among adults with cocaine abuse, the ACC tends to be over-reactive when they are presented with drug cues (Maas et al., 1998), and similarly, was over-reactive in the sample of adolescents with substance abuse, and under-reactive in the controls. Conversely, studies of youth with antisocial traits have found decreased activation in the ACC during tasks related to attention (Rubia et al., 2009), rewarded trials (Crowley et al., 2010), and others related to inhibition and emotion (see Hyde, Shaw, & Hariri, 2013; Waller et al., 2015). Cope et al. (2014) postulated that the decreased activation in the ACC for adults higher in psychopathy may be a result of feeling less conflicted than other substance abusers when thinking about using drugs.
The insula tends to be reactive among adults with substance abuse during drug craving and has been suggested as a biomarker to predict relapse (Koob & Volkow, 2010) but it had a significant negative response to drug cues in this sample of adolescents. The activation in the insula during the drug-cue task was not significantly associated with PCL-R total scores in the Cope et al. (2014) study, but clearly demonstrated negative association with both PCL Total and Factor 1 scores among our adolescent sample. Among youth with CU traits and serious behavioral issues, insula dysfunction is thought to be related to their emotional deficits but findings are inconsistent (Waller et al., 2015). It is reasonable to expect psychopathy would modulate responses in the insula because it is related to drive behavior and processing of stimuli that makes us comfortable or uncomfortable, which youth with CU and disruptive behavior are by definition less susceptible to experiencing. Of note, the insula had a negative response to the drug-cue pictures for both controls and the stimulant abuse group.
The pallidum is thought to be critical for further processing of the drug reward signal (Koob & Volkow, 2010) and is over-reactive in these tasks among individuals with drug addiction. Similarly, the caudate and putamen are associated with habit learning and are implicated in substance abuse and reward processing (Koob & Volkow, 2010). Factor 1 was negatively associated with activation in all of these regions. These findings are somewhat consistent with studies of reward processing, which have indicated clinical samples of youth with psychopathic traits are under responsive in the caudate and areas of the ventral striatum (Cohn et al., 2015; Finger et al., 2012). Taken as a whole, youth with psychopathic features, particularly CU traits (where the associations were the strongest), either do not experience the same preoccupation and compulsion for use of drugs that other substance abusers do, or simply have reduced responsiveness in areas of their reward system in general.
It is equally of interest to note which brain regions were not engaged during the craving task among the whole group of substance abusing adolescents, and which regions were not associated with psychopathic traits. Notably, psychopathic traits were not associated with activation in the OFC or areas of the prefrontal cortex in the two-factor ROI analyses or the exploratory whole brain analyses. Substance abuse researchers have shown the OFC to be critical in representing the reward value of stimuli (Hornak et al., 2004) and predictions of rewards (Schultz, Tremblay, & Hollerman, 2000). Therefore, it is strongly implicated in learning or habit-forming associations. In this sample as a whole, areas of the frontal cortex were engaged during cue-exposure but the OFC was not. Similarly, the nucleus accumbens thought to be associated with motivational action among substance abusers was not engaged in the sample as a whole. Exploratory analyses with the four-factor model indicated CU and antisocial traits were negatively associated with activity in the OFC and nucleus accumbens, indicating these regions were engaged. But again, these findings should be interpreted with caution in light of the potential for Type I error with the increasing number of tests.
The findings raise the question about developmental differences in the neural reactivity associated with addiction. The lack of association between psychopathic traits and activation in the prefrontal cortex among our adolescents may be due to developmental differences from psychopathic adults. Among adults, high scores on Factor 2 define a relatively homogenous group of abnormally impulsive and irresponsible individuals. Among adolescents and especially adolescents in institutions, however, scholars have postulated that high scores on Factor 2 (particularly the lifestyle traits) may be more common and less discriminating (Seagrave & Grisso, 2002), perhaps because the brain regions that govern decision-making are still maturing. Indeed, item response theory analyses have indicated these features are less discriminating among adolescents than adults (Vincent, 2002). Moreover, impulsivity and sensation seeking are associated with substance abuse and thus, were evident in the majority of this sample (mean Factor 2 scores were close to 15 out of a possible 18). Thus, the group of adolescents scoring high on Factor 2 could be a more heterogeneous group than in adults, some of which scored high simply as a function of their substance abuse. Moreover, some of the discrepant findings between adults and adolescents, particularly the strength of the association with CU traits, may be an artifact of CU traits accounting for more of the variance in our sample.
Limitations
One of the limitations with using an incarcerated sample for a substance abuse study is that the average length of abstinence was relatively long (approximately 4.6 months with a wide range). Neurological responses to cocaine craving decrease with longer lengths of abstinence, and increase shortly after use of cocaine (O’Brien, Childress, McLellan, & Ehrman, 1992). Although this sample still produced strong activation in response to drug cues and individuals reported significant craving, on average, it is plausible studies of youth with shorter lengths of abstinence, such as a substance abuse treatment sample, would uncover stronger associations between psychopathic traits and craving response. Moreover, use of an incarcerated sample means generally highly impulsive, irresponsible, sensation seeking adolescents. Use of a more normative adolescent sample with greater variability in these features may show a different pattern of associations between Factor 2 and the craving response. Several studies of normative adolescents have identified impulsivity and sensation seeking as two of the personality traits most predictive of substance abuse (Castellanos-Ryan & Conrod, 2012; Conrod, Pihl, Stewart, & Dongier, 2000).
It will be important for future research to determine if our findings are replicable using larger samples of young offenders and different samples of adolescents. Although our serious substance abuse sample was relatively homogenous with respect to relatively high levels of psychopathic traits and fairly extreme substance abuse histories, there was some variation in their drug use that could introduce some confounds. About half of the youth also had histories of opiate dependence. It is very difficult, if not impossible, to identify youth with stimulant abuse histories who have not also abused alcohol, marijuana, and other harder drugs. This does not differ from the Cope et al. (2014) adult sample, however, where there was also a mix of heroin and stimulant abusers. Future research with larger samples of adolescents should attempt to disentangle these effects. Although a sample of 40 participants is acceptable for a neuroimaging study, it is not optimal for including multiple covariates which decreases the likelihood of detecting small effects. Furthermore, we conducted separate analyses using the four-factor model in order to examine the relative contribution of CU traits versus interpersonal traits. As noted, these results are tenuous until research can replicate the findings with a larger sample.
There are some obvious challenges with interpretation of neuropsychological abnormalities in a sample of individuals with substance use histories. One issue is the question of whether the cognitive and affective deficits were preexisting or were a consequence of years of substance abuse. Substance abuse has been linked to abnormal activation in areas related to attentional control and executive function in both adults and adolescents (Volkow et al., 2002; and see Wetherhill & Tapert, 2013 for a review). Research with adults who abused cocaine found cocaine-induced changes at the neurotransmitter level in dopaminergic activity, such as selective decreased metabolic changes in the orbitofrontal and prefrontal cortex and in the basal ganglia (Volkow et al., 1992). One would expect the neurological changes as a result of chronic substance abuse to be much more pronounced in adults than adolescents, making the study of adolescents tantamount to our understanding of craving. Further, some of the differences noted between this study and Cope et al.’s (2014) findings may be a consequence of the years of chronic drug use among their psychopathic adults. We controlled for substance use severity by factoring in age of onset because we expected the duration of abuse to impact neural response.
Implications and Future Directions
There are a few implications of this study that warrant discussion. First, overall, the areas of neuronal activity in this adolescent substance abusing sample in response to drug cues had some differences from adult studies of addiction. Second, the presence of psychopathic traits was strongly correlated with the first age of regular substance use (r = −.39), and was stronger than correlations found with adults (r = 0.21 with number of years of regular use, Cope et al., 2014). This leads to the question of whether some of the behaviors of individuals who abuse substances simply mimic psychopathic features among adolescents. However, studies using more normative samples of adolescents have indicated that impulsivity and sensation seeking precede and predict substance abuse later (Castellanos-Ryan & Conrod, 2012; Conrod et al., 2000). Stimulation-seeking seems to be more important for substance use initiation (Prisciandaro, Korte, McRae-Clark, & Brady, 2012), and impulsivity is more important for transition to compulsive use (Khurana et al., 2015). Fortunately, in this study we found strong modulating effects for the affective features of psychopathy that were opposite of what is expected for substance users. This suggests that youth with both CU traits and behavioral features of psychopathy do not have a strong neural craving response and, therefore, may have different motivations for drug use and relapse than other adolescents who abuse substances.
Another timely implication is that there may be a need for ‘psychopathy-specific’ treatment of substance abuse, at least for youth who get involved with the law. Although they comprise a relatively small percentage of the population, the costs associated with individuals with psychopathy and co-morbid substance use disorders are great. In the offender arena, practitioners and researchers discovered they were unsuccessful in decreasing psychopaths’ offending and violence because the system was treating their behaviors despite their psychopathy rather than working with their psychopathy. The justice field has had some success in changing the violent and offending behaviors of adolescents with high psychopath scores using approaches more tailored to the psychopathic personality (Caldwell, Skeem, Salekin, & Van Rybroek, 2006). Moreover, there is a precedent for successful personality-targeted interventions for alcohol use prevention among adolescents (Concord et al., 2013).
There is a need for further study in a couple of areas to determine whether the design of targeted interventions for youth with CU traits or adults with psychopathy is warranted. First, it will be crucial to study the motivation of adolescents and adults with psychopathic traits for abusing substances. Finding relevant motivators to abstain from drug use that are more specific to youth with CU traits will undoubtedly be helpful and are likely to differ from other stimulant abusers. Second, it will be important to investigate the prevalence of CU traits in adolescent substance abuse treatment settings before one knows how useful such an approach would be outside of incarcerated settings. One crucial consideration in the creation of a psychopathy-specific treatment regimen will be that individuals with these personality traits are less likely to seek treatment than others because of their lack of emotional stress.
Supplementary Material
Acknowledgments
This study was funded by the National Institute of Drug Abuse (NIDA) grant K01 DA026502 (G.M.V., PI), the National Institute of Mental Health (NIMH) grant R01 MH071896 (K.A.K., PI) and National Institute of Child Health and Development (NICHD) grant R01 HD082257-01 (K.A.K, PI)
We are grateful to the staff and clients (and parents) at the Youth Diagnostic and Detention Facility and the New Mexico Youth and Families Department for their support and assistance in making this research possible.
Footnotes
Dr. Vincent, Dr. Cope, Dr. King, Mr. Nyalakanti, and Dr. Kiehl report no biomedical financial interests or potential conflicts of interest.
Ethical Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Anderson DA. The cost of crime. Foundations and Trends in Microeconomics. 2012;7:209–265. doi: 10.1561/0700000047. [DOI] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex: Functional contributions and dysfunction in psychopathy. Philosophical Transactions: Biological Sciences. 2008;363:2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Psychopathic traits from an RDoC perspective. Current Opinion in Neurobiology. 2015;30:79–84. doi: 10.1016/j.conb.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Breiter H, Gollub R, Weisskoff R, Kennedy D, Makris N, Berke J, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/S0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Budhani S, Blair RJR. Probabilistic response reversal in children with psychopathic tendencies: Success is a function of salience of contingency change. Journal of Child Psychology and Psychiatry. 2005;46:972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- Caldwell M, Skeem J, Salekin R, Van Rybroek G. Treatment response of adolescent offenders with psychopathy features: A 2-year follow-up. Criminal Justice & Behavior. 2006;33:571–596. doi: 10.1177/0093854806288176. [DOI] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi: 10.1046/j.1360-0443.1999.9433273.x. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Conrod PJ. Personality and substance misuse: evidence for a four-factor model of vulnerability. In: Verster JBK, Brady K, Galanter M, Conrod PJ, editors. Drug Abuse and Addiction in Medical Illness: Causes, Consequences, and Treatment. New York: Humana/Spring Press; 2012. pp. 47–62. [Google Scholar]
- Cauffman E, Kimonis ER, Dmitrieva J, Monahan KC. A multimethod assessment of juvenile psychopathy: Comparing predictive utility of the PCL:YV, YPI, and NEO PRI. Psychological Assessment. 2009;21:528–542. doi: 10.1037/a0017367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. The Mask of Sanity. 5th. St Louis, MO: Mosby; 1976. [Google Scholar]
- Conrod PJ, Pihl RO, Stewart SH, Dongier M. Validation of a system of classifying female substance abusers on the basis of personality and motivational risk factors for substance abuse. Psychology & Addiction Behavior. 2000;14:243–256. doi: 10.1037//0893-164x.14.3.243. [DOI] [PubMed] [Google Scholar]
- Cope LM, Vincent GM, Jobelius JL, Nyalakanti PK, Calhoun V, Kiehl KA. Psychopathic traits modulate brain responses to drug cues in incarcerated offenders. Frontiers in Human Neuroscience. 2014;8:1–16. doi: 10.3389/fnhum.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, et al. Risky decisions and their consequences: Neural processing by boys with antisocial substance disorder. PLoS One. 2010;5:e12835, 1–20. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Skelly LR, Kiehl KK. Brain response to empathy- eliciting scenarios involving pain in incarcerated individuals with psychopathy. JAMA Psychiatry. 2013;70:638–645. doi: 10.1001/jamapsychiatry.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry. 2011;168:152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BC, Tant AS, Tremba K, Kiehl KA. Assessment of psychopathic traits in an incarcerated adolescent sample: A methodological comparison. Journal of Abnormal Child Psychology. 2012;40(6):971–986. doi: 10.1007/s10802-012-9614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth A, Kosson D, Hare R. The Psychopathy Checklist: Youth Version. Toronto, Ontario: Multi-Health Systems; 2003. [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions on Medical Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Frick PJ. Developmental pathways to conduct disorder. Child and Adolescent Psychiatric Clinics of North America. 2006;15(2):311–331. doi: 10.1016/j.chc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of drug use and its association with DSM-IV drug abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1998;10:163–173. doi: 10.1016/S0899-3289(99)80131-X. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R. The Hare Psychopathy Checklist. Toronto, Ontario: Multi-Health Systems; 1991. [Google Scholar]
- Hare R. The Hare Psychopathy Checklist-Revised. Toronto, Ontario: Multi-Health Systems; 2003. [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. Journal of Abnormal Psychology. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill JF, Hart SD, Hare RD. Psychopathy and substance use. Journal of Personality Disorders. 1994;8:169–180. [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls E, Morris R, Bullock PR, et al. Reward-related reversal learning after surgical excisions in orbitofrontal and dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;3:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Hariri AR. Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: Review, integration, and directions for research. Developmental Review. 2013;33:168–223. doi: 10.1016/j.dr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Developmental Psychopathology. 1999;1:869–900. doi: 10.1017/S0954579499022369. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Experimentation versus progression in adolescent drug use: A test of an emerging neurobehavioral imbalance model. Development and Psychopathology. 2015;27:901–913. doi: 10.1017/S0954579414000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. doi: 10.1111/1469-8986.3720216. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Hoffman MB. The criminal psychopath: History, neuroscience, treatment, and economics. Jurimetrics. 2011;51:355–397. [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:64–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistico AMR, Salekin RT, DeCoster J, Rogers R. A large-scale meta-analysis relating the Hare measures of psychopathy to antisocial conduct. Law and Human Behavior. 2008;32:28–45. doi: 10.1107/s10979-007-9096-6. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Mailloux D, Forth A, Kroner D. Psychopathy and substance abuse in adolescent male offenders. Psychological Reports. 1997;80:529–530. [PubMed] [Google Scholar]
- Marsh AA, Finger E, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz ITN, Schechter JC, et al. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. Journal of Child Psychology & Psychiatry. 2013;54:900–910. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P, Hoeft F, Glover GH, Reiss AL. Methods and software for fMRI analysis for clinical subjects. Poster presented at the annual meeting of the Organization for Human Brain Mapping; San Francisco, CA. 2009. Jun, [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Girssom G, et al. The fifth edition of the addiction severity index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-S. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. The Journal of Neuroscience. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CS, Kosson DS, Forth AE, Hare RD. Factor structure of the Hare Psychopathy Checklist: Youth Version (PCL: YV) in incarcerated adolescents. Psychological Assessment. 2006;18:142–154. doi: 10.1037/1040-3590.18.2.142. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Annals of the New York Academy of Sciences. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, Koenigs M. Altered resting-state functional connectivity in cortical networks in psychopathy. Journal of Neuroscience. 2015;35:6068–6078. doi: 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquero AR, Farrington DP, Fontaine N, Vincent GM, Coid J. Childhood Risk, Offending Trajectories, and Psychopathy at Age 48 Years (in the Cambridge Study in Delinquent Development) Psychology, Public Policy, & the Law. 2012;18:577–598. doi: 10.1037/a0027061. [DOI] [Google Scholar]
- Prisciandaro JJ, Korte JE, McRae-Clark AL, Brady KT. Associations between behavioral disinhibition and cocaine use history in individuals with cocaine dependence. Addictive Behaviors. 2012;37:1185–1188. doi: 10.1016/j.addbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger R, Salmeron B, Ross T, Amen S, Sanfilipo M, Hoffmann R, et al. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage. 2005;26:1097–108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robins LN. Etiological implications in studies of childhood histories relating to antisocial personality. In: Hare RD, Schalling D, editors. Psychopathic behavior: Approaches to research. Chichester, England: Wiley; 1978. pp. 255–271. [Google Scholar]
- Roussey S, Toupin J. Behavioral inhibition deficits in juvenile psychopaths. Aggressive Behavior. 2000;26:413–424. doi: 10.1002/1098-2337(200011)26:6<413::AID-AB1>3.0.CO;2-Q. [DOI] [Google Scholar]
- Rubia K, Smith A, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. American Journal of Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rutherford MJ, Alterman AI, Cacciola JS, McKay JR. Validity of the Psychopathy Checklist-Revised in male methadone patients. Drug and Alcohol Dependence. 1997;44:143–149. doi: 10.1016/s0376-8716(96)01329-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seagrave D, Grisso T. Adolescent development and the measurement of juvenile psychopathy. Law and Human Behavior. 2002;26:219–239. doi: 10.1023/A:1014696110850. [DOI] [PubMed] [Google Scholar]
- Smith S, Newman J. Alcohol and drug abuse/dependence in psychopathic and nonpsychopathic criminal offenders. Journal of Abnormal Psychology. 1990;99:430–439. doi: 10.1037/0021-843X.99.4.430. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steadman HJ, Silver E, Monahan J, Appelbaum PS, Robbins PC, Mulvey EP, et al. A classification tree approach to the development of actuarial violence risk assessment tools. Law and Human Behavior. 2000;25:173–80. doi: 10.1023/A:1005478820425. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, et al. Brain circuits involved in emotional learning in antisocial behaviour and social phobia in humans. Neuroscience Letters. 2002;328:233–236. doi: 10.1016/S0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, DeBrito SA, et al. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous– unemotional traits. American Journal of Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Vincent GM. The Legitimacy of Psychopathy Assessments in Young Offenders: Contributions of Item Response Theory. Simon Fraser University; Burnaby, BC: 2002. Unpublished doctoral dissertation. [Google Scholar]
- Vincent GM. Psychopathy and violence risk assessment in youth. Child Psychiatric Clinics of North America. 2006;15(2):407–428. doi: 10.1016/j.chc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Vincent GM, Kimonis E, Clark A. Juvenile psychopathy: Appropriate and inappropriate uses in legal proceedings. In: Heilbrun K, DeMatteo D, Goldstein N, editors. APA Handbook of Psychology and Juvenile Justice. Washington, DC: APA Books; 2015. pp. 197–232. [DOI] [Google Scholar]
- Volkow ND, Fowler JS, Wang G, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow N, Hitzemann R, Wang G, Fowler J, Wolf A, Dewey S, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Ding YS, Logan J, et al. Relationship between psychostimulant induced “high” and dopamine transporter occupancy. Proceedings of the National Academy of Sciences USA. 1996;93:10388–10392. doi: 10.1073/pnas.93.19.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Murray L, Dotterer HL, Hyde LW. Neuroimaging approaches to understanding youth antisocial behavior. In: Toga AW, editor. Brain Mapping: An Encyclopedic Reference. Vol. 3. Academic Press; Elsevier; 2015. pp. 1001–1009. [DOI] [Google Scholar]
- Walsh Z, Allen LC, Kosson DS. Beyond social deviance: Substance use disorders and the dimensions of psychopathy. Journal of Personality Disorders. 2007;21:273–288. doi: 10.1521/pedi.2007.21.3.273. [DOI] [PubMed] [Google Scholar]
- Wetherhill R, Tapert SF. Adolescent brain development, substance use, and psychotherapeutic change. Psychology of Addiction Behavior. 2013;27:393–402. doi: 10.1037/a0029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Fowler KA, Sinclair S, Schechter JC, Majestic CM, Pine DS, et al. Disrupted expected value signaling in youth with disruptive behavior disorders to environmental reinforcers. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:579–588.e9. doi: 10.1016/j.jaac.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal Responses to cocaine cues: A neuro-cognitive analysis. Nature Neuroscience. 2004;3:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


