Abstract
Rationale
There is a clear need for discovery of effective medications to treat behavioral pathologies associated with alcohol addiction, such as chronic drinking.
Objective
The goal of this preclinical study was to assess effects of chronic alcohol drinking on the nucleus accumbens (NAcb) proteome to identify and validate novel targets for medications development.
Materials and methods
Two-dimensional difference in-gel electrophoresis (2D-DIGE) with matrix assisted laser desorption ionization tandem time-of-flight (MALDI-TOF/TOF) was used to assess effects of chronic voluntary home-cage (24-h access) alcohol drinking on the NAcb proteome of C57BL/6J mice. To extend these findings to a model of alcohol self-administration and reinforcement, we investigated potential regulation of the positive reinforcing effects of alcohol by the target protein glutathione S-transferase Pi 1 (GSTP1) using a pharmacological inhibition strategy in mice trained to self-administer alcohol or sucrose.
Results
Expression of 52 unique proteins in the NAcb was changed by chronic alcohol drinking relative to water control (23 upregulated, 29 downregulated). Ingenuity Pathway Analysis showed that alcohol drinking altered an array of protein networks associated with neurological and psychological disorders, molecular and cellular functions, and physiological systems and development. DAVID functional annotation analysis identified 9 proteins (SNCA, GSTP1, PRDX3, PPP3R1, EIF5A, PHB, PEBP1/RKIP, GAPDH, AND SOD1) that were significantly overrepresented in a functional cluster that included the Gene Ontology categories “response to alcohol” and “aging.” Immunoblots confirmed changes in Pebp1 (RKIP) and GSTP1 in NAcb with no change in amygdala or frontal cortex, suggesting anatomical specificity. Systemic inhibition of GSTP1 with Ezatiostat (0–30 mg/kg, i.p.) dose-dependently reduced the reinforcing effects of alcohol as measured by operant self-administration, in the absence of motor effects. Sucrose self-administration was also reduced but in a manner associated with nonspecific motor inhibition.
Conclusions
Protein expression profiling identified an array of proteins and networks in the NAcb, including GSTP1, that are novel molecular targets of chronic alcohol drinking. Pharmacological inhibition of GSTP1 significantly reduced the positive reinforcing effects of alcohol, which regulate repetitive use and abuse liability. The observation that this protein was both up-regulated after chronic drinking and that its inhibition could modulate the reinforcing properties of alcohol suggests that it is a key target for alcohol-related pathologies. Proteomic strategies combined with specific preclinical models has potential to identify and validate novel targets of alcohol that may be useful in the medical management of alcohol addiction.
Keywords: alcohol, addiction, drinking, operant, self-administration, reinforcement, reward, nucleus accumbens, 2D-DIGE, proteome, GSTP1, RKIP, SNCA, Neurogranin, pharmacotherapy
INTRODUCTION
Alcohol addiction is one of the most significant public health problems in modern society, but medical treatments are lacking. The National Epidemiologic Survey on Alcohol and Related Conditions recently found that repetitive 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder (AUD) are all increasing in the United States regardless of sex, age, racial/ethnicity, or socioeconomic status (Grant et al. 2017). This widespread pattern of repetitive alcohol use is associated with a DSM-5 AUD lifetime prevalence rate of 29.1% (Grant et al. 2015). Maladaptive use of alcohol contributes to over 200 disease and injury conditions, accounts for 5.9% of all global deaths (3.3 million in 2012), and represents 5.1% of the global burden of disease and injury (WHO 2014). Pharmacotherapy for alcohol abuse and dependence has potential to promote global health but current FDA approved medications are marginally effective, lack general efficacy, and suffer from low physician prescription and patient compliance rates (Bouza et al. 2004; Garbutt 2009; Heilig et al. 2011; Hersh et al. 1998; Kranzler 2000; Krystal et al. 2001). Thus, there is a clear need for discovery of novel, effective medications to treat behavioral pathologies, and other disease states, associated with alcohol addiction.
Medications development for alcohol addiction can be achieved through a variety of strategies. For instance, hypothesis-driven research could utilize knowledge of neural mechanisms mediating alcohol reward, craving, altered executive function, or other aspects of addiction to develop and test compounds acting on specific molecular and cellular targets (Litten et al. 2012). Similarly, unbiased screening methods, such as genomic or proteomic strategies, can be used with preclinical models to identify the spectrum of neural changes associated with specific disease states (Gorini et al. 2014). Identification of druggable targets can be followed with compound testing in preclinical models to determine potential therapeutic efficacy.
Here we utilized a three stage preclinical medications development approach to identify, validate, and test neural targets with potential efficacy for therapeutic regulation of chronic drinking. First, target identification was achieved using two-dimensional difference in-gel electrophoresis (2D-DIGE) to evaluate effects of chronic voluntary alcohol drinking on the nucleus accumbens (NAcb) proteome of C57BL/6J mice. We have successfully used this strategy to assess effects of voluntary drinking on the amygdala proteome, which identified site-specific activity of CaMKIIα and AMPA receptors as novel molecular mechanisms of the positive reinforcing effects of alcohol (Salling et al. 2016). In this study, we focused on the nucleus accumbens as a primary neural substrate of the positive reinforcing effects of alcohol (Hodge et al. 1997; Hodge et al. 1992; Samson and Hodge 1993). Second, target validation was accomplished by independent replication of the chronic drinking method followed by evaluation of specific target proteins in individual mice via immunoblot. Glutathione S-transferase P-1 (GSTP1) and phosphatidylethanolamine-binding protein 1 (PEBP1 or RKIP) were chosen for validation following identification of these proteins as constituents of the Gene Ontology (GO) cluster GO:0045471 entitled “response to ethanol.” Our goal was to further pursue 1 up- and 1 down-regulated protein. Third, compound testing was conducted to determine if pharmacological inhibition of GSTP1 using the selective inhibitor Ezatiostat (Telintra®, TLK199) would alter the positive reinforcing effects of alcohol using a well-characterized operant self-administration protocol (Faccidomo et al. 2009; Faccidomo et al. 2016; Faccidomo et al. 2015). The reinforcing effects of abused drugs represent a fundamental property of addiction liability and serve to maintain repetitive use over time. Thus, a pharmacological compound that reduces the reinforcing effects of a drug of abuse represents a highly significant candidate for further medications development. Based on the results of this three-stage preclinical medications development process of target identification, target validation, and compound testing, we propose that GSTP1 is a valid target for medications development and Ezatiostat, or related GSTP1 inhibitors, merit further testing for potential efficacy in reducing chronic alcohol use. Moreover, results show that GSTP1 is a key node in a variety of protein networks that regulate neurological diseases and basic molecular and cellular functions in the brain. Thus, manipulating GSTP1 has potential to restore homeostasis and reduce comorbidity. This proof of principle study suggests that neuroproteomic strategies have potential to identify therapeutic targets for specific behavioral pathologies in alcohol addiction, and that screening of other identified protein targets is warranted.
MATERIALS AND METHODS
Subjects
Male C57BL/6J mice (n = 50) were ordered from Jackson laboratories and arrived at 8 weeks old. Mice were housed singly in techniplast cages (12″×6.5″×7″) with continuous access to food and water. The colony room was maintained on a 12-hour reverse-light cycle (Dark: 7AM–7PM). All animals were treated in accordance with University of North Carolina at Chapel Hill and NIH guidelines for the Care and use of Laboratory Animals (Council 2011).
Experiment 1: Effects of alcohol drinking on the nucleus accumbens proteome
Home-Cage Alcohol Drinking
Home-cage drinking was conducted as described in (Besheer et al. 2006; Salling et al. 2016). Alcohol exposed mice had 24-h access to two graduated 10 mL drinking tubes containing alcohol vs. water for 28 days (Figure 1a). Alcohol concentration was increased during the first 4 days (days 1–2: 6% w/v; days 3–4: 10% w/v) and held constant thereafter (days 5–28: 20% w/v). Control mice had two water tubes. Volume consumed was recorded and tubes were refilled daily at 3 PM. Timecourse of drinking was assessed at 2-h intervals during the dark on day 14–15.
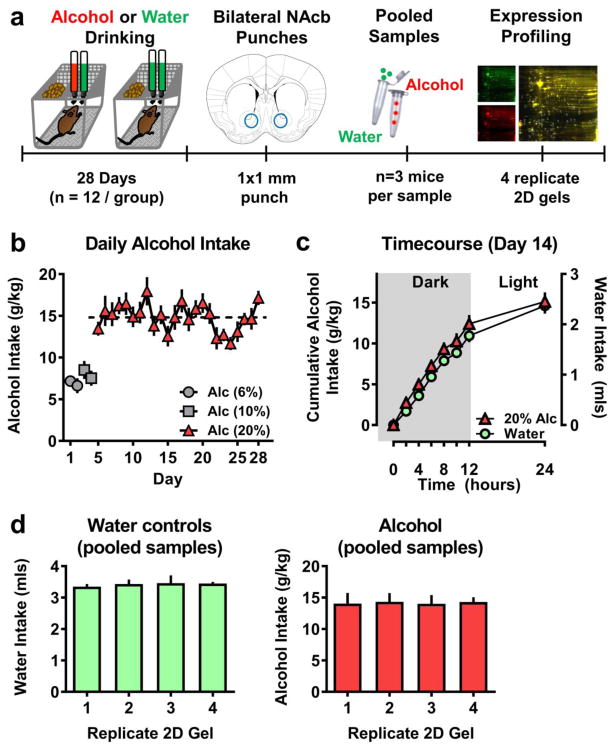
Figure 1. Experimental timeline and parameters of drinking for proteomic mice.
(a) Schematic representation of experimental procedure. After 28 days of alcohol or water home cage drinking, brains were sacrificed at T=0 (7AM) on the 29th day. Bilateral tissue punches were collected from the NAC and n=3 mice (6 punches) were pooled to form a sample. 4 replicate gels were run (4 alcohol and 4 water gels) for the proteomic analysis. (b) Daily alcohol intake (g/kg) plotted as a function of exposure day (n=12). Dashed line represents overall (days 5–28) average alcohol (20% v/v) intake of 14.86 g/kg. (c) Circadian pattern of water (green circle; mls/2hr) and alcohol (red triangle; g/kg/2-h) plotted as a function of time. Intake was measured for 24-h at 2-h intervals during the dark cycle. Time = 0 corresponds to 7AM on day 14 (n=12). (d) Average alcohol intake (g/kg) from the last 7 days plotted as a function of pooled sub-groups (n=3/group/gel). Data represent MEAN±SEM.
Protein Expression Profiling
Mice were deeply anesthetized with pentobarbital, perfused with 1M PBS, rapidly decapitated and brains were flash frozen in isopentane at 7AM on the 29th experimental day (Figure 1a). Plasma was collected at that time and blood alcohol content was measured using an Analox G-5 Analyzer (Analox Instruments, Lunenburg, MA). Mice from alcohol vs water (n=12) and water vs water control (n=12) groups that showed stable levels of intake over the 28-day exposure, and that had zero BAC at the time of tissue collection, were used for the 2D-DIGE experiment (Figure 1d). Bilateral punches (1 mm diameter × 1 mm thick) were taken from coronal sections of the nucleus accumbens. Nucleus accumbens punches from each mouse were pooled (n=3 mice per sample) to form a total of 4 alcohol and 4 water control samples, which were compared on 4 separate 2-D gels. Each pooled sample was formed by selecting subsets of mice that, when combined, showed no mean differences in alcohol or water intake. 2D-DIGE was performed as previously described (Agoglia et al. 2017; Salling et al. 2016). Briefly, samples were sent to Applied Biomics, Inc (Hayward, CA) for 2-D-DIGE analysis under established protocols. Image scans of each gel were obtained with Typhoon Trio (Amersham Biosciences, Buckinghamshire, UK). In-gel and cross-gel analyses were performed using DeCyder software 6.5 (GE-Healthcare, Buckinghamshire, UK).
For differential in-gel analysis (DIA), DeCyder identified individual spot boundaries by calculating area, peak height and peak slope of the fluorescent signal intensity for alcohol (red) and water (green). Spot volume (sum of pixel intensities within the spot boundary) was then calculated and expressed as a ratio (alcohol / water) for each spot on all 4 gels to indicate the relative change in signal. DeCyder represented the ratio in the range of 1 to ∞ for alcohol-induced increases in spot volumes and −1 to − ∞ for alcohol-induced decreases in spot volumes. Values between −1 and 1 were not represented; thus, a two-fold increase and decrease is represented by 2 and −2, respectively (not 2 and 0.5). Average ratio values (n=4 gels) are shown in Tables 1 and 2. For cross-gel comparisons, the DIA quantified protein spots as a function of an internal standard consisting of equal amounts of water and alcohol sample on each gel. Statistical comparisons of average alcohol vs water sample intensities between gels (n=4) were then conducted based on the relative change of each sample to its in-gel internal standard. This process effectively removes the system (gel-to-gel) variation enabling accurate quantitation of changes between samples. The log10 of this value (referred to as standardized log abundance) was used to aid scaling in graphical representation (Figure 2) and was employed in all statistical analyses.
Table 1.
List of proteins that showed a significant decrease in expression in nucleus accumbens tissue in alcohol drinking mice versus water-only controls. Proteins were identified by MALDI-TOF-TOF following 2D-DIGE analysis. Data are sorted in descending order of fold change (alcohol / water expression). P-values represent results of t-tests comparing me an standardized log abundance of protein expression from alcohol vs water groups (n=4 parallel 2D gels).
| Gene ID | Protein Name | Spot # | Cell Location | T-test p value | Fold Change |
|---|---|---|---|---|---|
| CNRIP1 | Cannabinoid receptor interacting protein 1 | 33 | Extracellular Space | 0.00000042 | −3.46 |
| SOD1 | Superoxide dismutase [Cu-Zn] | 49 | Cytoplasm | 0.0000072 | −2.60 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 15 | Cytoplasm | 0.01 | −2.43 |
| Pea15 | Astrocytic phosphoprotein PEA-15 | 52 | Cytoplasm | 0.000037 | −2.12 |
| Ndufa8 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 8 | 39 | Cytoplasm | 0.00011 | −2.01 |
| Ncald | Neurocalcin-delta | 31 | Cytoplasm | 0.000034 | −1.91 |
| Hpcal1 | Hippocalcin-like protein 1 | 32 | Cytoplasm | 0.012 | −1.87 |
| Ufc1 | Ubiquitin-fold modifier-conjugating enzyme 1 | 29 | Cytoplasm | 0.019 | −1.78 |
| Pfn2 | Profilin-2 | 59 | Cytoplasm | 0.019 | −1.74 |
| Fth1 | Ferritin heavy chain | 30 | Cytoplasm | 0.076 | −1.71 |
| Cfl2 | Cofilin-2 | 35 | Extracellular Space | 0.000077 | −1.70 |
| Ppp3r1 | Calcineurin subunit B type 1 | 53 | Cytoplasm | 0.012 | −1.64 |
| Pebp1 | Phosphatidylethanolamine-binding protein 1 (RKIP) | 23 | Cytoplasm | 0.0078 | −1.63 |
| Pcsk1n | ProSAAS | 43 | Extracellular Space | 0.016 | −1.63 |
| Uqcrc1 | Cytochrome b-c1 complex subunit Rieske, mitochondrial | 20 | Cytoplasm | 0.016 | −1.61 |
| Tuba1c | Tubulin alpha-1c chain | 44 | Cytoplasm | 0.022 | −1.61 |
| Ppp3r1 | Calcineurin subunit B type 1 | 51 | Cytoplasm | 0.0006 | −1.59 |
| Eif5a | Eukaryotic translation initiation factor 5A-1 | 47 | Cytoplasm | 0.00024 | −1.53 |
| Ndufb9 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 9 | 26 | Cytoplasm | 0.0000086 | −1.49 |
| Cfl1 | Cofilin-1 | 36 | Nucleus | 0.0012 | −1.49 |
| Ppib | Peptidyl-prolyl cis-trans isomerase B | 38 | Cytoplasm | 0.008 | −1.49 |
| Cyc1 | Cytochrome c1, heme protein, mitochondrial | 13 | Cytoplasm | 0.019 | −1.47 |
| Phb | Prohibitin | 12 | Nucleus | 0.05 | −1.46 |
| Uqcrc1 | Cytochrome b-c1 complex subunit Rieske, mitochondrial | 18 | Cytoplasm | 0.015 | −1.45 |
| Ran | GTP-binding nuclear protein Ran | 19 | Nucleus | 0.0000072 | −1.45 |
| Atp5h | ATP synthase subunit d, mitochondrial | 24 | Cytoplasm | 0.000014 | −1.44 |
| Eif1ax | Eukaryotic translation initiation factor 1A, X-chromosomal | 28 | Other | 0.0056 | −1.44 |
| Ppp3r1 | Calcineurin subunit B type 1 | 50 | Cytoplasm | 0.0014 | −1.42 |
| Acot7 | Cytosolic acyl coenzyme A thioester hydrolase | 8 | Cytoplasm | 0.018 | −1.38 |
| Nfu1 | NFU1 iron-sulfur cluster scaffold homolog, mitochondrial | 16 | Cytoplasm | 0.0034 | −1.37 |
| Cfl1 | Cofilin-1 | 37 | Nucleus | 0.0019 | −1.34 |
| Fth1 | Ferritin heavy chain | 27 | Cytoplasm | 0.0001 | −1.32 |
Table 2.
List of proteins that showed a significant increase in expression in nucleus accumbens tissue in alcohol drinking mice versus water-only controls. Proteins were identified by MALDI-TOF-TOF following 2D-DIGE analysis. Data are sorted in descending order of fold change (alcohol / water expression). P-values represent results of t-tests comparing mean standardized log abundance of protein expression from alcohol vs water groups (n=4 parallel 2D gels).
| Gene ID | Protein Name | Spot # | Cell Location | T-test p value | Fold Change |
|---|---|---|---|---|---|
| Nudt2 | Bis(5′-nucleosyl)-tetraphosphatase [asymmetrical] | 48 | Plasma Membrane | 0.000002 | 4.24 |
| Fam177a1 | Protein FAM177A1 | 10 | Other | 0.0000019 | 3.00 |
| Calm1 | Calmodulin 1 | 42 | Nucleus | 0.075 | 2.82 |
| Calml3 | Calmodulin-like protein 3 | 40 | Nucleus | 0.0018 | 2.62 |
| Tpi1 | Triosephosphate isomerase | 17 | Cytoplasm | 0.0000044 | 2.10 |
| Snca | Alpha-synuclein | 45 | Cytoplasm | 0.000096 | 1.59 |
| Gstp1 | Glutathione S-transferase P 1 | 21 | Cytoplasm | 0.000033 | 1.57 |
| Fn3krp | Ketosamine-3-kinase | 14 | Cytoplasm | 0.0001 | 1.54 |
| Tubb2a | Tubulin beta-2A chain | 1 | Cytoplasm | 0.026 | 1.52 |
| Uqcrc1 | Cytochrome b-c1 complex subunit 1, mitochondrial | 4 | Cytoplasm | 0.00074 | 1.51 |
| Gmfb | Glia maturation factor beta | 46 | Cytoplasm | 0.016 | 1.50 |
| Fam213b | Prostamide/prostaglandin F synthase | 25 | Cytoplasm | 0.025 | 1.45 |
| Nrgn | Neurogranin | 58 | Plasma Membrane | 0.033 | 1.41 |
| Tceb1 | Transcription elongation factor B polypeptide 1 | 54 | Cytoplasm | 0.026 | 1.39 |
| Pdcd5 | Programmed cell death protein 5 | 56 | Nucleus | 0.0066 | 1.38 |
| Zswim4 | Zinc finger SWIM domain-containing protein 4 | 55 | Other | 0.0038 | 1.36 |
| Atp5j | ATP synthase-coupling factor 6, mitochondrial | 60 | Cytoplasm | 0.0046 | 1.36 |
| Akr7a2 | Aflatoxin B1 aldehyde reductase member 2 | 7 | Cytoplasm | 0.0088 | 1.35 |
| Sh3bgrl2 | SH3 domain-binding glutamic acid-rich-like protein 2 | 61 | Nucleus | 0.027 | 1.34 |
| Tufm | Elongation factor Tu, mitochondrial | 5 | Cytoplasm | 0.0016 | 1.30 |
| Prdx3 | Thioredoxin-dependent peroxide reductase, mitochondrial | 22 | Cytoplasm | 0.0015 | 1.30 |
| Magoh | Protein mago nashi homolog | 57 | Nucleus | 0.012 | 1.28 |
| Ppp1ca | Serine/threonine-protein phosphatase PP1-alpha catalytic subunit | 6 | Cytoplasm | 0.021 | 1.26 |
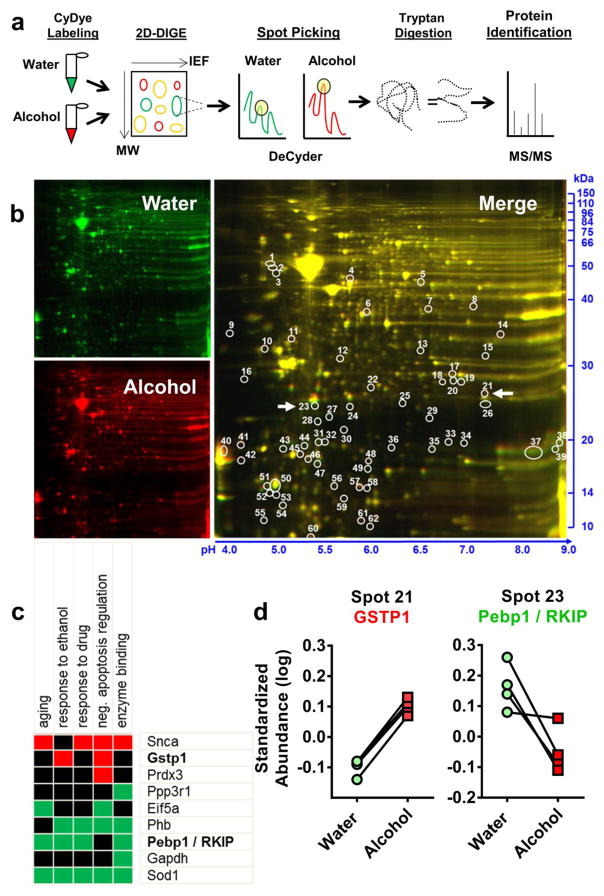
Figure 2. 2D-DIGE protein expression profiling of alcohol effects on nucleus accumbens with immunoblot confirmation.
(a) Schematic describing 2D-DIGE workflow. Alcohol exposed tissue from the nucleus accumbens was combined with Cy3 (red) and water controls with Cy2 (green) dye and run in 2D-DIGE, with protein separating in the y plane via molecular weight and the x plane via isoelectrical focusing (IEF). DeCyder software identifies protein spots with significantly different florescent signals. Selected spots are subjected to tryptan digestion and identified via tandem MALDI TOF/TOF mass spectrometry. (b) Representative 2D-DIGE gel showing water (green), alcohol (red) and the merged image generated by ImageQuant software. IEF is indicated on the x axis with pH values and molecular weight is indicated on the y axis in kDa. Circles indicate location of differentially expressed spots on the gel as detected by DeCyder Image analysis. Numeric markers correspond to Tables 1 & 2. White arrows correspond to proteins confirmed in Figure 5. (c) Statistically significant functional annotation cluster associated with the Gene Ontology term Biological Process, identified using the DAVID bioinformatics software. Cluster included 9 target proteins and “response to ethanol” was identified as a functional property of this cluster. GSTP1 and Pebp1/RKIP were included in 5 of 6 of the functions of this cluster and were followed for additional analysis. (d) Standardized log abundance of GSTP1 (Spot 21) demonstrating higher expression in alcohol (red squares) vs. water (green circles) exposed accumbens tissue. Standardized log abundance of Pebp1/RKIP (Spot 23) demonstrating reduced alcohol (red squares) vs. water (green circles) exposed accumbens tissue.
All protein spots that met the following a priori criteria (average change greater than 25%, change appeared in all 4 gels, significance determined by t-test, p < 0.05) were picked up by Ettan Spot Picker (GE-Healthcare, Buckinghamshire, UK), digested in-gel with trypsin, peptides were extracted, desalted and subjected to MALDI-TOF/TOF (Applied Biosystems, Foster City, CA). Resulting peptide masses and fragmentation spectra were submitted to GPS Explorer (V. 35) with the MASCOT search engine (Matrix Science, Boston, MA) to search the National Center for Biotechnology Information (NCBI) Reference Sequence (RefSeq) database for protein ID.
Bioinformatics
Initial functional annotation clustering was conducted using the Database for Annotation, Visualization and Integrated Discovery (DAVID; version 6.8) to identify potential overrepresentation of identified proteins and their co-association within the Gene Ontology (GO) category Biological Process (BP). In addition, protein identifiers, fold-change, and p-values from the proteomic analysis were uploaded to Ingenuity Pathway Analysis (IPA; QIAGEN Redwood City, CA) system for further dataset enrichment as described (Agoglia et al. 2017; Salling et al. 2016). The following parameters were used for Core Analysis: Ingenuity Knowledge Base (genes only); direct and indirect relationships; interaction networks; all data sources; experimentally observed confidence intervals; mouse (species).
Experiment 2: Independent confirmation of 2D-DIGE changes
To independently confirm a subset of 2D-DIGE changes, nucleus accumbens, prefrontal cortex, and amygdala tissue punches (1 × 1 mm) were obtained from additional mice (alcohol vs. water controls, n=8/group) that underwent the home-cage drinking protocol as described above. GSTP1 (R&D Systems; monoclonal mouse anti-GSTP1 antibody; 1:2500 dilution; 3% Normal Goat Serum blocking buffer) and RKIP (Cell Signaling; rabbit monoclonal anti-RKIP antibody; 1:2000 dilution; 5% Normal Goat Serum blocking buffer) protein expression were analyzed via Western Blot as reported previously (Agoglia et al. 2017; Salling et al. 2016; Wilkie et al. 2007). Optical density of each band at corresponding molecular weight was measured using ImageQuant (GE, Fairfield, CT) software by an experimenter blind to experimental condition. Values were then calculated as percent GAPDH or actin (loading control) and percent control (water) for each blot.
Experiment 3: Effects of GSTP1 Inhibition on Alcohol Self-Administration
Operant self-administration apparatus and procedure
Mouse alcohol self-administration sessions were conducted in 16 computer-controlled operant conditioning chambers (Med Associates, St. Albans, VT) as reported previously (Faccidomo et al. 2009; Faccidomo et al. 2016; Faccidomo et al. 2015; Salling et al. 2008). Each chamber contained 2 retractable levers. Responses on one lever (active lever) was reinforced by presentation of sucrose or sweetened alcohol (0.14 mL / 800 ms / reinforcer) into an adjacent drinking cup via syringe pump. Responses on the other lever (inactive lever) were recorded but produced no programmed consequences. One week after arrival in the colony, mice were provided access to 9% ethanol (v/v) + 2% sucrose (w/v) solution or water in their home cage. After 2 weeks of home-cage intake, separate groups of mice (n=8/group) were trained to lever press on a fixed-ratio 4 (FR4) schedule of sweetened alcohol reinforcement as described (Faccidomo et al. 2009). Subsequent 1-hr behavioral test sessions were conducted in the dark, between 0900 and 1500 h, 6 days/week. The drinking trough was checked at the end of each session to verify consumption of all delivered fluid.
GSTP1 Inhibition
To evaluate the potential regulation of alcohol self-administration by GSTP1, mice were first habituated to intraperitoneal (i.p) injections of DMSO via 7 injections 30-min prior to self-administration sessions. Subsequently, the GSTP1 inhibitor Ezatiostat (0, 1, 10, or 30 mg/kg, i.p.) was administered 30-min prior to alcohol self-administration sessions according to a Latin-Square design with no more than 2 injections per week.
Sucrose self-administration controls
A separate group of mice (n=8) was trained to self-administer sucrose (2% w/v) under the same behavioral protocol as used for the alcohol group. The dose effect curve for Ezatiostat (0 – 30 mg/kg, i.p.) was evaluated as described for alcohol self-administration
Locomotor activity
Spontaneous locomotor activity was assessed to determine if Ezatiostat-induced changes in operant behavior were due to nonspecific motor effects. These tests occurred in the operant self-administrating mice after completion of the self-administration dose effect curve. Ezatiostat (0, 10, or 30 mg/kg, i.p.) was administered 30-min prior to placement in Plexiglass activity monitor chambers (27.9 cm^2; ENV-510, Med Associates). Horizontal distanced traveled (cm) were recorded for 1-hour to correspond with the duration of self-administration sessions as described (Faccidomo et al. 2009).
Drugs
For home-cage drinking and operant self-administration, 95% ethyl alcohol was diluted with distilled water to produce either 6, 9, 10, or 20% v/v alcohol solution. The GSTP1 inhibitor Ezatiostat Hydrochloride(ethyl(2S)-2-amino-5-[[(2R)-3-benzylsulfanyl-1-[[(1R)-2-ethoxy-2-oxo-1-phenylethyl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoate;hydrochloride, ApexBio, Houston, TX) was dissolved in a 10% DMSO vehicle. Injection volume was 1ml/100g of body weight.
RESULTS
Experiment 1: Effects of alcohol drinking on the nucleus accumbens proteome
Alcohol Drinking
C57BL/6J mice consumed alcohol (20% v/v) vs. water under home-cage 24-h two-bottle choice conditions for 24 days. Total MEAN±SEM alcohol (20% v/v) intake over the 24-day access period was in the sub-dependence range of 14.86±0.33 g/kg/day (Figure 1b). Timecourse of drinking was measured on day 14 and showed a circadian pattern with most alcohol and water intake during the dark cycle (Figure 1c). Mice (n=12) were divided into four subgroups (n=3/group) for protein profiling. Subgroups were matched for total water and alcohol intake over the last 7 days of access (Figure 1d).
Differentially Expressed Proteins in the Nucleus Accumbens
At the onset of the dark cycle on Day 29, 12-h after alcohol access, mice were rapidly decapitated and brains were quickly frozen in −20° C isopentane (Figure 1a). In alcohol-exposed mice, Blood alcohol content at the time of brain collection was 0.0 mg/dL. Subsequently, brains were sliced on a cryostat (Leica) and circular tissue punches (1 × 1mm) were obtained from the prefrontal cortex, nucleus accumbens and amygdala. For proteomic analysis, NAcb tissue was pooled (n=3/sample/condition; Figure 1d) and whole-cell lysates were compared between water and alcohol exposed mice on 4 replicate gels via 2D-DIGE (Figure 2a). Differential in-gel image analysis (DIA) using DeCyder software identified 62 spots that met the a priori criterion for selection, and 55 were identified using MALDI TOF/TOF (Figure 2b; Table 1 & 2). DIA showed that expression ratios (alcohol/water) ranged from −3.46-fold to 4.24-fold with 32 proteins showing a significant decrease and 23 showing increased expression (Table 1 & 2). Cross-gel normalization was conducted via comparison of an internal standard made up of equal amounts of each sample (alcohol + water) on each gel.
Bioinformatics
Functional annotation clustering using the DAVID bioinformatics resource identified a single statistically significant cluster associated with the Gene Ontology term (GO-term) Biological Process that included 9 target proteins (Figure 2c). Response to ethanol (GO-term: 0045471) was prominent within the functional cluster with 4 identified proteins. Two proteins from the response to ethanol cluster (GSTP1 and PEBP1) were chosen for independent validation via immunoblot (see below). Standardized Abundance analysis (Log standardized abundance = 10log (vol Cy5 or Cy3/vol Cy2), showed that these proteins were consistently changed across the 2D gels when normalized to pooled control (alcohol + water samples; Cy2) (Figure 2d).
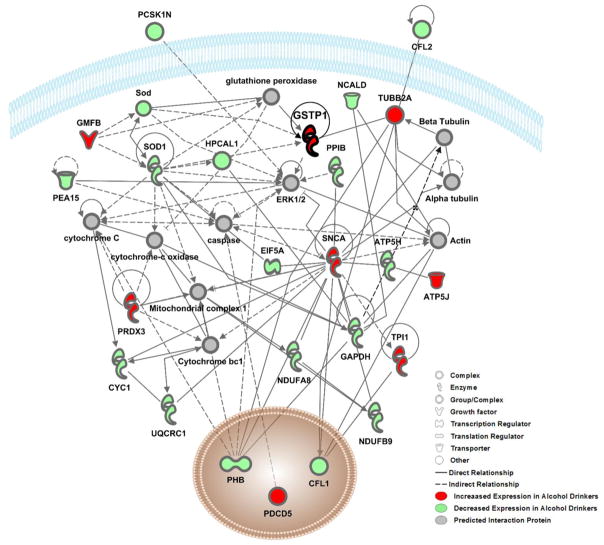
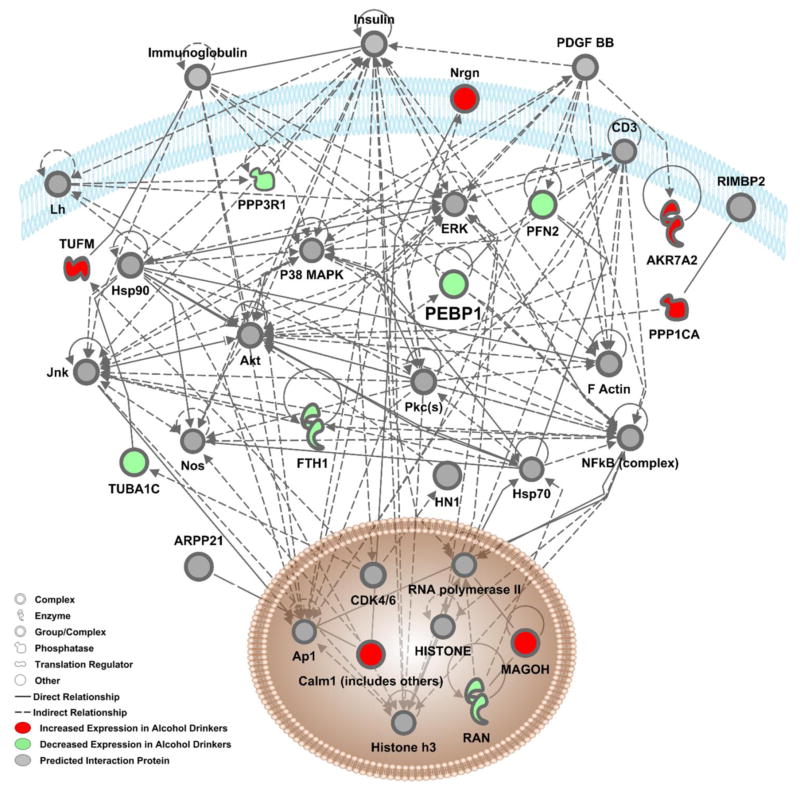
Further dataset enrichment was conducted using Ingenuity Pathway Analysis (IPA, Ingenuity Systems, Redwood City, CA). Protein ID’s, fold-change, and p-values were then submitted to IPA for Core Analysis. Results showed that cellular location of proteins was classified as follows: nucleus (9 proteins), cytoplasm (38 proteins), plasma membrane (2 proteins), extracellular space (3 protein) and other (3 proteins) (Table 1 & 2). Broadly, two networks of known protein-protein interactions related to Neurological disease, Organismal Injury and Psychological Disorders were identified (Figure 3 & 4; Table 3). Key functional clusters of these networks were identified by IPA and include disorders of the basal ganglia, cell-to-cell signaling, behavior, nervous system development and function, among others and the specific focus proteins that are associated with each function are noted in Table 3. Protein-protein interactions within these alcohol-sensitive networks were visualized using the IPA pathway designer and indicate key interaction proteins such as ERK1/2, which are known to be alcohol-sensitive (Figure 3 & 4; (Aroor and Shukla 2004; Faccidomo et al. 2009; Faccidomo et al. 2015; Girault et al. 2007; Jeanblanc et al. 2013; Ortiz et al. 1995; Radwanska et al. 2007). Network 1 (Figure 3) contains 24 alcohol-sensitive molecules and 10 statistically significant predicted interaction proteins. This network has a statistically significant p-score of 62 in IPA analysis (p-score represents the negative Log of the p-value (p-score = −log10 (P-value)) obtained from a hypergeometric test of overrepresentation calculated by right-tailed Fisher’s exact test.) Network 2 (Figure 4) contains 12 alcohol-sensitive molecules and 23 statistically significant interacting proteins.
Figure 3. Interaction Network 1 showing relationships between proteins in the highest scored network (P-score=62) identified by Ingenuity Pathway Analysis.
The color of nodes indicates increased-(red) or decreased-(green) expression of a protein in alcohol drinking mice as relative to water drinking controls. Proteins in grey nodes were not identified as being differentially expressed in our study but represent interacting proteins that were identified in the computationally generated networks. Black lines represent direct relationships between proteins and dashed lines represent indirect relationships. The interacting proteins and relationships were all identified based on the wealth of evidence stored in the IPA knowledge memory indicating relevance for this network.
Figure 4. Interaction Network 2 showing relationships between proteins in the second significantly scored network (P-score=25) identified by Ingenuity Pathway Analysis.
The color of nodes indicates increased-(red) or decreased-(green) expression of a protein in alcohol drinking mice as relative to water drinking controls. Proteins in grey nodes were not identified as being differentially expressed in our study but represent interacting proteins that were identified in the computationally generated networks. Black lines represent direct relationships between proteins and dashed lines represent indirect relationships. The interacting proteins and relationships were all identified based on the wealth of evidence stored in the IPA knowledge memory indicating relevance for this network.
Table 3.
Key functions of the known proteins identified in the two neural networks identified by Ingenuity Pathway Analysis (IPA). Arrows indicate direction of change in protein expression (↓ down-regulated, ↑ up-regulated) induced by alcohol drinking, as shown in Table 1 and 2. Protein clusters with multiple functions are noted by brackets. P values were derived in IPA by right-tailed Fisher Exact Test and indicate relative over-representation of proteins in a given function than what is expected by chance.
| Diseases and Disorders | P value | Proteins |
|---|---|---|
| Neurological & Psychological Disorders (Disorders of the Basal Ganglia) | 5.28E-11 | ATP5J↑, CYC1↓, FTH1↓, GAPDH↓, GSTP1↑, NDUFA8↓, Nrgn↑, PEBP1↓, PFN2↓, PPP3R1↓, RAN↓, SNCA↑, SOD1↓, TPI1↓, TUBA1C↓, TUBB2A↑, UQCRC1↓ |
| Skeletal and Muscular Disorders | 0.00094 | ATP5J↑, CYC1↓, FTH1↓, GAPDH↓, GSTP1↑, NDUFA8↓, Nrgn↑, PEBP1↓, PFN2↓, PPP3R1↓, RAN↓, SNCA↑, SOD1↓, TPI1↓, TUBA1C↓, UQCRC1↓ |
| Renal and Urological Disease | 0.00061 | GAPDH↓, GMFB↑, GSTP1↑, PEA15↓, PRDX3↑, SNCA↑, SOD1↓ |
|
| ||
| Molecular and Cellular Functions | P value | Proteins |
|
| ||
| Cell Death and Survival | 0.00043 | GAPDH↓, GMFB↑, GSTP1↑, PEA15↓, PPP3R1↓, PRDX3↑, SNCA↑, SOD1↓ |
| Cellular Assembly and Organization | 0.00092 | CFL1↓, CFL2↓, GAPDH↓, PFN2↓, PHB↓, PRDX3↑, SNCA↑, SOD1↓ |
| Cellular Compromise | 0.00054 | GMFB↑, PRDX3↑, SNCA↑, SOD1↓ |
| Cell-to Cell Signaling and Interaction | 0.00021 | CFL1↓, GAPDH↓, Nrgn↑, PPP3R1↓, SNCA↑ |
| Cell Morphology | 0.00029 | GAPDH↓, PRDX3↑, SNCA↑, SOD1↓ |
|
| ||
| Physiological System Development and Function | P value | Proteins |
|
| ||
| Behavior | 0.00023 | CFL1↓, PCSK1N↓, SNCA↑ |
| Embryonic Development | 4.64E-5 | GAPDH↓, PEA15↓, PPP3R1↓, PRDX3↑, SNCA↑, SOD1↓ |
| Nervous System Development and Function | 0.00092 | CFL1↓, Nrgn↑, PEBP1↓, PFN2↓, PPP3R1↓, SNCA↑, SOD1↓ |
| Tissue Morphology | 0.00092 | GSTP1↑, PEA15↓, PFN2↓, SNCA↑, SOD1↓ |
| Endocrine System Development and Function | 0.00085 | CFL1↓, PHB↓ |
Experiment 2: Independent confirmation of 2D-DIGE changes
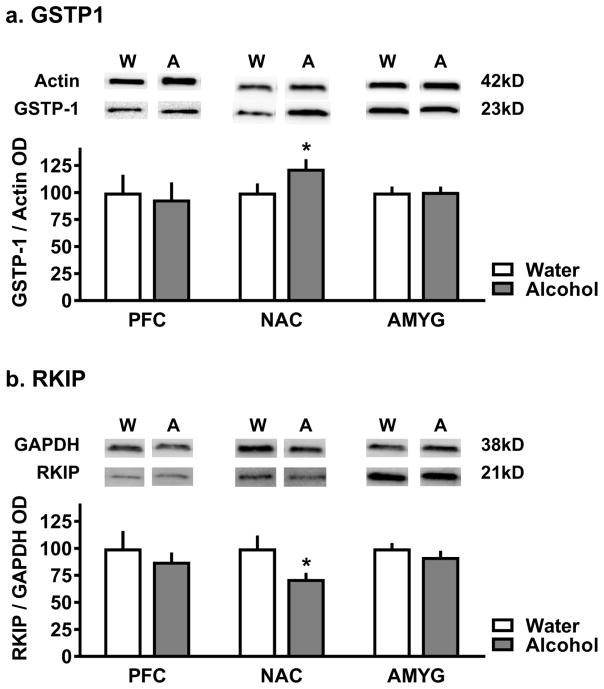
Immunoblot analysis in separate mice (n=8/group) confirmed that alcohol drinking (intake: 13.05±0.44 g/kg/24-h) increased GSTP1 protein expression relative to water controls (t(13)=1.79, p<0.05)) in nucleus accumbens tissue analyzed from individual mice (Figure 5a). Alcohol drinking also reduced RKIP expression relative to water controls (t(11)=2.03, p<0.05)) in the nucleus accumbens (Figure 5b). No alcohol-induced changes GSTP1 or RKIP expression were observed in prefrontal cortex or amygdala (Figure 5a–b), suggesting that the changes in expression for both of these target proteins was anatomically restricted to the nucleus accumbens.
Figure 5. Independent confirmation of 2D-DIGE changes.
a) Representative immunoblots (n = 8/group) and quantitative analysis showing that alcohol drinking significantly increased GSTP1 immunoreactivity relative to water control mice in nucleus accumbens but not prefrontal cortex (PFC) or amygdala (AMYG) samples. Data represent MEAN±SEM and are plotted relative to actin. Grey bars represent tissue from alcohol drinking mice and white bars represent tissue from water drinking mice. Asterisk indicates p ≤ 0.05, t-test. (b) Representative immunoblots (n = 8/group) and quantitative analysis showing that alcohol drinking significantly decreased Pebp1/RKIP immunoreactivity relative to water control mice in nucleus accumbens but not prefrontal cortex (PFC) or amygdala (AMYG) samples. Immunoblot data represent MEAN±SEM and are plotted relative to GAPDH.
Grey bars represent tissue from alcohol drinking mice and white bars represent tissue from water drinking mice. Asterisk indicates p ≤ 0.05, t-test.
Experiment 3: Effects of GSTP1 Inhibition on Alcohol Self-Administration
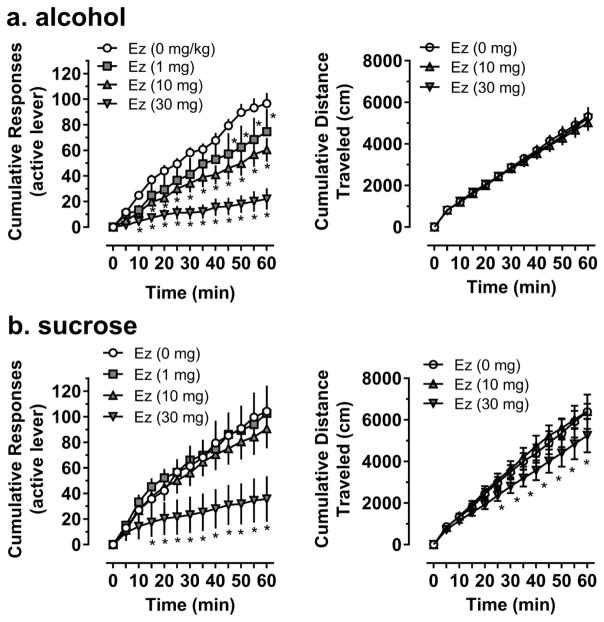
To extend the relevance of these findings, we sought to determine if GSTP1 activity regulates the positive reinforcing effects of alcohol. The GSTP1 inhibitor Ezatiostat (0 – 30 mg/kg, i.p.) was injected prior to operant self-administration sessions and effects on measures of operant self-administration were measured (Figure 6). A two-way repeated measures analysis of variance showed that alcohol-reinforced response rate increased as a function of time (F(12,72)=49.7, p<0.0001) (Figure 6a). Ezatiostat dose-dependently decreased alcohol-reinforced response rate (F(3,18)=9.7, p=0.0005) in a time-dependent manner as indicated by a significant interaction between drug dose and time (F(36,216)=4.9, p<0.0001) (Figure 6a). Dunnett’s multiple comparisons test showed that all doses of Ezatiostat produced reductions in alcohol-reinforced response rate starting at a range of time points during the session (Figure 6a, left). One way repeated measured analysis of variance revealed that alcohol intake, measured by dose consumed (g/kg) was dose-dependently reduced by Ezatiostat (F(3,18)=10.85, p=0.006; data not shown). Post hoc analysis revealed that the two highest doses of Ezatiostat (10 & 30 mg/kg) significantly reduced alcohol intake as compared to vehicle injection. Headpokes (F(3,18)=5.5, p=0.009) and inactive lever responses (F(3,18)=5.1, p=0.03), were all significantly reduced at 30 mg/kg Ezatiostat (data not shown). Importantly, these behaviors were unaffected by the lowest two doses of Ezatiostat suggesting that the drug doses that reduce alcohol self-administration measures were selective for those behaviors. Moreover, open-field locomotor activity was unaltered after administration of Ezatiostat confirming the absence of a non-specific effect on motor activity (Figure 6a, right). Preference for the alcohol lever ranged from 70–80% across all conditions and was unaffected by drug treatment. Together, these results point to the specificity of low doses of Ezatiostat in reducing alcohol-reinforced responding in a dose-dependent manner.
Figure 6. Effect of the GSTP1 inhibitor, Ezatiostat, on operant responding for alcohol and sucrose.
a) (Left panel) Dose-effect curve for Ezatiostat on alcohol-reinforced responding expressed as cumulative responses on the active lever. Symbols represent means for all doses and vertical bars represent SEM. Open circles represent responding 30 min after an i.p. injection of 10% DMSO. Ascending doses of the inhibitor are represented by squares (1mg/kg), upright triangle (10 mg/kg), upside down triangle (30 mg/kg), respectively. (Right panel) Cumulative distance traveled in an open field 30 min after an i.p. injection of vehicle (open circle), 10 mg/kg (upright triangle), 30 mg/kg (upside down triangle) Ezatiostat. Asterisks indicates significance from control, p ≤ 0.05, 2 way RM ANOVA, Dunnett’s post-hoc test. b) (Left panel) Dose-effect curve for Ezatiostat on sucrose-reinforced responding expressed as cumulative responses on the active lever. Symbols represent means for all doses and vertical bars represent SEM. Open circles represent responding 30 min after an i.p. injection of 10% DMSO. Ascending doses of the inhibitor are represented by squares (1mg/kg), upright triangle (10 mg/kg), upside down triangle (30 mg/kg), respectively. (Right panel) Cumulative distance traveled in an open field 30 min after an i.p. injection of vehicle (open circle), 10 mg/kg (upright triangle), 30 mg/kg (upside down triangle) Ezatiostat. Asterisks indicates significance from control, p ≤ 0.05, 2 way RM ANOVA, Dunnett’s post-hoc test.
To assess reinforcer specificity, effects of pre-session administration of Ezatiostat (0 – 30 mg/kg, i.p.) was tested in parallel behavior-matched controls. Sucrose-reinforced response rate increased as a function of time (F(12,72)=25.2, p<0.0001) in a manner qualitatively similar to sweetened alcohol response rate. Ezatiostat decreased sucrose-reinforced responding (F(3,18)=4.5, p=0.16) in a time-dependent manner (F(36,216)=5.1, p<0.0001). Dunnett’s multiple comparisons test showed that Ezatiostat produced dose-dependent reductions in sucrose-reinforced response rate only at the 30 mg/kg dose (Figure 6b, left). One way repeated measured analysis of variance revealed that headpokes (F(3,18)=5.0, p=0.03) and inactive lever responses (F(3,18)=6.5, p=0.01) were both significantly reduced at 30 mg/kg Ezatiostat (data not shown). Preference for the alcohol lever was approximately 80% for most doses. Locomotor activity increased as a function of time (f(12,72)=81, p<0.0001) and Ezatiostat (0–30 mg/kg, i.p.) reduced open-field locomotor activity (f(2,12)=4.4, p=0.037) in a time-dependent manner (F(24,144)=1.97, p<0.007). Dunnett’s multiple comparisons test showed that Ezatiostat (30 mg/kg) reduced locomotor activity during the 25 – 60 min interval (Figure 6, right). Together, the behavioral effects of Ezatiostat on both operant responding for sucrose and open field activity suggest that the reduction in sucrose-reinforced responding after administration of 30 mg/kg Ezatiostat was due to a non-specific locomotor effect in these mice.
DISCUSSION
Alcohol addiction is a major public health problem that impacts quality of life on a global scale (WHO 2014). The growing health, social, and economic impact of maladaptive alcohol use underscores the need for increased mechanistic understanding of alcohol addiction and development of targeted pharmacological therapeutics. One way to move the field forward is to identify novel biomarkers associated with specific behavioral pathologies in alcohol addiction and screen pharmacological compounds for potential efficacy (Litten et al. 2012; Litten et al. 2015). Here we used a neuroproteomic strategy to identify targets of chronic voluntary alcohol drinking in the NAcb, a key component of the reward pathway that is known to regulate the reinforcing effects of alcohol in rodents (Hodge et al. 1995; Hodge et al. 1997; Hodge et al. 1992; Hodge et al. 1993; Koob and Volkow 2016; Rassnick et al. 1992; Samson and Hodge 1993) and reflect craving in alcoholics (Heinz et al. 2009). Following target identification, we sought to determine if systemic inhibition of an identified target, GSTP1, regulates the reinforcing effects of alcohol, which are required for repetitive maladaptive use. Pharmacological therapeutics that reduce the reinforcing effects of alcohol may be useful in the medical management of repetitive use at various stages of the addiction cycle.
2D-DIGE protein expression profiling followed by MALDI-TOF/TOF identified 52 unique proteins with 100% confidence that were significantly down- or up-regulated in the NAcb (Tables 1 & 2, respectively) after 1 month of voluntary alcohol drinking. An overview of proteins targeted in the NAcb reveals that chronic alcohol drinking alters an array of protein networks that regulate various diseases and disorders, molecular and cellular functions, development, and behavior. Protein-set enrichment using IPA, identified statistically significant overrepresentation of clusters of alcohol-sensitive proteins in known disease states and various fundamental nervous system functions (Table 3). Functionally, the clusters are extraordinarily diverse, ranging from skeletal and muscular disorders to cell signaling, and endocrine development and function. Importantly, the cluster with the greatest statistical significance encompassed protein sets that have known functional involvement in Neurological and Psychological Disorders suggesting that alcohol use may alter the course of neurological and psychological disorders, such as diseases of the basal ganglia. Given the ubiquitous impact of alcohol on the NAcb proteome, a variety of medications development strategies are apparent. For example, pharmacological therapeutics might target protein networks that regulate a variety of functions, such as neurological diseases, cell death and survival, or cell-to-cell signaling.
To narrow the focus of this approach, we conducted a functional annotation analysis using DAVID bioinformatics tools that identified a cluster of proteins linked to the Gene Ontology term: “response to ethanol.” Within this cluster was GSTP1, the most prevalent member of the multifunctional glutathione S-transferase (GST) family of enzymes that serve major roles in cellular detoxification and signal transduction by conjugating target substrates to glutathione. GSTP1-1, binds and inhibits stress-activated c-Jun N-terminal kinase (JNK), a critical regulator of cell proliferation and apoptosis (Ruscoe et al. 2001). GSTP1 represents an interesting target for addiction therapeutics due to its role in oxidative stress, which can be induced by drugs of abuse including cocaine and alcohol (Uys et al. 2014). Moreover, cocaine increases GSTP1 expression in rat NAcb, and an inhibitor of GSTP1 blocks expression of cocaine-induced behavioral sensitization (Uys et al. 2011).
Due to the role of GSTP1 in tumorigenesis, this protein has emerged as a potential cancer therapeutic target and a variety of small molecule inhibitors are available. We tested effects of pre-session administration of Ezatiostat hydrochloride, a tripeptide analog of glutathione that is metabolized to TLK117 which crosses the blood brain barrier and can selectively inhibit GSTP1 catalytic activity with minimal effect on the related GSTα and −μ families {Ruscoe, 2001 #616;Laborde, 2010 #642}. Ezatiostat has been shown to stimulate the proliferation of myeloid precursors in preclinical models and has been tested in clinical trials for prevention of myelosuppression in myelodysplastic syndrome (Raza et al. 2009). After systemic administration, Ezatiostat dose-dependently reduced both alcohol-reinforced response rate and alcohol intake (g/kg) under operant self-administration conditions, which is direct evidence of reduced reinforcement function of alcohol. The two highest doses of Ezatiostat had no effect on locomotor activity in alcohol exposed mice, suggesting that the reduction in alcohol self-administration was not associated with nonspecific motor inhibition. Alternatively, under tests of reinforcer specificity, Ezatiostat reduced sucrose self-administration only at the highest dose tested, which also inhibited locomotor activity. The observation of a sedative effect in sucrose, but not alcohol, self-administering mice, suggests that alcohol self-administration may engender cross-tolerance to the locomotor, but not reinforcing effects of Ezatiostat. Our follow-up immunoblot results from Experiment 2 (Figure 5a) showed that the effect of alcohol on regulating levels of GSTP1 was restricted to the NAcb. Although systemic injection of Ezatiostat was likely inhibiting GSTP1 throughout the periphery and CNS, we know that GSTP1 is a target of alcohol in the NAcb and our data indicate that inhibiting this protein modulates the reinforcing effects of the drug. A future step to pin down the circuitry of this effect would be to microinject Ezatiostat directly in the NAcb. Overall, given the extensive development and testing of GSTP1 inhibitors in cancer therapeutics, this target may represent a viable candidate for cross-assessment in addiction and other neurological disorders.
Although, the mechanism(s) by which chronic alcohol drinking upregulated GSTP1 in the NAcb remain to be clarified, evidence suggests that GSTs may influence the development of alcohol addiction. For example, alcohol exposure can induce oxidative stress in a manner associated with increased GSH levels in rodent brain regions (Nordmann 1987; Reddy et al. 1999) and a gene microarray study found significant alterations in GSTA4 and GSTM1-5 expression in selectively bred ethanol-preferring (AA) rat cortex as compared to non-preferring (ANA) rats (Bjork et al. 2006). In addition, studies conducted on post-mortem human alcoholic brain tissue have identified oxidative stress as a common target of addiction in cortical regions (Flatscher-Bader et al. 2006). Thus, results of the present neuroproteomic approach are consistent with a growing understanding of the potential mechanistic role of glutathionylation and redox signaling in addiction (Uys et al. 2014) and behavioral response to drugs (Uys et al. 2011), and suggest that alcohol-induced upregulation of GSTP1 in the NAcb may reflect a general adaptation encompassing a variety of molecular functions that regulate the reinforcing effects of the drug during chronic use.
A feature of IPA analysis is that it identifies proteins that interact with the target dataset based on known protein-protein interactions from the literature. For both target networks, extracellular regulated protein kinase (ERK) was detected as an interaction node. Network 1 exhibited ERK interaction with 9 target proteins including GSTP1 and SNCA (Figure 3). In Network 2, ERK exhibited relationships with 12 interacting proteins and 1 target protein (PEBP1; Figure 4). The central involvement of ERK in both networks supports prior research from our group showing that (ERK) regulates several behavioral responses to alcohol, including the discriminative stimulus (Besheer et al. 2012) and positive reinforcing (Faccidomo et al. 2009; Faccidomo et al. 2015) effects of the drug. ERK activation is also associated with cue-induced reinstatement of alcohol-seeking behavior (Schroeder et al. 2008). The ERK signaling cascade transduces membrane receptor activity into long-term changes in gene expression through a series of kinase reactions involving Ras / Raf-1 / MEK and ERK. In the present study, chronic alcohol drinking directly decreased protein expression of the Raf-1 kinase inhibitor protein RKIP (PEBP1; Figure 5b), an effect that was restricted to the nucleus accumbens, showing that the ethanol-induced decrease in the expression of this protein is conserved to the NAcb under these conditions. This observation is consistent with evidence showing that chronic alcohol reduces RKIP expression in human neuroblastoma cells (Hellmann et al. 2009). Upon activation by PKC, RKIP dissociates from Raf-1 and phosphorylates G protein-coupled receptor kinase-2 (GRK-2), which blocks its activity. Since GRK-2 is inhibitory, blockade by RKIP disinhibits GPCR activity and facilitates signal transduction from Raf-1 to MEK (Lorenz et al. 2003; Trakul and Rosner 2005). In addition, RKIP controls activation of the nuclear NF-κB family (identified as an interacting protein in Network 2; Figure 4) of transcription factors that regulates expression of genes involved in immune function, stress response, and cancer (Karin and Greten 2005; Li and Verma 2002; Tang et al. 2010). Since RKIP is decreased in specific tumors and metastatic tissue, there is growing interest in developing approaches that restore RKIP or enhance interaction with RKIP target proteins in cancer therapy (Zeng et al. 2008). Similar strategies might be useful in medications development for alcohol addiction.
Of the 17 proteins that fell into the Neurological and Psychological Disorders cluster, many have been previously reported to be alcohol-sensitive, which is in strong agreement with results from the present study. For instance, alpha-synuclein (SNCA) was upregulated by 1.59-fold in the NAcb of alcohol drinking mice as compared to water controls. This is consistent with an array of clinical and preclinical studies linking SNCA to alcohol addiction (reviewed by (Janeczek and Lewohl 2013)), including evidence that that alcohol-preferring rats have higher levels of SNCA expression in the NAcb core (Pelkonen et al. 2010) and that αSYN transgenic mice show increased reinforcing effects of alcohol under operant self-administration conditions (Rotermund et al. 2017). It is of significance that SNCA was a key node in all protein clusters identified by IPA in the present study (Table 3). In addition to serving major roles in neural cell development and signaling, SNCA is a major causative gene in familial Parkinson’s disease (PD) and has been linked to dementia associated with PD, Alzheimer’s disease, and Lewy bodies disease (Irwin et al. 2013). Given this ubiquitous function of SCNA, alcohol-induced protein upregulation might influence basic molecular and cellular functions ranging from embryonic development to cell death and survival. Further work is warranted to evaluate SNCA as a target for medications development in alcohol addiction, and neurodegenerative diseases associated with alcohol abuse and alcoholism.
A number of additional alcohol-sensitive proteins were detected in the NAcb proteome that represent potential targets for medications development, including neurogranin (NGRN), calcineurin (PPP3R1), and calmodulin 1 and 3. NGRN is a calmodulin-binding protein that is highly expressed in neurons of addiction-associated brain regions including frontal cortex, hippocampus, amygdala, and striatum (Diez-Guerra 2010). NGRN is phosphorylated exclusively by PKCγ (Ramakers et al. 1999), a kinase long-known to regulate alcohol sensitivity and intake (Harris et al. 1995). At the synapse, increased NGRN expression localizes calmodulin at the neuronal membrane where binding to CAMKII lowers the threshold for long-term potentiation (Zhong and Gerges 2012). We have shown that CaMKII signaling in the amygdala is associated with increased glutamate synaptic activity and required for the reinforcing effects of alcohol (Salling et al. 2016). In the frontal cortex, CaMKII activity inhibits alcohol self-administration via top-down control (Faccidomo et al. 2016). Moreover, cue-induced reinstatement of CaMKII-T286 phosphorylation is upregulated in the NAcb (Salling et al. 2017), suggesting that alcohol-induced alterations in calcium signaling influence long-term behavioral pathologies associated with abstinence and relapse. Interestingly, NGRN is dephosphorylated by calcineurin (Seki et al. 1995), thus, increased calcineurin in the present study is consistent with an adaptive response to upregulated NGRN and associated signaling in the reward pathway. Overall, these data identify novel mechanisms by which altered calcium signaling in the NAcb might enhance the reinforcing effects of alcohol during initial stages of chronic drinking. Additional studies evaluating calmodulin, calcineurin and neurogranin as potential targets for medications development may shed new light on calcium-mediated regulation of behavioral pathologies in alcohol addiction.
In conclusion, the primary goal of this preclinical study was to increase understanding of global proteome-wide adaptations within the NAcb (e.g., reward pathway) that underlie chronic alcohol drinking. Results showed that chronic drinking alters protein expression in a wide array of networks that regulate basic functions of the nervous system and impact neurological and psychological disorders, such as Alzheimer’s and Parkinson’s diseases. These pathways, and specific protein nodes, represent a rich source of potential therapeutic targets for treatment of behavioral pathologies in alcohol addiction. We also sought to exploit this information in the continuing search for druggable targets that might be useful in the medical management of maladaptive chronic alcohol drinking. The protein GSTP1 was upregulated by alcohol drinking, and highly represented in protein clusters associated with neurological diseases, molecular and cellular functions, and physiological systems identified by bioinformatic analyses. Therefore, we chose to test the potential therapeutic efficacy of the GSTP1 inhibitor Ezatiostat, a small molecule compound that has been evaluated in clinical trials for various conditions including Myelodyplastic Syndrome, Chronic Neutropenia, and several forms of cancer. Inhibition of GSTP1 produced a dose-dependent reduction in the reinforcing effects of alcohol, indicating potential efficacy in reduction of chronic repetitive drinking, which is a hallmark of alcohol addiction. Additional preclinical studies are warranted to determine further efficacy in behavioral pathologies associated with dependence, such as relapse or escalated drinking during withdrawal. Overall, this study underscores the range of biological systems and processes that are altered by alcohol use, and strongly supports the need to discover biomarkers of specific behavioral pathologies and translate this information into targeted pharmacological therapeutic (Heilig and Leggio 2016).
Acknowledgments
This research was supported by NIAAA grants R37AA014983 and P60AA011065 to CWH.
References
- Agoglia AE, Holstein SE, Small AT, Spanos M, Burrus BM, Hodge CW. Comparison of the adolescent and adult mouse prefrontal cortex proteome. PLoS One. 2017;12:e0178391. doi: 10.1371/journal.pone.0178391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–64. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Cannady R, Grondin JJ, Hodge CW. Intra-amygdala inhibition of ERK(1/2) potentiates the discriminative stimulus effects of alcohol. Behav Brain Res. 2012;228:398–405. doi: 10.1016/j.bbr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Mole B, Hodge CW. GABAA receptor regulation of voluntary ethanol drinking requires PKCepsilon. Synapse. 2006;60:411–9. doi: 10.1002/syn.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork K, Saarikoski ST, Arlinde C, Kovanen L, Osei-Hyiaman D, Ubaldi M, Reimers M, Hyytia P, Heilig M, Sommer WH. Glutathione-S-transferase expression in the brain: possible role in ethanol preference and longevity. Faseb J. 2006;20:1826–35. doi: 10.1096/fj.06-5896com. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–28. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Council NR. Guide for the care and use of laboratory animals. 8. The National Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- Diez-Guerra FJ. Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life. 2010;62:597–606. doi: 10.1002/iub.357. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009;204:135–47. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Reid GT, Agoglia AE, Ademola SA, Hodge CW. CaMKII inhibition in the prefrontal cortex specifically increases the positive reinforcing effects of sweetened alcohol in C57BL/6J mice. Behav Brain Res. 2016;298:286–90. doi: 10.1016/j.bbr.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faccidomo S, Salling MC, Galunas C, Hodge CW. Operant ethanol self-administration increases extracellular-signal regulated protein kinase (ERK) phosphorylation in reward-related brain regions: selective regulation of positive reinforcement in the prefrontal cortex of C57BL/6J mice. Psychopharmacology (Berl) 2015;232:3417–30. doi: 10.1007/s00213-015-3993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatscher-Bader T, van der Brug MP, Landis N, Hwang JW, Harrison E, Wilce PA. Comparative gene expression in brain regions of human alcoholics. Genes Brain Behav. 2006;5(Suppl 1):78–84. doi: 10.1111/j.1601-183X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Garbutt JC. The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat. 2009;36:S15–23. quiz S24–5. [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gorini G, Harris RA, Mayfield RD. Proteomic approaches and identification of novel therapeutic targets for alcoholism. Neuropsychopharmacology. 2014;39:104–30. doi: 10.1038/npp.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017 doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc Natl Acad Sci U S A. 1995;92:3658–62. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–84. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Leggio L. What the alcohol doctor ordered from the neuroscientist: Theragnostic biomarkers for personalized treatments. Prog Brain Res. 2016;224:401–18. doi: 10.1016/bs.pbr.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–18. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann J, Rommelspacher H, Wernicke C. Long-term ethanol exposure impairs neuronal differentiation of human neuroblastoma cells involving neurotrophin-mediated intracellular signaling and in particular protein kinase C. Alcohol Clin Exp Res. 2009;33:538–50. doi: 10.1111/j.1530-0277.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- Hersh D, Van Kirk JR, Kranzler HR. Naltrexone treatment of comorbid alcohol and cocaine use disorders. Psychopharmacology (Berl) 1998;139:44–52. doi: 10.1007/s002130050688. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–93. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–91. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Haraguchi M. Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacol Biochem Behav. 1992;43:249–54. doi: 10.1016/0091-3057(92)90665-3. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Lewis RS, Erickson HL. Specific decreases in ethanol-but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205–930. Alcohol. 1993;10:191–6. doi: 10.1016/0741-8329(93)90034-l. [DOI] [PubMed] [Google Scholar]
- Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci. 2013;14:626–36. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeczek P, Lewohl JM. The role of alpha-synuclein in the pathophysiology of alcoholism. Neurochem Int. 2013;63:154–62. doi: 10.1016/j.neuint.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, Logrip ML, Janak PH, Ron D. BDNF-mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci. 2013;37:607–12. doi: 10.1111/ejn.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR. Pharmacotherapy of alcoholism: gaps in knowledge and opportunities for research. Alcohol Alcohol Suppl. 2000;35:537–47. doi: 10.1093/alcalc/35.6.537. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–9. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17:513–27. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39:579–84. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–9. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- Nordmann R. Oxidative stress from alcohol in the brain. Alcohol Alcohol Suppl. 1987;1:75–82. [PubMed] [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–97. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen A, Hiltunen M, Kiianmaa K, Yavich L. Stimulated dopamine overflow and alpha-synuclein expression in the nucleus accumbens core distinguish rats bred for differential ethanol preference. Journal of neurochemistry. 2010;114:1168–76. doi: 10.1111/j.1471-4159.2010.06844.x. [DOI] [PubMed] [Google Scholar]
- Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, Kaczmarek L. Alcohol Relapse Induced by Discrete Cues Activates Components of AP-1 Transcription Factor and ERK Pathway in the Rat Basolateral and Central Amygdala. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- Ramakers GM, Gerendasy DD, de Graan PN. Substrate phosphorylation in the protein kinase Cgamma knockout mouse. The Journal of biological chemistry. 1999;274:1873–4. doi: 10.1074/jbc.274.4.1873. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–8. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Raza A, Galili N, Smith S, Godwin J, Lancet J, Melchert M, Jones M, Keck JG, Meng L, Brown GL, List A. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood. 2009;113:6533–40. doi: 10.1182/blood-2009-01-176032. [DOI] [PubMed] [Google Scholar]
- Reddy SK, Husain K, Schlorff EC, Scott RB, Somani SM. Dose response of ethanol ingestion on antioxidant defense system in rat brain subcellular fractions. Neurotoxicology. 1999;20:977–87. [PubMed] [Google Scholar]
- Rotermund C, Reolon GK, Leixner S, Boden C, Bilbao A, Kahle PJ. Enhanced motivation to alcohol in transgenic mice expressing human alpha-synuclein. Journal of neurochemistry. 2017 doi: 10.1111/jnc.14151. [DOI] [PubMed] [Google Scholar]
- Ruscoe JE, Rosario LA, Wang T, Gate L, Arifoglu P, Wolf CR, Henderson CJ, Ronai Z, Tew KD. Pharmacologic or genetic manipulation of glutathione S-transferase P1–1 (GSTpi) influences cell proliferation pathways. The Journal of pharmacology and experimental therapeutics. 2001;298:339–45. [PubMed] [Google Scholar]
- Salling MC, Eastman CH, Psilos VR, Faccidomo K, Hodge SCW. Cue-induced reinstatement of alcohol-seeking is associated with increased CAMKII T286 phosphorylation in the reward pathway of mice. Pharmacol Biochem Behav. 2017 doi: 10.1016/j.pbb.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo S, Hodge CW. Nonselective suppression of operant ethanol and sucrose self-administration by the mGluR7 positive allosteric modulator AMN082. Pharmacol Biochem Behav. 2008;91:14–20. doi: 10.1016/j.pbb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Faccidomo SP, Li C, Psilos K, Galunas C, Spanos M, Agoglia AE, Kash TL, Hodge CW. Moderate Alcohol Drinking and the Amygdala Proteome: Identification and Validation of Calcium/Calmodulin Dependent Kinase II and AMPA Receptor Activity as Novel Molecular Mechanisms of the Positive Reinforcing Effects of Alcohol. Biol Psychiatry. 2016;79:430–42. doi: 10.1016/j.biopsych.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Hodge CW. The role of the mesoaccumbens dopamine system in ethanol reinforcement: studies using the techniques of microinjection and voltammetry. Alcohol Alcohol Suppl. 1993;2:469–74. [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–54. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki K, Chen HC, Huang KP. Dephosphorylation of protein kinase C substrates, neurogranin, neuromodulin, and MARCKS, by calcineurin and protein phosphatases 1 and 2A. Arch Biochem Biophys. 1995;316:673–9. doi: 10.1006/abbi.1995.1090. [DOI] [PubMed] [Google Scholar]
- Tang H, Park S, Sun SC, Trumbly R, Ren G, Tsung E, Yeung KC. RKIP inhibits NF-kappaB in cancer cells by regulating upstream signaling components of the IkappaB kinase complex. FEBS Lett. 2010;584:662–8. doi: 10.1016/j.febslet.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakul N, Rosner MR. Modulation of the MAP kinase signaling cascade by Raf kinase inhibitory protein. Cell Res. 2005;15:19–23. doi: 10.1038/sj.cr.7290258. [DOI] [PubMed] [Google Scholar]
- Uys JD, Knackstedt L, Hurt P, Tew KD, Manevich Y, Hutchens S, Townsend DM, Kalivas PW. Cocaine-induced adaptations in cellular redox balance contributes to enduring behavioral plasticity. Neuropsychopharmacology. 2011;36:2551–60. doi: 10.1038/npp.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, Mulholland PJ, Townsend DM. Glutathione and redox signaling in substance abuse. Biomed Pharmacother. 2014;68:799–807. doi: 10.1016/j.biopha.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Global status report on alcohol and health. WHO Press; Geneva: 2014. [Google Scholar]
- Wilkie MB, Besheer J, Kelley SP, Kumar S, O’Buckley TK, Morrow AL, Hodge CW. Acute ethanol administration rapidly increases phosphorylation of conventional protein kinase C in specific mammalian brain regions in vivo. Alcohol Clin Exp Res. 2007;31:1259–67. doi: 10.1111/j.1530-0277.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Imamoto A, Rosner MR. Raf kinase inhibitory protein (RKIP): a physiological regulator and future therapeutic target. Expert Opin Ther Targets. 2008;12:1275–87. doi: 10.1517/14728222.12.10.1275. [DOI] [PubMed] [Google Scholar]
- Zhong L, Gerges NZ. Neurogranin targets calmodulin and lowers the threshold for the induction of long-term potentiation. PLoS One. 2012;7:e41275. doi: 10.1371/journal.pone.0041275. [DOI] [PMC free article] [PubMed] [Google Scholar]