Abstract
The deleterious effects of alcohol use disorders (AUDs) on human health have been documented worldwide. The enormous socioeconomic burden coupled with lack of efficacious pharmacotherapies underlies the need for improved treatment strategies. At present, there is a growing body of preclinical evidence that demonstrates the potential of avermectins [ivermectin (IVM), selamectin (SEL), abamectin (ABM), and moxidectin (MOX)] in treatment of AUDs. Avermectins are derived by fermentation of soil micro-organism, Streptomyces avermitilis and have been extensively used for treatment of parasitic infections. From the mechanistic standpoint, avermectins are positive modulators of purinergic P2X4 receptors (P2X4Rs). P2X4Rs belong to P2X superfamily of cation permeable ion channels gated by adenosine-5’-triphosphate (ATP). Building evidence has implicated a role for P2X4Rs in regulation of ethanol intake and that ethanol can inhibit ATP-gated currents in P2X4Rs. Investigations using recombinant cell models and animal models of alcohol drinking have reported that IVM, ABM, MOX but not SEL, were able to antagonize the inhibitory effects of ethanol on P2X4Rs in vitro and reduce ethanol intake in vivo. Furthermore, IVM was shown to reduce ethanol consumption via P2X4R potentiation in vivo; supporting involvement of P2X4Rs in IVM’s anti-alcohol effects and that P2X4Rs can be used as a platform for developing novel anti-alcohol compounds. Taken together, these findings support the utility of avermectins as a novel class of drug candidates for treatment of AUDs.
Prevalence of Alcohol use disorders in the United States
Alcohol use disorders (AUDs) are one of the leading causes of mortality and morbidity in United States and worldwide, contributing to 3.8% of global deaths and 4.6% of disability-adjusted life years (DALYs) (Rehm et al. 2009). Alcoholism is also one of leading risk factors for diseases including cancer, diabetes and cardiovascular disorders (Costanzo et al. 2010; Rehm et al. 2003; Smyth et al. 2015) and is found to be co-morbid with severe mental disorders including bipolar disorder, major depressive disorder and post traumatic stress disorder (Grant et al. 2015; Regier et al. 1990). In the United States, the prevalence of AUDs increased from 12.7% in 2001–2002 (Grant et al. 2004) to 13.9% in 2012–2013, which equates to roughly 32 million individuals (Grant et al. 2015). During the 2006–2010 periods in the United States, the average annual alcohol attributable death (AAD) rate in the United States was 27.9 deaths per 100,000 and comprised nearly 9.8% of total deaths (Stahre et al. 2014). On account of significant contribution to deaths and premature mortality among the working age population, AUDs remain to be a massive economic burden. Economic costs (i.e. expenditure arising from productivity losses, criminal justice system, motor vehicle accidents) associated with alcoholism in the United States, increased from $223.5 billion in 2006 (Bouchery et al. 2011) to $249 billion in 2010 (Sacks et al. 2015). Furthermore, alcoholism remains to be a pervasive problem among the young population as it is regarded as the highest risk factor for DALYs in the young population aged 20–24 years, affecting approximately 9.7% of individuals within this age group (Mokdad et al. 2016). Moreover, binge drinking among youth population has attributed to deaths, self-inflicted injury, physical and sexual assault, memory loss, suicide attempts and poor academic performance (White and Hingson 2013). The detrimental effects of alcoholism on health across multiple age groups and the substantial economic losses linked to alcoholism, emphasizes the need to develop effective and useful treatment strategies to curb the disease.
Psychosocial intervention alone has very little impact on controlling behavioral symptoms of AUDs, with relapse rated as high as 70% since patient resume to drinking within a year (Finney et al. 1996; Swift 1999). However, the pharmacotherapy for treatment of AUDs has met with limited success over past several decades. At present, only 3 medications have been approved by FDA for AUDs which include disulfiram (Antabuse), naltrexone (Revia and Vivitrol) and acamprosate (Campral) (Castro and Baltieri 2004; Heilig and Egli 2006; Soyka and Lieb 2015; Soyka and Muller 2017). Disulfiram inhibits alcohol dehydrogenase and causes build-up of acetaldehyde, leading to adverse events that induce aversion to alcohol. The efficacy of disulfiram for treatment of AUDs has been met with negative criticism on account of lack of significant effects in various meta-analysis studies (Berglund et al. 2003; Heilig and Egli 2006). It is also widely accepted that efficacy of disulfiram is dependent upon the psychological attitude of patients and does not address the addiction problem (Hughes and Cook 1997). On the other hand, naltrexone (non-selective opioid antagonist) and acamprosate [partial agonist at N-methyl-D-aspartate receptors (NMDARs)] have been reported to negate the positive reinforcing effects of alcoholism, thereby reducing the craving and dependence (Bouza et al. 2004; O'Malley et al. 1992; Volpicelli et al. 1992). The utility of naltrexone as an effective anti-alcohol therapy has been somewhat controversial since naltrexone has been shown to reduce relapse rates as well as frequency and amount of drinking; but it does not significantly prolong abstinence, thus casting aspersions on its long-term benefits in the real world (Anton et al. 2001; Krystal et al. 2001; O'Malley et al. 1996). In addition, naltrexone is associated with severe adverse events including hepatotoxicity, asthenia and nausea, thus leading to high attrition rates in clinical trials (Croop et al. 1997). Acamprosate has been documented to exhibit significant effects on relapse rates and frequency of drinking in European trials, but its lack of efficacy in two double-blind studies in the United States raises skepticism about its potential as an anti-alcohol medication (Johnson 2008). While, acamprosate does have fewer serious adverse effects in comparison to naltrexone (Bouza et al. 2004), the complex dosing regimen of acamprosate (two 330 mg tablets, thrice a day), raises compliance issues with this drug (Heilig and Egli 2006). It is also hypothesized that acamprosate may preferentially induce abstinence in patients who have suffered from long term intoxication of ethanol and multiple withdrawal episodes (Heinz et al. 2003; Ulrichsen et al. 1998). The compliance issues, serious adverse events and methodological limitations with several of clinical studies associated with current drug therapy reinforces the need to discover and develop novel pharmacotherapies for effectively targeting the core phenomena of alcohol abuse and dependence.
Avermectins as a novel class of therapeutic agents for AUDs
The avermectins are group of semi-synthetic macrocyclic lactones derived from the soil micro-organism, Streptomyces avermitilis (Burg et al. 1979). They have been widely popular for their broad-spectrum efficacy against worms and ectoparasites in agricultural animals (Egerton et al. 1979; Ottesen and Campbell 1994). Avermectins exhibit their anti-parasitic activity via activation of glutamate gated Cl− channels in invertebrate nervous system (Wolstenholme and Rogers 2005). There is a now a growing body of literature that describes the ability of avermectins in modulation of ionotropic receptors in the central nervous system (CNS) that are typically involved in the cellular and behavioral effects of ethanol and could potentially represent a new class of anti-alcohol agents. In this review, we will shed light on in vitro and in vivo evidence of four such avermectins namely ivermectin (IVM), abamectin (ABM), selamectin (SEL) and moxidectin (MOX) that underlies their potential as novel anti-alcohol therapies.
Discovery and development of IVM
IVM, a semi-synthetic dihydro lactone derivative [22,23-dihydroavermectin B1a (80%) and B1b (20%)] was the first commercially available macrocyclic lactone antihelmintic used in the world of veterinary and human medicine (Crump and Omura 2011; Geary 2005; Omura 2008; Ottesen and Campbell 1994). IVM was discovered at the Kitasho Institute; Japan in collaboration with the US based Merck-Sharp-Dohme parasitology team in the mid-1970s (Crump and Otoguro 2005; Omura and Crump 2004). Initially, IVM was introduced to the world of veterinary medicine in 1981 on basis of its superiority over existing antihelmintics such as benzimidazoles, levamisole, pyrantel and its broad potency against a wide spectrum of nematodes and ectoparasites including roundworms, ticks, fleas and flies (Burg et al. 1979; Campbell et al. 1983; Egerton et al. 1979). IVM was truly considered a revolutionary drug at that point of time due to its unique pharmacological profile. That is, it inhibited glutamate Cl− channels in parasites or nematodes, leading to paralysis of pharyngeal and body wall muscles, as a result of which the parasites would die of starvation (Arena et al. 1991; Arena et al. 1995; Arena et al. 1992; Cully et al. 1994; Geary et al. 1993). In addition to its pharmacological profile, IVM possessed high lipophilicity, as a result of which, could be made available in wide variety of formulations including injectables, oral formulations, tropical formulations for treatment of livestock diseases (Geary 2005).
IVM was not used for treatment of human diseases until 1985, in the aftermath of a global effort initiated by the United Nations to treat tropical diseases that were a major public health concern in various endemic areas of Africa including onchocerciasis, a deadly infection caused by the filarial nematode, Onchocerca volvulus (Crump and Omura 2011). As a single annual dose of 150 µg/kg for onchocerciasis, IVM was effective in removing microfilariae from the skin lymphatics and inhibited the ability of female worms to generate more microfilariae, leading to extreme low levels of microfilaria for 12 months (Bennett et al. 1988; Remme et al. 1990; Taylor and Greene 1989). Notably, IVM did not cause any severe hypersensitive reactions or ocular damage that are generally associated with other existing therapies such as diethylcarbamazine and suramin (Dadzie et al. 1987; Taylor et al. 1986). In addition to onchocerciasis, IVM was also effective in treatment of other debilitating tropical diseases of socio-economic importance including lymphatic filariasis (Kumaraswami et al. 1988), Strongyloidiasis (Freedman et al. 1989; Richard-Lenoble et al. 2003) and Pediculosis (Dunne et al. 1991). Overall, a unique mechanism of action and excellent patient compliance made IVM a breakthrough drug in saving millions of lives in African countries where severe tropical diseases were an epidemic.
Pharmacological action of IVM on ion channels
a) Ligand gated ion channels (LGICs) belonging to Cys-loop superfamily
IVM has been demonstrated to be a positive allosteric modulator (PAM) of various ionotropic receptors that belong to the Cys-loop superfamily including the γ-amino-butyric acid receptors (GABARs) (Dawson et al. 2000; Krusek and Zemkova 1994; Schaeffer and Haines 1989; Sigel and Baur 1987), glycine receptors (GlyRs) (Graham et al. 1982; Shan et al. 2001) and nicotinic acetylcholine receptors (nAChRs) (Collins and Millar 2010; Krause et al. 1998) since IVM was shown to potentiate activity of these receptors in presence of the natural ligand and not independently.
Previous reports have shown that IVM can potentiate GABAARs-mediated Cl− currents as well as can reduce the desensitization of GABA-mediated currents in recombinant cell models (Dawson et al. 2000; Sigel and Baur 1987), cultured rat brain cortical neurons (Dawson et al. 2000) and mouse hippocampal embryonic neurons (Krusek and Zemkova 1994). IVM’s binding affinity for GABAARs seemed to be dependent upon the stoichiometry of the receptor since IVM’s ability to potentiate GABAARs was determined by the β, but not α or γ subunit composition in Xenopus oocytes (Dawson et al. 2000). At the behavioral level, IVM was shown to block anxiogenic effects induced by GABAergic antagonist, picrotoxin, in various animal models that measure anxiety including elevated-plus maze, open field and conflict behavior test, which is typical characteristic of GABAAR PAMs such as benzodiazepines (Spinosa et al. 2002).
IVM has been reported to be an unconventional modulator of GlyRs. Low concentrations of IVM (0.03 µM), exhibited strong potentiation of Cl− currents in presence of sub-saturating concentrations of glycine (25 µM), but high IVM concentrations (>0.1 µM) directly activated glycine-gated currents (Shan et al. 2001). These findings suggested a concentration-dependent-activity by IVM where it acted as a PAM at lower concentrations, but at higher concentrations it could serve as a direct agonist. Interestingly, IVM was reported to inhibit glycine-mediated Cl− currents when tested in cultured cortical neurons (Dawson et al. 2000), suggesting that IVM’s action on GlyRs may be subunit-dependent.
IVM has also been reported to act as a PAM of α7 nAChRs in the brain (Krause et al. 1998). IVM significantly increased ACh-mediated Ca2+ currents of nAchRs in Xenopus oocytes as well as in HEK 293 cells that naturally express human form of nAChRs by causing a significant increase in affinity of ACh for the receptors. This observed enhancement of Ca2+ currents was not accompanied by any changes in Cl− concentration in the cytoplasm, indicating that IVM directly potentiates nAChRs-mediated currents independent of Cl− concentration (Krause et al. 1998). Site directed mutagenesis studies have shown that certain amino acids in the four transmembrane (TM) domains (Ser 222 in TM1, Met 253 in TM2 and Ser 276 in TM3) of α7 nAChRs may play an important role in determining the modulatory effects of IVM on the receptors (Collins and Millar 2010).
b) LGICs belonging to purinergic P2X superfamily
The P2X superfamily of LGICs represent a group of cation-permeable channels that are adenosine-5’-triphosphate (ATP) (Khakh 2001; Khakh and North 2012; North 2002). At present, seven subunits of P2X receptors (P2XRs) (P2X1–P2X7) have been identified. The P2X4 receptors (P2X4Rs) are capable of forming either homotrimers or hetrotrimers with other P2XRs including P2X1 (Nicke et al. 2005), P2X6 (Le et al. 1998) and P2X7 (Guo et al. 2007). The expression of P2X4Rs in the CNS is ubiquitous since they are expressed on pre-, post and extra synaptic membranes on neuronal cells as well as various types of glial cells including astrocytes and microglia (Buell et al. 1996; Soto et al. 1996; Toulme et al. 2010; Toulme and Khakh 2012). P2X4Rs are postsynaptically expressed on CA1/CA3 pyramidal cells in the hippocampus (Le et al. 1998; Rubio and Soto 2001), the medium spiny neurons (MSNs) in the basal ganglia circuitry (striatum and ventral midbrain) (Amadio et al. 2007; Heine et al. 2007) and the purkinje cells of the cerebellar cortex (Le et al. 1998; Rubio and Soto 2001). P2X4Rs are also expressed on GABA releasing presynaptic terminals in the ventral tegmental area (VTA) (Xiao et al. 2008), the dopaminergic (DA) neurons of the striatum and ventral mesencephalon (Amadio et al. 2007), GABAergic neurons of arcuate nucleus (Arc) (Xu et al. 2016) and ventromedial nuclei (VMN) of the hypothalamus (Jo et al. 2011). P2X4Rs exhibit a high Ca2+ permeability in comparison to other P2XRs (Khakh and North 2012), thus giving it a crucial role in regulation of various physiological processes in the CNS. At the physiological level, P2X4Rs have been shown to modulate postsynaptic currents mediated by glutamate α-amino-3-hydroxy-5-methyl-isoxazole propionic acid receptors (AMPARs) and NMDARs in the CA1/CA3 region of hippocampus (Baxter et al. 2011; Sim et al. 2006) and GABAARs in various nuclei within the hypothalamus (such as Arc and VMN) (Jo et al. 2011; Xu et al. 2016) as well as modulate pre-synaptic neurotransmitter release (such as glutamate, GABA) in various brain regions and in spinal cord (Gu and MacDermott 1997; Jo and Schlichter 1999; Li et al. 1998).
Electrophysiological studies using recombinant cell systems investigating the effect of IVM on P2X4Rs found that IVM potentiated P2X4Rs-mediated currents in the presence of ATP, indicating that IVM is a PAM of P2X4Rs (Khakh et al. 1999; Priel and Silberberg 2004). The interaction of IVM with P2X4Rs was considered a complex one since; IVM exhibited two distinct effects on P2X4Rs function at different concentrations (Priel and Silberberg 2004). At low concentrations (0.3 µM), IVM increased the number of channel openings without any effect on single channel conductance or the duration of channel opening. However, at higher concentrations (>1 µM), IVM increased maximal current enhanced by saturating concentrations of ATP as well as reduced current deactivation state. A plausible explanation for this phenomenon is that higher concentrations of IVM interacts with additional sites on P2X4Rs which causes a reduction in deactivation state as well as increase the duration and probability of channel opening (Priel and Silberberg 2004).
Experiments using electrophysiological and membrane partitioning tools have shown IVM to partition into membrane, where the lactone ring interacts with both the TM1 and TM2 domains at the protein-lipid interface (Silberberg et al. 2007). It was proposed that the crevice formed at the interface between the two TM segments is hydrophobic in nature since mutations of moderately sized hydrophobic amino acids to tryptophan actually perturbed IVM’s affinity for the receptor (Silberberg et al. 2007). Site directed mutagenesis studies using alanine substitutions have shown that multiple amino acids in the extracellular domain (Trp 50, Thr 57, Ser 69, Val 60, Val 61) and in TM2 domain (Asn 338, Ser 341, Gly 342, Leu 346, Gly 347, Ala 349, Ile 356) are important in determining the binding interaction of IVM with P2X4Rs (Jelinkova et al. 2008; Jelinkova et al. 2006). Additionally, molecular modeling and mutagenesis experiments from our laboratory have shown that certain amino acids at interface of ectodomain and TM2 segment (Trp 46, Trp 50, Asp 331, Met 336) are involved in determining the selectivity of IVM to P2X4Rs (Asatryan et al. 2010).
In addition to P2X4Rs, recent reports suggested that IVM can also modulate the activity of P2X7 receptors (P2X7Rs). Using a recombinant expression system in HEK 293, that IVM was able to modulate activity of human but not murine P2X7Rs. (Norenberg et al. 2012). On the other hand, findings by two other groups suggested that IVM is able to modulate P2X7Rs in murine BV2 microglial cells which endogenously express P2X7Rs along with P2X4Rs (Asatryan et al. 2017; Raouf et al. 2007). These findings suggested that the disparities are due to the differences in the used cellular systems. In addition, based on the fact that BV2 cells endogenously express both P2XR subtypes, P2X4Rs and P2X7Rs, these findings may also suggest a potential physical interaction between the two receptors (Perez-Flores et al. 2015), which can affect responses to IVM.
Interaction between P2X4Rs and ethanol
Building evidence from various in vitro and in vivo investigations have suggested a role for P2X4Rs in modulation of ethanol dependent behavioral and cellular effects across a wide spectrum of cellular and animal models which will be discussed in detail in the following sections.
a) Ethanol inhibition of P2X4Rs in recombinant and mammalian expression systems
Among all the P2XRs, P2X4Rs are most ethanol sensitive receptors. Ethanol inhibits ATP-activated currents in P2X4Rs in a concentration-dependent manner (10 mM- 200 mM) in Xenopus oocyte systems (Asatryan et al. 2010; Davies et al. 2002; Popova et al. 2010). It was shown that similar results were also obtained in mammalian cell expression systems (for e.g. HEK 293 cells) and cultured neurons (Ostrovskaya et al. 2011). Chimeric methods such as site-directed mutagenesis studies from our laboratory have shown that certain amino acid residues on the ectodomain-TM2 interface including Asp 331and Met 336 play an important role in mediating interaction of ethanol with P2X4Rs (Popova et al. 2010). Notably, these same amino acids also play a role in regulating the interaction of IVM with P2X4Rs (Asatryan et al. 2010).
Patch clamp electrophysiological investigations in HEK 293 cells have provided us with some important mechanistic insights into interaction of ethanol with P2X4Rs. First, ethanol was found to block P2X4Rs in the open state without any alterations in kinetics (i.e. stabilization of desensitization state or ion permeability ratio). Second, the interaction of ethanol with P2X4Rs was much faster than ATP, in the sense that, reduction in the degree of ethanol inhibition was observed upon termination of both ATP and ethanol solution, suggesting that ethanol molecules get dissociated from P2X4Rs much before the unbinding of ATP occurs. In addition, the ability of ethanol to inhibit P2X4R function was independent of ATP concentration, indicating that ethanol may induce conformational changes in the structure of P2X4Rs, thus reducing the binding affinity of ATP. Moreover, in coherence with our findings from Xenopus oocyte experiments, alanine substitutions at Met 336 and Asp 331 significantly attenuated the antagonistic effects of ethanol on P2X4Rs in HEK 293 cells (Ostrovskaya et al. 2011).
b) Role of P2X4Rs in ethanol-dependent microglial functions
P2X4Rs have been reported to regulate ethanol-mediated responses in microglia (Gofman et al. 2014). Ethanol-induced suppression of phagocytosis in the presence of chemokine attractant, CX3CL1, was diminished in presence of P2X4R blockade, which suggests that ethanol-mediated phagocytosis is P2X4R-dependent (Gofman et al. 2014). P2X4Rs have also been shown to alter ethanol’s action on signaling cascades such as mitogen activated protein kinase/ extracellular regulated kinase (MAPK/ERK) and phosphatidylinositol 3-kinase (PI3K)-Akt pathways in microglial cells, which adds further evidence to role of P2X4Rs in ethanol-related microglial functions (Gofman et al. 2016).
c) Role of P2X4Rs in regulation of ethanol intake in animal models of alcohol drinking
Multiple in vivo investigations have established a link between P2X4Rs and ethanol consumption. For instance, a genomic study by Tabakoff et al., reported reduced p2rx4 mRNA expression in the whole brain of 28 recombinant mouse inbred strains that display a high preference for ethanol (Tabakoff et al. 2009). Similarly, in another study, reduced P2X4R expression was reported in five brain regions of inbred alcohol preferring rats (iP) in comparison to inbred alcohol non-preferring rats (iNP). The aforementioned studies suggest that reduced P2X4R expression can be a predisposing factor for high ethanol consumption (Kimpel et al. 2007). In agreement with this notion, we reported that P2X4R KO mice exhibited higher ethanol intake in comparison to their wildtype (WT) littermates without any changes in ethanol preference or water intake using a 24 hr two bottle choice paradigm (Wyatt et al. 2014), which is considered to mimic social drinking (Yoneyama et al. 2008). In addition to our social drinking models, P2X4R KO mice also exhibited significantly higher ethanol intake using drinking in dark (DID) procedure, which is a paradigm for binge drinking (Wyatt et al. 2014). To further strengthen our hypothesis that P2X4Rs play an important role in ethanol intake, shRNA-mediated knockdown of P2X4Rs in the nucleus accumbens (NAc) core significantly increased ethanol intake without causing any changes in ethanol preference or total fluid intake. This is the first study that puts forth a direct link between reduced P2X4R expression and increased ethanol intake. In contrast, there are other investigations that have reported an increased expression of p2rx4 gene in high alcohol drinking rats (HAD-2) in relation to low alcohol drinking rats (LAD-2) (McBride et al. 2012). In addition, increased p2rx4 gene expression was observed in the periaqueductal gray area (PAG) of midbrain, a site involved in processing fear and anxiety (McClintick et al. 2016) in adolescent male alcohol preferring (P) rats. In further support of this theory, lentivirus-mediated knockdown of P2X4Rs in the VTA significantly reduced ethanol intake in female HAD-2 rats (Franklin et al. 2015). These findings suggest that the relationship between P2X4R function and ethanol drinking behavior may be sex, species and brain region-dependent. Nevertheless, the overall theme that emerges from these investigations is that P2X4Rs have an important contribution to ethanol intake and manipulating P2X4R function via pharmacological agents can have a potential in treatment of alcohol addiction and abuse.
Preclinical investigation of IVM as novel therapy for AUD
a) IVM antagonized ethanol induced inhibition of P2X4Rs in vitro
The significant overlapping of binding sites of ethanol and IVM at the interface of ectodomain-TM2 segment led us to propose the hypothesis that IVM could possibly interfere with ethanol’s action on P2X4Rs. In support of our hypothesis, using two electrode voltage methodology in Xenopus oocytes, we demonstrated that IVM (0.5 µM) significantly attenuated the inhibitory effects of ethanol at multiple concentrations (25 mM to 100 mM), without inducing any changes in surface expression of P2X4Rs on the oocytes (Asatryan et al. 2010). Higher concentrations of IVM were able to antagonize high concentrations of ethanol (200 mM). Taking into consideration that 25 mM concentration of ethanol is 1.5 times the blood ethanol concentration (0.08%), which is regarded as legally intoxicating in the United States, these findings suggest that IVM does have the potential to negate ethanol induced behavioral effects in humans. The precise mechanism by which IVM antagonizes ethanol’s effects on P2X4Rs is not clearly understood. Since, IVM potentiates ATP currents independently; it was initially thought that IVM’s action on ethanol effects resulted from the synergy between IVM potentiation and ethanol inhibition. However, the data did not fit the additive model. Moreover, the absence of a parallel shift suggests that interaction of IVM with ethanol on P2X4Rs is not simply a representation of competitive antagonism (Asatryan et al. 2010). The involvement of same amino acids (Asp 331 and Met 336) in mediating the binding interaction of IVM and ethanol with P2X4Rs, led us to hypothesize that IVM competes with ethanol in the same binding pocket on P2X4Rs. In support of this hypothesis, substituting Met 336 in the TM 2 domain with bulky positively or negatively charged amino acids negated the effects of both IVM and ethanol on P2X4Rs, indicating that a charge at this site can disrupt the initiation of binding of IVM and ethanol to the channel. Additionally, size of the amino acid also plays a role in determining the binding affinity of IVM to P2X4Rs, since substitution of Met with small non polar residues increased IVM affinity to the receptor, but also decreased ethanol sensitivity (Asatryan et al. 2010).
To investigate interaction between Met 336 and other amino acids within the TM segments, a homology model of rat P2X4R built upon 3.1 A° X ray crystal structure of the zebrafish P2X4R (Kawate et al. 2009). Using this homology model, we identified a common binding pocket for both IVM and ethanol which comprised of Asp 331 and Met 336 in the TM 2 segment; Trp 46 and Trp 50 in the TM1 segment. Electrophysiological methods later confirmed that Trp 46 and not Trp 50 plays an important role in IVM and ethanol binding to the channel since, mutation at this site with non-aromatic amino acids significantly altered sensitivity of both IVM (0.5 µM) and ethanol (>100 mM) (Popova et al. 2013). Our molecular model of P2X4R also revealed Trp 46 to be in between Trp 42 and Trp 50 and the pi-pi interaction between Trp 42 and Trp 46 seem to be critical for interaction of both IVM and ethanol with P2X4Rs (Popova et al. 2013).
b) IVM reduces ethanol intake and preference in vivo
Collectively, findings from in vitro investigations suggest that IVM interferes with ethanol’s action on P2X4Rs in vivo and this can subsequently lead to reduction in ethanol drinking behavior. In support of this hypothesis, acute administration of IVM (1.25–10 mg/kg) was demonstrated to significantly reduce ethanol intake as well as preference in male and female C57BL/6J mice across a wide variety of alcohol drinking paradigms that mimic social drinking, binge drinking and alcohol seeking behavior (Asatryan et al. 2014; Wyatt et al. 2014; Yardley et al. 2012; Yardley et al. 2015; Yardley et al. 2014). The anti-alcohol effects of IVM were significant at 9 hr post administration, which correlates well with the Tmax (8–10 hr) as well as plasma half-life (8–12 hr) of IVM (Yardley et al. 2012). Furthermore, the reduction in ethanol intake caused by lowest effective dose of IVM (2.5 mg/kg) correlated well with brain concentration of IVM, which was 0.28 ng*hr/mg of tissue, indicating that the decrease in ethanol intake is a direct consequence of IVM’s ability to penetrate into the brain and not due to some indirect effect of IVM. Changes in ethanol intake were not accompanied by significant changes in water intake or body weight or food intake suggesting that IVM does not have any deleterious impact on physiology of drinking or overall health of the mice. The reduction in ethanol preference in the 24 hr two bottle choice paradigm (Yardley et al. 2012) as well as ethanol self-administration in the self operant chamber technique (Kosten 2011; Yardley et al. 2012) indicates a role for IVM in negating the positive reinforcement effects of ethanol drinking as well as the motivation to seek ethanol. In further support of IVM’s ability to diminish ethanol reinforcement, IVM was also found to reduce saccharin consumption in the 24 hr two bottle choice paradigm, indicating that IVM can block the chemosensory properties of reward of stimuli (Yardley et al. 2012). In addition to studies undertaken in C57BL/6J mice, IVM was also demonstrated to reduce ethanol intake, but not sucrose, in male and female HAD-1 and HAD-2 rats (Franklin et al. 2015). Overall, these studies suggest a potential for IVM in negating alcohol consumption and reinforcement across a wide variety of animal models of alcohol drinking.
The aforementioned findings were, however, obtained from acute IVM administration. AUD is a chronic disease and hence, requires long term treatment to control the behavioral manifestations without causing any adverse events. Thus, the effects of repeated dosage of IVM on ethanol drinking behavior were also investigated in mice. A 7 day treatment of IVM (2.5 mg/kg) via intraperitoneal (i.p.) route induced significant reduction in ethanol intake and preference in two bottle choice paradigm without any overt signs of toxicity in female C57BL/6J mice (Yardley et al. 2012). Additionally, 10 day treatment of IVM (3 mg/kg) via i.p. route also caused a significant decrease in ethanol intake as well as preference in female C57BL/6J mice, without any significant changes in fluid intake or body weight (Yardley et al. 2014). The selection of this dose is based on its correspondence to a dose shown to be safe in humans. Pharmacokinetic analysis revealed IVM to have a Cmax of 0.73 ng*hr/mg in the brain at 32 hr post 10 day injection, which correlates well with the anti-alcohol effects of IVM.
The effectiveness of IVM in attenuating multiple forms of ethanol drinking behavior via the i.p. route has been well documented. However, to further reinforce the argument of IVM being repositioned as novel therapeutic agent for AUD, it would have to be delivered via a more sustainable, less stressful route to ensure patient compliance. In support of this notion, administration of IVM in the form of fast dissolving oral strips (0.21mg) over a 30 day period also resulted in significant reduction of ethanol intake as well as preference, without any significant changes in total fluid intake, in the 24 hr two bottle choice paradigm (Yardley et al. 2015). These changes in ethanol drinking behavior were accompanied by no histopathological toxicities in the brain, liver and kidney, indicating that IVM does not induce any toxicity via oral administration (Yardley et al. 2015). Thus, oral administration of IVM was found to be effective in decreasing alcohol consumption without inducing any serious adverse effects in mice or altering the morphology of organs, supporting its potential as a safe therapeutic agent that can be given on chronic basis for treatment of AUDs.
The argument of repurposing IVM as an anti-alcohol therapeutic agent is further supported by previous findings that have shown IVM to not elicit any adverse effects across a wide spectrum of behavioral paradigms that assess emotional, cognitive and perceptual function (Bortolato et al. 2013). IVM did exhibit anxiolytic properties in some of anxiety-related paradigms such as elevated plus maze and marble burying, which are reminiscent of GABAARs potentiation (Bortolato et al. 2013). However, unlike GABAAR PAMs such as benzodiazepines, IVM does not elicit any rewarding effects independently (Bortolato et al. 2013). Notably, single doses of IVM of 120 mg administered to humans in a clinical trial, did not induce any adverse effects in humans and were well tolerated (Guzzo et al. 2002). Overall, the anti-alcohol effects of IVM coupled with its safety profile suggested a new use for utility of IVM as a safe, well tolerated and efficacious medication for treatment of AUDs.
In the context of mechanism of action, IVM-mediated reduction in ethanol intake is, in part, P2X4R-dependent since the degree of reduction in ethanol consumption was significantly diminished in male P2X4R KO mice (Wyatt et al. 2014). This finding is considered critical since, it added to the evidence that P2X4R potentiation induced by IVM would lead to changes in ethanol’s action, leading to attenuation of ethanol–induced behavioral effects. However, the precise mechanism by which IVM-mediated P2X4R potentiation leads to reduction in ethanol intake remains elusive. To date, ethanol has been shown to inhibit pre-synaptic P2XRs at the GABA releasing synaptic terminals in the VTA, inhibiting GABA release onto the DA neurons and subsequently increasing the firing of VTA DA neurons (Xiao et al. 2008). This study identified an indirect role for P2X4Rs in modulation of DA neuronal activity in the VTA. In this context, based on our evidence in vitro, one could hypothesize that IVM antagonizes the inhibitory effects of ethanol on P2X4Rs, resulting in enhanced GABA release into presynapses and subsequent inhibition of DA neurons. Inhibition of DA system would eventually counteract the craving for ethanol. To support this hypothesis, future patch clamp electrophysiological studies on VTA DA neurons or DA neurons in other brain sites integral to mesolimbic circuitry (such as NAc or dorsal hippocampus) are necessary using IVM and specific P2X4R antagonists/ P2X4R KO mice, to delineate the exact role of P2X4Rs in regulation of DA neuronal activity in the reward circuitry and how its physiological function can be altered in presence of IVM and/or ethanol. To incorporate more evidence into interaction between IVM and DA system in the mesolimbic circuitry, IVM was reported to modulate signaling molecules integral to DA neurotransmission in the ventral striatum such as dopamine and cyclic-AMP regulated phosphoprotein of 32 kDa (DARPP-32) via P2X4R potentiation as well as a similar trend with respect to cyclic-AMP response element binding protein (CREB) phosphorylation in the same brain region (Khoja et al. 2016). Considering that alterations in phosphorylation of DARPP-32 and CREB have been associated in regulation of ethanol drinking behavior (Nuutinen et al. 2011; Pandey et al. 1999; Pandey et al. 2004), IVM may be attenuating ethanol drinking behavior via its effects on DA-dependent signaling cascades in regions integral to drug reward circuitry.
Preclinical evidence supporting IVM-related macrocyclic lactones as anti-alcohol therapeutic agents
a) ABM
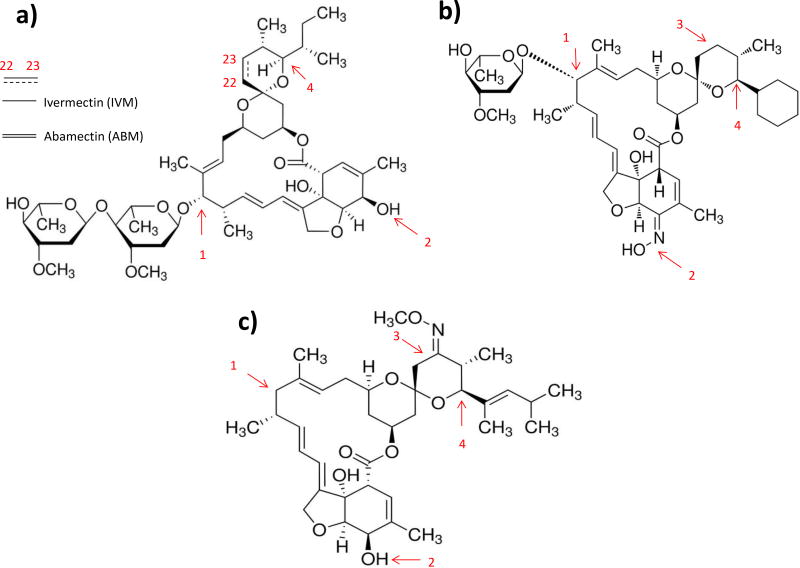
ABM is structurally similar to IVM, except that ABM possesses a double bond at C22–C23 of the spiroketal unit and IVM is a product of chemical hydrogenation at C22–C23 of ABM (Fig 1). Expectedly, ABM (0.5 µM–1 µM) significantly potentiated ATP-gated currents in P2X4Rs in Xenopus oocytes, to a similar degree as IVM. However, higher concentrations of ABM (≥ 3 µM) exhibited a greater potency than IVM in enhancement of P2X4Rs-mediated currents and in fact, increased P2X4Rs activity even in the absence of ATP, indicating that ABM acts as a direct agonist at higher concentrations (Asatryan et al. 2014). In addition to P2X4Rs, ABM (0.5 µM – 3 µM), significantly potentiated GABAARs-mediated currents to a similar extent as IVM, but was less potent than IVM at higher concentrations (10 µM) (Asatryan et al. 2014). Additionally, ABM antagonized the inhibitory properties of ethanol in a similar manner to IVM. 0.5 µM ABM significantly antagonized the inhibitory effects of ethanol (100 mM) on ATP-induced currents (Asatryan et al. 2014).
Figure 1.
Structural differences between IVM or ABM (a), SEL (b) and MOX (c) may play an important role in determining their pharmacological effects and pharmacokinetic properties. ABM possesses a double bond at C22–C23 position of spiroketal unit and IVM is an outcome of chemical hydrogenation of ABM (a). 1) IVM possesses two saccharide moieties; SEL contains one saccharide moiety and MOX does not have any saccharide moiety. 2) SEL has an oxime group instead of the hydroxyl group of the tetrahydro-benzofuran unit in IVM, ABM and MOX. 3) MOX contains a methoxime group at C23 position and this position is hydrogenated in remaining avermectins. Lastly, 4) IVM and ABM has isopropyl/isobutyl group, SEL has a cyclohexyl ring and MOX contains a dimethyl-butyl group at C25 position.
ABM (5 mg/kg. i.p.) significantly reduced voluntary ethanol intake as well as preference, but increased water intake (Asatryan et al. 2014). The decrease in ethanol consumption upon ABM treatment could be linked to reduced preference for ethanol, suggesting that ABM can block the positive reinforcing effects of ethanol. However, the extent of reduction of ABM was lesser compared to IVM, even though, ABM achieved a higher concentration than IVM in the brain (40 ng × hr/ml v/s 1.81 ng × hr/ml) (Asatryan et al. 2014). Thus, the discrepancies in ethanol reduction between the two avermectins cannot be simply explained by differences in brain concentrations and activity at target receptors.
b) SEL
In contrast to ABM, SEL exhibits major structural differences with IVM (Fig 1). SEL possesses a cyclohexyl ring in place of isobutyl moiety that is present in IVM. SEL has one saccharide moiety instead of two which is the case for IVM. SEL possesses an unsaturated ketoxime group instead of allylic hydroxyl group that is found on IVM (Fig 1). The presence of one saccharide moiety reduces the affinity of P-glycoprotein (P-gp) for SEL, since saccharide moiety can determine the affinity of a compound for P-gp (Lespine et al. 2007).
The structural differences with IVM, may contribute to SEL’s poor activity at P2X4Rs as well as its inability to attenuate the inhibitory effects of ethanol. 10 µM SEL exhibited similar potency as 0.5 µM concentrations of IVM and ABM in terms of P2X4R potentiation. SEL was able to potentiate ATP-activated currents in P2X4Rs in a concentration dependent manner upto 30 µM, but its magnitude of response was still much less in comparison to IVM and ABM (Asatryan et al. 2014). Additionally, SEL’s action on GABAARs was much weaker than that of IVM and ABM at concentrations greater than 1 µM (Asatryan et al. 2014). On account of its weak potentiation on P2X4Rs, SEL (10 µM) did not exhibit any antagonistic effect on ethanol induced inhibition of P2X4Rs (Asatryan et al. 2014).
In correlation with in vitro evidence, SEL (5 mg/kg., i.p.) did not cause any reduction in ethanol intake or preference in the 24 hr two bottle choice paradigm (Asatryan et al. 2014). The lack of any significant changes in ethanol intake upon SEL treatment is not due to inability to penetrate into the brain since SEL was able to attain higher concentrations than IVM in the brain (Asatryan et al. 2014). But rather, due to inability of SEL to antagonize the effects ethanol on either P2X4Rs or GABAARs. Thus, the structural changes in SEL such as absence of one more saccharide moiety and presence of ketoxime group may possibly underlie SEL’s lack of efficacy in reducing ethanol consumption.
c) MOX
Recently, the Davies group has begun a new effort on the development of MOX for treatment of AUDs. Part of this effort is based on improved CNS safety profile MOX versus IVM and thus, more suitable pharmacotherapy for AUDs. First, MOX is weaker substrate for P-gp transporter than IVM and less dependence on P-gp for removal from the brain (Janko and Geyer 2013; Menez et al. 2012). Second, in agreement with published studies, our investigation also showed that MOX has lower potency on GABAARs as compared to IVM (Huynh et al. 2017). Together, these results suggest that the use of MOX as a long-term treatment for AUDs will less likely lead to complications arising from toxic brain accumulation due to a deficiency in P-gp function and/or drug-drug interaction with other concurrent (P-gp substrate) medications and from excessive stimulation of GABAARs that can result in CNS depression and potentially coma.
Recent electrophysiological and behavioral evidence from our laboratory have supported MOX as an effective anti-alcohol agent. Similar to IVM and ABM, MOX at multiple concentrations (0.5 µM and 1 µM) significantly potentiated P2X4R-mediated currents in Xenopus oocytes (Huynh et al. 2017). Furthermore, MOX (0.5 µM) significantly attenuated the inhibitory effects of 25 mM, but not 50 mM, ethanol on P2X4Rs (Huynh et al. 2017), providing us with first piece of evidence regarding the anti-alcohol potential of MOX. Notably, the same concentration of IVM was able to attenuate the antagonistic properties of higher concentrations of ethanol (>50 mM) (Asatryan et al. 2010).
In coherence with our in vitro findings, MOX significantly reduced ethanol intake in both male and female C57BL/6J mice across wide spectrum of alcohol drinking paradigms (Huynh et al. 2017). Dose response studies revealed MOX to exert greater efficacy than IVM on ethanol consumption. In a time course study, MOX elicited significant reduction in ethanol intake after 4 hr, which is much faster than that of IVM (Huynh et al. 2017). To date, MOX-mediated reduction in ethanol consumption has not been assessed in P2X4R KO mice, which would be a crucial study since MOX is a PAM on several other LGICs in the CNS including GABAARs, GlyRs and nAchRs (Menez et al. 2012; Wolstenholme and Rogers 2005), all of which can contribute to MOX-mediated attenuation of ethanol drinking behavior. Furthermore, it would add evidence that P2X4Rs are the main targets through which avermectins can exhibit their anti-alcohol effects in vivo.
The structural differences between MOX and IVM (Fig 1) may underlie the discrimination in time course effect and degree in reduction of ethanol consumption between the two avermectins. MOX does not possess any saccharide moieties. There is presence of methoxime on MOX, but not on IVM. Lastly, MOX contains a dimethyl butyl substituent at C25 position whereas IVM has a isopropyl group at that position. The presence of these substituents may add to the lipophilicity of MOX, which may partially contribute to faster penentration of MOX across blood brain barrier (BBB).
MOX is currently under clinical development for onchocerciasis (as an alternative to IVM) with excellent safety signals and no adverse events reported to date (Cotreau et al. 2003; Korth-Bradley et al. 2012). The significant attenuation on ethanol drinking behavior, coupled with superior physiochemical properties and lack of adverse events, support MOX as a safe, tolerable, and effective therapeutic agent for treatment of AUDs.
Overall summary and conclusion
The findings reported, herewith, demonstrate the potential of avermectins in treatment of alcohol addiction via positive modulation of P2X4Rs. IVM, ABM and MOX, but not SEL, attenuated the inhibitory effects of ethanol on P2X4Rs as well as significantly decrease ethanol consumption across multiple alcohol drinking paradigms. The lack of potency of SEL in negating the behavioral effects of ethanol suggests that certain key moieties in the structure of avermectins play a crucial role in deciding the efficacy of these compounds. The correlation between our in vitro and in vivo investigations indicate that the effects of avermectins on ethanol induced inhibition on P2X4Rs in vitro can be a good predictor of their ability to reduce ethanol intake in vivo. Considering that the avermectins are also positive modulators of GABAARs, GlyRs and nAChRs (plausible targets for ethanol action), a plausible interaction between P2X4Rs and these ionotropic receptors may be associated with pharmacological effects of avermectins in vivo. Taken together, we identify P2X4Rs as a novel platform for development of novel pharmacological agents that target P2X4Rs and on basis of that, avermectins represent as a new class of potential anti-alcohol agents.
Acknowledgments
ACKNOWLEDGEMENTS AND CONFLICTS OF INTEREST DISCLOSURE:
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant R01 AA022448 (D.L.D) and USC School of Pharmacy. D.L.D and L.A. are inventors on a patent for the use of IVM for treatment alcohol use disorders.
LIST OF ABBREVIATIONS
- AADs
alcohol attributable deaths
- ABM
abamectin
- ACh
acetylcholine
- AMPARs
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- Arc
arcuate nucleus
- ATP
adenosine-5’-triphosphate
- AUDs
alcohol use disorders
- CNS
central nervous system
- CREB
cyclic-AMP response element binding protein
- DA
dopamine
- DALYs
disability adjusted life years
- DARPP-32
dopamine and cyclic-AMP regulated phosphoprotein of 32kDa
- DID
drinking in the dark
- GABARs
γ-amino butyric acid receptors
- GlyRs
glycine receptors
- HAD 1/LAD 1, HAD-2/LAD-2
replicate lines of high alcohol / low alcohol drinking rats
- iP
inbred alcohol preferring rats
- iNP
inbred alcohol non-preferring rats
- i.p.
intraperitoneal
- IVM
ivermectin
- LGICs
ligand gated ion channels
- MAPK/ERK
mitogen activated protein kinase/extracellular regulated kinase
- MOX
moxidectin
- MSNs
medium spiny neurons
- NAc
nucleus accumbens
- nAChRs
nicotinic acetylcholine receptors
- NMDARs
N-methyl-D-aspartate receptors
- P
alcohol preferring rats
- PAG
periaqueductal gray area
- PAM
positive allosteric modulator
- PI3-K
phosphatidylinositol 3-kinase
- P2XRs
P2X receptors
- P2X4Rs
P2X4 receptors
- P2X7Rs
P2X7 receptors
- P2X4R KO
P2X4 knockout
- P-gp
P-glycoprotein
- SEL
selamectin
- TM
transmembrane
- VTA
ventral tegmental area
- VMN
ventromedial nucleus
- WT
wildtype
References
- Amadio S, Montilli C, Picconi B, Calabrei P, Volont C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: An immunohistological study. Purinergic signalling. 2007;3:389–398. doi: 10.1007/s11302-007-9069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham PK, Waid LR, Malcolm RJ, Dias JK, Roberts JS. Posttreatment results of combining naltrexone with cognitive-behavior therapy for the treatment of alcoholism. J Clin Psychopharmacol. 2001;21:72–7. doi: 10.1097/00004714-200102000-00013. [DOI] [PubMed] [Google Scholar]
- Arena JP, Liu KK, Paress PS, Cully DF. Avermectin-sensitive chloride currents induced by Caenorhabditis elegans RNA in Xenopus oocytes. Molecular pharmacology. 1991;40:368–74. [PubMed] [Google Scholar]
- Arena JP, Liu KK, Paress PS, Frazier EG, Cully DF, Mrozik H, Schaeffer JM. The mechanism of action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. The Journal of parasitology. 1995;81:286–94. [PubMed] [Google Scholar]
- Arena JP, Liu KK, Paress PS, Schaeffer JM, Cully DF. Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation by avermectin. Brain research Molecular brain research. 1992;15:339–48. doi: 10.1016/0169-328x(92)90127-w. [DOI] [PubMed] [Google Scholar]
- Asatryan L, Ostrovskaya O, Lieu D, Davies DL. Ethanol differentially modulates P2X4 and P2X7 receptor activity and function in BV2 microglial cells. Neuropharmacology. 2017;128:11–21. doi: 10.1016/j.neuropharm.2017.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Popova M, Perkins DI, Trudell JR, Alkana RL, Davies DL. Ivermectin antagonizes ethanol inhibition in P2X4 receptors. Journal of Pharmacology And Experimental Therapeutics. 2010;334:720–728. doi: 10.1124/jpet.110.167908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Yardley MM, Khoja S, Trudell JR, Huynh N, Louie SG, Petasis NA, Alkana RL, Davies DL. Avermectins differentially affect ethanol intake and receptor function: Implications for developing new therapeutics for alcohol use disorders. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17:907–916. doi: 10.1017/S1461145713001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur J Neurosci. 2011;34:213–20. doi: 10.1111/j.1460-9568.2011.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, Williams JF, Dave V. Pharmacology of ivermectin. Parasitol Today. 1988;4:226–8. doi: 10.1016/0169-4758(88)90163-9. [DOI] [PubMed] [Google Scholar]
- Berglund M, Thelander S, Salaspuro M, Franck J, Andreasson S, Ojehagen A. Treatment of alcohol abuse: an evidence-based review. Alcoholism, clinical and experimental research. 2003;27:1645–56. doi: 10.1097/01.ALC.0000090144.99832.19. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Yardley M, Khoja S, Godar SC, Asatryan L, Finn DA, Alkana RL, Louie SG, Davies DL. Pharmacological insights into the role of P2X4 receptors in behavioral regulation: lessons from ivermectin. Int J Neuropsychopharmacology. 2013;16:1059–1070. doi: 10.1017/S1461145712000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic Costs of Excessive Alcohol Consumption in the U.S., 2006. Amer J Prevent Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99:811–28. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors: an emerging channel family. The European journal of neuroscience. 1996;8:2221–8. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Burg RW, Miller BM, Baker EE, Birnbaum J, Currie SA, Hartman R, Kong YL, Monaghan RL, Olson G, Putter I, Tunac JB, Wallick H, Stapley EO, Oiwa R, Omura S. Avermectins, new family of potent anthelmintic agents: producing organism and fermentation. Antimicrob Agents Chemother. 1979;15:361–7. doi: 10.1128/aac.15.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WC, Fisher MH, Stapley EO, Albers-Schonberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science. 1983;221:823–8. doi: 10.1126/science.6308762. [DOI] [PubMed] [Google Scholar]
- Castro LA, Baltieri DA. The pharmacologic treatment of the alcohol dependence. Rev Bras Psiquiatr. 2004;26(Suppl 1):S43–6. doi: 10.1590/s1516-44462004000500011. [DOI] [PubMed] [Google Scholar]
- Collins T, Millar NS. Nicotinic acetylcholine receptor transmembrane mutations convert ivermectin from a positive to a negative allosteric modulator. Molecular pharmacology. 2010;78:198–204. doi: 10.1124/mol.110.064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation. 2010;121:1951–9. doi: 10.1161/CIRCULATIONAHA.109.865840. [DOI] [PubMed] [Google Scholar]
- Cotreau MM, Warren S, Ryan JL, Fleckenstein L, Vanapalli SR, Brown KR, Rock D, Chen CY, Schwertschlag US. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol. 2003;43:1108–15. doi: 10.1177/0091270003257456. [DOI] [PubMed] [Google Scholar]
- Croop RS, Faulkner EB, Labriola DF. The safety profile of naltrexone in the treatment of alcoholism. Results from a multicenter usage study. The Naltrexone Usage Study Group. Archives of general psychiatry. 1997;54:1130–5. doi: 10.1001/archpsyc.1997.01830240090013. [DOI] [PubMed] [Google Scholar]
- Crump A, Omura S. Ivermectin, 'wonder drug' from Japan: the human use perspective. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2011;87:13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump A, Otoguro K. Satoshi Omura: in pursuit of nature's bounty. Trends Parasitol. 2005;21:126–32. doi: 10.1016/j.pt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LHT, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Dadzie KY, Bird AC, Awadzi K, Schulz-Key H, Gilles HM, Aziz MA. Ocular findings in a double-blind study of ivermectin versus diethylcarbamazine versus placebo in the treatment of onchocerciasis. Br J Ophthalmol. 1987;71:78–85. doi: 10.1136/bjo.71.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcoholism, clinical and experimental research. 2002;26:773–778. [PubMed] [Google Scholar]
- Dawson GR, Wafford KA, Smith A, Marshall GR, Bayley PJ, Schaeffer JM, Meinke PT, McKernan RM. Anticonvulsant and adverse effects of avermectin analogs in mice are mediated through the gamma-aminobutyric acid A receptor. Journal of Pharmacology And Experimental Therapeutics. 2000;295:1051–1060. [PubMed] [Google Scholar]
- Dunne CL, Malone CJ, Whitworth JA. A field study of the effects of ivermectin on ectoparasites of man. Trans R Soc Trop Med Hyg. 1991;85:550–1. doi: 10.1016/0035-9203(91)90255-w. [DOI] [PubMed] [Google Scholar]
- Egerton JR, Ostlind DA, Blair LS, Eary CH, Suhayda D, Cifelli S, Riek RF, Campbell WC. Avermectins, new family of potent anthelmintic agents: efficacy of the B1a component. Antimicrob Agents Chemother. 1979;15:372–8. doi: 10.1128/aac.15.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney JW, Hahn AC, Moos RH. The effectiveness of inpatient and outpatient treatment for alcohol abuse: the need to focus on mediators and moderators of setting effects. Addiction. 1996;91:1773–96. discussion 1803–20. [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, Bell RL, McBride WJ. Involvement of Purinergic P2X4 Receptors in Alcohol Intake of High-Alcohol-Drinking (HAD) Rats. Alcoholism, clinical and experimental research. 2015;39:2022–31. doi: 10.1111/acer.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DO, Zierdt WS, Lujan A, Nutman TB. The efficacy of ivermectin in the chemotherapy of gastrointestinal helminthiasis in humans. J Infect Dis. 1989;159:1151–3. doi: 10.1093/infdis/159.6.1151. [DOI] [PubMed] [Google Scholar]
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends in Parasitology. 2005;21:530–532. doi: 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Geary TG, Sims SM, Thomas EM, Vanover L, Davis JP, Winterrowd CA, Klein RD, Ho NF, Thompson DP. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp Parasitol. 1993;77:88–96. doi: 10.1006/expr.1993.1064. [DOI] [PubMed] [Google Scholar]
- Gofman L, Cenna JM, Potula R. P2X4 receptor regulates alcohol-induced responses in microglia. J Neuroimmune Pharmacol. 2014;9:668–78. doi: 10.1007/s11481-014-9559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofman L, Fernandes NC, Potula R. Relative Role of Akt, ERK and CREB in Alcohol-Induced Microglia P2X4R Receptor Expression. Alcohol and alcoholism. 2016;51:647–654. doi: 10.1093/alcalc/agw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D, Pfeiffer F, Betz H. Avermectin B1a inhibits the binding of strychnine to the glycine receptor of rat spinal cord. Neuroscience letters. 1982;29:173–6. doi: 10.1016/0304-3940(82)90349-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou P, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug and alcohol dependence. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–53. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4 / P2X7 heteromeric receptors. Molecular pharmacology: mol. 2007 doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- Guzzo CA, Furtek CI, Porras AG, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, Hsieh JY, Lasseter KC. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. The Journal of Clinical Pharmacology. 2002;42:1122–1133. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heine C, Wegner A, Grosche J, Allgaier C, Illes P, Franke H. P2 receptor expression in the dopaminergic system of the rat brain during development. Neuroscience. 2007;149:165–81. doi: 10.1016/j.neuroscience.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Heinz A, Lober S, Georgi A, Wrase J, Hermann D, Rey ER, Wellek S, Mann K. Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol and alcoholism. 2003;38:35–9. doi: 10.1093/alcalc/agg005. [DOI] [PubMed] [Google Scholar]
- Hughes JC, Cook CC. The efficacy of disulfiram: a review of outcome studies. Addiction. 1997;92:381–95. [PubMed] [Google Scholar]
- Huynh N, Arabian N, Naito A, Louie S, Jakowec MW, Asatryan L, Davies DL. Preclinical development of moxidectin as a novel therapeutic for alcohol use disorder. Neuropharmacology. 2017;113:60–70. doi: 10.1016/j.neuropharm.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janko C, Geyer J. Moxidectin has a lower neurotoxic potential but comparable brain penetration in P-glycoprotein-deficient CF-1 mice compared to ivermectin. J Vet Pharmacol Ther. 2013;36:275–84. doi: 10.1111/j.1365-2885.2012.01424.x. [DOI] [PubMed] [Google Scholar]
- Jelinkova I, Vavra V, Jindrichova M, Obsil T, Zemkova HW, Zemkova H, Stojilkovic SS. Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflugers Arch. 2008;456:939–950. doi: 10.1007/s00424-008-0450-4. [DOI] [PubMed] [Google Scholar]
- Jelinkova I, Yan Z, Liang Z, Moonat S, Teisinger J, Stojilkovic SS, Zemkova H. Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem Biophys Res Commun. 2006;349:619–625. doi: 10.1016/j.bbrc.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Jo YH, Donier E, Martinez A, Garret M, Toulme E, Boue-Grabot E. Cross-talk between P2X4 and gamma-aminobutyric acid, type A receptors determines synaptic efficacy at a central synapse. The Journal of biological chemistry. 2011;286:19993–20004. doi: 10.1074/jbc.M111.231324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Biochemical Pharmacology. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Reviews, Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76:51–69. doi: 10.1016/j.neuron.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neuroscience. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoja S, Shah V, Garcia D, Asatryan L, Jakowec MW, Davies DL. Role of purinergic P2X4 receptors in regulating striatal dopamine homeostasis and dependent behaviors. Journal of neurochemistry. 2016;139:134–48. doi: 10.1111/jnc.13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpel MW, Strother WN, McClintick JN, Carr LG, Liang T, Edenberg HJ, McBride WJ. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol. 2007;41:95–132. doi: 10.1016/j.alcohol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth-Bradley JM, Parks V, Patat A, Matschke K, Mayer P, Fleckenstein L. Relative Bioavailability of Liquid and Tablet Formulations of the Antiparasitic Moxidectin. Clin Pharmacol Drug Dev. 2012;1:32–7. doi: 10.1177/2160763X11432508. [DOI] [PubMed] [Google Scholar]
- Kosten TA. Pharmacologically targeting the P2rx4 gene on maintenance and reinstatement of alcohol self-administration in rats. Pharmacology Biochemistry and Behavior. 2011;98:533–538. doi: 10.1016/j.pbb.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Ivermectin: A positive allosteric effector of the alpha 7 meuronal nicotinic acetylcholine receptor. Molecular pharmacology. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- Krusek J, Zemkova H. Effect of ivermectin on gamma-aminobutyric acid-induced chloride currents in mouse hippocampal embryonic neurones. European journal of pharmacology. 1994;259:121–8. doi: 10.1016/0014-2999(94)90500-2. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA Veterans Affairs Naltrexone Cooperative Study G. Naltrexone in the treatment of alcohol dependence. The New England journal of medicine. 2001;345:1734–9. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Kumaraswami V, Ottesen EA, Vijayasekaran V, Devi U, Swaminathan M, Aziz MA, Sarma GR, Prabhakar R, Tripathy SP. Ivermectin for the treatment of Wuchereria bancrofti filariasis. Efficacy and adverse reactions. JAMA : the journal of the American Medical Association. 1988;259:3150–3. [PubMed] [Google Scholar]
- Le KT, Babinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le KT, Villeneuve P, Ramjaun AR, McPherson PS, Beaudet A, Seguela P. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience. 1998;83:177–90. doi: 10.1016/s0306-4522(97)00365-5. [DOI] [PubMed] [Google Scholar]
- Lespine A, Martin S, Dupuy J, Roulet A, Pineau T, Orlowski S, Alvinerie M. Interaction of macrocyclic lactones with P-glycoprotein: Structure-affinity relationship. Eur J Pharm Sci. 2007;30:84–94. doi: 10.1016/j.ejps.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Li P, Calejesan AA, Zhuo M. ATP P2x receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. Journal of neurophysiology. 1998;80:3356–60. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, McClintick JN, Ding ZM, Hyytia P, Colombo G, Edenberg HJ, Lumeng L, Bell RL. Gene expression in the ventral tegmental area of 5 pairs of rat lines selectively bred for high or low ethanol consumption. Pharmacology, biochemistry, and behavior. 2012;102:275–85. doi: 10.1016/j.pbb.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, McBride WJ, Bell RL, Ding ZM, Liu Y, Xuei X, Edenberg HJ. Gene Expression Changes in Glutamate and GABA-A Receptors, Neuropeptides, Ion Channels, and Cholesterol Synthesis in the Periaqueductal Gray Following Binge-Like Alcohol Drinking by Adolescent Alcohol-Preferring (P) Rats. Alcoholism, clinical and experimental research. 2016;40:955–68. doi: 10.1111/acer.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menez C, Sutra JF, Prichard R, Lespine A. Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (−/−) mice and effects on mammalian GABA(A) channel activity. PLoS neglected tropical diseases. 2012;6:e1883. doi: 10.1371/journal.pntd.0001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, El Bcheraoui C, Moradi-Lakeh M, Kyu HH, Barber RM, Wagner J, Cercy K, Kravitz H, Coggeshall M, Chew A, O'Rourke KF, Steiner C, Tuffaha M, Charara R, Al-Ghamdi EA, Adi Y, Afifi RA, Alahmadi H, AlBuhairan F, Allen N, AlMazroa M, Al-Nehmi AA, AlRayess Z, Arora M, Azzopardi P, Barroso C, Basulaiman M, Bhutta ZA, Bonell C, Breinbauer C, Degenhardt L, Denno D, Fang J, Fatusi A, Feigl AB, Kakuma R, Karam N, Kennedy E, Khoja TA, Maalouf F, Obermeyer CM, Mattoo A, McGovern T, Memish ZA, Mensah GA, Patel V, Petroni S, Reavley N, Zertuche DR, Saeedi M, Santelli J, Sawyer SM, Ssewamala F, Taiwo K, Tantawy M, Viner RM, Waldfogel J, Zuniga MP, Naghavi M, Wang H, Vos T, Lopez AD, Al Rabeeah AA, Patton GC, Murray CJ. Global burden of diseases, injuries, and risk factors for young people's health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:2383–401. doi: 10.1016/S0140-6736(16)00648-6. [DOI] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92:925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Sobottka H, Hempel C, Plotz T, Fischer W, Schmalzing G, Schaefer M. Positive allosteric modulation by ivermectin of human but not murine P2X7 receptors. British journal of pharmacology. 2012;167:48–66. doi: 10.1111/j.1476-5381.2012.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Nuutinen S, Kiianmaa K, Panula P. DARPP-32 and Akt regulation in ethanol-preferring AA and ethanol-avoiding ANA rats. Neuroscience letters. 2011;503:31–6. doi: 10.1016/j.neulet.2011.08.002. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, Rounsaville B. Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Archives of general psychiatry. 1996;53:217–24. doi: 10.1001/archpsyc.1996.01830030039007. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Archives of general psychiatry. 1992;49:881–7. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Omura S. Ivermectin: 25 years and still going strong. Int J Antimicrobial Agents. 2008;31:91–98. doi: 10.1016/j.ijantimicag.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Omura S, Crump A. The life and times of ivermectin - a success story. Nat Rev Microbiol. 2004;2:984–9. doi: 10.1038/nrmicro1048. [DOI] [PubMed] [Google Scholar]
- Ostrovskaya O, Asatryan L, Wyatt L, Popova M, Li K, Peoples R, Alkana R, Davies D. Ethanol is a fast channel inhibitor of purinergic P2X4 receptors. J Pharm Exp Ther. 2011;337:171–179. doi: 10.1124/jpet.110.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottesen EA, Campbell WC. Ivermectin in human medicine. J Antimicrob Chemother. 1994;34:195–203. doi: 10.1093/jac/34.2.195. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Mittal N, Lumeng L, Li TK. Involvement of the cyclic AMP-responsive element binding protein gene transcription factor in genetic preference for alcohol drinking behavior. Alcoholism, clinical and experimental research. 1999;23:1425–34. [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H, Xu T. Partial deletion of the cAMP response element-binding protein gene promotes alcohol-drinking behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:5022–30. doi: 10.1523/JNEUROSCI.5557-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Flores G, Levesque SA, Pacheco J, Vaca L, Lacroix S, Perez-Cornejo P, Arreola J. The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochemical and Biophysical Research Communications. 2015;467:484–490. doi: 10.1016/j.bbrc.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Popova M, Asatryan L, Ostrovskaya O, Wyatt RL, Li K, Alkana RL, Davies DL. A point mutation in the ectodomain-transmembrane 2 interface eliminates the inhibitory effects of ethanol in P2X4 receptors. J Neurochem. 2010;112:307–317. doi: 10.1111/j.1471-4159.2009.06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova M, Trudell J, Li K, Alkana R, Davies D, Asatryan L. Tryptophan 46 is a site for ethanol and ivermectin action in P2X4 receptors. Purinergic signalling. 2013 doi: 10.1007/s11302-013-9373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Silberberg SD. Mechanism of Ivermectin Facilitation of Human P2X4 Receptor Channels. The Journal of General Physiology. 2004;123:281–293. doi: 10.1085/jgp.200308986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf R, Chabot-Doré AJ, Ase AR, Blais D, Séguéla P. Differential regulation of microglial P2X4 and P2X7 ATP receptors following LPS-induced activation. Neuropharmacology. 2007;53:496–504. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA : the journal of the American Medical Association. 1990;264:2511–8. [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98:1209–28. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Remme J, De Sole G, Dadzie KY, Alley ES, Baker RH, Habbema JD, Plaisier AP, van Oortmarssen GJ, Samba EM. Large scale ivermectin distribution and its epidemiological consequences. Acta Leiden. 1990;59:177–91. [PubMed] [Google Scholar]
- Richard-Lenoble D, Chandenier J, Gaxotte P. Ivermectin and filariasis. Fundam Clin Pharmacol. 2003;17:199–203. doi: 10.1046/j.1472-8206.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. Journal of Neuroscience. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med. 2015;49:e73–e79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Schaeffer JM, Haines HW. Avermectin binding in Caenorhabditis elegans. A two-state model for the avermectin binding site. Biochem Pharmacol. 1989;38:2329–38. doi: 10.1016/0006-2952(89)90473-5. [DOI] [PubMed] [Google Scholar]
- Shan Q, Haddrill JL, Lynch JW. Ivermectin, an unconventional agonist of the glycine receptor chloride channel. J Biol Chem. 2001;276:12556–12564. doi: 10.1074/jbc.M011264200. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R. Effect of avermectin B1a on chick neuronal gamma-aminobutyrate receptor channels expressed in Xenopus oocytes. Molecular pharmacology. 1987;32:749–52. [PubMed] [Google Scholar]
- Silberberg SD, Li M, Swartz KJ. Ivermectin interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron. 2007;54:263–274. doi: 10.1016/j.neuron.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. Journal of Neuroscience. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth A, Teo KK, Rangarajan S, O'Donnell M, Zhang X, Rana P, Leong DP, Dagenais G, Seron P, Rosengren A, Schutte AE, Lopez-Jaramillo P, Oguz A, Chifamba J, Diaz R, Lear S, Avezum A, Kumar R, Mohan V, Szuba A, Wei L, Yang W, Jian B, McKee M, Yusuf S Investigators P. Alcohol consumption and cardiovascular disease, cancer, injury, admission to hospital, and mortality: a prospective cohort study. Lancet. 2015;386:1945–54. doi: 10.1016/S0140-6736(15)00235-4. [DOI] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Gomez-Hernandez JM, Hollmann M, Karschin C, Stuhmer W. P2X4: an ATP-activated ionotropic receptor clonned from rat brain. Proc Natl Acad Sci USA. 1996;93:3684–3688. doi: 10.1073/pnas.93.8.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Lieb M. Recent Developments in Pharmacotherapy of Alcoholism. Pharmacopsychiatry. 2015;48:123–35. doi: 10.1055/s-0035-1547237. [DOI] [PubMed] [Google Scholar]
- Soyka M, Muller CA. Pharmacotherapy of alcoholism - an update on approved and off-label medications. Expert Opin Pharmacother. 2017;18:1187–1199. doi: 10.1080/14656566.2017.1349098. [DOI] [PubMed] [Google Scholar]
- Spinosa HS, Stilck SRAN, Bernardi MM. Possible anxiolytic effects of ivermectin in rats. Vet Res Commun. 2002;26:309–321. doi: 10.1023/a:1016094726033. [DOI] [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift RM. Drug therapy for alcohol dependence. The New England journal of medicine. 1999;340:1482–90. doi: 10.1056/NEJM199905133401907. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, Koob G, Richardson H, Kechris K, Bell RL, Hübner N, Heinig M, Pravenec M, Mangion M, Legault L, Dongier M, Conigrave KM, Whitfield JB, Saunders JB, Grant B, Hoffman PL. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biology. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, Greene BM. The status of ivermectin in the treatment of human onchocerciasis. Am J Trop Med Hyg. 1989;41:460–6. doi: 10.4269/ajtmh.1989.41.460. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Murphy RP, Newland HS, White AT, D'Anna SA, Keyvan-Larijani E, Aziz MA, Cupp EW, Greene BM. Treatment of onchocerciasis. The ocular effects of ivermectin and diethylcarbamazine. Arch Ophthalmol. 1986;104:863–70. doi: 10.1001/archopht.1986.01050180097039. [DOI] [PubMed] [Google Scholar]
- Toulme E, Garcia A, Samways D, Egan TM, Carson MJ, Khakh BS. P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J Gen Physiol. 2010;135:333–53. doi: 10.1085/jgp.200910336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulme E, Khakh BS. Imaging P2X4 receptor lateral mobility in microglia: regulation by calcium and p38 MAPK. The Journal of biological chemistry. 2012;287:14734–48. doi: 10.1074/jbc.M111.329334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichsen J, Haugbol S, Brandt CF, Allerup P, Hemmingsen R. Irreversibility of kindled alcohol-withdrawal behaviour in rats. Alcohol and alcoholism. 1998;33:230–43. doi: 10.1093/oxfordjournals.alcalc.a008387. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Archives of general psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- White A, Hingson R. The burden of alcohol use: excessive alcohol consumption and related consequences among college students. Alcohol research : current reviews. 2013;35:201–18. doi: 10.35946/arcr.v35.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme AJ, Rogers AT. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl):S85–95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
- Wyatt LR, Finn DA, Khoja S, Yardley MM, Asatryan L, Alkana RL, Davies DL. Contribution of P2X4 receptors to ethanol intake in male C57BL/6 mice. Neurochemical research. 2014;39:1127–39. doi: 10.1007/s11064-014-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhou C, Li K, Davies DL, Ye JH. Purinergic type 2 receptors at GABAergic synapses on ventral tegmental area dopamine neurons are targets for ethanol action. Journal of Pharmacology And Experimental Therapeutics. 2008;327:196–205. doi: 10.1124/jpet.108.139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernstein AM, Wong A, Lu XH, Khoja S, Yang XW, Davies DL, Micevych P, Sofroniew MV, Khakh BS. P2X4 Receptor Reporter Mice: Sparse Brain Expression and Feeding-Related Presynaptic Facilitation in the Arcuate Nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:8902–20. doi: 10.1523/JNEUROSCI.1496-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley M, Wyatt L, Khoja S, Asatryan L, Ramaker MJ, Finn DA, Alkana RL, Huynh N, Louie SG, Petasis NA, Bortolato M, Davies DL. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology. 2012;63:190–201. doi: 10.1016/j.neuropharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Huynh N, Rodgers KE, Alkana RL, Davies DL. Oral delivery of ivermectin using a fast dissolving oral film: Implications for repurposing ivermectin as a pharmacotherapy for alcohol use disorder. Alcohol. 2015;49:553–9. doi: 10.1016/j.alcohol.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Neely M, Huynh N, Asatryan L, Louie SG, Alkana RL, Davies DL. Multiday administration of ivermectin is effective in reducing alcohol intake in mice at doses shown to be safe in humans. Neuroreport. 2014;25:1018–23. doi: 10.1097/WNR.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]