Abstract
Cilia are surface-exposed organelles that dynamically concentrate signaling molecules to organize sensory, developmental and homeostatic pathways. Entry and exit of signaling receptors is germane to the processing of signals and the molecular machines for entry and exit have strated to emerge. The IFT-A complex and its membrane recruitment factor Tulp3 complex promotes the entry of signaling receptors into cilia while the BBSome and its membrane recruitment factor Arl6GTP ferry activated signaling receptors out of cilia. Ciliary exit is a surprisingly complex process entailing passage through a first diffusion barrier at the transition zone, diffusion inside an intermediate compartment and crossing of a periciliary diffusion barrier. The two barriers may organize a privileged compartment where activated signaling receptors transiently reside.
Introduction
Sorting of membrane proteins between compartments is overwhelmingly carried out by coat complexes that recognize targeting determinants exposed on the cytoplasmic domains of cargoes. While canonical coats package cargoes into carrier vesicles, a distinguishing feature of ciliary trafficking is the absence of vesicular intermediates (Fig. 1A). Instead, membrane proteins are trafficked within cilia by lateral transport within the plane of the ciliary membrane. Three multiprotein complexes harboring domains related to coat complexes are the lead actors in ciliary trafficking [1] and assemble into intraflagellar transport (IFT) trains that move up and down cilia under the power of the microtubule motors kinesin-II and dynein 2. The IFT-A and IFT-B complexes were discovered based on their decreased ciliary abundance upon inactivation of kinesin-II in the single cell model organism Chlamydomonas reinhardtii [2]. A third coat-like complex, the BBSome, was identified as an obligate complex of eight Bardet-Biedl syndrome proteins by tandem affinity purification of BBS4 [3–5]. Bardet-Biedl Syndrome (BBS), the first syndromic ciliopathy to be defined, is characterized by retinal degeneration, polydactyly, cystic kidneys, obesity, anosmia and male infertility [6]. The combination of a large genetic diversity (22 genes account for >80% of all known patient mutations) with a nearly indistinguishable disease presentation suggested the existence of a rich molecular pathway that now appears to converge on the BBSome [7]. Unlike IFT-A or IFT-B which can readily be separated into sub-complexes [8,9], the BBSome remains octameric in all tested conditions including 2.5M NaCl [10] and deletion of most subunits collapses the complex [4,11–13]. The BBSome has been purified from sources as varied as cultured mammalian cells [3], bovine retina [10], Chlamydomonas [14], Paramecium [15] and Trypanosoma [16] and was likely present in the last common ancestor of eukaryotes. The BBSome may have been missed in the initial discovery of IFT-A and IFT-B because its ciliary abundance in Chlamydomonas is nearly one order of magnitude lower than IFT-B [14] and because its entry into cilia may not require Kinesin-II [17]. However, the BBSome associates with IFT trains in nearly every organism examined and, if not for historical considerations, the BBSome may have been termed IFT-C.
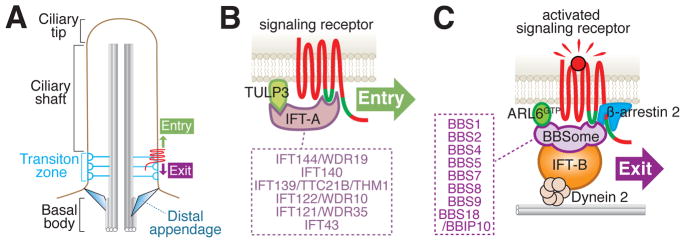
Figure 1.
IFT-A mediates constitutive ciliary entry and the BBSome is in charge of regulated exit from cilia. A. Schematic of relevant ciliary structures. B. Direct binding of the BBSome to the third intracellular loop and the C-terminal tail of various GPCRs has been shown by [10,31,37]. A role for dynein 2 and IFT-B in exit is supported by multiple lines of evidence [17,38]. The IFT-B/Dynein 2 interaction is speculated from the proximity of IFT-B to the microtubule in immuno-electron microscopy [67] and nanoscopy reconstructions [28]. Finally, β-arrestin 2 enables the specific retrieval of activated signaling receptors [34,49,50]. C. Direct binding of IFT-A to the ciliary targeting signal inside the third intracellular loop (green line) of SSTR3 was demonstrated by [34]. Little support is currently available for Kinesin-II and IFT-B participating in the entry of membrane proteins into cilia [45,46] and these complexes were not included in the entry diagram. The subunit composition of IFT-A and BBSome is listed in the boxes.
Importance of the BBSome in signaling
While BBSome subunits are conserved throughout evolution of ciliated organisms, they are lost in organisms that use cilia solely for motility [18], underscoring an intimate connection between the BBSome and signaling. Congruently, bbs mutants fail cilium-based signaling in every organism examined to date. Unlike IFT-A or IFT-B mutants that typically display pronounced structural abnormalities of the cilium, BBS mutants assemble mostly normal cilia [7,13,14,16,19], thus suggesting a more direct role for the BBSome in ciliary signaling. Cilium-based signaling is best exemplified by the Hedgehog (Hh) pathway, which patterns limbs and neural tube. During the course of Hedgehog signaling, the dynamic accumulation of signaling receptors inside cilia alters the ciliary environment to modify the activity of the Gli2 and Gli3 transcription factors. Of particular interest, the GPCR Gpr161 disappears from cilia during Hh signaling and the GPCR Smoothened (Smo) accumulates in cilia upon pathway activation. There is a multiplicity of evidence for Hedgehog signaling abnormalities when BBSome function is reduced. In zebrafish, BBSome depletion increases Hh signaling in fins, resulting in skeletal patterning abnormalities [20]. In mice, mutations in genes encoding BBSome subunits exacerbate the Hedgehog-related patterning defects (polydactyly, exencephaly) of cilia-related mutants [21–23]. In cultured cells, the transcriptional output of the Hh pathway is reduced by 25% in BBSome mutants [22] and all BBSome subunits have been identified as highly ranked hits in recent genome-wide CRISPR screens for Hedgehog regulators [24,25]. These observations strongly suggest that the post-axial polydactyly observed in BBS patients arises from altered Hedgehog signaling in the human limb bud.
Trypanosoma and Leishmania are bloodborne parasites that undergo complex interactions with host cell types to establish a persistent infection and evade the immune system. Surprisingly, in the absence of BBSome function, Trypanosoma and Leishmania are unable to mount an infection [16,19], supporting the existence of a cilium-based signaling pathway that participates in host infection. Finally, Chlamydomonas bbs mutants fail to swim away from noxious light dosages [14].
The BBSome as a coat adaptor for IFT-B
Biochemical reconstitution studies demonstrated that the small Arf-like GTPase Arl6 (encoded by the BBS3 gene) becomes membrane-associated once bound to GTP and recruits the BBSome, its sole effector, onto synthetic membranes where polymerization of a BBSome/Arl6 coat ensues [10]. These properties echo the assembly of canonical coats such as clathrin/AP-1 and Arf1 on endosome membranes with the important distinction that the BBSome coat does not deform membranes. Clathrin coats exemplify the division of labor between coat adaptor (AP-1/2/4) and cage layer (clathrin). Adaptor layer captures cargoes and initiate cargo clustering through amorphous polymerization [26,27]. The ensuing recruitment of the cage layer leads to lattice polymerization, membrane deformation and culminates in the packaging of cargoes in an individualized carrier vesicle. Similarly, the BBSome directly recognizes the cytoplasmic determinants of membrane proteins but, in contrast to canonical coats, BBSome coat polymerization does not promote membrane deformation [10]. At present the most logical cage complex for the BBSome and IFT-A is IFT-B itself and unpublished super-resolution microscopy supports the existence of a bilayered IFT-B/BBSome coat [28]. A major question posed by these studies was where BBSome coats are assembled within the cell and what trafficking step they mediate.
What is the trafficking step mediated by the BBSome?
The first ciliary membrane proteins whose localization pattern was altered by the BBSome were the Somatostatin Receptor 3 (Sstr3) and the Melanocortin concentrating hormone receptor 1 (Mchr1), two GPCRs normally localized to neuronal cilia and that fail to localize to cilia of primary neurons defective for BBSome or Arl6 function [10,29,30]. The direct and specific recognition of the third intracellular loop of Sstr3 (which encodes a ciliary targeting signal) by the BBSome lent support to a direct role for the BBSome in mediating ciliary entry of Sstr3 [10,31]. A function of the BBSome in ciliary import was strengthened by similar findings of decreased Polycystin 1 (PC1) and Smo levels in cilia of BBSome- or Arl6-depleted cells and by interactions between the BBSome and the cytoplasmic tail of PC1 and Smo [31–33].
A requirement for the BBSome in the exit of the membrane-associated protein phospholipase D (PLD) from Chlamydomonas cilia shed a counterpoint to the ciliary import function of the BBSome [14,17]. The BBSome exit model was reinforced by contemporaneous studies in mammalian cells that described a requirement for the BBSome and Arl6 in the signal-dependent exit from cilia of the dopamine receptor 1 (Drd1), Sstr3, Gpr161 and the Hh receptor Patched 1 [21,22,34–37] (Fig. 1B). Smo represents an unusual case for ciliary signaling receptors in that it undergoes signal-dependent accumulation in cilia. The findings of elevated ciliary Smo levels in unstimulated cells defective for dynein 2, BBSome or Arl6 suggest that the BBSome and dynein 2 constitutively remove Smo from cilia [30,36,38]; a reduction in BBSome-dependent exit from cilia upon Hh pathway activation may thus be responsible for the signal-dependent ciliary accumulation of Smo [34]. While binding of the Chlamydomonas BBSome to PLD has yet to be demonstrated, the mammalian BBSome was shown to recognize the cytoplasmic tails or loops of Smo, Sstr3, Gpr161, Drd1 and Patched [10,22,31,32,34,37]. A recent study of BBSome-binding motifs in ciliary signaling receptors identified a high level of degeneracy centered around motifs rich in basic and aromatic residues [31]. The BBSome may thus select a wide range of cargoes for ciliary removal, as suggested by systematic profiling studies of ciliary proteomes that collectively found over 100 proteins membrane proteins accumulating but relatively few depleted in Bbs mutant mammalian cilia [39,40]. In final support of the BBSome role in ciliary exit, studies in nematodes support a role for the BBSome in ciliary exit of signaling receptors [41].
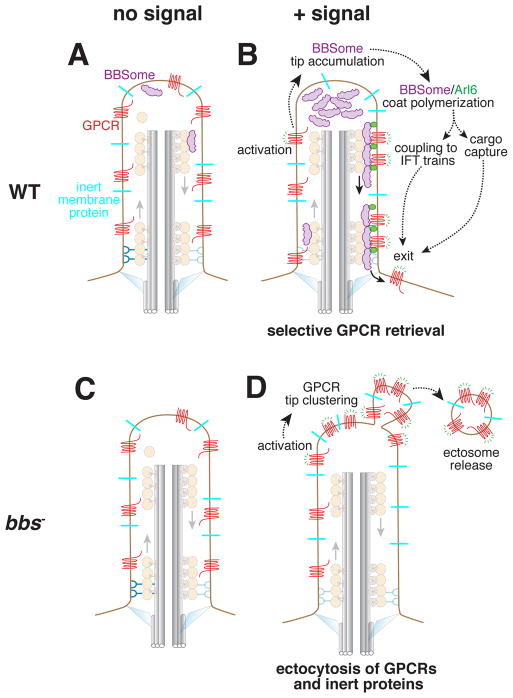
While it is formally possible that the BBSome mediates both ciliary entry and exit in a context- and cargo-specific manner, a more parsimonious model can now be proposed that limits BBSome function to ciliary exit. Real-time imaging of signaling receptors exit from cilia revealed that activated GPCRs that fail BBSome-mediated retrieval are packaged into extracellular vesicles at the tip of cilia through a process termed signal-dependent ectocytosis [35] (Fig. 2C–D). While ectocytosis packages specific receptors in extracellular vesicles, it does not fully exclude ciliary membrane proteins thus resulting non-specific losses from cilia. The decreased ciliary levels of signaling receptors in Bbs mutants may thus be due to pervasive ectocytosis rather than import defects [35].
Figure 2.
Conventional and unconventional pathways for exit from cilia. A and C. No exit takes place in the absence of GPCR stimulation. B. Activation of ciliary Gαi-coupled GPCRs (e.g. Sstr3 or Smo) leads to the accumulation of BBSomes at the tip and the Arl6-dependent formation of large and processive retrograde BBSome trains. The requirement for Arl6 in the formation of large trains suggests that these trains are the macroscopic correlates of BBSome/Arl6GTP coats. BBSome-mediated retrieval only removes the activated GPCRs from cilia while leaving ciliary bystanders behind. D. When BBSome or Arl6 function is abrogated, signal-dependent ectocytosis leads to specific removal of activated GPCRs and the non-specific loss of ciliary bystanders [35]. This observation offers a cogent explanation for the loss of several membrane proteins form Bbs mutant cilia.
What are the complexes that mediate entry into cilia?
A role of the BBSome in import is further challenged by several publications that have made a persuasive case for IFT-A mediating entry of signaling receptors into cilia [34,42–44] (Fig. 1C). Tulp3, the membrane-recruitment factor of IFT-A, is required for the constitutive entry of nearly every ciliary membrane protein tested amongst a panel representing sixteen GPCRs, the polycystin complex PC1/PC2 and the cystoprotein Pkhd1 [43]. The IFT-A complex subunits Ift121 and Ift144 are required for constitutive accumulation of several GPCRs in cilia [42] and Ift144 is required for signal-dependent accumulation of Smo and the constitutive entry of Gpr161 in cilia [44]. Physical interactions between IFT-A and the ciliary targeting signals of Sstr3 or Pkhd1 [34,42,43] strongly suggest that IFT-A directly recognizes membrane proteins and ferries them into cilia with the help of its membrane-recruitment factor Tulp3 (Fig. 1C).
Surprisingly, deletion of Ift139 results in a failure of Gpr161 and Smo to exit cilia [44]. One possible interpretation is that the Ift139-less IFT-A complex is still competent to import GPCRs into cilia but fails to release its cargoes once they have reached their destination. The sharing of a common recognition determinant on Sstr3 by IFT-A and the BBSome [10,31,34] supports the necessity of a release from IFT-A before BBSome can remove this GPCR from cilia.
Intriguingly, kinesin-II is not required for the signal-dependent entry of the adhesion molecule SAG1 into Chlamydomonas flagella [45] and a recent preprint suggests that ciliary entry of Smo may not require IFT-B [46]. It is thus conceivable that the IFT-A/Tulp3 complex alone mediates the entry of membrane proteins into the ciliary shaft (Fig. 1C) and that the IFT-A/Tulp3/cargo complexes subsequently become engaged to anterograde IFT-B trains inside cilia.
The BBSome is an active participant in the signal-dependent retrieval of GPCRs
Co-movement of BBSome with IFT-B in both anterograde and retrograde directions has been observed in Chlamydomonas [14], mammalian cells [34,36,47] and nematode [48]. The constitutive intracilary transport of the BBSome suggests that its cargoes specifically latch onto retrograde BBSome trains either constitutively (PLD) or in a regulated manner (Gpr161, Sstr3). Congruently, processive retrograde trajectories of single Sstr3 molecules disappear in the absence of Arl6 while their anterograde movements remain unaffected [34]. How do GPCRs specifically latch onto retrograde but not anterograde BBSome trains?
While small BBSome trains form constitutively (Fig. 2A), cAMP-dependent signaling downstream of Sstr3 or Smo promotes the formation of large and highly processive retrograde BBSome trains that remove Sstr3 and Gpr161 from cilia [34] (Fig. 2B). Since Arl6 is required for the signal-dependent doubling in retrograde BBSome train size, we propose that local generation of Arl6GTP at the tip promotes the formation of BBSome/Arl6 coats that effectively capture cargoes and stably attach to retrograde IFT trains. The formation of large BBSome trains enhances exit rates of activated GPCRs but is not sufficient to remove inactive GPCRs from cilia, strongly suggesting that another element of the retrieval machinery helps to read out the state of GPCR activation. Because retrieval of activated GPCRs requires the conformational sensor β-arrestin 2 [34,49] and β-arrestin 2 is recruited to cilia upon GPCR activation [49,50], β-arrestin 2 is likely to assist in the recruitment of activated GPCRs to large retrograde BBSome trains.
Interestingly, PLD requires the BBSome for both anterograde and retrograde intraciliary movements [51]. It is conceivable that constitutive cargoes such as PLD latch onto both anterograde and retrograde BBSome-containing IFT trains because constitutive cargoes form high affinity interactions with the BBSome regardless of Arl6-dependent coat formation. Yet attachment to anterograde BBSome trains is not sufficient for entry and by extension, retrograde BBSome trains must have unique properties that enable PLD exit from cilia.
The transition zone is breached by GPCRs associated with BBSome/Arl6 trains
At the base of cilia, the transition zone (TZ) separates the membrane of the ciliary shaft from the plasma membrane by establishing a diffusion barrier for membrane proteins [52]. Single molecule tracking of activated GPCRs revealed that Arl6 is required for retrograde transport and TZ crossing [34] (Fig. 3). We consider two models for how BBS/Arl6 trains may assist cargo passage through the TZ. First, the icebreaker model makes the simple assumption that stable association between cargoes and moving IFT trains is sufficient to power through a zone of high resistance [51]. An alternative model posits that the Arl6/BBSome trains endow their cargoes with unique barrier-crossing properties. This second model is distantly reminiscent of karyopherins and their bound cargoes crossing the hydrogel interior of the nuclear pore complex while inert proteins are unable to permeate through the hydrogel [53].
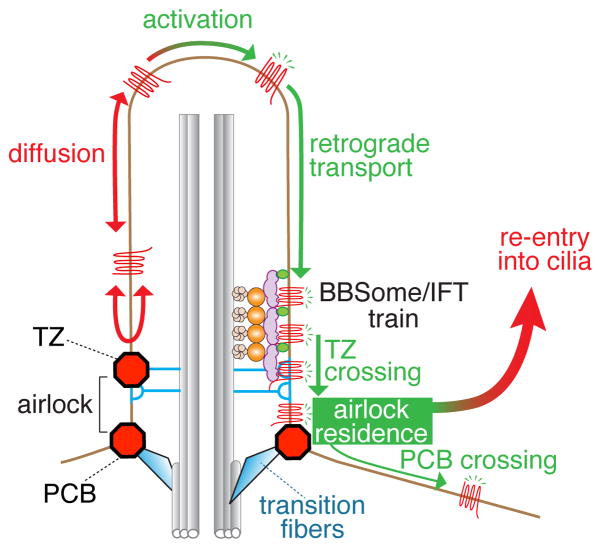
Figure 3.
Two barriers must be crossed for activated GPCRs to exit cilia. The first barrier at the transition zone is crossed by associating with BBSome/IFT trains while the mechanisms for crossing the second barrier remain unknown [34]. Between the transition zone (TZ) and the periciliary barrier (PCB) lies the airlock where GPCR that have crossed the TZ reside for a few seconds before either re-entring cilia (99.5% of the time) or crossing the PCB (0.5% of the time).
Two barriers are better than one
After crossing the TZ, activated GPCRs spend several seconds in an intermediate compartment bounded by the TZ on one side and a newly discovered periciliary diffusion barrier (PCB) on the other (Fig. 3). Because exit from cilia is only completed once GPCRs cross the PCB, this intermediate compartment is akin to an airlock. Perplexingly, 99.5% of airlock visits result in the GPCR returning to the ciliary shaft. Is there a functional benefit to this exceedingly inefficient and energetically costly exit process? Urine production in mammals provides a similar example of a vastly inefficient disposal process with 180 L of plasma filtered through the glomeruli every day yielding 1.5 L of urine. Filtering the entire plasma volume every 15 min allows kidneys to precisely and rapidly control the composition of body fluids [54]. Similarly, by providing the cilium with a checkpoint before exit is complete, the airlock may offer a way to rapidly sample candidate cargoes for exit. The relatively slow rates of exit that result from this rather inefficient strategy (it takes over 2h to empty the cilium of Gpr161 but only 5 min to endocytose the entire plasma membrane pool of β adrenergic receptor) add considerable inertia the ciliary composition, a possible advantage for developmental signaling pathways that integrate inputs over large time scales.
An additional benefit of transiently housing activated signaling receptors in the airlock may be to organize signaling cascades. Work by the Sung and Christensen labs found that, upon activation, the insulin-like growth factor 1 (IGF1) and the transforming growth factor β (TGF-β) transiently re-localize to a compartment at the base of cilia non-overlapping with axonemal markers, reminiscent of the airlock. Furthermore, activated IGF1R and TGF-βR are proposed to organize downstream signaling in the intermediate compartment [55,56]. Similarly, the phosphoinositides PI(4,5)P2 and PI(3,4,5)P3 are dynamically enriched in a compartment that may correspond to the airlock [57].
What constitutes the periciliary barrier?
A recent preprint has described a membranous region located between the tip of the transition fibers that the Liao lab named distal appendage matrix (DAM) [58]. The dimensional similarities between the DAM and the PCB are striking and the leakage of Smo and Sstr3 out of cilia in cells depleted of the DAM component FBF1 suggests that the PCB may be equivalent to the DAM. Studies in C.elegans further support a role for FBF1 in building a ciliary gate [59].
Alternatively, the airlock may correspond to the ciliary pocket, an invagination of the plasma membrane that ensheathes the base of the cilium [60]. The ciliary pocket contacts the tip of the transition fibers at its base and constricts around the shaft of the cilium. This model is attractive because the measured width of the ciliary pocket (480–500 nm, [58,61]) is in good agreement with our measurement of airlock width and the major ultrastructural localization of the BBSome in trypanosomes is at invaginated pits along the ciliary pocket [16]. The ciliary pocket is an active zone of endocytosis in a variety of organisms [60–62] and exocytosis is exclusively directed to the pocket in trypanosomes [62]. These observations suggest that the pocket may act as a waystation between the cilium proper and the rest of the cell. A molecularly defined cytoskeletal structure found at the collar in trypanosomes [63] may limit the diffusion of membrane proteins [62]. The presence of actin filaments near the collar in mammalian cells [61] thus raises the possibility that the collar may defines the periciliary diffusion barrier. This model implies that pocket-less cilia that emerge from the apical surface of some epithelial cells [60] may lack the periciliary barrier and that exchanges between plasma and ciliary barrier may be less restricted in these cells. Pocket-less cilia may thus enable more rapid exchanges of membrane proteins between ciliary and plasma membrane.
Conclusions and Perspectives
Regardless of the nature of the PCB, we do not presently know how GPCRs cross this second barrier. As GPCRs randomly diffuse within the airlock, they have likely already detached from BBSome/Arl6 coats [34]. The near-complete immobilization of GPCRs for 5 s immediately before exit suggests the possibility of capture by clathrin-coated pits, in agreement with the pocket hypothesis. Tracking of single GPCRs after they exit cilia in conjunction with imaging of clathrin and if the PCB will be required to answer these questions
The molecular mechanism of TZ crossing represents one of the most funcdamental and fascinating questions in ciliary biology. The well-studied membrane diffusion barriers at the yeast bud neck, the axon initial segment and the tight junction are impassable barriers that must be crossed by a vesicular intermediate [64]. The crossing of the TZ by select GPCRs suggests that the molecular assembly of a highly selective barrier at the TZ will reveal new concepts in diffusion barriers. The molecular description of the transition zone has seen tremendous progress in the past 6 years [52]. In particular, recent developments in multicolor super-resolution imaging [58,65] and electron tomography [28,66] give hope that a high resolution molecular model of the transition zone will soonemerge to serve as a foundation for dynamic studies of TZ crossing.
Acknowledgments
I apologize to those whose work was omitted due to space limitations. Research in the lab is supported by NIH grants R21HD087126 and R01GM089933. This work was made possible, in part, by NEI EY002162 - Core Grant for Vision Research and by a Research to Prevent Blindness unrestricted grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Dam TJP, Townsend MJ, Turk M, Schlessinger A, Sali A, Field MC, Huynen MA. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc Natl Acad Sci U A. 2013;110:6943–6948. doi: 10.1073/pnas.1221011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 4.Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15:854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Scheidecker S, Etard C, Pierce NW, Geoffroy V, Schaefer E, Muller J, Chennen K, Flori E, Pelletier V, Poch O, et al. Exome sequencing of Bardet-Biedl syndrome patient identifies a null mutation in the BBSome subunit BBIP1 (BBS18) J Med Genet. 2014;51:132–136. doi: 10.1136/jmedgenet-2013-101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet EJHG. 2013;21:8–13. doi: 10.1038/ejhg.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weihbrecht K, Goar WA, Pak T, Garrison JE, DeLuca AP, Stone EM, Scheetz TE, Sheffield VC. Keeping an Eye on Bardet-Biedl Syndrome: A Comprehensive Review of the Role of Bardet-Biedl Syndrome Genes in the Eye. Med Res Arch. 2017:5. doi: 10.18103/mra.v5i9.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taschner M, Weber K, Mourão A, Vetter M, Awasthi M, Stiegler M, Bhogaraju S, Lorentzen E. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 2016 doi: 10.15252/embj.201593164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katoh Y, Terada M, Nishijima Y, Takei R, Nozaki S, Hamada H, Nakayama K. Overall Architecture of the Intraflagellar Transport (IFT)-B Complex Containing Cluap1/IFT38 as an Essential Component of the IFT-B Peripheral Subcomplex. J Biol Chem. 2016;291:10962–10975. doi: 10.1074/jbc.M116.713883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Yu D, Seo S, Stone EM, Sheffield VC. Intrinsic Protein-Protein Interaction-mediated and Chaperonin-assisted Sequential Assembly of Stable Bardet-Biedl Syndrome Protein Complex, the BBSome. J Biol Chem. 2012;287:20625–20635. doi: 10.1074/jbc.M112.341487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, et al. BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci. 2013;126:2372–2380. doi: 10.1242/jcs.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou G, Koga M, Blacque OE, Murayama T, Ohshima Y, Schafer JC, Li C, Yoder BK, Leroux MR, Scholey JM. Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol Biol Cell. 2007;18:1554–1569. doi: 10.1091/mbc.E06-09-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valentine MS, Rajendran A, Yano J, Weeraratne SD, Beisson J, Cohen J, Koll F, Van Houten J. ParameciumBBS genes are key to presence of channels in Cilia. Cilia. 2012;1:16. doi: 10.1186/2046-2530-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langousis G, Shimogawa MM, Saada EA, Vashisht AA, Spreafico R, Nager AR, Barshop WD, Nachury MV, Wohlschlegel JA, Hill KL. Loss of the BBSome perturbs endocytic trafficking and disrupts virulence of Trypanosoma brucei. Proc Natl Acad Sci U A. 2016;113:632–637. doi: 10.1073/pnas.1518079113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechtreck KF, Brown JM, Sampaio JL, Craft JM, Shevchenko A, Evans JE, Witman GB. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J Cell Biol. 2013;201:249–261. doi: 10.1083/jcb.201207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettencourt-Dias M, Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB. Stepwise evolution of the centriole-assembly pathway. J Cell Sci. 2010;123:1414–1426. doi: 10.1242/jcs.064931. [DOI] [PubMed] [Google Scholar]
- 19.Price HP, Paape D, Hodgkinson MR, Farrant K, Doehl J, Stark M, Smith DF. The Leishmania major BBSome subunit BBS1 is essential for parasite virulence in the mammalian host. Mol Microbiol. 2013;90:597–611. doi: 10.1111/mmi.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tayeh MK, Yen H, Beck JS, Searby CC, Westfall TA, Griesbach H, Sheffield VC, Slusarski DC. Genetic interaction between Bardet-Biedl syndrome genes and implications for limb patterning. Hum Mol Genet. 2008;17:1956–1967. doi: 10.1093/hmg/ddn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee LE, Garcia-Gonzalo FR, Bowie RV, Li C, Kennedy JK, Ashrafi K, Blacque OE, Leroux MR, Reiter JF. Conserved Genetic Interactions between Ciliopathy Complexes Cooperatively Support Ciliogenesis and Ciliary Signaling. PLoS Genet. 2015;11:e1005627. doi: 10.1371/journal.pgen.1005627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum Mol Genet. 2012;21:1945–1953. doi: 10.1093/hmg/dds004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetz SC, Bangs F, Barrington CL, Katsanis N, Anderson KV. The Meckel syndrome-associated protein MKS1 functionally interacts with components of the BBSome and IFT complexes to mediate ciliary trafficking and hedgehog signaling. PLoS ONE. 2017;12:e0173399. doi: 10.1371/journal.pone.0173399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breslow DK, Hoogendoorn S, Kopp AR, Morgens DW, Vu BK, Kennedy MC, Han K, Li A, Hess GT, Bassik MC, et al. A CRISPR-based screen for Hedgehog signaling provides insights into ciliary function and ciliopathies. Nat Genet. 2018 doi: 10.1038/s41588-018-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pusapati GV, Kong JH, Patel BB, Krishnan A, Sagner A, Kinnebrew M, Briscoe J, Aravind L, Rohatgi R. CRISPR Screens Uncover Genes that Regulate Target Cell Sensitivity to the Morphogen Sonic Hedgehog. Dev Cell. 2018;44:113–129. e8. doi: 10.1016/j.devcel.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinrichsen L, Meyerholz A, Groos S, Ungewickell EJ. Bending a membrane: How clathrin affects budding. Proc Natl Acad Sci U S A. 2006;103:8715–8720. doi: 10.1073/pnas.0600312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkhatib N, Bresteau E, Baschieri F, Rioja AL, Niel G, van Vassilopoulos S, Montagnac G. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science. 2017;356:eaal4713. doi: 10.1126/science.aal4713. [DOI] [PubMed] [Google Scholar]
- 28.Robichaux MA, Potter VL, Zhang Z, He F, Schmid MF, Wensel TG. Defining the Layers of a Sensory Cilium with STORM and Cryo-Electron Nanoscopies. bioRxiv. 2017 doi: 10.1101/198655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, Bugge K, Stone EM, Rahmouni K, Sheffield VC. Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc Natl Acad Sci U A. 2011;108:20678–20683. doi: 10.1073/pnas.1113220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Klink BU, Zent E, Juneja P, Kuhlee A, Raunser S, Wittinghofer A. A recombinant BBSome core complex and how it interacts with ciliary cargo. eLife. 2017;6:e27434. doi: 10.7554/eLife.27434. Mapping at of BBSome-binding motifs at amino acid resolution on candidate BBSome cargoes reveals a remarkable diversity of binding motifs distributed throughout the intracellular loops and tails. [W/F/Y][K/R] emerges as a consensus BBSome-binding motif. The degeneracy in BBSome-binding motifs suggests a vast range of BBSome cargoes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo S, Zhang Q, Bugge K, Breslow D, Searby CC, Nachury MV, Sheffield VC. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 2011;7:e1002358. doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su X, Driscoll K, Yao G, Raed A, Wu M, Beales PL, Zhou J. Bardet–Biedl syndrome proteins 1 and 3 regulate the ciliary trafficking of polycystic kidney disease 1 protein. Hum Mol Genet. 2014;23:5441–5451. doi: 10.1093/hmg/ddu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Ye F, Nager AR, Nachury MV. BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J Cell Biol. 2018 doi: 10.1083/jcb.201709041. Imaging of BBSomes trains and single GPCRs demonstrates that GPCR signaling triggers the formation of large BBSome trains that ferry GPCRs through the transition zone. A second barrier after the transition zone hinders receptors that have passed through the transition zone from exiting cilia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Nager AR, Goldstein JS, Herranz-Pérez V, Portran D, Ye F, García-Verdugo JM, Nachury MV. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell. 2017;168:252–263. e14. doi: 10.1016/j.cell.2016.11.036. The discovery of signal-dependent ectocytosis in cells defective for BBSome function provides a relatively straightforward interpretation for the loss of membrane proteins from Bbs mutant cilia; this is because signal-dependent ectocytosis is only partly selective and removes bystanders as well as activated GPCRs from cilia. This paper highlights the importance of live cell imaging in assessing the ciliary dynamics of membrane proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow D, Gygi SP, Nachury MV. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell. 2014;31:265–278. doi: 10.1016/j.devcel.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci CMLS. 2011;68:2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ocbina PJR, Anderson KV. Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn Off Publ Am Assoc Anat. 2008;237:2030–2038. doi: 10.1002/dvdy.21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV. Proteomics of Primary Cilia by Proximity Labeling. Dev Cell. 2015;35:497–512. doi: 10.1016/j.devcel.2015.10.015. The proteomics of IMCD3 cilia reveals several membrane proteins accumulating in cilia of Bbs19/Ift27−/− cilia but none depleted in agreement with a role for the BBSome in ciliary exit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Datta P, Allamargot C, Hudson JS, Andersen EK, Bhattarai S, Drack AV, Sheffield VC, Seo S. Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet-Biedl syndrome. Proc Natl Acad Sci U A. 2015;112:E4400–9. doi: 10.1073/pnas.1510111112. The proteomics of photoreceptor cilia from Bbs17/Lztfl1−/− mice reveals 138 proteins accumulating but only 8 proteins depleted. This systematic study lends strong support to the hypothesis that the BBSome only functions in export from cilia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Q, Zhang Y, Wei Q, Huang Y, Li Y, Ling K, Hu J. BBS4 and BBS5 show functional redundancy in the BBSome to regulate the degradative sorting of ciliary sensory receptors. Sci Rep. 2015;5:11855. doi: 10.1038/srep11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Fu W, Wang L, Kim S, Li J, Dynlacht BD. Role for the IFT-A Complex in Selective Transport to the Primary Cilium. Cell Rep. 2016;17:1505–1517. doi: 10.1016/j.celrep.2016.10.018. These three papers mount a persuasive case for the IFT-A/TULP3 complex being the importer of membrane proteins into cilia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Badgandi HB, Hwang S, Shimada IS, Loriot E, Mukhopadhyay S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol. 2017;216:743–760. doi: 10.1083/jcb.201607095. These three papers mount a persuasive case for the IFT-A/TULP3 complex being the importer of membrane proteins into cilia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Hirano T, Katoh Y, Nakayama K. Intraflagellar transport-A complex mediates ciliary entry and retrograde trafficking of ciliary G protein-coupled receptors. Mol Biol Cell. 2017;28:429–439. doi: 10.1091/mbc.E16-11-0813. These three papers mount a persuasive case for the IFT-A/TULP3 complex being the importer of membrane proteins into cilia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belzile O, Hernandez-Lara CI, Wang Q, Snell WJ. Regulated Membrane Protein Entry into Flagella Is Facilitated by Cytoplasmic Microtubules and Does Not Require IFT. Curr Biol CB. 2013;23:1460–1465. doi: 10.1016/j.cub.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eguether T, Cordelieres FP, Pazour GJ. Uncoupling Intraflagellar Transport and Primary Cilia Formation Demonstrates Deep Integration of IFT in Hedgehog Signaling. bioRxiv. 2017 doi: 10.1101/226324. [DOI] [Google Scholar]
- 47.Williams CL, McIntyre JC, Norris SR, Jenkins PM, Zhang L, Pei Q, Verhey K, Martens JR. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat Commun. 2014;5:5813. doi: 10.1038/ncomms6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blacque OE, Reardon MJ, Li C, McCarthy J, Mahjoub MR, Ansley SJ, Badano JL, Mah AK, Beales PL, Davidson WS, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal K, Hwang S, Somatilaka B, Badgandi H, Jackson PK, DeFea K, Mukhopadhyay S. Smoothened determines β-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol. 2016;212:861–875. doi: 10.1083/jcb.201506132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green JA, Schmid CL, Bley E, Monsma PC, Brown A, Bohn LM, Mykytyn K. Recruitment of β-Arrestin into Neuronal Cilia Modulates Somatostatin Receptor Subtype 3 Ciliary Localization. Mol Cell Biol. 2015;36:223–235. doi: 10.1128/MCB.00765-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Liu P, Lechtreck KF. The Bardet–Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export. Proc Natl Acad Sci. 2018 doi: 10.1073/pnas.1713226115. Co-imaging of PLD and BBSome in Chlamydomonas flagella demonstrates that the BBSome mediates anterograde and retrograde IFT of PLD but is only required for exit of PLD form cilia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Gonzalo FR, Reiter JF. Open Sesame: How Transition Fibers and the Transition Zone Control Ciliary Composition. Cold Spring Harb Perspect Biol. 2017;9:a028134. doi: 10.1101/cshperspect.a028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt HB, Görlich D. Transport Selectivity of Nuclear Pores, Phase Separation, and Membraneless Organelles. Trends Biochem Sci. 2016;41:46–61. doi: 10.1016/j.tibs.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Hall JE. Guyton and Hall Textbook of Medical Physiology. 13e. Saunders; 2015. [Google Scholar]
- 55.Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MPR, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, et al. TGF-β Signaling Is Associated with Endocytosis at the Pocket Region of the Primary Cilium. Cell Rep. 2013;3:1806–1814. doi: 10.1016/j.celrep.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Yeh C, Li A, Chuang J-Z, Saito M, Cáceres A, Sung C-H. IGF-1 activates a cilium-localized non-canonical Gβ signaling pathway that regulates cell cycle progression. Dev Cell. 2013;26:358–368. doi: 10.1016/j.devcel.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dyson JM, Conduit SE, Feeney SJ, Hakim S, DiTommaso T, Fulcher AJ, Sriratana A, Ramm G, Horan KA, Gurung R, et al. INPP5E regulates phosphoinositide-dependent cilia transition zone function. J Cell Biol. 2017;216:247–263. doi: 10.1083/jcb.201511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Yang TT, Chong WM, Wang W-J, Mazo G, Tanos B, Chen Z, Tran MNT, Chen Y-D, Weng RR, Huang C-E, et al. Architecture of mammalian centriole distal appendages accommodates distinct blade and matrix functional elements. bioRxiv. 2017 doi: 10.1101/193474. This tour-de-force in multi-color STORM imaging localizes 10 proteins previously localized to the distal centrioles at super-resolution. A complete architecture of the transition fibers reveals that one protein, FBF1, is unexpectedly located between the tip of the fibers. Deletion of FBF1 results in the leakage of SSTR3 and SMO form cilia, suggesting a gate function for FBF1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Q, Zhang Y, Schouteden C, Zhang Y, Zhang Q, Dong J, Wonesch V, Ling K, Dammermann A, Hu J. The hydrolethalus syndrome protein HYLS-1 regulates formation of the ciliary gate. Nat Commun. 2016;7:12437. doi: 10.1038/ncomms12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benmerah A. The ciliary pocket. Curr Opin Cell Biol. 2013;25:78–84. doi: 10.1016/j.ceb.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- 62.Field MC, Carrington M. The trypanosome flagellar pocket. Nat Rev Microbiol. 2009;7:775–786. doi: 10.1038/nrmicro2221. [DOI] [PubMed] [Google Scholar]
- 63.Lacomble S, Vaughan S, Gadelha C, Morphew MK, Shaw MK, McIntosh JR, Gull K. Three-dimensional cellular architecture of the flagellar pocket and associated cytoskeleton in trypanosomes revealed by electron microscope tomography. J Cell Sci. 2009;122:1081–1090. doi: 10.1242/jcs.045740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trimble WS, Grinstein S. Barriers to the free diffusion of proteins and lipids in the plasma membrane. J Cell Biol. 2015;208:259–271. doi: 10.1083/jcb.201410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi X, Garcia G, III, Van De Weghe JC, McGorty R, Pazour GJ, Doherty D, Huang B, Reiter JF. Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat Cell Biol. 2017;71:79–1188. doi: 10.1038/ncb3622. [DOI] [PubMed] [Google Scholar]
- 66.Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG. Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell. 2012;151:1029–1041. doi: 10.1016/j.cell.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sedmak T, Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J Cell Biol. 2010;189:171–186. doi: 10.1083/jcb.200911095. [DOI] [PMC free article] [PubMed] [Google Scholar]