Abstract
Excessive stress exposure often leads to emotional dysfunction, characterized by disruptions in healthy emotional learning, expression, and regulation processes. A prefrontal cortex (PFC)-amygdala circuit appears to underlie these important emotional processes. However, limited human neuroimaging research has investigated whether these brain regions underlie the altered emotional function that develops with stress. Therefore, the present study used functional magnetic resonance imaging (fMRI) to investigate stress-induced changes in PFC-amygdala function during Pavlovian fear conditioning. Participants completed a variant of the Montreal Imaging Stress Task (MIST) followed (25 minutes later) by a Pavlovian fear conditioning task during fMRI. Self-reported stress to the MIST was used to identify three stress-reactivity groups (Low, Medium, and High). Psychophysiological, behavioral, and fMRI signal responses were compared between the three stress-reactivity groups during fear conditioning. Fear learning, indexed via participant expectation of the unconditioned stimulus during conditioning, increased with stress reactivity. Further, the High stress-reactivity group demonstrated greater autonomic arousal (i.e., skin conductance response, SCR) to both conditioned and unconditioned stimuli compared to the Low and Medium stress-reactivity groups. Finally, the High stress group did not regulate the emotional response to threat. More specifically, the High stress-reactivity group did not show a negative relationship between conditioned and unconditioned SCRs. Stress-induced changes in these emotional processes paralleled changes in dorsolateral, dorsomedial, and ventromedial PFC function. These findings demonstrate that acute stress facilitates fear learning, enhances autonomic arousal, and impairs emotion regulation, and suggests these stress-induced changes in emotional function are mediated by the PFC.
Neural mechanisms of stress-induced changes in Pavlovian fear conditioning
Excessive and prolonged stress exposure often disrupts healthy emotional function and can ultimately lead to the development of an anxiety disorder. Anxiety-related disorders are linked to neurobiological alterations in brain circuitry that produce broad and long-lasting changes in behavior and cognition (Christoffel, Golden, & Russo, 2011). For example, high levels of stress exposure can lead to emotion dysfunction, in part, by disrupting emotion learning, memory, and regulation processes (Maier & Watkins, 1998). Pavlovian fear conditioning is a popular procedure often used to assess fear learning and emotion regulation. However, little human neuroimaging research has investigated the neural substrates that underlie stress-induced changes in fear learning and emotion regulation. Comprehensive knowledge of the neural circuitry that mediates stress-related changes in healthy emotion function is essential to understanding stress-related disorders. Thus, an important challenge now facing the field is to determine the neural substrates that mediate the detrimental impact of stress on emotional function.
Pavlovian fear conditioning is an effective and popular paradigm often used in both human and animal models to study emotional learning, memory, and regulation (Maren, 2001). Furthermore, Pavlovian conditioning is ideal for human studies of emotion because the neural mechanisms of fear conditioning are relatively well established across species (Kim & Jung, 2006). In a typical Pavlovian fear conditioning study, an originally neutral stimulus is repeatedly paired with an aversive threat (unconditioned stimulus; UCS) that elicits a reflexive emotional response (unconditioned response; UCR). The neutral stimulus becomes a conditioned stimulus (CS) that elicits a conditioned response (CR), once the CS−UCS association is formed. Thus, the CR serves as an index of associative fear learning and expression. During differential fear conditioning, one CS (CS+) is paired with the UCS while a second CS (CS−) is presented alone. Changes in the response to the CS+ (warning signal) and the CS− (safety signal) serve as an index of fear learning. For example, differential emotional responses to the warning vs safety signal serve as an index of anticipatory fear learning. Further, there are several approaches that can be used to index emotion regulation. For example, because organisms must flexibly learn that a warning signal can become a safety signal (i.e. during extinction), extinction learning (i.e., decreased CR once the CS+ and UCS are no longer paired) provides a measure of inhibitory control of emotion (Raio & Phelps, 2015).
A second approach to measuring emotion regulation is known as conditioned UCR diminution. Conditioned UCR diminution is demonstrated by a diminished emotional response (i.e. the UCR) when the UCS is predictable (e.g., the UCS is preceded by the CS+) compared to when the UCS is unpredictable (e.g., the UCS is preceded by the CS− or is presented alone). This conditioned reduction in the UCR to a predictable UCS provides a continuous measure of the ability to regulate the emotional response to threat (i.e. the UCS). Thus, differences in the emotional response to a predictable UCS compared to an unpredictable UCS serve as an index of fear regulation (Dunsmoor, Bandettini, & Knight, 2008; Harnett et al., 2015; Knight, Waters, King, & Bandettini, 2010; Wood, Ver Hoef, & Knight, 2012). These differences in the emotional response to predictable and unpredictable UCS presentations cannot be explained by a simple non-associative learning process (i.e., habituation [Baxter, 1966; Goodman, Harnett, & Knight, 2018; Knight, Lewis, & Wood, 2011]). Specifically, changes in the UCR are greater to a predictable UCS than unpredictable UCS over an equivalent number of trials. Further, the anticipatory response to the warning signal (increased CR to CS+) facilitates UCR diminution (decreased UCR to the predictable UCS) but does not facilitate decreases in the UCR to the unpredictable UCS (Knight, Lewis, & Wood, 2011; Wood et al., 2012). Accordingly, an inverse relationship between the CR and the UCR appears to reflect inhibitory emotion processes that arise from anticipatory fear learning. Therefore, investigations of conditioned UCR diminution may provide novel insight into the alterations of healthy emotion learning and expression that characterize stress-related disorders (Dretsch et al., 2016; Linnman, Zeffiro, Pitman, & Milad, 2011; Wood, Kuykendall, Ver Hoef, & Knight, 2013; Wood et al., 2012).
Exposure to acute stress facilitates fear learning and impairs emotion regulation (Antov, Wolk, & Stockhorst, 2013; Raio & Phelps, 2015). Under normal levels of acute stress, it is adaptive to reallocate neural resources to increase fear learning at the expense of executive functions, including emotion regulation (Hermans, Henckens, Joels, & Fernandez, 2014). Accordingly, an improved ability to learn warning cues that predict threat should provide a selective advantage to survival. However, the development of anxiety disorders results from the dysfunction of normal fear processes (Rosen & Schulkin, 1998). Therefore, one approach to better understanding anxiety disorders is examining stress-induced dysfunction of healthy fear learning and emotion regulation. Stress-related disruption of fear learning and emotion regulation may underlie maladaptive emotional function and lead to anxiety disorder (Beckers, Krypotos, Boddez, Effting, & Kindt, 2013; Rau, DeCola, & Fanselow, 2005). Thus, excessive stress could play a causal role in the development of anxiety via disruptions of normal fear processes. Converging evidence from both human and animal research has demonstrated that stress exposure impacts subsequent fear learning. For example, rats pre-exposed to acute stress demonstrate greater conditioned emotional responses during subsequent fear conditioning (Rau & Fanselow, 2009; Shors, Weiss, & Thompson, 1992). In addition, the intensity of physical stressors (e.g., electric shock) mediates the strength of conditioned fear (Rau & Fanselow, 2009; Shors & Servatius, 1997). Furthermore, conditioned emotional responses in rats pre-exposed to acute stress show greater resistance to extinction (Rau & Fanselow, 2009), suggesting stress disrupts emotion regulation processes. Findings from human studies of stress-induced changes in fear conditioning and emotion regulation, while scarce, are consistent with animal studies. For example, stress pre-exposure increases expectations of threat (Bentz et al., 2013) and elicits larger conditioned emotional responses (Jackson, Payne, Nadel, & Jacobs, 2006). Likewise, stress exposed groups show greater resistance to extinction (vs non-stressed groups), consistent with the view that stress disrupts fear regulation processes (Antov et al., 2013; Jackson et al., 2006).
Normal, healthy fear learning and emotion regulation are supported by a PFC-amygdala network (Hartley & Phelps, 2010; Ochsner & Gross, 2005; Wood et al., 2012; Wood et al., 2015). Specifically, prior fear conditioning studies have shown that the dorsomedial PFC (dmPFC), dorsolateral PFC (dlPFC), and ventromedial PFC (vmPFC) modulate the amygdala activity that underlies fear learning and expression (Knight, Nguyen, & Bandettini, 2005; Knight et al., 2010; Lang et al., 2009; Marschner, Kalisch, Vervliet, Vansteenwegen, & Buchel, 2008; Wheelock et al., 2014; Wood et al., 2013). Accordingly, dysfunction of PFC-amygdala circuitry during fear conditioning appears to mediate alterations of healthy emotional learning and expression. Given that stress reactivity varies with fear conditioning (Raio & Phelps, 2015) and PFC-amygdala circuitry underlies healthy emotion learning and expression (Knight et al., 2005), the stress-related disruption of Pavlovian fear conditioning is likely mediated by dysfunction of PFC-amygdala circuitry. The view that stress-related disruption of fear conditioning is mediated by PFC-amygdala function is further bolstered by evidence that there is considerable overlap in the brain regions that mediate fear conditioning and stress reactivity [for review see: (Hermans et al., 2014)]. Therefore, dysfunction of the PFC-amygdala circuit may be an important mechanism of disrupted fear learning and emotion regulation following stress. Although the overlapping brain circuitry implicated in stress and fear conditioning studies has been established, neuroimaging studies have just begun to assess the interaction between these processes.
Few prior human neuroimaging studies have investigated the neural substrates of fear learning following an acute stressor (Merz et al., 2013; Vogel et al., 2015). This limited prior work suggests PFC-amygdala circuitry underlying fear conditioning varies with stress reactivity (i.e., cortisol, skin conductance response [SCR], heart rate, self-report) (Merz et al., 2013; Vogel et al., 2015). Furthermore, amygdala connectivity during conditioning appears to increase as a function of cortisol availability (manipulated via pharmacological blockade of mineralocorticoid receptors) (Vogel et al., 2015). Thus, cortisol reactivity appears to play an important role in alterations of PFC-amygdala function during fear conditioning. Although these findings provide valuable knowledge regarding the neural substrates that mediate the effect of acute stress exposure on anticipatory fear learning, these investigations did not focus on stress-related changes in emotion regulation (Antov et al., 2013; Jackson et al., 2006; Raio & Phelps, 2015). Accordingly, a comprehensive assessment of the relationship between stress reactivity, anticipatory fear learning, and regulatory control is needed to bridge an important gap in the current understanding of brain regions that mediate stress-related changes in emotion regulation.
The current study used a psychosocial stress and Pavlovian fear conditioning paradigms to investigate the neural processes that mediate the impact of stress exposure on fear learning and emotion regulation. We hypothesized that enhanced anticipatory fear learning and weaker emotion regulation (as indexed by conditioned UCR diminution), following stress exposure, would be mediated by alterations in PFC-amygdala function. Because prior studies have shown an interaction between anticipatory responses and conditioned UCR diminution (Dunsmoor, Bandettini, & Knight, 2007; Knight et al., 2011; Wood et al., 2012), we also assessed the relationship between CRs and UCRs as a function of stress reactivity. We hypothesized that the effect of learning on UCR diminution would be differentially mediated by stress reactivity.
Method
Participants
120 right-handed volunteers (53 males, 67 females, mean age = 20.15 years, age range 18–23 years) participated in a functional magnetic resonance imaging (fMRI) study using a variant of the Montreal Imaging Stress Task [MIST; (Dedovic et al., 2005; Goodman et al., 2016; Wheelock et al., 2016)] followed by a Pavlovian fear conditioning task (Harnett et al., 2015). All participants provided written informed consent as approved by the University of Alabama at Birmingham Institutional Review Board.
Task design
MIST
Participants completed a modified version of the MIST, a psychosocial stress task, using methods described in prior work (Goodman et al., 2016; Wheelock et al., 2016). Briefly, participants completed math problems during two separate blood-oxygen-level dependent (BOLD) fMRI scans to assess Control and Stress conditions of the task (see Supplementary Materials S1.1 for further details).
Approximately 25 minutes after the MIST, participants began the fear conditioning task. Prior research indicates a 25 minute delay between the MIST and the Pavlovian fear conditioning task is sufficient for the stress-induced effects of the MIST to affect the subsequent conditioning task (Hermans et al., 2014; Raio & Phelps, 2015).
Pavlovian Fear Conditioning
Two pure tones (700 Hz and 1300 Hz; counterbalanced) served as the CS+ and CS− during the conditioning procedure in the current study. The CS+ always (24 trials at a 100% paring rate) co-terminated with the unconditioned stimulus (UCS; 100-dB white noise, 0.5 s duration), while the CS− (24 trials) was presented without the UCS. The UCS was also presented alone on 24 trials (UCS alone). A total of 72 trials (18 s inter-trial interval) were presented during two fMRI conditioning scans (36 trials per scan; 12 CS+, 12 CS−, 12 UCS alone trials), each lasting approximately 15 minutes. Trial order was pseudorandomly determined, without replacement, such that no more than two of any trial type (CS+, CS−, and UCS alone) were consecutively presented. Statistical analyses focused on comparisons of responses to the CS+ vs CS− and CS+UCS (i.e., UCS that followed the CS+) vs UCS alone as described in prior work (Harnett et al., 2015; Knight et al., 2011; Knight, Waters, & Bandettini, 2009; Knight et al., 2010; Wood et al., 2013; Wood et al., 2015).
Psychophysiological and Behavioral Analysis
MIST
Following the completion of the MIST, participants completed separate self-report questionnaires for the Control and Stress scans (for details, see Wheelock et al., 2016). For each scan, participants rated the applicability of eight statements (e.g.,” I felt overwhelmed”) on a scale between 1–5. Thus, total scores ranged between 8 (low stress) and 40 (high stress) for each scan. Self-reported stress scores for the Control and Stress scans were transformed by squaring each participant’s stress ratings (Stress2, Control2) to determine individual differences in stress reactivity. A tertile split using the transformed differential scores (Stress2 - Control2) was then used to separate participants into Low, Medium, and High stress-reactivity groups.
Heart Rate
Heart rate (HR) during Stress and Control scans was assessed as a manipulation check of peripheral stress reactivity. HR was measured using an MR compatible photoplethysmograph placed on the index finger of the non-dominant hand. HR was recorded at 50Hz using a Siemens Physiological Monitoring Unit. QRSTool (Allen, Chambers, & Towers, 2007) was used to identify peak pulse waveforms within each HR timeseries and CMetX (Allen et al., 2007) was used to calculate the average beats per minute (BPM) during Contol and Stress scans.
UCS Expectancy
UCS expectancy was collected during conditioning as an index of participants’ moment-to-moment expectation of UCS presentation. Participants were instructed to rate their expectation of UCS presentation using a rating bar on a 0 (certain the UCS would not be presented) to 100 (certain the UCS would be presented) scale using previously described methods (Knight & Wood, 2011). The expectancy rating bar was visible throughout the entirety of the conditioning scans. UCS expectancy responses were recorded at a 40 Hz sampling rate, and calculated as the average expectancy rating during the 1 s before UCS onset and the equivalent period of time during CS− trials.
Skin Conductance Response
An MR compatible psychophysiological monitoring system (Biopac Systems; Goleta, CA) was used to collect SCR data. SCR was measured (10 kHz sampling rate) using a pair of disposable radio-translucent electrodes (1 cm diameter, Biopac Systems) attached to the thenar and hypothenar eminences of participant’s left palm during the Pavlovian fear conditioning task. All SCR data were preprocessed using Biopac Acknowledge 4.1 software. A 1 Hz low pass filter was applied and data were resampled at 250 Hz. Conditioned and unconditioned SCR amplitudes were calculated from response onset to response peak during the 10 s following stimulus (i.e., CS or UCS, respectively) onset. Participants were considered nonresponders and their SCR data were excluded if peak amplitudes did not exceed 0.05 μS in any of the 4 stimulus conditions (CS+, CS−, UCS alone, CS+UCS).
Fear Conditioning and Emotion Regulation
UCS expectancy and psychophysiological (SCR) responses were used to index fear learning and emotion regulation during conditioning. Conditioned responses to warning (CS+) versus safety cues (CS−) served as an index of fear learning. In contrast, responses to predictable (CS+UCS) vs unpredictable (UCS alone) threats served as an index of emotion regulation. 2 × 3 mixed model ANOVAs were used to test for within-subject effects of stimulus type (CS+ vs. CS− or CS+UCS vs. UCS alone), and between-subject effects of stress reactivity (Low, Medium, High), as well as the interaction between stimulus type and stress reactivity on UCS expectancy and SCR (see Supplementary Materials S1.2 for further details).
Relationship between CR and UCR
Multiple regression analysis was used to investigate whether the linear relationship between the CR and UCR varied between stress-reactivity groups (see Supplementary Materials S1.3 for further details). A correlation analysis was performed on the CR and UCR data for each group individually to characterize any differences in the linear relationships between the stress-reactivity groups.
BOLD Imaging Analysis of Pavlovian Fear Conditioning
Scanning Acquisition Parameters
BOLD fMRI was acquired on a 3T Siemens Allegra scanner using a gradient-echo echoplanar (EPI) sequence (TR=2000 ms, TE=30ms, FOV=24 cm, matrix=64×64, slice thickness=4 mm). High resolution anatomical images (MPRAGE) served as an anatomical reference (T1 weighted, TR=2300 ms, TE=3.9 ms, FOV=25.6 cm, matrix=256×256, slice thickness=1 mm, 0.5 mm gap). Although EPI data were acquired during both the MIST and fear conditioning tasks, fMRI data from the MIST have been reported previously (Wheelock et al., 2016; Goodman et al., 2016) and are not included in the current report.
MRI Data Preprocessing and 1st Level Analysis
Functional MRI data from the conditioning task were preprocessed using the AFNI software package (Cox, 1996). Functional MRI data were slice time corrected, motion corrected, and coregistered to the MPRAGE (see Supplementary Materials S1.4 for further details). For first-level analyses, activity was modeled with a gamma variate hemodynamic response function with reference waveforms for all stimuli (i.e., CS+, CS−, CS+UCS, UCS alone) and regressors to account for participant head motion and joystick movement.
Group-level Analysis
A voxel-wise hypothesis-driven analysis was completed to assess whether the neural activity during fear conditioning varied with stress reactivity using a linear mixed effects analysis (3dLME in AFNI) to test for a within-subject effect of stimulus type (CS+ vs. CS− or CS+UCS vs. UCS alone), and a between-subject effect of stress reactivity (Low, Medium, High), as well as the interaction between stimulus type and stress reactivity on the BOLD signal. Based on prior work, analyses were restricted to the amygdala, hippocampus, parahippocampal gyrus, PFC, inferior parietal lobule (IPL), cingulate, and insula using an anatomical mask (AFNI Talairach & Tournoux Atlas) to reduce the number of voxel-wise comparisons (Dunsmoor et al., 2007; Harnett et al., 2015; Knight et al., 2010; Wood et al., 2013). A voxel-wise threshold of p < 0.05 (corrected) was used to reduce family-wise error (FWE) (see Supplementary Materials S1.5 for further details). An exploratory whole brain supplementary analysis was also completed to determine whether other brain regions, that were not included in the hypothesis driven analysis, may mediate the impact of stress on emotional learning and regulation (see Supplementary Materials S1.5 for further details).
Relationship between CR and UCR
CRs (responses to the CS+) were extracted from functionally defined ROIs of the interaction between stress group and CS+ (warning cue) vs. CS− (safety cue) in order to index individual differences in the anticipatory response to warning cues (CR) across activated brain regions (see Supplementary Materials S1.6 for further details). To assess whether the relationship between the CR and UCR in these brain regions varied as a function of stress reactivity, a linear mixed-effects (3dLME in AFNI) analysis identified voxels with a linear relationship between CS+ (warning cue) and CS+UCS (predictable threat) responses that varied by stress-reactivity group (Low, Medium, High). The resulting areas of activation from this analysis identified brain regions that demonstrated a relationship between CR and UCR amplitude that varied with stress reactivity (see Supplementary Materials S1.6 for further details).
Positive and Negative Affect Schedule
After scanning concluded, participants completed the Positive and Negative Affect Schedule [PANAS; (Watson, Clark, & Tellegen, 1988)] (see Supplementary Materials S1.7 for further details). Negative affect scores were calculated and compared across stress-reactivity groups to ensure that the results of the current study did not simply reflect inter-individual variability in pre-existing emotional traits of participants. Accordingly, a one-way ANOVA comparing total negative affect scores across groups assessed this possible alternative explanation.
Results
Psychophysiological and Behavioral Results
MIST
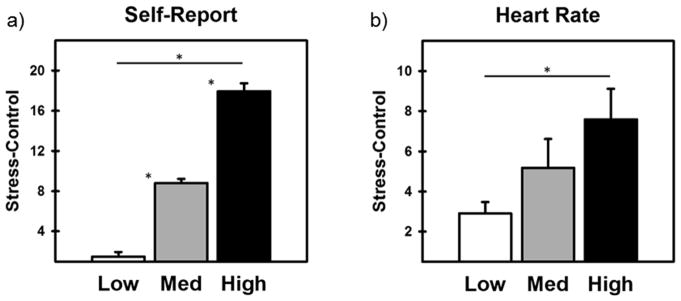
Participants’ self-reported stress ratings for the Control and Stress scans of the MIST were calculated based on previously published work (Wheelock et al., 2016). Participants’ differential stress scores (Stress - Control) were normally distributed (M= 9.33, range: −6 to 28). A tertile split of the transformed differential self-reported stress scores for the Control and Stress scans were used to separate participants into Low (n=40), Medium (n=39), and High (n=41) stress-reactivity groups (Figure 1). A one-way ANOVA comparing participants’ differential self-reported stress to the MIST (Stress - Control) confirmed that self-reported stress ratings varied across the stress-reactivity groups [F(2, 119) = 207.70, p < 0.001]. Bonferroni corrected post-hoc analysis confirmed that self-reported stress was the lowest in the Low stress-reactivity group (M = 1.48, SE = 0.46), intermediate in the Medium stress-reactivity group (M = 8.81, SE = 0.41), and highest in the High stress-reactivity group (M = 17.95, SE = 0.79; all ps < 0.001; Figure 1a). A one-way ANOVA comparing participants’ differential HR response to MIST scans (Stress – Control) confirmed that the peripheral emotional response to stress varied across the three stress-reactivity groups [F(2,55) = 3.58, p<0.05; Figure 1b]. Post-hoc analysis confirmed that the differential HR responses were greater for the High stress than Low stress group (p < 0.05). No other post-hoc group comparisons yielded significant results (all ps > 0.18).
Figure 1.
Differential (Stress - Control) self-reported stress and heart rate (HR) during the MIST for Low, Medium, and High stress-reactivity groups. a) Increases in self-reported stress from Low to Medium to High demonstrated increased stress reactivity across groups. b) HR was measured as a manipulation check and demonstrated that the differential HR (Stress - Control) increased across Low to High stress-reactivity groups. Asterisk indicates a significant difference (p < 0.05; corrected).
UCS Expectancy
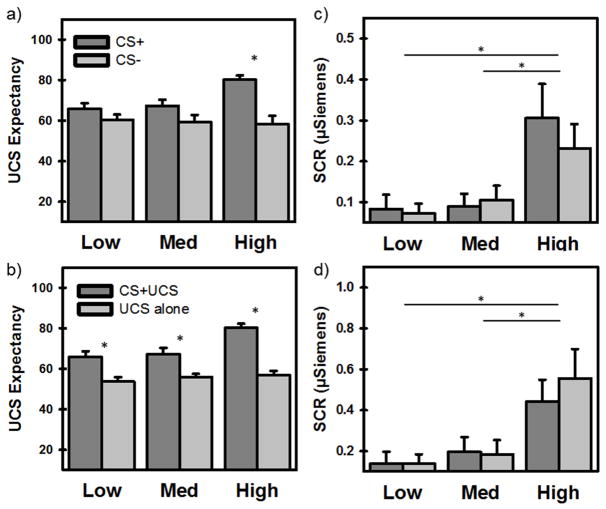
A 2 × 3 mixed model ANOVA revealed significant differences in participants’ UCS (i.e., threat) expectancy (Figure 2a) based on stimulus type (CS+ vs CS−) [F(1, 117) = 24.71, p < 0.001], but not by group (Low, Medium, High) [F(2, 117) = 2.19, p = 0.12]. However, there was a significant interaction of stimulus type and group [F(2, 117) = 4.65, p < 0.05]. Posthoc comparisons revealed that UCS expectancy was greater during the CS+ than CS− in the High (but not Low and Medium) stress-reactivity group (p < 0.001). Posthoc comparisons also revealed that UCS expectancy was greater in the High group than Medium and Low stress-reactivity groups during the CS+ (both ps < 0.01). No other posthoc comparisons were significant. A supplementary trial-by-trial analysis confirmed that, compared to Medium and Low reactivity groups, the High stress reactivity group demonstrated more rapid and robust discrimination (i.e., difference in slopes of CS+ vs CS− across conditioning trials) for UCS expectancy (See Supplementary Materials S2.1 and Figure S1).
Figure 2.
Differential responses to stimulus events (CS+ vs CS− or CS+UCS vs UCS alone) separated by stress-reactivity groups (Low, Medium, High) for each behavioral and psychophysiological measure (UCS expectancy, SCR). a) The High group (but not Low and Medium) reported greater UCS expectancy to the CS+ than CS−. b) All groups reported greater UCS expectancy prior to UCSs that were predictable (CS+UCS) compared to UCSs that were unpredictable (UCS alone). c) Average SCRs to the CSs (CS+ and CS−) were greater for the High than Medium and Low stress-reactivity groups. Medium and Low groups did not differ. d) Average SCRs to the UCSs (CS+UCS and UCS alone) were greater for the High than Medium and Low groups. Medium and Low groups did not differ. Asterisk indicates a significant difference (p < 0.05; corrected).
Another 2 × 3 mixed model ANOVA revealed significant differences in participants’ UCS expectancy prior to predictable (CS+UCS) vs. unpredictable (UCS alone) threat (Figure 2b). The results revealed a significant main effect of stimulus type [F(1, 117) = 99.68, p < 0.001] and a main effect of group [F(2, 117) = 5.50, p < 0.01]. Further, there was a significant interaction of stimulus type and group [F(2, 117) = 5.99, p < 0.01]. Posthoc comparisons revealed that UCS expectancy was greater in the High than Medium and Low stress-reactivity groups (both ps < 0.05) and was greater during predictable (CS+UCS) than unpredictable (UCS alone) trials for all three stress-reactivity groups (all ps < 0.01). Posthoc comparisons also revealed that UCS expectancy ratings of the CS+UCS were greater in the High than Medium and Low stress-reactivity groups (both ps < 0.01). No other posthoc comparisons were significant. Taken together with UCS expectancy during CS+ vs CS− presentations, these findings demonstrate enhanced fear learning in High stress-reactivity participants.
Skin conductance response
A 2 × 3 mixed model ANOVA revealed significant differences in participants’ SCRs to CSs during conditioning (Figure 2c) based on group [F(2, 84) = 5.05, p < 0.01], but not stimulus type [F(1, 84) = 0.21, p = 0.65]. There was no interaction between stimulus type and group [F(2, 84) = 1.03, p = 0.36]. Posthoc comparisons revealed that SCRs to CSs were greater in the High group than the Medium and Low stress-reactivity groups (both ps < 0.05). No other posthoc comparisons were significant. A supplementary trial-by-trial analysis revealed no significant differences between stress reactivity groups in discrimination across trials (i.e., difference in slopes of CS+ vs CS− across conditioning trials) for SCR (See Supplementary Materials S2.2).
Another 2 × 3 mixed model ANOVA revealed significant differences in participants’ SCRs to UCSs during conditioning (Figure 2d) by group [F(2, 84) = 6.30, p < 0.01], but not stimulus type [F(1, 84) = 2.29, p = 0.13]. There was no interaction between stimulus type and group [F(2, 84) = 2.36, p = 0.10]. Posthoc comparisons revealed that SCRs to UCSs were greater in the High group than Medium and Low stress-reactivity groups (both ps < 0.05). No other posthoc comparisons were significant. Taken together, these SCR results demonstrate greater autonomic arousal in High stress-reactivity participants during Pavlovian fear conditioning.
Relationship between CR and UCR
Multiple regression analysis revealed that there was a significant negative relationship between SCRs to the CS+ and CS+UCS [F(1, 76) = 6.78, p < 0.05]. However, the slope of the negative relationship between the CR and UCR varied across the stress-reactivity groups [F(5, 76) = 3.76, p < 0.05]. To further describe the variability in the relationship between the CR and UCR, separate correlation analyses were completed for each group. There was a negative relationship between the CR and UCR for all participants, when combined across groups (r = −.29, p < 0.05) (Figure 3a). However, while the Low (r = −.64, p < 0.001; Figure 3b) and Medium (r = −.77, p < 0.001; Figure 3c) stress-reactivity groups showed a negative relationship between the CR and UCR, no relationship between the CR and UCR was observed in the High stress-reactivity group (r =.07, p = 0.77; Figure 3d). These findings suggest that the typical negative relationship between the CR and UCR is not observed in High stress-reactivity participants.
Figure 3.
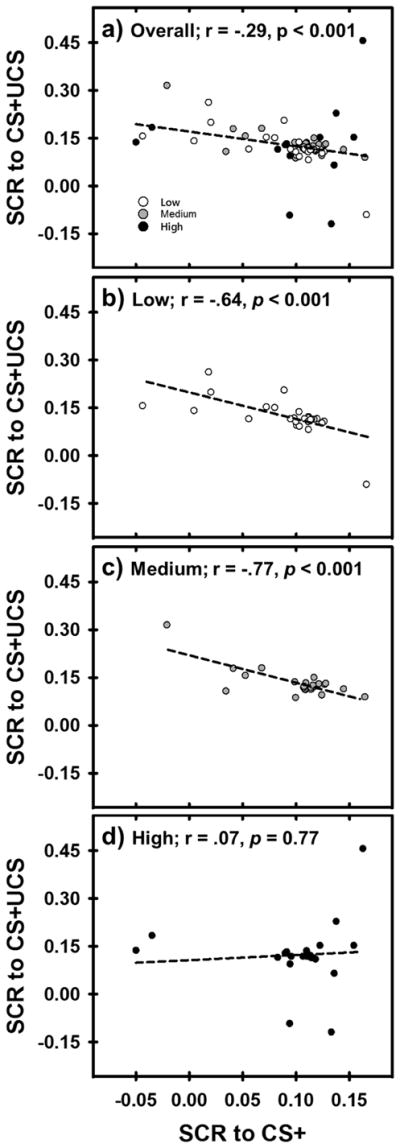
The relationship between SCRs to the CS+ and CS+UCS. Dashed lines reflect the correlation between the CR and UCR in each panel. Panel a) shows the negative CR and UCR relationship for all participants, collapsed across stress-reactivity groups (Low, Medium, High). Panels b–c) show the negative CR and UCR relationship in Low and Medium stress-reactivity groups. Panel d) shows no relationship between the CR and UCR in the High stress-reactivity group.
Functional MRI: Stimulus type × Stress-reactivity Group
CS × stress-reactivity group
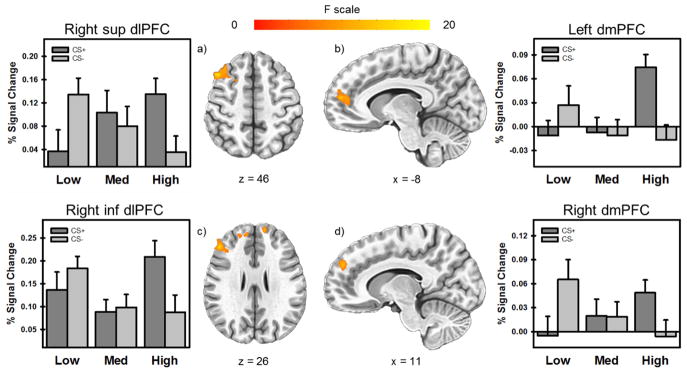
The 3dLME analysis of fMRI data revealed significant activation in the dlPFC, dmPFC, insula, and IPL for the within-subject effect of stimulus type (CS+ vs. CS−) and in the ventrolateral PFC (vlPFC), dlPFC, and dmPFC for the between-subject effect of stress reactivity (Low, Medium, High) (p < 0.05, corrected) (Table S1). The interaction between stimulus type and stress-reactivity group revealed that differential neural responses to CS+ and CS− varied across stress-reactivity groups in the dlPFC, dmPFC, and anterior cingulate cortex (ACC) (p < 0.05, corrected) (Table S1; Figure 4). Results from the supplementary whole brain LME analysis are reported in the supplementary materials (S2.3, Table S2, Figure S2).
Figure 4.
Clusters of significant activation for the interaction between stimulus type (CS+ vs CS) and stress-reactivity groups (High, Medium, Low). Differential neural responses to CS+ and CS− varied across stress-reactivity groups in the dlPFC and dmPFC (a–d). As stress reactivity increased, responses to CS+ showed a corresponding increase. Alternatively, as stress reactivity increased, responses to CS− demonstrated a corresponding decrease.
UCS × stress-reactivity group
The 3dLME analysis revealed significant activation in the dlPFC, dmPFC, vmPFC, vlPFC, ACC, mid cingulate, and insula for the within-subject effect of stimulus type (CS+UCS vs. UCS alone), and within the dmPFC, posterior cingulate, and mid cingulate for the between-subject effect of stress reactivity (Low, Medium, High) (p < 0.05, corrected) (Table S3). No significant activations were observed for the interaction between stimulus type and stress-reactivity group. Results from the supplementary whole brain LME analysis are reported in the supplementary materials (S2.4, Table S4).
Functional MRI: Stress-reactivity group × relationship between CR and UCR
CRs (brain activity elicited by CS+) within each of the 4 functional ROIs (displayed in Figure 4a–d) were compared with UCRs (brain activity elicited by CS+UCS) on a voxel-wise basis to determine whether the relationship varied across stress-reactivity groups (Low, Medium, High) using AFNIs 3dLME program. Two of these functional ROIs were located within the right dlPFC anatomical boundaries. Thus, these two regions are distinguished as the right superior (Figure 4a) and inferior (Figure 4c) dlPFC, based on their relative positions in the brain. The CR elicited by the CS+ (extracted using 3dROIstats in AFNI) from each of the functional ROIs were included in four separate LME models along with stress-reactivity group (Low, Medium, High) as independent variables, while the UCR during the CS+UCS was included as the dependent variable. Areas of significant activation resulting from the main effect of CR and the interaction of CR and stress-reactivity group for each of the four LME analyses are reported in separate sections below.
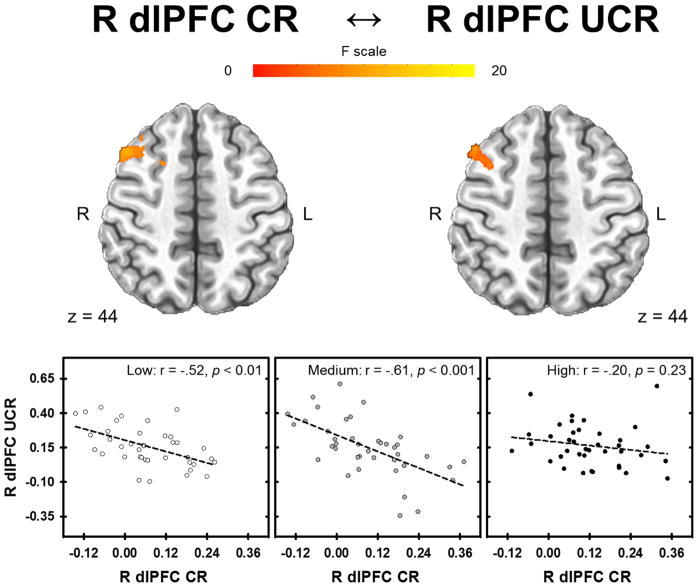
Right superior dlPFC
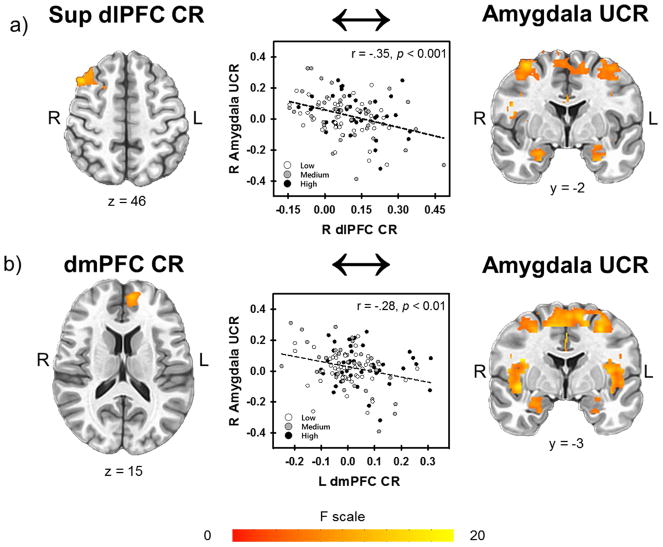
The 3dLME analysis revealed a negative linear relationship between CR within the right superior dlPFC (1348 mm3, peak voxel 42, 17, 46; displayed in Figure 4a) and the UCR within the dlPFC, dmPFC, vlPFC, vmPFC, ACC, mid cingulate, insula, IPL, and amygdala (Figure 5a) (Table S5). The inverse relationship between CR and UCR varied across stress-reactivity groups in the dlPFC, dmPFC, and ACC (Figure 6). All areas of significant activation for the main effect of CR and the interaction with stress-reactivity group were significant at p < 0.05 (corrected) (Table S5).
Figure 5.
Relationship between CR and UCR activity. Regardless of group, CRs in a) the right dlPFC and b) the left superior dmPFC were inversely related to UCRs in the amygdala.
Figure 6.
Relationship between CR and UCR activity that varied with stress-reactivity group. CRs in the right superior dlPFC were inversely related to UCRs in a similar right dlPFC region for the Low and Medium group. Only the High group showed no relationship between CRs in the right superior dlPFC and UCRs in the right dlPFC region.
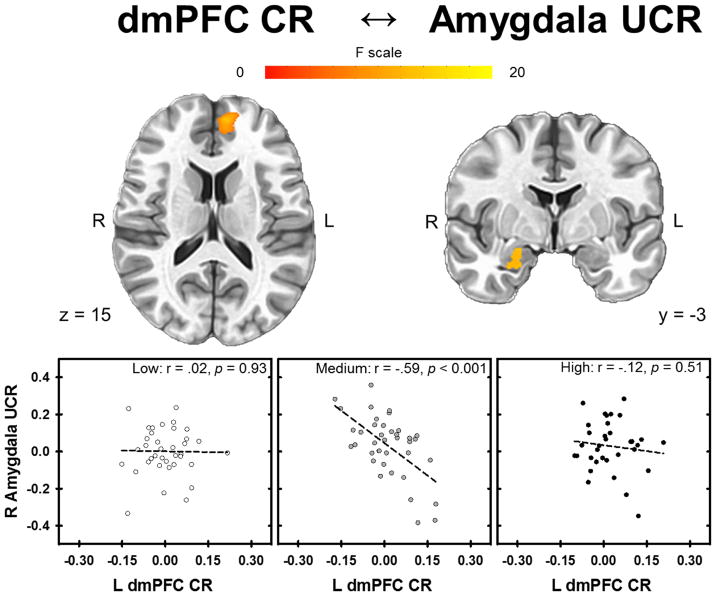
Left dmPFC
The 3dLME analysis demonstrated the left dmPFC CR (1193 mm3, peak voxel −8, 49, 15; displayed in Figure 4b) was negatively related to the UCR in the dlPFC, dmPFC, vlPFC, vmPFC, ACC, mid cingulate, insula, IPL, and amygdala (Figure 5b) (Table S6). The negative relationship between CR and UCR varied across stress-reactivity groups in the dlPFC, dmPFC, vmPFC, ACC, and amygdala (Figure 7). All clusters of significant activation for the main effect of CR and the interaction with stress-reactivity group were significant at p < 0.05 (corrected) (Table S6).
Figure 7.
Relationship between the CR and UCR that varied with stress-reactivity groups. CRs in the left dmPFC were inversely related to UCRs in the amygdala only for the Medium stress-reactivity group. The Low and High stress-reactivity groups showed no relationship between CRs to the CS+ in the left dmPFC and UCRs in the amygdala.
Right inferior dlPFC
The 3dLME analysis demonstrated the right inferior dlPFC CR (895 mm3, peak voxel 45, 31, 26; displayed in Figure 4c) was negatively related to the UCR in the dlPFC, dmPFC, vlPFC, vmPFC, ACC, mid cingulate, insula, IPL, and amygdala (Table S7). The negative relationship between CR and UCR varied across stress-reactivity groups in the dlPFC, dmPFC, vlPFC, ACC, insula, and IPL. All clusters of significant activation for the main effect of CR and the interaction with stress-reactivity group were significant at p < 0.05 (corrected) (Table S7).
Right dmPFC
The 3dLME analysis demonstrated the right dmPFC CR (753 mm3, peak voxel 11, 47, 29; displayed in Figure 4d) was negatively related to the UCR in the dlPFC, dmPFC, vlPFC, vmPFC, ACC, mid cingulate, insula, IPL, and amygdala (Table S8). The negative relationship between CR and UCR varied across stress-reactivity groups in the dlPFC, dmPFC, vmPFC, and ACC. All clusters of significant activation for the main effect of CR and the interaction with stress-reactivity group were significant at p < 0.05 (corrected) (Table S8).
Supplementary Analysis
An exploratory whole brain supplementary 3dLME analysis was also completed to determine whether other brain regions, that were not included in the hypothesis-driven analysis may, mediate the impact of stress reactivity on the effect of the CR on the UCR. Results from the supplementary whole brain LME analysis are reported in the supplementary materials (S2.5; Figure S3; Table S9).
Positive and Negative Affect Schedule
A one-way ANOVA compared total negative affect scores across stress-reactivity groups (Low, Medium, High). The ANOVA yielded no significant effect of Group [F(2, 118) = 1.05, p = 0.36]. This finding demonstrated that stress-reactivity groups did not differ in negative affect.
Discussion
Although the behavioral effects of acute stress on fear learning, emotional expression, and emotion regulation have been widely studied (Antov et al., 2013; Jackson et al., 2006; Raio & Phelps, 2015; Rau & Fanselow, 2009; Shors & Servatius, 1997; Shors et al., 1992), the neurobiology that underlies the stress-induced changes in these processes has received less attention. Therefore, new knowledge of the neural circuitry that underlies stress-related changes in healthy emotion function is critical to understanding disorders that arise from stress exposure. The current study investigated stress-induced changes in fear learning, emotional expression, and emotion regulation to better understand the neural mechanisms that underlie the impact stress has on emotional function. Our findings suggest that high stress reactivity is linked to enhanced fear learning (e.g. UCS expectancy), greater autonomic arousal (e.g. SCR), and disrupted emotion regulation (e.g. no relationship between CR and UCR). We found that dlPFC and dmPFC activity during fear conditioning varied with stress reactivity. Further, the relationship between the CR within these dorsal PFC regions and the UCR within the insula, IPL, ACC, amygdala, and other PFC regions varied with stress reactivity. These findings indicate acute stress exposure alters fear learning, emotional expression, and emotion regulation processes, and suggest the PFC, insula, IPL, and amygdala mediate the impact stress has on these processes. Thus, stress-induced alterations in this neural circuit appear to play an important role in stress-induced changes in emotional learning, expression, and regulation.
Fear Learning
A primary objective of the current study was to assess stress-induced changes in behavior during fear conditioning and determine whether underlying neural activity varied with stress reactivity. Thus, our initial analyses were designed to determine whether fear learning varied with stress reactivity. Overall, UCS expectancy was greater during the CS+ than CS− following stress exposure (Figure 2a). This finding demonstrates that fear conditioning was supported by the current study. Comparisons of brain activation during CS+ versus CS− trials in the current study (Table S1) suggests that this fear learning was supported by activity within the PFC, IPL, and insula. This finding is consistent with prior research that has demonstrated these brain regions play an important role in fear learning processes (Delgado, Nearing, Ledoux, & Phelps, 2008; Dunsmoor, Prince, Murty, Kragel, & LaBar, 2011; Knight et al., 2005; Knight, Smith, Stein, & Helmstetter, 1999; Knight et al., 2009). Additionally, we observed that differential UCS expectancy (CS+ vs CS−) increased as stress reactivity increased. This finding demonstrates fear conditioning is enhanced as stress reactivity increases. Consistent with our findings, prior research has found that stress reactivity influences fear conditioning. Specifically, stress exposure appears to facilitate subsequent fear learning in rats (Rau & Fanselow, 2009; Shors & Servatius, 1997; Shors et al., 1992) and humans (Antov et al., 2013; Bentz et al., 2013; Jackson et al., 2006). Further, we observed differential PFC (i.e., dlPFC, dmPFC, ACC) activation during warning (CS+) vs safety cues (CS−) that varied with stress reactivity. Prior human neuroimaging research has also demonstrated that the PFC underlies changes in fear conditioning following acute stress (Merz et al., 2013; Vogel et al., 2015). Taken together, comparisons of stress reactivity and fear conditioning in the current study suggest that stress-enhanced fear learning is associated with corresponding changes in PFC activation. Thus, findings from the current study suggest that stress-induced changes in PFC function are an important mechanism that underlies the stress-related changes in fear conditioning.
Emotional Expression and Arousal
In the current study, acute psychosocial stress reactivity varied with emotional expression during the subsequent fear conditioning task. More specifically, as stress reactivity to the MIST increased, psychophysiological (SCR) conditioned and unconditioned responses increased during fear conditioning. Further, the psychophysiological response paralleled activation within the dlPFC, dmPFC, vlPFC, mid cingulate, and PCC. Thus, these brain regions may underlie the stress-induced differences in emotional arousal observed between stress-reactivity groups in the present study. One alternative explanation is that corresponding changes in emotion expression, psychosocial stress reactivity, and emotional brain function are related to differences in trait anxiety. For example, prior conditioning research indicates that high anxiety is associated with greater conditioned responses (Nielsen & Petersen, 1976; Pitman & Orr, 1986; Schwerdtfeger, 2006; Thayer, Friedman, Borkovec, Johnsen, & Molina, 2000). Further, other research has demonstrated that an individual’s anxiety level affects their emotional response to aversive events (Cook, Davis, Hawk, Spence, & Gautier, 1992; Grillon, Ameli, Foot, & Davis, 1993). Prior findings from our laboratory also suggest that anxiety level enhances conditioned and unconditioned responses (Knight et al., 2011; Wheelock et al., 2014; Wood et al., 2012). Therefore, in the current study, we compared trait negative affect (PANAS scores) between the stress-reactivity groups to assess the impact that general negative affect has on stress reactivity and emotional expression. Our analysis revealed no differences in negative affect across the three stress-reactivity groups. Therefore, our findings suggest that acute stress exposure leads to increased emotion expression independent of negative affect. Furthermore, changes in PFC activity that corresponded with these differences in emotional expression suggest that these brain regions may underlie stress-induced increases in emotional expression.
Emotion Regulation
We found that stress reactivity during the MIST varied with emotion regulation during the subsequent fear conditioning task. Emotion regulation was indexed via learning-related reductions in the emotional response to threat, a process known as Pavlovian conditioned UCR diminution. Specifically, there is typically a negative relationship between the CR and the UCR during fear conditioning such that as the CR increases, the UCR decreases. Therefore, the negative relationship that is typically observed between the CR and UCR was used to index inhibitory emotion processes that arise from fear learning. Thus, our analysis was designed to determine whether the negative relationship between the CR and UCR was influenced by stress reactivity. Overall, SCRs demonstrated a negative linear relationship between the CR and UCR following stress exposure (Figure 3a). Our neuroimaging results suggest that this inhibitory process was supported by CR activity within the dlPFC and dmPFC that modulated UCR activity within the dlPFC, dmPFC, vlPFC, vmPFC, anterior cingulate cortex, mid cingulate, insula, IPL, and amygdala (Figure 5 and Tables S5–8). Prior research suggests these brain regions are important for emotion regulation processes (Knight et al., 2010; Lang et al., 2009; Marschner et al., 2008; Wheelock et al., 2014; Wood et al., 2013; Wood et al., 2012). Further, prior work from our laboratory has shown that increased anticipatory PFC activity during the CS+ is associated with decreased threat-elicited (i.e., UCS-evoked) activity in the PFC, IPL, and amygdala (Wood et al., 2012). In the current study, CR activity within the PFC was negatively related to UCR activity within the PFC, cingulate, insula, IPL, and amygdala. Further, the negative relationship between the CR and UCR in these brain regions paralleled the negative relationship between the CR and UCR in the SCRs during fear conditioning. These findings suggest that these brain regions support important inhibitory emotion processes that arise from fear learning.
To determine whether stress reactivity alters emotion regulation, the linear relationship between the CR and UCR was compared between Low, Medium, and High stress-reactivity groups. SCRs for the Low and Medium, but not High, stress-reactivity groups demonstrated an inverse relationship between the CR and UCR (Figure 3b–d). These findings suggest that high stress reactivity disrupts emotion regulation. Prior research supports our findings, suggesting stress exposure increases resistance to extinction, an index of emotion regulation (Antov et al., 2013; Jackson et al., 2006; Raio & Phelps, 2015). Further, our findings suggest that PFC activity underlies the stress-related disruption of inhibitory emotion processes that arise from fear learning. Specifically, the relationship between PFC CRs and UCR activity in other brain regions varied with stress reactivity. More specifically, increased dlPFC and dmPFC CR activity was associated with decreased UCR activity in the dlPFC, dmPFC, ACC, insula, IPL, and amygdala for the Medium stress-reactivity group. However, the High stress-reactivity group did not consistently demonstrate a relationship between the CR and UCR within these brain regions (Tables S5–8 and Figures 6–7). Alternatively, the Low stress-reactivity group demonstrated a relationship between the PFC CR and PFC UCR (Figure 6), but not the amygdala UCR (Figure 7). Because the relationship between the CR and UCR reflects inhibitory emotion processes that arise from fear learning, decreased fear learning in the Low stress-reactivity group may explain the absence of a negative relationship between PFC CR activity and amygdala UCR activity. Specifically, the Low stress reactivity group did not report differential UCS expectancies (CS+ vs CS−), unlike the Medium and High stress-reactivity groups. Thus, inconsistent relationships between the CR and UCR in the Low stress-reactivity group may arise from the absence of fear learning, rather than impaired emotion regulation. In contrast, the impaired emotion regulation in the High stress-reactivity group cannot be explained by poor fear learning given that fear learning increased as stress reactivity increased (Figure 2). Given that increased CRs should be associated with decreased UCRs, the findings from the current study suggest that high stress reactivity subsequently disrupts the process by which anticipatory PFC activity diminishes the response to the imminent threat, despite enhanced fear learning.
Taken together, comparisons of stress reactivity and emotion regulation in the current study suggest that stress disrupts neural processes, leading to disruptions in emotion regulation. Specifically, we observed stress-induced changes in the dlPFC and dmPFC anticipatory (i.e., CR) activity. Further, these changes in anticipatory activity were associated with changes in threat-elicited (i.e., UCR) activity within the PFC, insula, IPL, and amygdala. Thus, findings from the current study provide novel evidence that stress-induced changes in anticipatory dorsal PFC activity are important mechanisms that link acute stress exposure to subsequent disruptions in emotion regulation.
Conclusions
The current study provides novel evidence that PFC, cingulate, insula, IPL, and amygdala function underlies stress-induced changes in emotional learning, expression, and regulation processes. Specifically, the present results demonstrate that greater stress reactivity is associated with enhanced fear conditioning, increased emotional expression, and reduced emotion regulation. Enhanced fear conditioning appears to be mediated by stress-induced changes in dmPFC, dlPFC, and ACC activity during fear conditioning, while the increased emotional expression appears to be mediated by stress-induced changes in dmPFC, PCC, and mid cingulate responses to threat. The disruptions observed in emotion regulation appear to be mediated by stress-induced changes in the relationship anticipatory dlPFC and dmPFC activity has with threat-elicited activity within the PFC, ACC, insula, IPL, and amygdala. Given the PFC-amygdala circuit supports emotional learning, expression, and regulation processes (Hartley & Phelps, 2010; Ochsner & Gross, 2005; Wood et al., 2012; Wood et al., 2015), the present findings link changes in the function of brain regions that underlie these emotional processes to stress-induced changes in emotional behavior. Thus, the present study provides a new mechanistic understanding of the impact stress has on emotion-related neural systems, and in turn, emotional behavior.
Supplementary Material
Acknowledgments
This research was funded by the National Institute of Mental Health of the National Institutes of Health [grant number MH098348].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen Chambers, Towers The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol Psychol. 2007;74(2):243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Antov, Wolk, Stockhorst Differential impact of the first and second wave of a stress response on subsequent fear conditioning in healthy men. Biol Psychol. 2013;94(2):456–468. doi: 10.1016/j.biopsycho.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Baxter R. Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of Experimental Psychology. 1966;71(3):447. doi: 10.1037/h0022977. [DOI] [PubMed] [Google Scholar]
- Beckers, Krypotos, Boddez, Effting, Kindt What’s wrong with fear conditioning? Biol Psychol. 2013;92(1):90–96. doi: 10.1016/j.biopsycho.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Bentz, Michael, Wilhelm, Hartmann, Kunz, vonRohr, de Quervain Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology. 2013;38(7):1186–1197. doi: 10.1016/j.psyneuen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Christoffel, Golden, Russo Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22(5):535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, Davis, Hawk, Spence, Gautier Fearfulness and startle potentiation during aversive visual stimuli. Psychophysiology. 1992;29(6):633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cox AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dedovic, Renwick, Mahani, Engert, Lupien, Pruessner The Montreal Imaging Stress Task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J Psychiatry Neurosci. 2005;30(5):319–325. [PMC free article] [PubMed] [Google Scholar]
- Delgado, Nearing, Ledoux, Phelps Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretsch, Wood, Daniel, Katz, Deshpande, Goodman, … Knight Exploring the Neurocircuitry Underpinning Predictability of Threat in Soldiers with PTSD Compared to Deployment Exposed Controls. Open Neuroimag J. 2016;10:111–124. doi: 10.2174/1874440001610010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor, Bandettini, Knight Impact of continuous versus intermittent CS−UCS pairing on human brain activation during Pavlovian fear conditioning. Behav Neurosci. 2007;121(4):635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Dunsmoor, Bandettini, Knight Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40(2):811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor, Prince, Murty, Kragel, LaBar Neurobehavioral mechanisms of human fear generalization. Neuroimage. 2011;55(4):1878–1888. doi: 10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AM, Harnett NG, Knight DC. Pavlovian conditioned diminution of the neurobehavioral response to threat. Neuroscience and Biobehavioral Reviews. 2018;84:218–224. doi: 10.1016/j.neubiorev.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, Wheelock, Harnett, Mrug, Granger, Knight The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon, Ameli, Foot, Davis Fear-potentiated startle: relationship to the level of state/trait anxiety in healthy subjects. Biological Psychiatry. 1993;33(8–9):566–574. doi: 10.1016/0006-3223(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Harnett, Wheelock, Wood, Ladnier, Mrug, Knight Affective state and locus of control modulate the neural response to threat. Neuroimage. 2015;121:217–226. doi: 10.1016/j.neuroimage.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, Phelps Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, Henckens, Joels, Fernandez Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37(6):304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Jackson, Payne, Nadel, Jacobs Stress differentially modulates fear conditioning in healthy men and women. Biol Psychiatry. 2006;59(6):516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kim, Jung Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, Lewis, Wood Conditioned diminution of the unconditioned skin conductance response. Behav Neurosci. 2011;125(4):626–631. doi: 10.1037/a0024324. [DOI] [PubMed] [Google Scholar]
- Knight, Nguyen, Bandettini The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26(4):1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight, Smith, Stein, Helmstetter Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. Neuroreport. 1999;10(17):3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- Knight, Waters, Bandettini Neural substrates of explicit and implicit fear memory. Neuroimage. 2009;45(1):208–214. doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, Waters, King, Bandettini Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49(1):843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, Kroll, Lipinski, Wessa, Ridder, Christmann, … Flor Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29(4):823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman, Zeffiro, Pitman, Milad An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol Mood Anxiety Disord. 2011;1(1):8. doi: 10.1186/2045-5380-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, Watkins Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105(1):83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Maren Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Marschner, Kalisch, Vervliet, Vansteenwegen, Buchel Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28(36):9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz, Wolf, Schweckendiek, Klucken, Vaitl, Stark Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology. 2013;38(11):2529–2541. doi: 10.1016/j.psyneuen.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Nielsen, Petersen Electrodermal correlates of extraversion, trait anxiety and schizophrenism. Scandinavian Journal of Psychology. 1976;17(2):73–80. doi: 10.1111/j.1467-9450.1976.tb00214.x. [DOI] [PubMed] [Google Scholar]
- Ochsner, Gross The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pitman, Orr Test of the conditioning model of neurosis: differential aversive conditioning of angry and neutral facial expressions in anxiety disorder patients. Journal of Abnormal Psychology. 1986;95(3):208–213. doi: 10.1037//0021-843x.95.3.208. [DOI] [PubMed] [Google Scholar]
- Raio, Phelps The influence of acute stress on the regulation of conditioned fear. Neurobiol Stress. 2015;1:134–146. doi: 10.1016/j.ynstr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau, DeCola, Fanselow Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29(8):1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rau, Fanselow Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12(2):125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Rosen, Schulkin From normal fear to pathological anxiety. Psychol Rev. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger Trait anxiety and autonomic indicators of the processing of threatening information: a cued S1–S2 paradigm. Biological psychology. 2006;72(1):59–66. doi: 10.1016/j.biopsycho.2005.07.008. S0301-0511(05)00130-4 [pii] [DOI] [PubMed] [Google Scholar]
- Shors, Servatius The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol Learn Mem. 1997;68(1):92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Shors, Weiss, Thompson Stress-induced facilitation of classical conditioning. Science. 1992;257(5069):537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Thayer, Friedman, Borkovec, Johnsen, Molina Phasic heart period reactions to cued threat and nonthreat stimuli in generalized anxiety disorder. Psychophysiology. 2000;37(3):361–368. [PubMed] [Google Scholar]
- Vogel, Klumpers, Krugers, Fang, Oplaat, Oitzl, … Fernandez Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology. 2015;40(4):947–956. doi: 10.1038/npp.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, Clark, Tellegen Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wheelock, Harnett, Wood, Orem, Granger, Mrug, Knight Prefrontal cortex activity is associated with biobehavioral components of the stress response. Frontiers in human neuroimaging. 2016 doi: 10.3389/fnhum.2016.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock, Sreenivasan, Wood, Ver Hoef, Deshpande, Knight Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage. 2014;102:904–912. doi: 10.1016/j.neuroimage.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, Kuykendall, Ver Hoef, Knight Neural Substrates Underlying Learning-Related Changes of the Unconditioned Fear Response. The open neuroimaging journal. 2013;7:41. doi: 10.2174/1874440001307010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood Ver Hoef, Knight Neural mechanisms underlying the conditioned diminution of the unconditioned fear response. Neuroimage. 2012;60(1):787–799. doi: 10.1016/j.neuroimage.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Wood Wheelock, Shumen Bowen, Ver Hoef, Knight Controllability modulates the neural response to predictable but not unpredictable threat in humans. Neuroimage. 2015;119:371–381. doi: 10.1016/j.neuroimage.2015.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.