Abstract
The hippocampus is a structure that is critical for memory. Previous studies have shown that age-related differences in specialization along the longitudinal axis of this structure (i.e., subregions) and within its internal circuitry (i.e., subfields) relate to age-related improvements in memory in school-age children and adults. However, the influence of age on hippocampal development and its relations with memory ability earlier in life remains under-investigated. This study examined effects of age and sex on hippocampal subregion (i.e., head, body, tail) and subfield (i.e., subiculum, CA1, CA2-4/DG) volumes, and their relations with memory, using a large sample of 4- to 8-year-old children. Results examining hippocampal subregions suggest influences of both age and sex on the hippocampal head during early childhood. Results examining subfields within hippocampal head suggest these age effects may arise from CA1, whereas sex differences may arise from subiculum and CA2-4/DG. Memory ability was not associated with hippocampal subregion volume but was associated with subfield volume. Specifically, within the hippocampal head, relations between memory and CA1 were moderated by age; in younger children bigger was better, whereas in older children smaller was superior. Within the hippocampal body, smaller CA1 and larger CA2-4/DG contributed to better memory performance across all ages. Together, these results shed light on hippocampal development during early childhood and support claims that the prolonged developmental trajectory of the hippocampus contributes to memory development early in life.
Keywords: development, memory, hippocampus, early childhood
Introduction
The hippocampus is a complex structure comprised of multiple subfields (cornu ammonis areas 1–4, dentate gyrus, and subiculum) that are disproportionately distributed along the longitudinal axis (head, body, tail; Insausti & Amaral, 2012; Poppenk, Evensmoen, Moscovitch, & Nadel, 2013). Previous work examining the development of the hippocampus in school-aged children (~8 years of age and older) and adolescents has identified age- and sex-related differences in volumes of both subregions (i.e., head, body, tail; Daugherty, Bender, Raz, & Ofen, 2016; Demaster, Pathman, Lee, & Ghetti, 2013; Gogtay et al., 2006; Riggins, Blankenship, Mulligan, Rice, & Redcay, 2015; Schlichting, Guarino, Schapiro, Turk-browne, & Preston, 2016) and subfields (CA1–4, DG, subiculum; Daugherty et al., 2016; Lee, Ekstrom, & Ghetti, 2014; Tamnes et al., 2014). Age-related differences arise from multiple sources including: neurogenesis, synaptic growth, dendritic arborization, pruning, vascularisation and myelination (Benes & Tamminga, 1994; Huttenlocher, 1990; Lenroot & Giedd, 2006). These changes have been shown to have functional relevance, as many of these studies also linked age-related differences in hippocampal volume to age-related differences in cognitive abilities such as memory (see Ghetti & Bunge, 2012 for review) and language (e.g., Lee, Nordahl, Amaral, Lee, Solomon, & Ghetti, 2015). Sex differences may partially arise from effects of sex hormones as well as their collaboration with neurotransmitters and other intra- and extracellular mediators (Marrocco & McEwen, 2016; McEwen, 2010; Scharfman & MacLusky, 2017). Sex differences are important to document across development as they are thought to be associated with observed sex differences in age of onset, prevalence, and symptomatology observed in many neurodevelopmental disorders (Giedd, Castellanos, Rajapakse, Vaituzis, & Rapoport, 1997).
To date, few studies have examined development of the hippocampus early in life (prior to 8 years) and its implications for memory. This is particularly unfortunate as 1) neuroanatomical data from nonhuman primate studies suggest early childhood is a period of important developmental change in the hippocampus (e.g., Lavenex & Banta Lavenex, 2013; Serres, 2001) and 2) behavioral studies in children suggest early childhood is a period of dramatic improvement in memory (e.g., Bauer et al., 2012; Drummey & Newcombe, 2002; Riggins, 2014; Sluzenski, Newcombe, & Kovacs, 2006). Although theorists propose these developmental phenomena are linked (Bauer, 2006; Josselyn & Frankland, 2012; Lavenex & Banta Lavenex, 2013; Nadel & Moscovitch, 1997), empirical data are lacking.
The present study sought to address this gap by 1) systematically examining effects of age and sex on hippocampal subregions and subfields in 4- to 8-year-old children and 2) probing whether any observed differences in sex or age relate to memory ability during this developmental period. In order to set the stage for the current study, we first review findings from previous studies in humans examining age- and sex-differences in hippocampal subregions and their relations with memory, followed by findings in humans examining hippocampal subfields pertinent to these associations. Finally, findings from neuroanatomical data in nonhuman primates and behavioral studies of memory development in young children are reviewed, as this literature provided the primary motivation and hypotheses for the current study.
Subregions
Previous research suggests total hippocampal volume increases during childhood and is greater in boys compared to girls (e.g., Brown & Jernigan, 2012; Hu, Pruessner, Coupe, & Collins, 2013; Uematsu et al., 2012). However, hippocampal subregions (distributed along the longitudinal axis) show different developmental trajectories. Gogtay and colleagues (2006) first documented these regional differences in a longitudinal study, showing that between 4 to 25 years of age, anterior regions (i.e., head) decreased in size (particularly in the right hemisphere), whereas posterior regions (i.e., body and tail) increased in size (particularly in the left hemisphere). In addition, qualitative differences were observed between males and females, but statistical comparison was not possible due to the limited sample size. Furthermore, few scans were obtained during early childhood; the average age when participants were first scanned was 13 years.
Since then, cross sectional-studies have corroborated regional differences in hippocampal volume in school-aged children and adults and extended this work by relating these differences to memory performance (Daugherty et al., 2016; Demaster et al., 2013; Riggins et al., 2015; Schlichting et al., 2016). Overall, these studies suggest age-related differences in hippocampal subregion volume, with differential changes occurring along the longitudinal axis as children move into adolescence and adulthood. Nonlinear changes have been observed in the hippocampal head, with the smallest volumes found in adults. In addition, volume of the hippocampal head has been shown to relate to performance on memory tasks, although the direction of this effect (positive or negative relation) varies across studies/age groups (cf. Riggins et al., 2015; Schlichting et al., 2016).
Finally, although most of these previous studies include sex as a covariate in the analyses, only one directly examined sex differences in hippocampal subregion volumes and reported that none were observed (Daugherty et al., 2016).
Subfields
With advances in imaging methodology, research has begun to document the effects of age and sex on hippocampal subfield (CA1–4, dentate gyrus, subiculum) development (Daugherty, Flinn, & Ofen, 2017; Krogsrud et al., 2014; Lee et al., 2014; Schlichting et al., 2016; Tamnes et al., 2014). Across these studies, hippocampal subfields (typically CA1 and CA3-4/DG), showed different patterns of change (i.e., increases or decreases in volume) depending on the study and the age groups under investigation. However, across studies, volumetric differences have been related to memory performance. In two studies focusing on older children and adults (Lee et al., 2014; Daugherty et al., 2017), CA3/DG volume in the body was positively associated with memory, whereas in two studies including younger children (Schlichting et al., 2016; Tamnes et al., 2014) it was CA1 volume that was related to memory (although the direction of this effect varied between these studies).
In a sample closest to the age range of the present study, 244 4- to 22-year-old individuals, Krogsrud et al. (2014) reported increased volume in CA1, CA2/3, CA4/ DG, presubiculum, subiculum, and fimbria measured throughout the head and body of the hippocampus between 4 to ~15 years, followed by little age-related change beyond that point. Memory was not assessed. In a sample recruited from the same cohort, Tamnes et al. (2014) examined subfield development longitudinally in 85 individuals aged 8–21 years using 170 scans. They also investigated relations with memory. Nearly all subfields showed decreases in volume across development. Greater CA1 and CA2–3 volume was related to better memory performance (a finding similar to Schlichting et al., 2016).
Of these studies, many included sex as a covariate. Of those that directly examined sex differences in hippocampal subfield volumes, Daugherty et al. (2017) reported no significant effects, Schlichting et al. (2016) reported an interaction between sex and age for the subiculum in the hippocampal head, and both Tamnes et al. (2014) and Krogsrud et al. (2014) reported larger volumes in males for CA1, CA2/3, CA4/DG, and subiculum, that were driven mainly by participants under 13 years of age.
Early childhood
Of the above-mentioned studies, only three included participants younger than 6 years of age (Krogsrud et al., 2014; Riggins et al., 2015; Tamnes et al., 2014), yet none of them examined this younger age group systematically. Neuroanatomical data obtained from human and nonhuman primate tissue samples suggest developmental changes may be substantial in the hippocampus during early childhood (Eckenhoff & Rakic, 1988; Lavenex & Banta Lavenex, 2013; Serres, 2001), which some researchers propose underlie age-related changes in cognitive abilities (e.g., memory, spatial navigation) observed in this developmental stage (Bauer, 2006; Josselyn & Frankland, 2012; Lavenex & Banta Lavenex, 2013). However, to date, no studies have systematically examined hippocampal structure or its relation to cognition in humans during early childhood.
Of the cognitive abilities thought to improve due to the maturation of specific hippocampal subfields, laboratory-based studies of memory during early childhood have identified the ability to bind details of an event together and later recall these details as a significant source of change. For example, using a cohort-sequential design, Riggins (2014) examined developmental changes in children’s memory for novel facts and the sources from whom those facts were learned. Results showed that memory for facts improved between 4 to 10 years of age in a linear fashion. Memory for details of these facts (i.e., the source from whom they were learned), which is thought to reflect binding, showed the greatest rates of improvement between 5 – 7 years of age. This change was evident not only in the memory for the source of the facts but also in the types of errors children made. With age, children’s errors transitioned from those thought to be due to metacognitive abilities (e.g., guessing) to errors in episodic memory specificity (e.g., knowing the fact was learned in the laboratory but being unable to recall from whom it was learned). Similar findings of age-related improvements in memory during early childhood have been reported across multiple labs using several different memory paradigms (e.g., Bauer et al., 2012; Ngo, Newcombe, & Olson, 2017; Sluzenski et al., 2006).
The goal of the current study was to systemically examine hippocampal development in early childhood and link age- and sex-related hippocampal differences to memory performance.
Method
Effects of age and sex on hippocampal subregion volumes (i.e., head, body, tail) were examined using T1 scans from sample of 186 4- to- 8-year-old children. These results were further probed via analysis of hippocampal subfields (subiculum, CA1, CA2-4/DG) obtained from ultra-high resolution T2 scans in a subset of the same children (n = 153). Memory was assessed using the novel fact paradigm described above (Riggins, 2014).
Participants
A total of 200 4- to 8-year-old children (100 male, 100 female, average age 6.29 years, SD = 1.49) participated in the present study, which is part of an ongoing longitudinal investigation examining brain and memory development in early childhood. Younger age groups were oversampled to ensure enough useable data would be available and because participants were being followed longitudinally. Of these children, 193 provided useable data for the memory assessment, 186 provided useable T1 scans for assessment of subregions and 153 provided useable T2 scans for assessment of subfields. Despite attrition, the distribution of age and sex was comparable in the subregion and subfield samples. For subregions (T1 scans) there were 89 males and 97 females (average age at time of scan 6.28 years, SD = 1.47; with 46 4 year olds (23 male), 39 5 year olds (17 male), 41 6 year olds (26 male), 30 7 year olds (13 male), and 30 8 year olds (10 male)). For subfields (T2 scans) there were 69 male and 84 female (average age at time of scan was 6.38 years, SD = 1.50; with 37 4 year olds (16 male), 25 5 year olds (9 male), 36 6 year olds (24 male), 28 7 year olds (12 male), 27 8 year olds (8 male)).
Procedures
Children visited the laboratory twice, approximately 7 days apart (mean = 7.13 days, SD = 2.62).
Memory
During the first visit to the lab, children were taught novel facts (e.g., “A group of rhinos is called a crash”) from one of two different sources, a female adult (“Abby”) and a male-voiced puppet (“Henry”), via digital videos. The children learned 6 facts from each source for a total of 12 facts. Presentation of facts was blocked by source, where children first learned 6 facts from one source followed by 6 facts from the other source, and the order of blocks was randomly assigned across participants. There were 3 lists of facts. Each list consisted of unique facts that were similar across lists (e.g., “A group of kangaroos is called a mob” or “A group of goats is called a tribe”). These lists were randomly assigned across participants. Children were told to pay attention to the facts as they would be tested on the facts the following week but were not told that they would be tested on the source of the facts. Before each fact, children were asked if they knew the fact (e.g., “Do you know what a group of rhinos is called?”). If they answered correctly, that fact was excluded at testing and an additional novel fact from the list from the same source was presented. Each source had 8 possible facts to account for the possibility that children would know 1 or 2 of the facts. If a child knew 3 or more facts from one source, the total number of facts the child was tested on was reduced (but this was rare, n = 4).
When children returned to the lab for their second visit, they were tested on their memory for the facts and their sources. Children were asked to answer 22 trivia questions and to tell the experimenter where they had learned the answers to those trivia questions. They were told that they had learned some of the questions the week before from either “Abby” or “Henry,” some they might have learned outside the laboratory (e.g., from a teacher or parent), and some they may not know. The children had learned 6 of the 22 facts presented from “Abby,” 6 from “Henry,” 5 were facts commonly known by children (e.g., “What color is the sky?”), and 5 were facts that children typically would not know (e.g., “What is the colored part of your eye called?”). Each list of 22 facts had two random presentation orders, and these orders were counterbalanced across participants. Children were instructed to ask the experimenter for “hints” (i.e., multiple-choice options) if they did not know an answer to a question.
Each question was asked (e.g., “What is a group of rhinos called?”) and the child was given the opportunity to answer freely. If the child indicated they did not know the answer, they were given four pre-determined multiple-choice options (e.g., mob, crash, herd, or school). Once the child had given an answer either during free recall or multiple-choice, the experimenter asked where or from whom the child had learned the information. Again, children were given the opportunity to answer freely, and if they indicated they did not know where they had learned it, they were given five multiple choice options: parent, teacher, girl in the video, puppet in the video, or just knew/guessed.
The main dependent measure of interest was the proportion of questions for which the child accurately recalled both the fact and the source of the fact (i.e., source memory conditionalized on fact memory) as this is thought to reflect the binding of the fact and source. Consistent with previous research, memory for individual facts was also examined as were the errors children made regarding source judgments. Three types of errors occurred: children indicating they guessed or always knew the fact (termed guessed/knew errors), children indicating a person outside the experiment taught them (termed extra-experimental errors, e.g., teacher, parent, television, book) or children indicating the wrong experimental source taught them the fact (termed intra-experimental errors, i.e., indicating “Abby” taught them when in reality it was “Henry” or vice versa,). Note: One child did not provide data for the memory task due to experimenter error.
IQ
Indices of intelligence were obtained using subtests from age-appropriate standardized intelligence tests (i.e., Wechsler Intelligence Scale for Children-Fourth Edition, or WISC, and the Wechsler Preschool and Primary Scale of Intelligence, or WPPSI). Scaled scores from the block design subtest, which reflects visual-spatial intelligence, were obtained for use as covariates in analyses including memory performance to control for general differences in intelligence. One child was not administered the IQ test; 7 children were administered the IQ test the previous year.
MRI
All participants completed training in a mock scanner before MR data acquisition in order to become acclimated to the scanner environment and receive motion feedback. Participants were scanned in a Siemens 3.0-T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions, Erlangen, Germany) using a 32-channel coil. Structural data were collected using a high-resolution T1 magnetization-prepared rapid gradient-echo (MPRAGE) sequence consisting of 176 contiguous sagittal slices (voxel size: .9 mm isotropic, TR=1900 ms, TE=2.32ms, 900ms inversion time, 9° flip angle, pixel matrix= 256 × 256). In addition, ultra-high resolution structural scans were obtained of medial temporal lobe (MTL) with a T2-weighted fast spin echo sequence (voxel size: .4×.4×2mm, TR=4120ms, TE=41ms, 24 slices, 149° flip angle).
Subregions
Hippocampal subregion volumes (head, body, and tail) for both left and right hemispheres were extracted using Freesurfer v5.1 (surfer.nmr.mgh.harvard.edu; Fischl, 2012) and refined using Automatic Segmentation Adapter Tool (ASAT, nitrc.org/projects/segadapter; Wang et al., 2011). ASAT is a freely available tool designed to correct systematic errors in segmentation requiring as few as ten manually traced examples (Lee et al., 2015). To train the ASAT, the hippocampi for ten subjects were manually traced using boundaries set forth by the “EADC-ADNI Harmonized Protocol for Manual Hippocampal Segmentation” (Frisoni et al., 2015). Two subjects from each age group were randomly selected for manual tracing. It was required that these scans had clear visibility of the hippocampus in both hemispheres to be used as a training case. Following recommended methods (Lee et al., 2015), the following parameters were used to train ASAT: 4 × 4 × 4 voxel sampling radius, 50% sampling rate, 500 training iteration and dilation radius of 2 voxels. Manual edits were then performed on the hippocampus in the right (n=7) or left (n=17) hemisphere or both (n=2) to correct minor over or under-inclusions using the “EADC_ADNI Harmonized Protocol for Manual Hippocampal Segmentation” as a reference (Frisoni et al., 2015). The hippocampus was then divided into head, body, and tail subregions using manual identification of standard anatomical landmarks. The uncal apex served as the border between the head and body (Weiss, Dewitt, Goff, Ditman, & Heckers, 2005). The boundary between the body and tail was identified as the slice at which the fornix separates from the hippocampus and becomes clearly visible (Watson et al., 1992). Raters were blind to participant age and sex. Reliability for identification of these landmarks indicated 94.60% agreement within 1 slice and 99.99% agreement within 2 slices. Intra-class correlation coefficients (ICCs) were high and ranged from .897 – .985.
Subfields
Existing protocols for manual tracing of hippocampal subfields were reviewed (n =21, see Yushkevich et al., 2015). Protocols developed for T2-weighted images with resolution similar to our data and collected from 3T scanners were compared. Although several exist, only one protocol (La Joie et al., 2010) yielded the subfields of interest (CA1, CA2-4/DG, and subiculum) in both the head and the body of the hippocampus. Thus, we adopted these criteria for identification of hippocampal subfields. Tracing guidelines for the original protocol were based on Duvernoy (1998) and Harding (1998). A brief review of the protocol is below, with a focus on minor adjustments to the La Joie et al. (2010) protocol. Hippocampal subfield volumes were identified in the head and body of the hippocampus in both left and right hemispheres. Although there is disagreement regarding the ability to segment subfield boundaries in the hippocampal head using MRI, the current protocol focused on three large ROIs, collapsing across smaller subfields that tend to be more problematic. Moreover, Dice Similarity Coefficients (DSC) are calculated separately for head and body to ensure adequate reliability of the assessments. Consistent with previous literature, subfield volumes were not derived for the hippocampal tail due to its small size and the difficulty of accurately identifying subfield boundaries. In each subregion, three subfields were identified: subiculum, CA1, and a combination region of CA2-4/dentate gyrus (CA2-4/DG). Although the latter region combines multiple subfields, it includes both of the “late” developing subfields (CA3 and DG) and CA2, which is relatively small in size.
First, image contrast was adjusted so that white matter (WM) appeared black and cerebrospinal fluid (CSF) appeared white. Tracings were performed on slices perpendicular to the long axis of the hippocampus and began on the slice where the hippocampus first appeared. To determine the beginning of the hippocampus, sagittal slices were used according to EADC-ADNI Harmonized Hippocampal tracing Protocol (Frisoni et al., 2015). Consistent with La Joie et al. (2010), the fimbria was excluded from the ROIs; however, the alveus was included as it served as a more reliable landmark when determining the border (as the WM/CSF contrast was much more obvious than the WM/GM contrast). Similar to La Joie et al. (2010), we identified 7 different slice types that were used for manual segmentation. Table 1 describes each slice type, its defining features, the typical number of slices per subject, and both outer and internal boundaries. Additional details can be found in La Joie et al. (2010).
Table 1.
Slice types and features, based on La Joie et al., 2010.
| Slice type | Typical number of slices |
Defining feature |
Outter Boundaries | Internal boundaries (if novel or changed from previous slices) |

|
|---|---|---|---|---|---|
| a. Head – anterior, subiculum only | 1 to 2 | In head, but no SRLM | alveus superiorly (inclusive), parahippocampal WM inferiorly, CSF laterally | n/a |
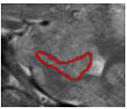
|
| b. Head – anterior, subiculum and CA1 | 1 to 2 | In head, SRLM | alveus superiorly (inclusive), parahippocampal WM inferiorly, CSF laterally | The subiculum was separated from CA1 by
the darker band (representing the stratum lacunosummoleculare - SRLM) and white voxels of CSF from uncal sulcus |
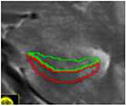
|
| c. Head – with digitations | 2 to 3 | In head, clear “digits” | alveus superiorly (inclusive), parahippocampal WM inferiorly, CSF laterally | CA1 was traced on both the medial and
lateral ends of the superior/ dorsal GM band, while the middle digitation (or medial digit when only 2 were present) of this superior GM band corresponded to the CA2-4/DG subfield. Vertical lines are traced to separate CA1 from other subfields on this slice |
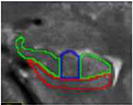
|
| d. Head – post digitations | 1 to 2 | In head, post digits | alveus superiorly (inclusive), parahippocampal WM inferiorly, CSF laterally | CA1 was traced on the lateral end of the
superior/dorsal GM band. In agreement with Joie et al., 2010 and Harding et al., 1998, the CA1/subiculum border was not constant along the medial–lateral axis: in the most anterior slices of the body of the hippocampus, most of the inferior part of the hippocampus was considered as subiculum, while in the most posterior slice, CA1 progressed medially |
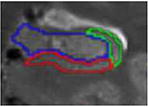
|
| e. Head – uncal apes | 1 | Last slice in head, uncal apex visible | alveus superiorly (inclusive), parahippocampal WM inferiorly, CSF laterally | same as above |
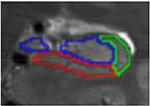
|
| f. Body | 9 to 10 | post uncal apex | alveus superiorly (inclusive), parahippocampal WM inferiorly, CSF laterally, CSF medially | Vestigial hippocampal sulcus used as the infero-medial border of the CA2-4/DG subfield |
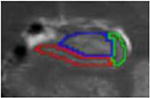
|
| g. Body – last slice | 1 | slice before fornix separates | alveus superiorly (inclusive), parahippocampal WM inferiorly, CSF laterally, CSF medially | CAl/subiculum border is placed from the
intersection between CA2-4/DG and subiculum subfields to the adjacent parahippocampal gyrus white matter |
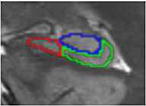
|
Two raters (FG, TR) blinded to the identity and age of the subjects independently traced 10 cases (2 from each of the 5 age groups) bilaterally. DSCs were calculated to determine overlap and were as follows for each subregion and subfield: subiculum-head = .75, subiculum-body = .73, CA1-head = .72, CA1-body = .78, CA2-4/DG-head = .82, CA2-4/DG-body = .85. Intra-rater reliability was also assessed; DSCs were follows: subiculum-head = .75, subiculum-body = .73, CA1-head = .70, CA1-body = .78, CA2-4/DG-heaad = .81, CA2-4/DG-body = .87. DSC values above 0.7 are typically considered acceptable for agreement (Zijdenbos, Dawant, Margolin, & Palmer, 1994); as such, overlap between the two raters indicated agreement.
One rater (FG) then traced an additional 10 cases (again, 2 from each age group). These segmentations were combined with the 10 cases used for manual reliability (i.e., 20 total) and input into Automatic Segmentation of Hippocampal Subfields software (ASHS, Yushkevich, Pluta, et al., 2015). This yielded a study-specific template, which was subsequently used to generate hippocampal subfield volumes for the entire sample. All resulting segmentations were checked manually for quality. Segmentations with clear errors were omitted from further analysis. No manual edits were made on the remaining segmentations, but it was noted that variability was greater in the head than the body due to greater variability of the underlying neuroanatomy of this region (Ding & Van Hoesen, 2015). During this quality check process, demarcation of head and body boundaries was performed using the anatomical landmarks described above.
In order to ensure that any observed effects were not the result of differences in brain size, subregion and subfield volumes were adjusted to control for differences in intracranial volume (ICV) using an analysis of covariance approach (Raz et al., 2005; Van Petten, 2004). Brain extraction was conducted separately in 6 toolboxes including ANTs, AFNI, FSL, BSE, ROBEX, and SPM8. The voxels extracted by at least four toolboxes were included in the brain mask (see Tillman et al., 2017 for similar approach). Exploration of ICV values indicated significant independent influences of age (β = .302, p < .001) and sex (β = −.365, p < .001) on total brain size (adjusted R2 = .196, F(2, 183) = 23.599, p < .001). Preliminary analyses examining relations between regional volumes and ICV for each age/gender group revealed heterogeneity of this relation (e.g., left hippocampal head predicted by ICV: 4-year-old females b = 257.839, SE = 80.437; 7-year-old females b = −6.66, SE = 118.524, z = 1.85, p < .05), thus corrections were carried out for each age group separately, using age and sex to estimate ICV values (adjusted volume = raw volume – b * (ICV – predicted ICV), see Keresztes et al., 2017). To account for the possibility that any observed effects were simply a product of this adjustment, results were examined for native volumes first and then for adjusted volumes. Only the latter are reported.
Statistical analysis
Effects of age and sex on hippocampal volume
Data were collapsed across hemispheres and examined for outliers, which were removed for analysis. All predictor variables were mean-centered and sex was dummy coded. Interaction terms were calculated by multiplying mean-centered Age by dummy-coded Sex. Linear regressions were conducted using Age, Sex, and Age × Sex interactions to predict volumes of the following three subregions: head, body, and tail. Exploratory analyses examined the possibility of non-linear effects using Age2 and Age2 × Sex interactions; however, these showed no evidence of nonlinear effects (according to Akaike information criterion or AIC values and the lack of significance of these terms).
Follow-up regressions using the same predictors (Age, Sex, and Age × Sex) were conducted to predict volumes of the following hippocampal subfields: subiculum, CA1, and CA2-4/DG in both the head and body separately. Exploratory analyses examined the possibility of non-linear effects using Age2 and Age2 × Sex interactions. A non-linear model was identified as a better fit for CA1 in the hippocampal head based on lower AIC values for this model.
Effects of sex and age on memory performance
Linear regressions were conducted using Age, Sex, and Age × Sex interactions to predict memory for facts, memory for the source of the facts and the three types of errors made: guessed/knew responses, extra-experimental errors, and intra-experimental errors.
Relations between hippocampal volume and memory
Relations between the hippocampus and memory were examined using linear regression. The following variables were entered as predictors of performance on the memory task: Age, Sex, IQ, volumes for either subregions (head, body, and tail) or subfields (subiculum, CA1, CA2-4/DG) in both the head and body, plus interactions between these volumes and age. When interactions with age were observed, they were plotted for illustration purposes only using values 1 standard deviation above and 1 standard deviation below the mean age. IQ was included as a covariate to ensure any observed effects were not due to difference in overall intelligence.
Results
To preview, results suggest age- and sex-related differences are present in the head of the hippocampus during early childhood. Examination of subfields within the hippocampal head showed effects of sex on both subiculum and CA2-4/DG, whereas age effects were apparent in CA1. Within the hippocampal head, relations between memory and CA1 were moderated by age; in younger children bigger was better, in older children smaller was superior. In addition, within the hippocampal body, smaller CA1 and larger CA2-4/DG both contributed to better memory performance across all ages.
Age- and Sex-effects on Hippocampal Subregions
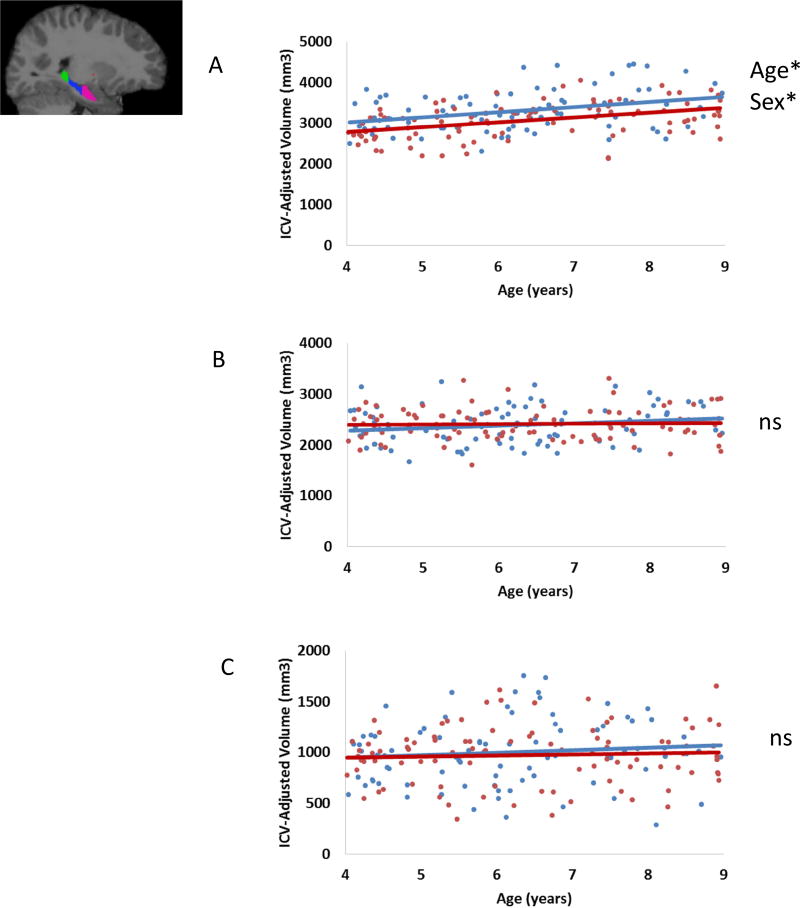
Figure 1 and Table 2 summarizes the observed effects of Age and Sex on hippocampal subregion volumes. Results of analyses are presented for hippocampal head, followed by the body and the tail.
Figure 1.
ICV-adjusted subregion volumes as a function of age for males and females in the A) head (pink), B) body (blue), and C) tail (green). (In scatterplots, blue indicates male participants, red indicates female participants, *denotes p < .05, ns = not significant).
Table 2.
Summary of regression analyses predicting hippocampal subregion volumes (n=186)
| Predictor variables |
Head | Body | Tail |
|---|---|---|---|
|
| |||
| β | β | β | |
| Age | .371*** | .026 | .052 |
| Sex | .265*** | −.028 | .053 |
| Age × Sex | 0.011 | .124 | .124 |
|
| |||
| Adj. R2 | .182*** | .005 | −.007 |
| F | 14.601*** | 1.331 | .569 |
p < .05
p < .01
p < .001
Results of the regression indicated the model explained 19.6% of variance in hippocampal head volume. Both Age and Sex significantly predicted hippocampal head volume. Volume increased with age and volume in males was greater than volume in females. The interaction between Age and Sex was not significant.
Results indicated this model did not explain a significant amount of variance in hippocampal body volume nor in the tail.
Distribution of Subfields across Subregions
One possible reason that age and sex differences emerged in the head of the hippocampus, as opposed to the body or tail, is that different proportions of hippocampal subfields may be found in this subregion compared to the more posterior subregions. Previous research in adults has suggested that hippocampal subfields are disproportionally distributed along the longitudinal axis. Specifically, in adults, the largest part of the dentate gyrus is found within the hippocampal body, whereas the largest part of CA1–2 is found within the hippocampal head (Malykhin, Lebel, Coupland, Wilman, & Carter, 2010). However, such differential distribution has not been documented in children. To examine this question, we computed proportion values for each ICV-adjusted subfield within each subregion.
To explore differences in the distribution of the subfields along the longitudinal axis, we conducted a 2 Subregion (head, body) × 3 Subfield (subiculum, CA1, CA2-4/DG) RM-ANOVA with Age and Sex as covariates. This revealed an interaction between Subregion and Subfield (F(2,276) = 10.856, p < .001). Follow-up paired t-tests suggested the proportion of the subiculum in the head (.34) was greater than that in the body (.26), t(140) = 22.342, p < .001; the proportion of CA1 was smaller in the head (.26) than the body (.30), t(140) = −9.40, p < .001, and the proportion of CA2-4/DG was smaller in the head (.39) than in the body (.44), t(140) = 22.342, p < .001. These results suggest, similar to adults, subfields are disproportionately distributed along the long axis of the hippocampus.
Age- and Sex-Effects on Hippocampal Subfields
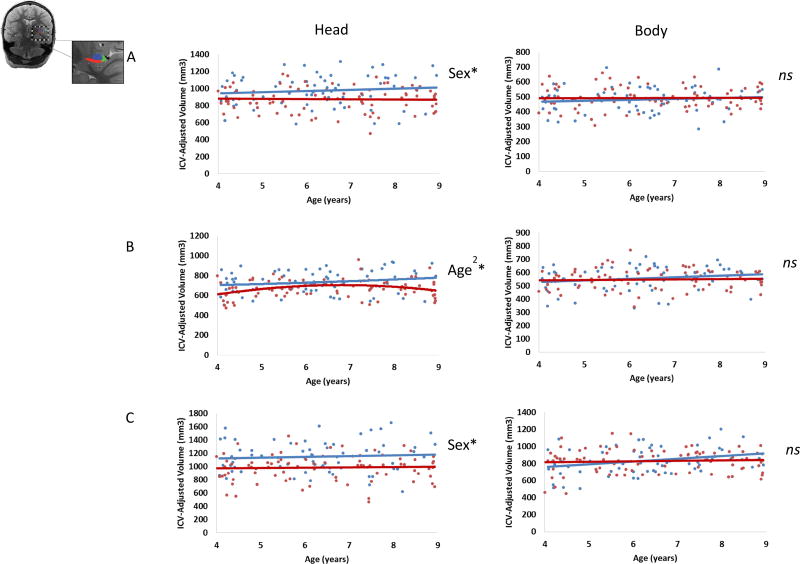
Regression analyses were used to examine effects of Age and Sex on each subfield (subiculum, CA1, and CA2-4/DG) within each subregion. Results are presented grouped by subregion (head, body). Figure 2 provides a summary of the effects of Age and Sex on hippocampal subfield volume.
Figure 2.
Effects of Age and Sex on A) subiculum (red), B) CA1 (green), and C) CA2-4/DG (blue) volumes in both the head (left column) and body (right column). (In scatterplots, blue indicates male participants, red indicates female participants, *denotes p < .05, ns = not significant).
Head
Results (summarized in Table 3) indicated main effects of Sex in the subiculum and CA2-4/DG within the head of the hippocampus. For the subiculum, this model explained 9.3% of the variance. Sex was a significant predictor, with greater volumes in males than in females; however, neither Age nor the Age × Sex interaction was significant. For CA2-4/DG, this model explained 13.0% of the variance. Sex was a significant predictor, with greater volumes in males than in females; however, neither Age nor the Age × Sex interaction was significant.
Table 3.
Summary of regression analyses predicting hippocampal head subfield volumes (n=153).
| Head | |||
|---|---|---|---|
|
| |||
| Predictor variables |
Subiculum | CA1 | CA2- 4/DG |
|
| |||
| β | β | β | |
| Age | −.022 | .153 | .028 |
| Sex | .299*** | .148 | .360*** |
| Age × Sex | .092 | .033 | .031 |
| Age2 | -- | −.23* | -- |
| Age2 × Sex | -- | .031 | -- |
|
| |||
| Adj. R2 | .075** | .105*** | .112*** |
| F | 5.087** | 4.56*** | 7.193*** |
p < .05
p < .01
p ≤ .001, -- not applicable
Results indicated a nonlinear model was a better fit for CA1 volume within the hippocampal head. This model explained 13.5% of the variance. Age2 was a significant predictor of volume. Neither Sex, Age, Age × Sex, nor the Age2 × Sex interaction were significant.
Body
In the hippocampal body, the model did not predict volume of subiculum (adjusted R2 = −.009, F(3,147) = .537, p = .657), CA1 (adjusted R2 = .002, F(3,145) = 1.122, p = .342), or CA2-4/DG (adjusted R2 = .024, F(3,147) = 2.215, p = .089).
Age- and Sex-Effects on Memory
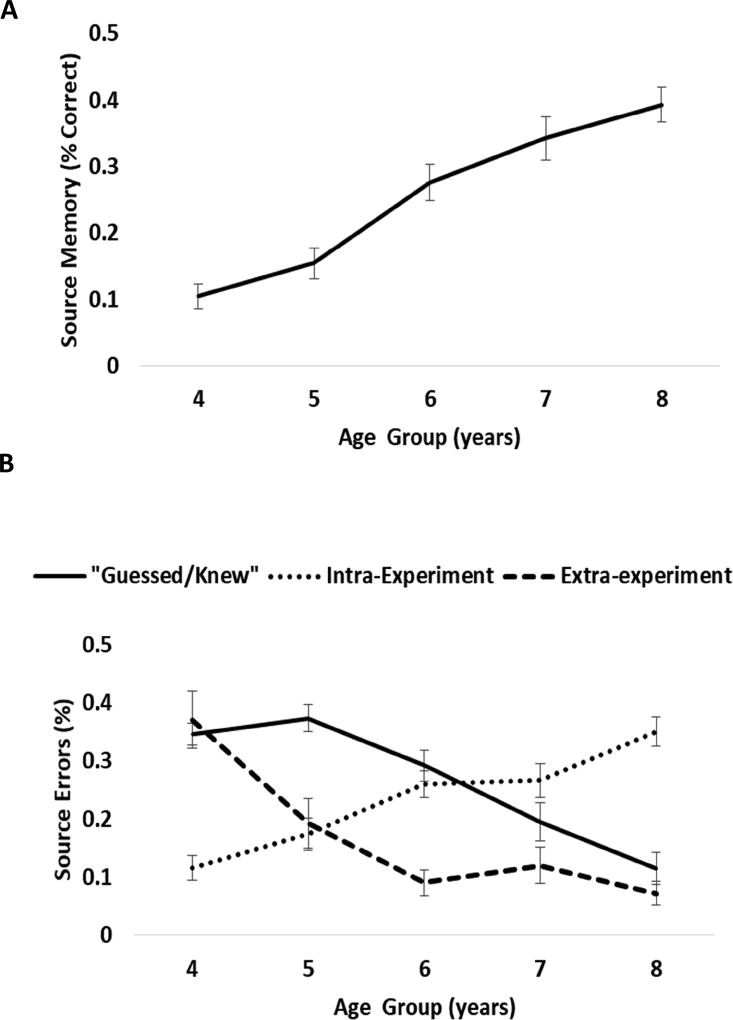
Results suggested effects of Age, but not Sex or interactions with Sex, for all memory measures (Figure 3, Table 4). Age was positively related to children’s ability to recall novel facts after a 1-week delay and the source from whom the facts were learned. Age was negatively related to children’s “guess/knew” responses and nominations of extra-experimental sources. Intra-experimental errors increased with age.
Figure 3.
Age-related differences in A) source memory and B) the types of source errors.
Table 4.
Summary of regression analyses predicting memory via age and sex (n=193).
| Predictor variables |
Fact Memory |
Source Memory |
“Guessed/Knew” responses |
Extra- experimental errors |
Intra- experimental errors |
|---|---|---|---|---|---|
|
| |||||
| β | β | β | β | β | |
| Age | .666*** | .641*** | −.421*** | −.392*** | .543*** |
| Sex | .212 | .303 | −.482 | .025 | .330 |
| Age × Sex | −.266 | −.328 | .512 | −.077 | −.255 |
|
| |||||
| Adj. R2 | .375*** | .326*** | .100*** | .152*** | .230*** |
| F | 39.380*** | 32.004*** | 8.142*** | 12.435*** | 20.115*** |
p < .05
p < .01
p ≤ .001
Relations between Hippocampal Volumes and Memory
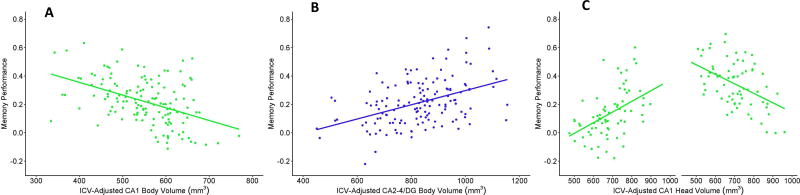
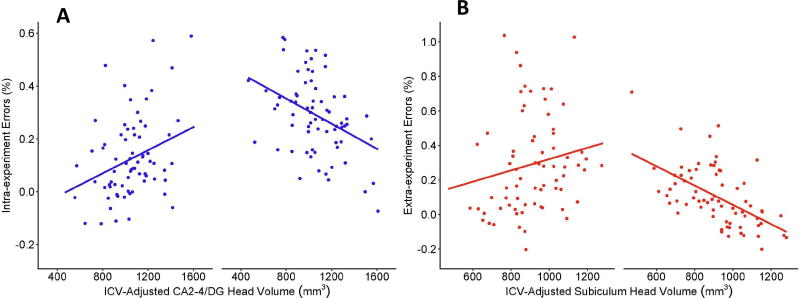
Relations between hippocampal volumes and source memory are summarized in Table 5. There were no significant relations between hippocampal subregion volumes and memory for facts (βs= −.062−.057, ps = .376–.883). However, the model examining relations between subfield volumes and source memory accuracy explained 46.1% of the variance. After accounting for main effects of Age, IQ and Sex, significant predictors included CA1 in the body, CA2-4/DG in the body, and the interaction between CA1 in the head and Age. As illustrated in Figure 4, better memory performance was associated with decreased CA1 (Figure 4A) and increased CA2-4/DG (Figure 4B) subfield volumes in the hippocampal body for all children. In addition, better memory performance in young children was associated with larger CA1 volume in the hippocampal head, whereas better memory performance in older children was associated with smaller CA1 volume in the hippocampal head (Figure 4C).
Table 5.
Summary of regression analyses predicting memory via hippocampal subfields (n=153)
| Predictor variables | Source Memory |
Intra- experimental errors |
Extra- experimental errors |
|---|---|---|---|
|
| |||
| β | β | β | |
| Age | .495*** | .476*** | −0.329*** |
| Sex | .021 | .070 | −.110 |
| IQ | .138* | .038 | −.084 |
| Head - subiculum | .122 | .187 | −.094 |
| Head - CA1 | −.008 | −.079 | .030 |
| Head - CA2-4/DG | .034 | −.036 | −.078 |
| Head - subiculum × Age | .196 | .227 | −.268* |
| Head - CA1 × Age | −.417*** | .012 | .203 |
| Head - CA2-4/DG × Age | −.053 | −.303* | .207 |
| Body - subiculum | .100 | .020 | −.047 |
| Body - CA1 | −.286** | −.014 | .154 |
| Body - CA2-4/DG | .332** | .042 | −.170 |
| Body - subiculum × Age | −.061 | −.034 | .109 |
| Body - CA1 × Age | .180 | −.260 | −.069 |
| Body - CA2-4/DG × Age | −.079 | .091 | .221 |
|
| |||
| Adj. R2 | 0.397*** | .270*** | .177*** |
| F | 7.135*** | 4.444*** | 3.003*** |
p < .05
p < .01
p ≤ .001
Figure 4.
Relations between memory performance and A) CA1 volume in the body, B) CA2-4/DG volume in the body and C) CA1 volume in the head for younger (-1 SD from mean, left) and older (+1 SD from mean, right) participants.
Relations were also observed between hippocampal subfield volumes and the types of errors children made on the memory task (Figure 5). First, the model examining relations between subfield volumes and intra-experimental errors (i.e., recollecting the fact was learned in the lab but a failure to recall exactly which source state the fact) explained 34.8% of the variance. After accounting for main effects of Age (, IQ and Sex, the only significant predictor was the interaction between CA2-4/DG volume in the head and Age. Intra-experimental errors were associated with increased CA2-4/DG volume in younger children and smaller CA2-4/DG volumes in older children (Figure 5A). Second, the model examining relations between subfield volumes and extra-experimental errors (i.e., stating the fact was learned from a source external to the lab) explained 26.5% of the variance. After accounting for main effects of Age, IQ and Sex, the only significant predictor was the interaction between subiculum volume in the head and Age. Extra-experimental errors were associated with increased subiculum volume in younger children, and smaller subiculum volumes in older children (Figure 5B). Finally, the model examining relations between subfield volumes and “guessed/knew” errors was not significant (adjusted R2 = .071, F(15,125) = 1.718, p = .055).
Figure 5.
Relations between A) intra-experimental errors and CA2-4/DG in the head for younger (-1 SD from mean, left) and older (+1 SD from mean, right) participants, and B) extra-experimental errors and subiculum volume in the head for younger (-1 SD from mean, left) and older (+1 SD from mean, right) participants.
Discussion
The goal of the present study was to systemically examine effects of age and sex on hippocampal subregion and subfield volumes during early childhood and their relations with memory performance. Results suggest age- and sex-related differences are present in the hippocampal head even after adjusting for individual differences in ICV. Within the head, sex effects were observed in subiculum and CA2-4/DG and age effects were observed in CA1. Hippocampal subfield volumes also showed relations with memory. Specifically, within the hippocampal head, relations between memory and CA1 were moderated by age; in younger children bigger was better, in older children smaller was superior. Within the hippocampal body, smaller CA1 and larger CA2-4/DG both contributed to better memory performance across all ages. In addition, subfield volumes were also related to errors children made on the task. Intra-experimental errors were related to CA2-4/DG volume in the head, whereas extra-experimental errors were related to subiculum volume in the head. These findings can be interpreted in light of the fact that intra-experimental errors reflect episodic memory processing (i.e., children need to recall that the fact was learned in the laboratory setting), whereas extra-experimental errors draw on semantic knowledge (e.g., where does one commonly learn information). Finally, “guessed/knew” responses did not relate to volume, likely because these responses are more related to more global metacognitive abilities. Overall, these findings are consistent with previous studies in older individuals and with neuroanatomical data from non-human primate tissue samples, which suggest prolonged development of hippocampal subfields (Eckenhoff & Rakic, 1988; Lavenex & Banta Lavenex, 2013; Serres, 2001). However, the present findings add specificity to previous results and shed some light on why early childhood may be a time of rapid change in memory ability.
In terms of volume, age-related differences were observed in children within a relatively narrow 5-year period. Most previous studies showing age-related differences in volume tend to examine a wider age range (e.g., children to adults or 4 to 22 years). However, this finding is not surprising given that it is consistent with research in nonhuman primates suggesting early childhood is a period of significant change in the hippocampus (Lavenex & Banta Lavenex, 2013). The neurobiological processes underlying age-related differences likely include synaptic growth, dendritic arborization, pruning, vascularization and myelination (Benes et al., 1994; Huttenlocher, 1990; Lenroot & Giedd, 2006). Postnatal neurogenesis likely also contributes to these differences, although it is thought to be largely restricted to DG (Cayre et al., 2009; Toni et al., 2008). According to animal models, immature cells continue to accrue within the DG postnatally and elevated rates of dendritic development and synapse formation persist until at least 5 years of age in humans (Eckenhoff & Rakic, 1988; Lavenex & Banta Lavenex, 2013; Serres, 2001). During early childhood, neuronal connections between granule cells of the dentate gyrus and pyramidal neurons of Ammon’s horn form, which alter the functional circuits of the hippocampus and regions located downstream from the dentate gyrus, particularly CA3 (see Lavenex & Banta Lavenex, 2013 for recent review). These processes, and others, may have contributed to volume differences in our sample.
Results also suggest that these observed differences have functional significance, as volumes were related to memory in an age-dependent manner. Specifically, similar to previous reports, CA1 in the head was related to memory. In younger children, larger volumes were related to better memory, a finding similar to Riggins et al. (2015) and Tamnes et al. (2014). In older children, smaller volumes were related to better memory, a finding similar to Schlichting et al. (2017). Additionally, in the present study, relations between memory and volume were also observed in CA2-4/DG within the body. Similar to Lee et al. (2014), Daugherty et al. (2016), and Tamnes et al. (2014), larger volumes were related to better memory performance. However, a novel finding in the present study was that CA1 in the body negatively related to memory performance. This finding may have emerged because of the novel age group under investigation, the specific nature of the memory task utilized, or a combination of these and other methodological factors.
Sex differences observed in subiculum and CA2-4/DG in the head of the hippocampus are consistent with the previous studies that suggest sex differences exist in hippocampal subfields, particularly in young children (Krogsrud et al., 2014; Tamnes et al., 2014). Similar to these reports, volumes were greater in males than females. However, sex was not related to memory performance. This finding is consistent with our behavioral results, which suggest no differences between males and females in performance on the memory task. Findings of sex differences may be related to the plethora of receptors for sex hormones (estrogen and androgen) within the hippocampus, as well as other non-hormonal factors (Geidd et al., 1996; Marrocco & McEwen, 2016; McEwen, 2010; Scharfman & MacLusky, 2016).
Findings of age-related differences in memory ability within this developmental period were strikingly similar to previous reports (e.g., Drummey & Newcombe, 2000; Riggins, 2014). Memory increased as a function of age, with the greatest increases between 5–6 years of age. In addition, the type of errors children made also changed in the expected manner. Young children were more likely to nominate extra-experimental sources or to indicate they guessed/knew the answer, whereas older children were more likely to recall that the fact had been discussed in the research setting and were more likely to make intra-experimental errors (i.e., forgetting exactly which of the sources was associated with that fact). This is consistent with the vast literature on children’s memory development that suggests that the ability to bind details of an event together and recall these details later in life is what matures during memory development (Bauer et al., 2012; Drummey & Newcombe, 2002; Riggins, 2014; Sluzenski, Newcombe, & Kovacs, 2006).
Overall, findings from hippocampal subregions and subfields highlight the importance of examining the hippocampus in as much detail as possible (i.e., at the level of subfields), as age- and sex-related differences in volume and relations with memory were most apparent at this level of analysis. Studies that examine the hippocampus at the level of subregions collapse across functionally distinct circuits in subfields and are therefore at-risk for missing effects unique to specific subfields.
Despite the fact that significant age- and sex- effects were observed on hippocampal subfield volumes in the hippocampal head, these factors often only accounted for a modest proportion of variance. Moreover, sex and age did not account for much of the variance in the volume of hippocampal body or tail. This suggests the presence of other substantial influences on hippocampal development during this period (e.g., stress, parental care, sleep). These factors should be identified and considered in subsequent studies. Similarly, although measures of hippocampal subfields accounted for a fair amount of variation in memory performance (46%), it is certain that other neural regions, particularly cortical regions such as prefrontal cortex and posterior parietal cortex also contribute to memory during this period (see Ghetti & Bunge, 2012 for review).
Strengths of the present investigation include the novel age range investigated, the large sample size examined, and the exploration of both subregions and subfields in the same individuals. Moreover, identification of subfields in both the head and body of the hippocampus is notable as the combined use of manual and automated tracing methodologies enhances the reproducibility of the methods. Despite these strengths, weaknesses include the cross-sectional design, the imbalance of males and females at different ages, lack of data on subfield volumes in the hippocampal tail, and the exclusive focus on the hippocampus.
This study is the first to report age- and sex-effects on hippocampal subregion and subfield development during early childhood. Results suggest both age- and sex-related effects, however only the former were related to memory ability. These findings add unique insight into a period characterized by dramatic changes in both brain development and cognitive behavior.
Acknowledgments
The authors declare no competing financial interest. We thank the members of the Neurocognitive Developmental Lab at the University of Maryland and the Maryland Neuroimaging Center for their support in data collection and the participants and their families for their participation. This research was supported by the National Institutes of Health (grant HD079518, awarded to T.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauer PJ. Remembering the Times of Our Lives. Remembering the Times of our Lives: Memory in Infancy and Beyond. 2006 https://doi.org/10.4324/9781315785226.
- Bauer PJ, Doydum AO, Pathman T, Larkina M, Güler OE, Burch M. It’s all about location, location, location: Children’s memory for the “ where” of personally experienced events. Journal of Experimental Child Psychology. 2012;113(4):510–522. doi: 10.1016/j.jecp.2012.06.007. https://doi.org/10.1016/j.jecp.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Tamminga CA. Human brain receptors, VII: Cortical GABA-ergic interneurons. American Journal of Psychiatry. 1994;151(8):1104. doi: 10.1176/ajp.151.8.1104. [DOI] [PubMed] [Google Scholar]
- Brown TT, Jernigan TL. Brain development during the preschool years. Neuropsychology Review. 2012 doi: 10.1007/s11065-012-9214-1. https://doi.org/10.1007/s11065-012-9214-1. [DOI] [PMC free article] [PubMed]
- Daugherty AM, Bender AR, Raz N, Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26(2):220–228. doi: 10.1002/hipo.22517. https://doi.org/10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Flinn R, Ofen N. NeuroImage Hippocampal CA3-dentate gyrus volume uniquely linked to improvement in associative memory from childhood to adulthood. NeuroImage. 2017 Dec;153:75–85. doi: 10.1016/j.neuroimage.2017.03.047. 2016. https://doi.org/10.1016/j.neuroimage.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaster D, Pathman T, Lee JK, Ghetti S. Structural Development of the Hippocampus and Episodic Memory: Developmental Differences Along the Anterior / Posterior Axis. 2013 doi: 10.1093/cercor/bht160. https://doi.org/10.1093/cercor/bht160. [DOI] [PubMed]
- Ding SL, Van Hoesen GW. Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto- and chemoarchitecture. Journal of Comparative Neurology. 2015;523:2233–2253. doi: 10.1002/cne.23786. [DOI] [PubMed] [Google Scholar]
- Drummey AB, Newcombe NS. Developmental changes in source memory. 2002;4:502–513. [Google Scholar]
- Duvernoy HM. The human hippocampus, functional anatomy, vascularization, and serial sections with MRI. 2. Spinger: Berlin; 1998. [Google Scholar]
- Eckenhoff MF, Rakic P. Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life span of the rhesus monkey. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1988;8(8):2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012 doi: 10.1016/j.neuroimage.2012.01.021. https://doi.org/10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed]
- Frisoni GB, Jack CR, Bocchetta M, Bauer C, Frederiksen KS, Liu Y, Winblad B. The EADC-ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: Evidence of validity. Alzheimer’s and Dementia. 2015;11(2):111–125. doi: 10.1016/j.jalz.2014.05.1756. https://doi.org/10.1016/j.jalz.2014.05.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience. 2012 doi: 10.1016/j.dcn.2012.05.002. https://doi.org/10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1997;21(8):1185–1201. doi: 10.1016/s0278-5846(97)00158-9. https://doi.org/10.1016/S0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16(8):664–672. doi: 10.1002/hipo.20193. https://doi.org/10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Harding A. Variation in hippocampal neuron number with age and brain volume. Cerebral Cortex. 1998;8(8):710–718. doi: 10.1093/cercor/8.8.710. https://doi.org/10.1093/cercor/8.8.710. [DOI] [PubMed] [Google Scholar]
- Hu S, Pruessner JC, Coupe P, Collins DL. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. NeuroImage. 2013;74:276–287. doi: 10.1016/j.neuroimage.2013.02.032. https://doi.org/10.1016/j.neuroimage.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. https://doi.org/10.1016/0028-3932(90)90031-I. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. Hippocampal Formation. In The Human Nervous System. 2012:896–942. https://doi.org/10.1016/B978-0-12-374236-0.10024-0.
- Josselyn SA, Frankland PW. Infantile amnesia: A neurogenic hypothesis. Learning & Memory. 2012;19(9):423–433. doi: 10.1101/lm.021311.110. https://doi.org/10.1101/lm.021311.110. [DOI] [PubMed] [Google Scholar]
- Keresztes A, Bender AR, Bodammer NC, Lindenberger U, Shing YL, Werkle-Bergner M. Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proceedings of the National Academy of Sciences, 201710654. 2017 doi: 10.1073/pnas.1710654114. https://doi.org/10.1073/pnas.1710654114. [DOI] [PMC free article] [PubMed]
- Krogsrud SK, Tamnes CK, Fjell AM, Amlien I, Grydeland H, Sulutvedt U, Walhovd KB. Development of hippocampal subfield volumes from 4 to 22 years. Human Brain Mapping. 2014;35(11):5646–5657. doi: 10.1002/hbm.22576. https://doi.org/10.1002/hbm.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Joie R, Fouquet M, Mézenge F, Landeau B, Villain N, Mevel K, Chételat G. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. NeuroImage. 2010;53(2):506–514. doi: 10.1016/j.neuroimage.2010.06.024. https://doi.org/10.1016/j.neuroimage.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behavioural Brain Research. 2013 doi: 10.1016/j.bbr.2013.02.007. https://doi.org/10.1016/j.bbr.2013.02.007. [DOI] [PubMed]
- Lee JK, Ekstrom AD, Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. https://doi.org/10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Lee JK, Nordahl CW, Amaral DG, Lee A, Solomon M, Ghetti S. Assessing hippocampal development and language in early childhood: Evidence from a new application of the automatic segmentation adapter tool. Human Brain Mapping. 2015 doi: 10.1002/hbm.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. https://doi.org/10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Marrocco J, McEwen BS. Sex in the brain: Hormones and sex differences. Dialogues in Clinical Neuroscience. 2016;18(4):373–383. doi: 10.31887/DCNS.2016.18.4/jmarrocco. https://doi.org/10.12032/TMR201705034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: Mechanisms of neuronal remodeling. Annals of the New York Academy of Sciences 1204. 2010;(SUPPL.1):38–59. doi: 10.1111/j.1749-6632.2010.05568.x. https://doi.org/10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997 doi: 10.1016/s0959-4388(97)80010-4. https://doi.org/10.1016/S0959-4388(97)80010-4. [DOI] [PubMed]
- Ngo CT, Newcombe NS, Olson IR. The ontogeny of relational memory and pattern separation. Developmental Science. 2017 doi: 10.1111/desc.12556. https://doi.org/10.1111/desc.12556. [DOI] [PMC free article] [PubMed]
- O’Keefe J, Nadel L. Précis of O’Keefe & Nadel’s The hippocampus as a cognitive map. Behavioral and Brain Sciences. 1979;2(4):487–494. https://doi.org/10.1017/S0140525×00063949. [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. https://doi.org/10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. https://doi.org/10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Developmental Psychology. 2014;50(2):449–59. doi: 10.1037/a0033622. https://doi.org/10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Blankenship SL, Mulligan E, Rice K, Redcay E. Developmental Differences in Relations Between Episodic Memory and Hippocampal Subregion Volume During Early Childhood. Child Development. 2015;86(6):1710–1718. doi: 10.1111/cdev.12445. https://doi.org/10.1111/cdev.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Sex differences in hippocampal area CA3 pyramidal cells. Journal of Neuroscience Research. 2017 doi: 10.1002/jnr.23927. https://doi.org/10.1002/jnr.23927. [DOI] [PMC free article] [PubMed]
- Schlichting ML, Guarino KF, Schapiro AC, Turk-browne NB, Preston AR. Hippocampal Structure Predicts Statistical Learning and Associative Inference Abilities during Development. 2016;i:37–51. doi: 10.1162/jocn_a_01028. https://doi.org/10.1162/jocn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2001. pp. 45–58. [Google Scholar]
- Sluzenski J, Newcombe NS, Kovacs SL. Binding, Relational Memory, and Recall of Naturalistic Events: A Developmental Perspective. 2006;32(1):89–100. doi: 10.1037/0278-7393.32.1.89. https://doi.org/10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Engvig A, Grydeland H, Krogsrud SK, Østby Y, Fjell AM. Regional hippocampal volumes and development predict learning and memory. Developmental Neuroscience. 2014;36(3–4):161–174. doi: 10.1159/000362445. https://doi.org/10.1159/000362445. [DOI] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, Shackman AJ. Intrinsic functional connectivity of the central extended amygdala. bioRxiv. 2017 doi: 10.1002/hbm.23917. (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H. Developmental Trajectories of Amygdala and Hippocampus from Infancy to Early Adulthood in Healthy Individuals. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0046970. https://doi.org/10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004 doi: 10.1016/j.neuropsychologia.2004.04.006. https://doi.org/10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed]
- Wang H, Das SR, Suh JW, Altinay M, Pluta J, Craige C, Yushkevich PA. A learning-based wrapper method to correct systematic errors in automatic image segmentation: Consistently improved performance in hippocampus, cortex and brain segmentation. NeuroImage. 2011;55(3):968–985. doi: 10.1016/j.neuroimage.2011.01.006. https://doi.org/10.1016/j.neuroimage.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Leroux G. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42(9):1743–1743. doi: 10.1212/wnl.42.9.1743. https://doi.org/10.1212/WNL.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Dewitt I, Goff D, Ditman T, Heckers S. Anterior and posterior hippocampal volumes in schizophrenia. Schizophrenia Research. 2005;73(1):103–112. doi: 10.1016/j.schres.2004.05.018. https://doi.org/10.1016/j.schres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Amaral RSC, Augustinack JC, Bender AR, Bernstein JD, Boccardi M, Zeineh MM. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: Towards a harmonized segmentation protocol. NeuroImage. 2015;111:526–541. doi: 10.1016/j.neuroimage.2015.01.004. https://doi.org/10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding SL, Gertje EC, Wolk DA. Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Human Brain Mapping. 2015;36(1):258–287. doi: 10.1002/hbm.22627. https://doi.org/10.1002/hbm.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging. 1994;13(4):716–724. doi: 10.1109/42.363096. https://doi.org/10.1109/42.363096. [DOI] [PubMed] [Google Scholar]