Abstract
The p53 transcription factor is mutated in over half of human cancers, and p53-null mice are highly predisposed to cancer, highlighting p53’s essential role in tumor suppression. Studies in mouse models have revealed that p53 cell cycle arrest and apoptosis responses to acute DNA damage signals are dispensable for tumor suppression, prompting a search for new mechanisms underlying p53-mediated cancer suppression. p53 responds to other types of stress signals and regulates a host other cellular processes, including maintenance of genomic stability, metabolism, stemness, non-apoptotic cell death, migration/invasion, and cell signaling, any or all of which could be fundamental for suppressing carcinogenesis. The ability of p53 to govern numerous transcriptional programs and cellular functions likely explains its potent tumor suppressor activity.
Introduction
The gene encoding the p53 transcription factor is mutated in over half of all human cancers, reflecting its crucial role in preventing cancer [1]. This key role as a tumor suppressor is supported by observations from mouse models, where p53 inactivation results in a rapid, fully-penetrant tumor predisposition [2]. Early studies on p53 revealed that it plays a fundamental role in stress responses, especially in triggering cell cycle arrest or apoptosis in response to acute DNA damage signals [1,3]. The p53-mediated cell cycle arrest response was envisaged to allow cells an opportunity to arrest to repair DNA damage before proceeding through the cell cycle and to thereby prevent the propagation of oncogenic mutations, while the apoptotic response was proposed to eliminate damaged or neoplastic cells. p53 was shown to trigger these responses by inducing specific downstream target genes including the CDK inhibitor p21, and the pro-apoptotic Bcl-2 family members Puma and Noxa, which are important for DNA-damage-induced cell cycle arrest and apoptosis, respectively [1,3]. These responses provided reasonable initial explanations for the mechanisms underlying p53-mediated tumor suppression. In support of such mechanisms, evidence from various mouse tumor models has suggested that p53 restricts proliferation and triggers apoptosis in developing tumors [reviewed in 3]. As will be described in this review, however, the picture of p53-mediated tumor suppression is much more complex than envisaged originally.
p53 Acute DNA Damage Responses Are Dispensable for Tumor Suppression
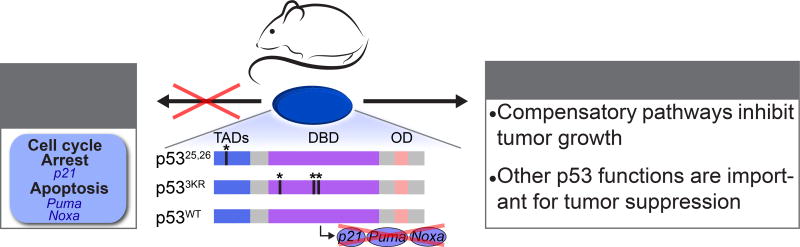
In recent years, a series of studies interrogating the requirement of p53 acute DNA damage programs for cancer suppression has challenged the importance of these responses for p53-mediated tumor suppression. The first set of such studies used mice expressing temporally-regulatable versions of p53 to demonstrate that the presence of p53 during exposure to acute DNA damage is dispensable for inhibiting tumorigenesis, and that instead the ability of p53 to respond to oncogenic signals as tumors are developing is most fundamental for tumor suppression [4–6]. This idea was elaborated upon by analyses of p53 knock-in mouse strains expressing mutants in the first, second or both of two p53 transcriptional activation domains (TADs). Analysis of the TAD1/2 p5325,26,53,54 mutant showed first that transcriptional activation potential is critical for p53-mediated tumor suppression [7]. Moreover, these studies revealed a selective activity of the p5325,26 TAD1 mutant, which cannot mount responses to acute DNA damage yet is completely effective as a tumor suppressor, indicating that the capacity of p53 to drive cell cycle arrest or apoptosis in response to acute genotoxic stress is dispensable for tumor suppression and that the transcriptional programs responsible for acute DNA damage responses and tumor suppression are distinct. This notion was supported by the generation of another mouse knock-in strain expressing the p533KR mutant, in which 3 acetylation sites in the p53 DNA binding domain were mutated, rendering the protein unable to induce classical p53 target genes and responses to DNA damage [8]. This protein was nonetheless able to suppress spontaneous tumor development in mice. Finally, an additional study focused on the key effectors for DNA-damage-induced cell cycle arrest and apoptosis – p21, Noxa, and Puma [9]. Mice lacking these three proteins displayed defective DNA damage responses yet again were not prone to spontaneous tumor development. Collectively, these studies prompted a paradigm shift in the field, as they questioned the significance of the p53 responses to acute DNA damage for tumor suppression, and opened the door to the search for new mechanisms underlying p53-mediated tumor suppression.
If Not Acute DNA Damage Responses, Then What?
If the p53 pathways important for acute DNA damage responses are nonessential for tumor suppression, then how does p53 work? As mentioned, there is ample evidence from various mouse tumor models that p53 inhibits cell division and induces apoptosis in tumors in vivo, yet the aforementioned studies suggest that p53-dependent acute DNA damage responses and tumor suppression can be uncoupled. How can these discrepancies be reconciled? We will consider several potential explanations, which are not necessarily mutually exclusive (Figure 1):
Plasticity in p53 pathways. It may be that when p53 DNA damage response pathways are perturbed, compensatory pathways allow p53 to still function to suppress cancer. Thus, in such a scenario, the DNA damage response pathways are not unimportant, but there is sufficient robustness in the system to compensate for their disruption. Importantly, cell cycle arrest and apoptosis responses to acute DNA damage are effectively perturbed in the models described above, thus any compensation in developing tumors would have to rely on other pathways.
Cell-type or context-specific differences. It may be that classical p53 DNA damage response pathways are unimportant in some contexts, but are essential for the suppression of some tumor types driven by specific initiating events or originating in particular tissues. Indeed, p21 and Puma are required for tumor suppression in certain instances, although even in these cases, they only account for a fraction of p53 activity [10,11].
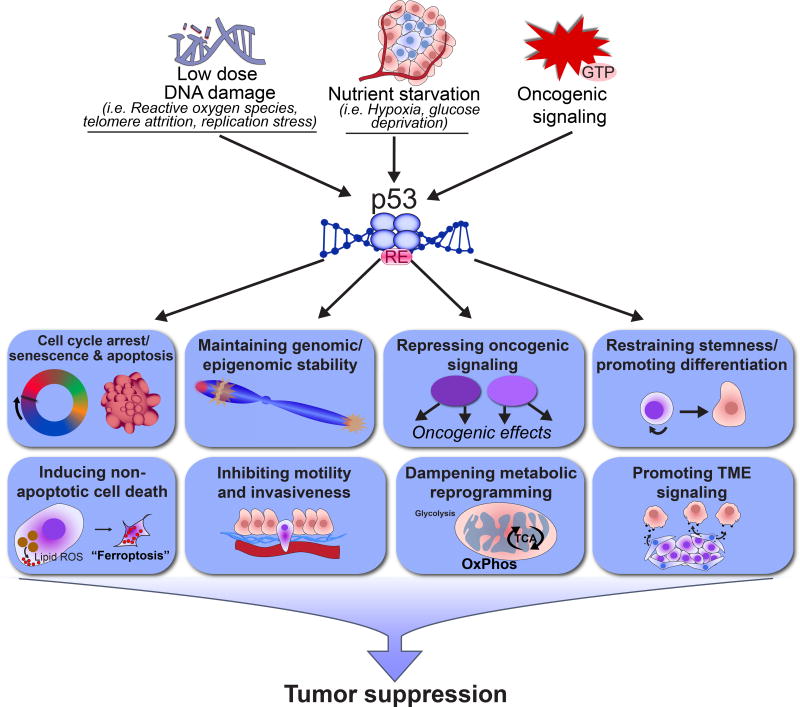
Importance of other non-canonical p53 activities for tumor suppression. It may be that acute DNA damage response pathways are truly dispensable for tumor suppression per se, and that instead, other p53 signaling pathways or p53 cellular functions are more critical for tumor suppression (Figure 2). For example, activation of p53 by stress signals more relevant to the tumor microenvironment, such as chronic low-dose DNA damage or hypoxia could be relevant to tumor suppression (Box 1). It could also be that the ability of p53 to regulate other cellular processes, such as genomic stability, metabolism, and stemness is most critical for tumor suppression (see below). However, it is important to note that p53-mediated regulation of cellular processes must ultimately impact tumor growth, and therefore would be expected to have an effect on proliferation and survival, but presumably through transcriptional networks distinct from those used during acute DNA damage responses. This concept is consistent with the observed effects of p53 on proliferation index and cell viability in tumor models in vivo [reviewed in 3]. In addition, the fact that p53, rather than individual p53 target genes, is so commonly mutated in cancer suggests that p53 likely controls a multitude of cellular programs that coordinately suppress cancer. We will briefly summarize what is known about these other non-canonical functions next, to highlight the variety of cellular processes p53 can regulate.
Figure 1. p53 acute DNA damage responses and tumor suppression.
Analysis of different p53 mouse strains, including the p53 transactivation domain 1 Trp5325,26 mutant strain, the acetylation site Trp533KR mutant strain, and the triple knockout p21−/−;uma−−;Noxa−/− strain, demonstrated that p53-induced cell cycle arrest and apoptosis responses to acute DNA damage are dispensable for tumor suppression. Instead, there may be compensatory pathways that are engaged in the absence of acute DNA damage response pathways to allow tumor suppression. Alternatively, acute DNA damage response pathways may be truly dispensable for tumor suppression, and other p53 signaling pathways and downstream p53 functions are responsible for p53 tumor suppressor activity. TAD- transactivation domain, DBD- DNA binding domain, OD- oligomerization domain.
Figure 2. Overview of p53 signaling pathways in tumor suppression.
Different stress stimuli relevant to tumor development in vivo, such as chronic low-dose DNA damage, nutrient starvation, and oncogenic signaling activate p53. In response to such signals, p53 binds to specific DNA response elements (REs) and regulates gene expression programs to modulate different cellular processes, thereby leading to tumor suppression.
Box 1. p53 Responses to Chronic Stress Signals.
Many studies have focused on understanding p53 responses to acute DNA damage and the short-term effects on cell cycle progression or survival in the face of such potent stress signals. Such studies have defined p21 as a key mediator of cell cycle arrest and Puma and Noxa as critical mediators of apoptosis. While these approaches may be very relevant for addressing mechanisms of therapeutic responses, they may not be the best strategy for understanding p53 function in tumor suppression, where chronic, lower-dose stresses may be more relevant for eliciting p53 responses in nascent tumors. These include cell-intrinsic stresses such as low-dose chronic DNA damage resulting from telomere attrition, accumulation of ROS, and replication fork collapse, all of which can emerge as incipient cancers proliferate. Indeed, the idea that p53 does respond to some type of DNA damage during tumor suppression is consistent with studies detailing the activation of DNA damage cascades during the genesis of human tumors [46]. p53 may also be activated by cell-extrinsic microenvironmental stresses such as hypoxia and nutrient starvation, which can be triggered by the abnormal vascularization of tumors.
Some evidence supports the idea that in conditions of chronic stress, p53 may act through pathways distinct from those mapped in studies of responses to acute genotoxic stress. Specifically, comparative analyses of the mechanisms of p53-induced cell cycle arrest in response to acute and chronic DNA damage revealed that the p19Arf protein is critical only in the latter case, highlighting mechanistic differences in the pathways [47]. In addition, hypoxia-induced, p53-dependent apoptosis has been proposed to rely predominantly on p53 repression function [48]. While p53 target genes involved in chronic responses remain to be interrogated, these studies underscore the idea that acute and chronic responses have disparate aspects. In future studies, it will be crucial to map pathways of p53 action in contexts most closely recapitulating p53 tumor suppressor function in vivo.
An Array of Cellular Functions Regulated by p53
Beyond inducing cell cycle arrest, senescence, and apoptosis in response to acute DNA damage, p53 also regulates various other aspects of cellular behavior (Figure 2). Given the plethora of target genes that p53 regulates, the ability of p53 to control many cellular processes is anticipated, and the coordinate regulation of many different gene expression programs presumably underlies p53’s potent tumor suppressor activity. Specifically, p53 has been implicated in the following processes:
Maintaining Genomic Stability
p53 has long been known as the “guardian of the genome” based on its role in acute DNA damage responses. However, beyond this role, an explosion of recent studies has shown that p53 can maintain genomic integrity through additional mechanisms. First, p53 transactivates various DNA repair genes and directly controls different forms of DNA repair, including mismatch repair, base excision repair, and nucleotide excision repair [12]. Second, p53 responds to abnormal ploidy by driving cell cycle arrest, which unlike DNA damage-induced arrest, is triggered by the Hippo pathway, yet is still mediated at least in part via p21 [13]. Without p53, tetraploid cells continue to divide, giving rise to cells with whole-chromosome aneuploidies and structural rearrangements, fueling carcinogenesis. Third, recent reports suggest that p53 promotes genomic integrity preemptively by enhancing DNA replication fork progression, as p53 deficiency provokes replication fork collapse and genomic instability [14,15]. Different mechanisms have been proposed for this p53 function, one linked to transactivation of Mdm2, which itself promotes replication fork progression [14], and the other to p53 preventing DNA topological stress caused by transcription, which perturbs fork progression [15]. Finally, a number of studies have suggested that p53 preserves genomic integrity by restricting the movement of transposons and repetitive elements, which might reflect an evolutionary function of p53 [16,17]. Accordingly, it was shown recently that in Drosophila or zebrafish lacking p53, as well as in p53-deficient cancers, retrotransposon expression is de-repressed. While not well-understood at the molecular level, one experiment suggested that p53 induces H3K9me3 marks to dampen retrotransposon expression. Notably, the inability of p53 human tumor-derived mutants to restrain mobile element expression bolsters the idea that this activity could represent a key tumor suppression mechanism. Important in vivo support for the idea that loss of p53-enforced genome stabilization promotes cancer comes from studies of p53-null mice. These mice primarily develop thymic lymphomas, which display a high frequency of copy number variations that drive specific oncogenic events important for tumorigenesis [18].
Maintaining Epigenetic Stability
p53 has also been described as a guardian of the epigenome [19,20]. Recent studies in embryonic stem (ES) cells showed that p53 represses the de novo DNA methyltransferases Dnmt3a and 3b and activates Tet1 and Tet2, enzymes that promote DNA demethylation. p53 contributes to DNA methylation homeostasis and preserves clonal homogeneity, as p53-deficient ES cells exhibited globally elevated DNA methylation as well as enhanced intra-clonal heterogeneity. Similar global hypermethylation in thymii of thymic lymphoma-prone p53-null mice supports the idea that such destabilization of the epigenome could contribute to tumorigenesis [20].
Dampening Metabolic Reprogramming
Cancer cells typically undergo metabolic reprogramming to ensure adequate production of essential building blocks fundamental for rapid growth [21]. p53 dampens this cellular rewiring by inhibiting glycolysis through repression of genes such as the Glut1 and Glut4 glucose transporters and by promoting mitochondrial oxidative phosphorylation via activation of genes such as Sco2 [22]. p53 also helps to limit a damaging oxidative environment, by inducing genes involved in antioxidant programs, including sestrins. Notably, the aforementioned tumor suppression-competent p533KR mutant retains the capacity to activate certain metabolic and antioxidant target genes, supporting the idea that these functions could contribute to p53-mediated tumor suppression [8]. p53 further promotes cellular homeostasis by inducing autophagy, which results in degradation of damaged organelles and proteins, and has been associated with transformation suppression [23]. Importantly, the ability of p53 to regulate metabolism also helps to enhance cell survival, thus the full complexity of the p53 metabolic role in cancer remains to be elucidated [22].
Restraining Stemness/Promoting Differentiation
p53 limits a variety of aspects of stem cell behavior, including proliferation and self-renewal, and enhances differentiation [24,25]. The significance of this program in cancer is underscored by the observation that p53 mutation promotes a stem cell signature in breast and lung cancers [26]. In addition, p53 limits cellular reprogramming, as in the generation of induced pluripotent stem cells (iPSCs) from differentiated cells, again supporting the idea that an aspect of p53 tumor suppressor function is to restrain plasticity and promote differentiation [27]. Indeed, p53 mutations similar to those arising in cancer are observed during the generation of human iPSCs in culture [28]. Beyond acting through the target genes p21 and miR34a [29,30], p53 can also restrict cellular plasticity through another target gene, the non-coding RNA Neat1. Consistent with observations that Neat1 is induced during differentiation in various cell types [31], recent studies have revealed that Neat1 safeguards the proper expression of differentiation programs and blocks transformation of pancreatic acinar and ductal cells into premalignant lesions in a mouse pancreatic cancer model [32].
Inducing Non-apoptotic Cell Death
Efforts to elucidate how the tumor suppression-competent p533KR mutant might suppress cancer led to the identification of ferroptosis, an iron-dependent, non-apoptotic form of cell death as a potential mechanism for p53-mediated tumor suppression [33]. Like wild-type p53, p533KR suppresses the expression of SLC7A11, a cystine/glutamate antiporter whose inhibition triggers ferroptosis by causing reduced glutathione production and a consequent accumulation of detrimental lipid ROS. While p533KR can induce ferroptosis and suppress cancer, p534KR, a mouse p53 mutant bearing an additional lysine mutation in residue 98, cannot induce ferroptosis or suppress xenograft tumor growth, correlating ferroptosis and tumor suppression [34]. Moreover, a p53 polymorphic variant, p53S47, is deficient in regulating ferroptosis genes, inducing ferroptosis, and suppressing tumorigenesis, again linking these processes [35]. However, other studies suggest that p53 may delay, rather than promote, ferroptosis, suggesting a potential context-dependency to this pathway [36].
Inhibiting Motility and Invasiveness
p53 restricts migration and invasion in in vitro systems, suggesting a potential role for p53 in impeding malignant progression [37]. One mechanism through which p53 inhibits migration is by attenuating epithelial-mesenchymal transition (EMT) by transactivating miR-200c and miR34a, microRNAs capable of silencing EMT drivers, including Zeb1 and Snail [38,39]. In addition, p53 restrains RhoA/ROCK signaling, which is critical for migration and invasion, through transcriptional activation of RhoE and Notch [37]. p53 also hinders the formation of invadopodia, structures that degrade the extracellular matrix to permit invasion, by transactivating miR-143 and Cald1 [37]. Interestingly, mouse models suggest that lung tumors are more metastatic without p53, but that additional stochastic events beyond p53 loss must occur to enable metastasis [40]. Thus, additional studies are needed to better understand p53 function in these processes in different in vivo contexts.
Promoting TME Signaling
p53 stimulates the secretion of molecules that direct changes in the tumor microenvironment. For example, p53 signals to immune cells to infiltrate tumors and assist with tumor suppression by attacking and clearing tumor cells [41,42]. In addition, p53 inhibits angiogenesis by such mechanisms as transactivating Thrombospondin1, thus attenuating tumor growth and metastasis [43].
Repressing Oncogenic Signaling
One potential mechanism through which p53 might exert pleiotropic effects on cell behavior is through regulation of pathways that themselves exert widespread effects. For example, p53 has been shown to restrain the oncoprotein c-myc, through activation of miR-145 [44]. Recent studies have also demonstrated that p53 transactivates Ptpn14 to inhibit the oncoprotein Yap, a Hippo pathway effector that promotes various pro-tumorigenic phenotypes such as proliferation, migration and invasion [45].
Integrating the Pieces of the Puzzle
The aforementioned studies demonstrate that p53 regulates many processes that could in principle contribute to tumor suppression (Figure 3). Understanding the relative contributions of these different effects of p53 to tumor suppression requires the identification of specific p53 target genes involved in each of these pathways and a genetic interrogation of such components for cancer suppression. Adding to the complexity of deciphering tumor suppression pathways is the notion that p53 governs a coordinated program in which multiple functions are co-regulated to enforce tumor suppression, a compelling hypothesis but one that is difficult to test from a technical point of view. If p53 does indeed activate various cooperating pathways to impede tumorigenesis, then its loss would have highly pleiotropic effects and explain why p53 is so commonly mutated in cancer, while mutations in p53 target genes are less frequent. It is also possible that there may be tissue-specificity in terms of which p53-regulated responses are most crucial for tumor suppression in a given setting, requiring analyses to be performed in a diversity of tumor contexts. Deconvoluting the molecular basis for p53-mediated tumor suppression thus remains a complex but fascinating endeavor which will ultimately fuel new therapeutic development.
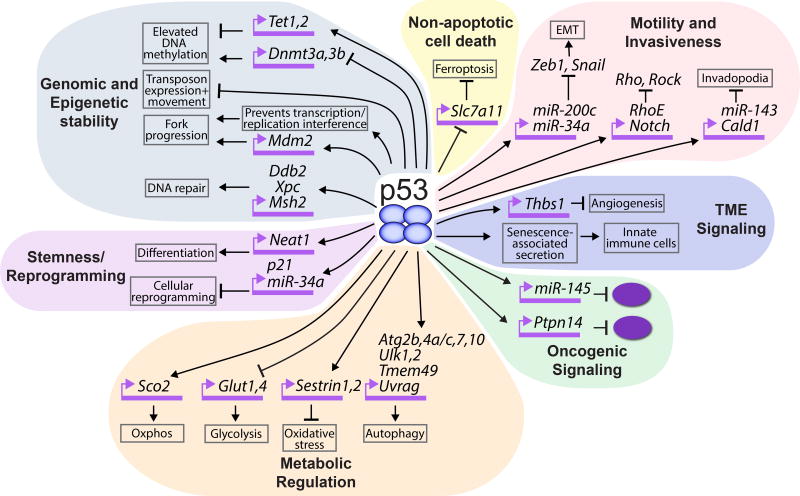
Figure 3. Detailed schematic of non-canonical p53-regulated cellular processes and p53 target genes implicated in these processes.
The various non-canonical p53-modulated cell biological processes that may contribute to tumor suppression are depicted. The figure focuses on p53-regulated pathways described in the text and some direct p53 target genes involved in these pathways; it is not meant to depict every reported gene that may participate in each pathway.
Highlights.
p53 responses to acute DNA damage signals are dispensable for tumor suppression.
p53 transcriptional activity is critical for tumor suppression.
p53 responds to diverse stresses and regulates a variety of cellular processes.
Tumor suppression likely relies on p53 coordinately regulating various processes.
Acknowledgments
We thank Anthony Boutelle, Kathryn Bieging-Rolett, Alyssa Kaiser, and Liz Valente for critical reading of the manuscript. We apologize to those whose work was not cited due to space constraints. This work was supported by an NCI R35 CA197591 grant to L.D.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lane D, Levine A. p53 Research: the past thirty years and the next thirty years. Cold Spring Harb Perspect Biol. 2010;2:a000893. doi: 10.1101/cshperspect.a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia PB, Attardi LD. Illuminating p53 function in cancer with genetically engineered mouse models. Semin Cell Dev Biol. 2014;27:74–85. doi: 10.1016/j.semcdb.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinkal G, Parikh N, Donehower LA. Timed Somatic Deletion of p53 in Mice Reveals Age-Associated Differences in Tumor Progression. PLOS ONE. 2009;4:e6654. doi: 10.1371/journal.pone.0006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Efeyan A, Garcia-Cao I, Herranz D, Velasco-Miguel S, Serrano M. Tumour biology: Policing of oncogene activity by p53. Nature. 2006;443:159–159. doi: 10.1038/443159a. [DOI] [PubMed] [Google Scholar]
- 6.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 7.Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, Broz DK, Basak S, Park EJ, McLaughlin ME, et al. Distinct p53 Transcriptional Programs Dictate Acute DNA-Damage Responses and Tumor Suppression. Cell. 2011;145:571–583. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor Suppression in the Absence of p53-Mediated Cell-Cycle Arrest, Apoptosis, and Senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valente Liz J, Gray Daniel HD, Michalak Ewa M, Pinon-Hofbauer J, Egle A, Scott Clare L, Janic A, Strasser A. p53 Efficiently Suppresses Tumor Development in the Complete Absence of Its Cell-Cycle Inhibitory and Proapoptotic Effectors p21, Puma, and Noxa. Cell Reports. 2013;3:1339–1345. doi: 10.1016/j.celrep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci U S A. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valente LJ, Grabow S, Vandenberg CJ, Strasser A, Janic A. Combined loss of PUMA and p21 accelerates c-MYC-driven lymphoma development considerably less than loss of one allele of p53. Oncogene. 2016;35:3866–3871. doi: 10.1038/onc.2015.457. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat Rev Mol Cell Biol. 2005;6:44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- 13.Ganem Neil J, Cornils H, Chiu S-Y, O’Rourke Kevin P, Arnaud J, Yimlamai D, Théry M, Camargo Fernando D, Pellman D. Cytokinesis Failure Triggers Hippo Tumor Suppressor Pathway Activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Klusmann I, Rodewald S, Müller L, Friedrich M, Wienken M, Li Y, Schulz-Heddergott R, Dobbelstein M. p53 Activity Results in DNA Replication Fork Processivity. Cell Reports. 2016;17:1845–1857. doi: 10.1016/j.celrep.2016.10.036. This paper reports that p53 activation can enhance replication fork progression, a mechanism through which p53 can promote genomic stability. [DOI] [PubMed] [Google Scholar]
- 15•.Yeo CQ, Alexander I, Lin Z, Lim S, Aning OA, Kumar R, Sangthongpitag K, Pendharkar V, Ho VH, Cheok CF. p53 Maintains Genomic Stability by Preventing Interference between Transcription and Replication. Cell Rep. 2016;15:132–146. doi: 10.1016/j.celrep.2016.03.011. This paper describes a new function of p53- the coordination between transcription and replication - which is important for promoting normal replication fork progression. [DOI] [PubMed] [Google Scholar]
- 16••.Wylie A, Jones AE, D'Brot A, Lu W-J, Kurtz P, Moran JV, Rakheja D, Chen KS, Hammer RE, Comerford SA, et al. p53 genes function to restrain mobile elements. Genes & Development. 2015;30:64–77. doi: 10.1101/gad.266098.115. Using Drosophila, zebrafish, and human cancer cells, Wylie et al. show that p53 can restrict retrotransposon activity, thereby preserving genome integrity and suggesting a mechanism for tumor suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine AJ, Ting DT, Greenbaum BD. P53 and the defenses against genome instability caused by transposons and repetitive elements. Bioessays. 2016;38:508–513. doi: 10.1002/bies.201600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudgeon C, Chan C, Kang W, Sun Y, Emerson R, Robins H, Levine AJ. The evolution of thymic lymphomas in p53 knockout mice. Genes & Development. 2014;28:2613–2620. doi: 10.1101/gad.252148.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tovy A, Spiro A, McCarthy R, Shipony Z, Aylon Y, Alton K, Ainbinder E, Furth N, Tanay A, Barton M, et al. p53 is essential for DNA methylation homeostasis in naïve embryonic stem cells, and its loss promotes clonal heterogeneity. Genes & Development. 2017;31:959–972. doi: 10.1101/gad.299198.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park IY, Sohn BH, Choo JH, Joe CO, Seong JK, Lee YI, Chung JH. Deregulation of DNA methyltransferases and loss of parental methylation at the insulin-like growth factor II (Igf2)/H19 loci in p53 knockout mice prior to tumor development. J Cell Biochem. 2005;94:585–596. doi: 10.1002/jcb.20263. [DOI] [PubMed] [Google Scholar]
- 21.Ward Patrick S, Thompson Craig B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- 23.Kenzelmann Broz D, Mello SS, Bieging KT, Jiang D, Dusek RL, Brady CA, Sidow A, Attardi LD. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes & Development. 2013;27:1016–1031. doi: 10.1101/gad.212282.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivos DJ, Mayo LD. Emerging Non-Canonical Functions and Regulation by p53: p53 and Stemness. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17121982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConnell AM, Yao C, Yeckes AR, Wang Y, Selvaggio AS, Tang J, Kirsch DG, Stripp BR. p53 Regulates Progenitor Cell Quiescence and Differentiation in the Airway. Cell Rep. 2016;17:2173–2182. doi: 10.1016/j.celrep.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno H, Spike BT, Wahl GM, Levine AJ. Inactivation of p53 in breast cancers correlates with stem cell transcriptional signatures. Proc Natl Acad Sci U S A. 2010;107:22745–22750. doi: 10.1073/pnas.1017001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krizhanovsky V, Lowe SW. Stem cells: The promises and perils of p53. Nature. 2009;460:1085–1086. doi: 10.1038/4601085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkle FT, Ghosh S, Kamitaki N, Mitchell J, Avior Y, Mello C, Kashin S, Mekhoubad S, Ilic D, Charlton M, et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017 doi: 10.1038/nature22312. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YJ, Lin C-P, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol. 2011;13:1353–1360. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagawa S, Hirose T. Paraspeckle nuclear bodies--useful uselessness? Cell Mol Life Sci. 2012;69:3027–3036. doi: 10.1007/s00018-012-0973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Mello SS, Sinow C, Raj N, Mazur PK, Bieging-Rolett K, Broz DK, Imam JFC, Vogel H, Wood LD, Sage J, et al. Neat1 is a p53-inducible lincRNA essential for transformation suppression. Genes & Development. 2017;31:1095–1108. doi: 10.1101/gad.284661.116. In this paper the authors identify Neat1 as a p53-induced lincRNA and demonstrate that Neat1 deficiency enhances transformation. Neat1 function is associated with its ability to preserve differentiation in oncogene-expressing cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Jiang L, Kon N, Li T, Wang S-J, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. This article shows that p53 can induce ferroptosis, an alternative cell death mechanism that could contribute to tumor suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao Y, Gu W. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016;17:366–373. doi: 10.1016/j.celrep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jennis M, Kung CP, Basu S, Budina-Kolomets A, Leu JI, Khaku S, Scott JP, Cai KQ, Campbell MR, Porter DK, et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30:918–930. doi: 10.1101/gad.275891.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Reports. 2017;20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 37.Muller PAJ, Vousden KH, Norman JC. p53 and its mutants in tumor cell migration and invasion. The Journal of Cell Biology. 2011;192:209–218. doi: 10.1083/jcb.201009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang C-J, Chao C-H, Xia W, Yang J-Y, Xiong Y, Li C-W, Yu W-H, Rehman SK, Hsu JL, Lee H-H, et al. p53 regulates epithelial-mesenchymal transition (EMT) and stem cell properties through modulating miRNAs. Nature cell biology. 2011;13:317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim NH, Kim HS, Li XY, Lee I, Choi HS, Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER, et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol. 2011;195:417–433. doi: 10.1083/jcb.201103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caswell DR, Chuang C-H, Yang D, Chiou S-H, Cheemalavagu S, Kim-Kiselak C, Connolly A, Winslow MM. Obligate Progression Precedes Lung Adenocarcinoma Dissemination. Cancer Discovery. 2014;4:781–789. doi: 10.1158/2159-8290.CD-13-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 44.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo Y-Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proceedings of the National Academy of Sciences. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Mello SS, Valente LJ, Raj N, Seoane JA, Flowers BM, McClendon J, Bieging-Rolett KT, Lee J, Ivanochko D, Kozak MM, et al. A p53 super-tumor suppressor reveals a tumor suppressive p53-Ptpn14-Yap axis in pancreatic cancer. Cancer Cell. 2017 doi: 10.1016/j.ccell.2017.09.007. Provisionally accepted. This study uncovers a p53-Ptpn14-Yap axis that is integral to p53-mediated tumor suppression, connecting p53 to the Hippo pathway and providing support for the idea that Yap activation in tumors is associated with p53 loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 47.Bieging-Rolett KT, Johnson TM, Brady CA, Beaudry VG, Pathak N, Han S, Attardi LD. p19(Arf) is required for the cellular response to chronic DNA damage. Oncogene. 2016;35:4414–4421. doi: 10.1038/onc.2015.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochemical and Biophysical Research Communications. 2005;331:718–725. doi: 10.1016/j.bbrc.2005.03.154. [DOI] [PubMed] [Google Scholar]