Abstract
Study of the clinical effects of combination therapy for malaria is aided by the ability to measure concentrations of individual partner drugs. Existing methods for measurement of the antimalarial drug lumefantrine (LF) in dried blood spots (DBS) on filter paper rely on chemical pretreatment of the paper to facilitate drug elution. However, in the absence of pretreatment, DBS may still offer some utility for semi-quantitative measurements and pharmacokinetic-pharmacodynamic (PK-PD) analyses. We present a method for semi-quantitation of LF in DBS on untreated filter paper using liquid chromatography tandem mass spectrometry. Optimal recovery was achieved by extraction with acetone-water-formic acid (90:5:5). The range of quantitation was 100–20,000 ng/ml. Mean intra- and inter-day accuracy values were 86.6% (coefficient of variation [CV]: 10.1%) and 91.8% (CV: 16.1%), therefore we propose the assay as semi-quantitative. Clinical application was demonstrated in exploratory PK-PD analyses of a drug efficacy trial of artemether-lumefantrine in children with uncomplicated falciparum malaria using post-treatment day 7 samples, parasite clearance times estimated from serial blood smears, and recurrence of malaria out to 35 days. The median day 7 concentration among children (n=71) was 111 ng/ml (interquartile range: 100–194 ng/ml). We used a truncated calibration curve of 100–5,000 ng/ml for calculations due to low observed concentrations. Calculations using the full calibration curve yielded similar values (+1% avg. deviation). Controlling for participant age, gender, and parasite burden, each log increase in LF day 7 concentration corresponded to a decrease of 7.1 h in mean parasite clearance time (95% confidence interval: 0.1–14.3 h, P=0.05). A nested case-control study of participants (n=18) with and without recurrent malaria showed mean post-treatment day 7 concentrations of 181 ng/ml and 235 ng/ml, respectively, but the difference was not significant (P=0.64). A method for semi-quantitation of LF from post-treatment day 7 collections of DBS on untreated filter paper demonstrated clinical application in exploratory PK-PD analyses of parasite clearance and reinfection. Use of DBS will endure in certain study settings by virtue of their ease of collection and resilience. Their utility should continue to be explored as our instruments gain in sensitivity and as clinical pharmacology inquiries are pursued to the field.
Keywords: Lumefantrine, antimalarial drugs, malaria, clinical pharmacology, dried blood spots, LC-MS/MS
1. Introduction
Malaria remains endemic in 91 countries and territories, infecting over 200 million people and causing nearly a half-million deaths in 2016, mainly due to infection with Plasmodium falciparum in young children in sub-Saharan Africa [1]. Control and elimination of malaria are achieved primarily through vector control with indoor residual spraying and distribution of insecticide-treated bed nets, and case management with artemisinin-based combination therapies (ACTs) [2]. In the absence of a sufficiently effective vaccine, drugs remain a keystone in control and elimination programs. Evaluations of the pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of ACTs are aided by the ability to determine concentrations of the individual constituent drugs in treated individuals. In the resource-limited settings where malaria is prevalent, solutions that eschew reliance on electricity, multistep processing, and cold-chain transport are desirable. Whole blood collected as dried blood spots (DBS) on filter paper offers a means of producing resilient field samples.
Among current ACTs, artemether-lumefantrine (AL) is the most widely deployed. Typical peak concentrations of lumefantrine (LF) seen clinically range from 4,000–9,000 ng/ml and median day 7 concentrations are reported between 216–528 ng/ml [3–7], with lower concentrations observed in younger children and in assays of DBS matrices on paper [5, 6]. The terminal elimination half-life of LF is 3–4 days, and prior PK-PD studies estimated a threshold day 7 concentration of LF for protection against re-infection ranging from 175 to 280 ng/ml [4, 8, 9].
Highly hydrophobic, LF adsorbs to filter paper used for DBS collections; existing methods of extraction from DBS therefore rely on pretreatment of the collection card with tartaric or phosphoric acid to promote drug elution [10, 11]. However, determinations of LF using DBS on filter paper may provide useful PK data even if pretreatment was not done. Therefore, as annex to a therapeutic efficacy trial of AL conducted in rural Zambia from 2014–2015, we developed a method for LF determinations from DBS on untreated filter paper and applied it for a pilot PK-PD analysis of malaria parasite clearance and reinfection following standard AL therapy.
2. Materials and methods
2.1. Chemicals and materials
LF was acquired from AK Scientific, Inc. (California, USA) with a production date of September 2, 2013. The deuterated LF (LF-D9) internal standard was provided as a gift from Novartis Pharma. Co. (New Jersey, USA), prepared in 50% acetonitrile and 0.5% formic acid on May 25, 2010. Acetone, acetonitrile, HPLC-grade water, and ammonium formate were purchased from Fisher Scientific (New Jersey, USA) and ethyl acetate, formic acid, and Whatman 903 protein saver cards from Sigma-Aldrich (Missouri, USA). All solvents were HPLC grade, and chemicals were ACS reagents. Human whole blood with EDTA was purchased from Biological Specialty, Co. (Colmar, Pennsylvania, USA).
2.2. Calibration curve standards and quality control samples
Calibration curve standards and quality control samples were prepared in 3 batches at approximately 3 months, 2 months, and 1–3 days prior to assay. Standards of 100, 200, 500, 1,000, 5,000, 10,000 and 20,000 ng/ml were prepared from spiked whole blood applied in 25 or 50 μl aliquots to Whatman 903 protein saver filter paper with drying times of 3–18 h. Quality controls of 300, 3,000 and 17,000 ng/ml were similarly prepared. All samples were kept in clear plastic storage bags with desiccant. The 3- and 2-month-old samples were stored at −80 °C and then ambient temperature for 1 to 3 weeks, and the 1- to 3-day-old samples were kept at ambient temperature in clear bags with desiccant.
2.3. Clinical specimens
We obtained DBS from patients enrolled in a therapeutic efficacy trial of AL in Zambia, collected 7 days after the first treatment dose. Consent was obtained from parents or legal guardians of participating children according to an Institutional Review Board-approved protocol. Trial results will be published in a separate manuscript. Briefly, 100 children aged 6 to 59 months with uncomplicated Plasmodium falciparum malaria were treated with the standard 6-dose regimen of AL dosed according to weight. Children were enrolled over the 8-month period December 2014 to July 2015. Indoor residual spraying of participants’ households was prevalent and bed net use was common (98% and 87%). Finger prick capillary blood was spotted onto Whatman 903 protein saver filter paper and allowed to dry at ambient temperature and humidity for 3–4 h. Blood was spotted in volumes of approximately 25–50 μl per spot at intervals of 6 h for 48 h following start of treatment then weekly for 5 weeks. They were stored with desiccant at −20°C until July 2016, at which time they were transported with desiccant at ambient temperature to the laboratory and placed in −80 °C storage after approximately four weeks outside of frozen storage. Assays were done using the 3-month-old calibrators and quality controls except where noted.
2.4. Extraction procedure
A 3 mm paper punch was made from the DBS using a handheld 3 mm round hole puncher (Fiskars Brands, Inc., Middleton, WI, USA). The punch and 10 μl of LF-D9 (100 ng/ml) were added to a 1.5 ml Eppendorf tube. The mixture was extracted with different combinations of water and organic solvents: 50% acetonitrile with 0.5% formic acid, acetonitrile with 5% formic acid, 50% acetone with 5% ZnSO4 and 5% formic acid, ethyl acetate with 5% formic acid, methanol with 5% formic acid, acetone (50%, 90% and 100%) with 5% formic acid, and methoxyethoxy ethanol with 5% formic acid. Rotation (5–30 min) was compared to vortex mixing (5–30 min), with and without sonication (3 min). All samples were centrifuged for 3 min at 25,000 × g. The liquid phase was transferred in volumes of 20–40 μl to autosampler vials for liquid chromatography tandem mass spectrometry (LC-MS/MS).
2.5. Analytical procedure
Instrument and chromatographic conditions were adopted from a published method for plasma samples [12]. Briefly, the LC-MS/MS instrumentation comprised twin PE 200 micro-LC pumps and PE autosampler (Perkin-Elmer, Connecticut, USA) and the API 2000 triple quadrupole MS system (AB Sciex, Ontario, Canada). A Zorbax C8 column (50 × 2.1 mm, 5 μm; Agilent Technologies Inc., California, USA) was used for chromatographic separation. Solvent A was aqueous ammonium formate 10 mM, pH 4.0, and solvent B was acetonitrile with 0.1% formic acid. Elution by gradient LC was done according to the schedule 0–1 min: 50% solvent B, 1–4 min: 50–100% solvent B, 4–6 min: 100% solvent B, 6–6.1 min: 100–50% solvent B, 6.1–8 min: 50% solvent B. Injection volumes were 10 μl and the flow rate was 0.4 ml/minute with LF retention times of 3.5 min. The precursor-product ion pairs were mass-to-charge ratios (m/z) of 528→510 for LF and m/z 537→519 for the internal standard. Chromatographic data were analyzed using Analyst 1.6.2 (Danaher Co., Washington, DC, USA).
2.6. Method validation and assessments
Validation was performed by evaluating back-calculated concentrations of the calibrator (standard curve) samples, intra- and inter-day precision and accuracy of control samples, matrix effect, and extraction recovery. The calibration curve was validated in three runs with seven concentrations over several days at ambient temperature. To assess storage recovery, we compared control samples stored for 1 day, 6 days, and 3 months to freshly prepared samples. We assessed for variation due to differences in weight among punches by examining weight as a covariate in statistical models and by comparing different punches of the same or similar weight to those of different weights. We assessed for differences due to location of the punch by comparing results of punches taken from the center of a DBS to those taken from the periphery of the same DBS. Given variations in DBS volumes that occurred during patient collections, we spotted 25 μl quality control samples and compared them to 50 μl spots.
2.7. Matrix effect and recovery
Matrix effect was evaluated by comparing the peak area-under-the-intensity-curve of LF in 90% acetone with 5% formic acid (set 1) to the peak area of LF spiked into blank DBS extract (set 2) at the same concentration. Recovery was assessed by comparing the peak area of LF extracted from DBS (set 3) to those in set 2, using a calculated estimate of 3.125 μl of blood per 3 mm DBS punch.
2.8. Clinical application to a therapeutic efficacy trial
LF concentrations were correlated with parasite clearance time and prevalence of reinfection in exploratory PK-PD analyses of trial participants. Parasite clearance time was determined by microscopic examination of blood smears collected every 6 h and reinfection was determined by microscopy at weekly follow-up visits. Detectable concentrations of LF below the limit of quantitation, 100 ng/ml, were assigned a value equal to the half the limit (50 ng/ml, n=30) and concentrations above the limit were assigned the value of the limit (5,000 ng/ml, n=1). Correlations between LF concentration and participant characteristics (age, weight, gender, hemoglobin level, nutritional status) and specimen characteristics (time in storage, weight) were explored. To assess for evidence of inoculum effect (i.e., diminished drug efficacy due to high parasitemia), the interaction between LF exposure and initial parasite density was modeled. For the analysis of drug concentrations and malaria reinfection, a nested case-control design was used. Children who were reinfected within the 5-week follow-up period (cases) were matched by age, gender, and bed net use to children enrolled on the same day (controls).
2.9. Statistical analysis
Continuous and binary variables were compared using Student’s t test and the chi-squared test, respectively. Data were fitted to unadjusted and adjusted linear regression models. Nested models were compared using the F test. A threshold of significance of P=0.05 was used. Statistical analyses were done using Stata 14.0 (StataCorps, College Station, Texas, USA).
3. Results
3.1 Extractions of LF from DBS on untreated filter paper
Maximum recovery (31%) was by extraction with 90 μl 90% acetone with 5% formic acid, vortex mixing for 30 min followed by sonication for 3 min, and centrifugation. Other methods achieved recoveries of 26–29%. After processing, the sample was clear russet with paper fibers adhered to the tube surface.
3.2. Assay validation and assessment
Representative chromatograms are depicted in Fig. 1. The range of the calibration curve was 100–20,000 ng/ml and accuracy of the back-calculated concentrations, expressed as percent deviation from nominal values, was between −23 and 15% (mean −2.9%) and precision (coefficient of variation) was 8.2 to 26% (Table 1). Intra-day precision was 4–20% (mean 10%), and inter-day precision was 14–19% (mean 16%) (Table 2). The assay demonstrated least reliable performance at high concentrations (17,000 ng/ml), with several runs outside the FDA recommended thresholds for accuracy and precision of 15% (Table 2). Location of the punch (center or periphery), blood volume (25 or 50 μl), and weight of the punch did not significantly impact LF measurements. Recovery decreased rapidly and to a large extent with storage. Six days of storage at ambient temperature with desiccant reduced yield by 61–90%, and three months of storage reduced yield by 84–91% (Table 3). The difference between recovery at six days and three months was not statistically significant (P > 0.40 for all concentrations, Student’s t test) but was significant between one day of storage and longer storage times (P < 0.05).
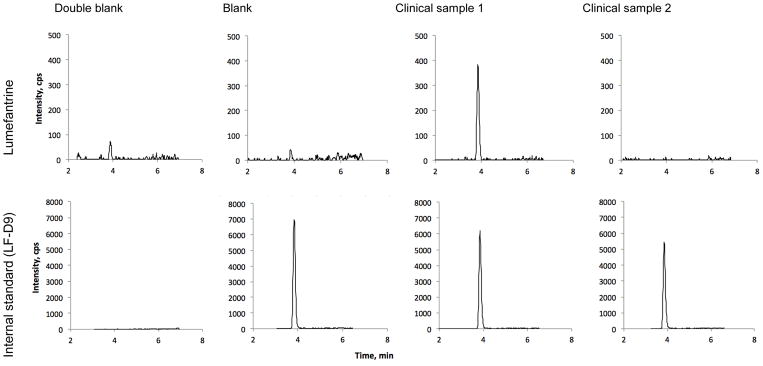
Fig. 1.
Representative chromatograms of lumefantrine and the internal standard (deuterated lumefantrine, LF-D9) for double blank dried blood spot, single blank, and two clinical samples of 184 ng/ml (1) and 0 ng/ml (2).
Table 1.
Standard curve precision and accuracy of mean back-calculated concentrations for lumefantrine in dried blood spots on untreated filter paper
| Measure | Nominal concentration (ng/ml) | Slope | Intercept | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 100 | 200 | 500 | 1,000 | 5,000 | 10,000 | 20,000 | ||||
| Mean (ng/ml) | 103 | 176 | 384 | 1,100 | 4,660 | 11,500 | 18,800 | 0.0012 | −0.0285 | 0.9892 |
| CV (%) | 13.9 | 12.3 | 8.2 | 25.7 | 6.5 | 15.4 | 9.7 | 0.0008 | 0.0256 | 0.0077 |
| Accuracy (%) | 2.8 | −11.8 | −23.1 | 9.8 | −6.9 | 14.9 | −6.2 | |||
| No. | 3 | 3 | 3 | 3 | 2 | 3 | 3 | |||
Table 2.
Intra- and inter-day precision and accuracy of lumefantrine concentrations from dried blood spots on untreated filter paper
| Nominal (ng/ml) | Mean (ng/ml) | Standard deviation (ng/ml) | Coefficient of variation (%) | Deviation from nominal value (%) | No. of replicates* |
|---|---|---|---|---|---|
| Intra-day | |||||
| 100 | 88–108 | 8–14 | 8.0–13.4 | −12 to 8 | 4–5 |
| 300 | 211–294 | 8–27 | 4.0–9.5 | −30 to −2 | 4–6 |
| 3,000 | 2,512–3,595 | 191–300 | 7.6–8.4 | −16 to −20 | 4–6 |
| 17,000 | 12,900–15,200 | 1,469–2,599 | 9.7–20.1 | −24 to −11 | 4–5 |
| Inter-day | |||||
| 100 | 99 | 14 | 13.9 | −1 | 14 |
| 300 | 270 | 42 | 15.4 | −10 | 16 |
| 3,000 | 2,841 | 539 | 19.0 | −5 | 14 |
| 17,000 | 14,178 | 2,254 | 15.9 | −17 | 9 |
Three batches of 4–6 replicates for each intra-day run. A subgroup of inter-day high concentration replicates was removed from validation calculations because of incorrect concentrations due to human error.
Table 3.
Recovery of lumefantrine from stored dried blood spots (n=4)
| Concentration, ng/ml | Mean recovery (%) by storage time (CV) | ||
|---|---|---|---|
| 1 day | 6 days | 3 months* | |
| 300 | 32 (11) | 10 (11) | 9 (28) |
| 3,000 | 88 (11) | 39 (38) | 16 (7.7) |
| 17,000 | 54 (13) | 17 (17) | 11 (3.0) |
n=2
CV, coefficient of variation.
For our analysis of day 7 clinical samples, accuracy of low concentration quality controls (100, 150 and 500 ng/ml) was ±3% and precision ranged from 1 to 3%, for medium concentrations (3,000 and 5,000 ng/ml) accuracy and precision were ±10% and 4 to 10%, and for the high concentration (17,000 ng/ml) they were ±19% and 8%. Due to unreliability of the high concentration quality control (±19%) and because clinical sample concentrations were well below the upper limit of the standard curve, for our analysis of the day 7 clinical specimens we used a range of quantitation of 100–5,000 ng/ml with higher or lower results considered beyond the limit of quantitation, or undetected if the peak area of the unknown sample was less than three times that of the blank. We conducted a sensitivity analysis using a quantitation range of 100–20,000 ng/ml which yielded similar results deviating on average by +1%
3.3. Matrix effect
Ion suppression was observed at low LF concentrations but was compensated for by use of the LF-D9 internal standard. The normalized matrix effect was not significant (Table 4).
Table 4.
Matrix effect and recovery of lumefantrine from six-day-old dried blood spots stored at ambient temperature (n=4)
| Concentration, ng/ml | Matrix effect, % | Recovery, % | |||
|---|---|---|---|---|---|
|
|
|
||||
| LF | IS | IS normalized | LF | IS | |
| 300 | 72.1 | 70.2 | 103 | 10.0 | 89.1 |
| 3,000 | 74.4 | 73.7 | 101 | 38.8 | 91.0 |
| 17,000 | 90.8 | 89.9 | 101 | 16.5 | 93.2 |
LF, lumefantrine. IS, internal standard.
3.4. Exploratory PK-PD analyses
Of the 81 enrolled trial participants who completed follow-up, 67 contributed specimens for day 7 LF determinations. DBS specimens had been stored for a period of time ranging from 16 to 24 months at the time of assay. None of the examined participant- or specimen-related characteristics (participant age, weight, sex, hemoglobin concentration, nutritional status; specimen time in storage, weight) were found to be significantly associated with LF concentration in simple and multivariable regressions (Table 5).
Table 5.
Associations of participant and specimen characteristics with post-treatment day 7 log lumefantrine concentrations
| Covariate | β coefficient | 95% confidence interval | P value |
|---|---|---|---|
| Participant age, months | 0.01 | −0.02, 0.05 | 0.41 |
| Participant weight, kg | −0.06 | −0.25, 0.15 | 0.58 |
| Female gender | −0.14 | −0.44, 0.16 | 0.35 |
| Hemoglobin, g/dl | −0.03 | −0.14, 0.08 | 0.64 |
| Nutritional status, Z-score | 0.07 | −0.22, 0.36 | 0.64 |
| Specimen age, weeks | −0.01 | −0.03, 0.02 | 0.59 |
| Specimen weight, kg | 0.76 | −0.17, 1.68 | 0.11 |
Estimated from the adjusted linear regression model. Interpretation of the β coefficient for gender is the difference in log lumefantrine concentration between girls and boys. Interpretation of the β coefficient for all other covariates is change in log lumefantrine concentration per unit change in the covariate.
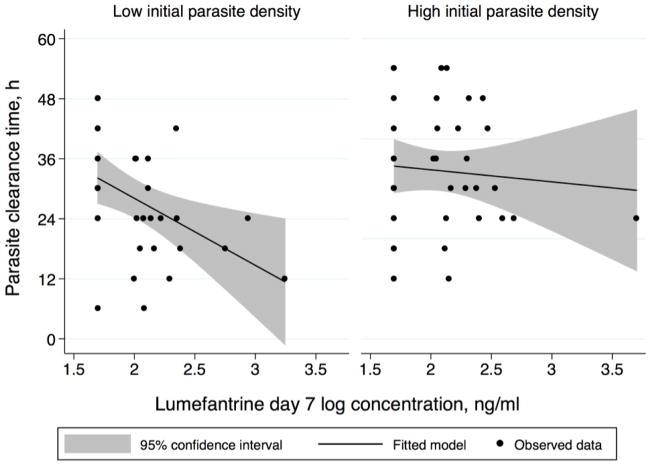
Greater LF exposure was significantly associated with shorter parasite clearance time in unadjusted and adjusted analyses. Controlling for age, sex, and initial parasite burden, each log base 10 increase in LF day 7 concentration corresponded with a decrease of 7.1 h in mean parasite clearance time (95% confidence interval [CI]: 0.1–14.3 h, P=0.05). Among children with parasitemia lower than the median, parasite clearance was 13.4 h faster for each magnitude increase in day 7 LF concentration (95% CI: 3.5 to 23.3 h, P<0.01), compared to 2.4 h faster per log-concentration increase for those with dense parasitemia greater than or equal to the median (95% CI: 11.9 to −7.1 h, P=0.61) (F test P=0.03) (Fig. 2).
Fig. 2.
Indirect drug effect of the antimalarial lumefantrine (LF) on parasite clearance stratified by burden of infection. The figures depict a pharmacokinetic-pharmacodynamic profile of parasite clearance time and LF exposure stratified by participants with low (below the median) and high (equal to or above the median) initial parasite densities. Adjusted for age and sex, children with parasitemia lower than the median cleared parasites 13.4 h faster for each magnitude increase in LF day 7 concentration (95% confidence interval [CI]: 3.5 to 23.3 h, P<0.01) compared to 2.4 h faster per magnitude increase in those with parasitemia greater than or equal to the median (95% CI: 11.9 to −7.1 h, P=0.61) (F test P=0.03).
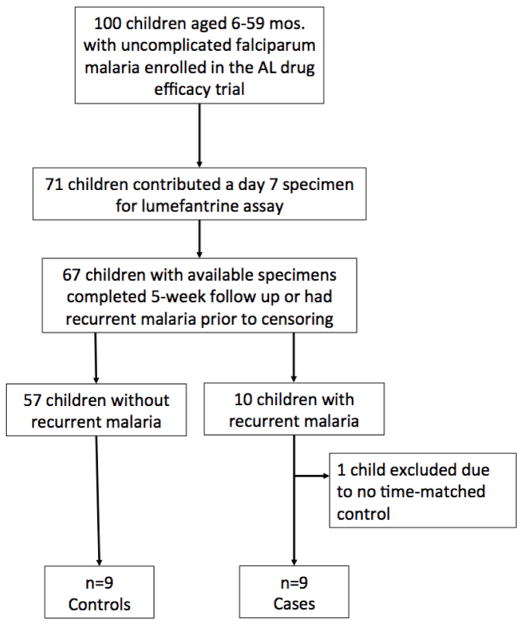
Nine children with malaria reinfection were included in the nested case-control study (Fig. 3). Mean post-treatment day 7 LF concentrations were 181 ± 158 ng/ml in cases and 235 ± 276 ng/ml in controls. The difference was not significant (P=0.64) (Table 6).
Fig. 3.
Flowchart of trial enrollment and selection for inclusion in the nested case-control study. AL, artemether-lumefantrine.
Table 6.
Results of nested case-control study of time-matched participants with and without recurrent parasitemia
| Characteristic | Cases n = 9 |
Controls n = 9 |
P value |
|---|---|---|---|
| Age, mos., mean (SD) | 27 (16) | 34 (17) | 0.32 |
| Female gender, n (%) | 6 (67) | 5 (56) | 0.96 |
| Weight, kg, mean (SD) | 11.4 (2.6) | 12 (2.9) | 0.54 |
| Net use, n (%) | 9 (100) | 9 (100) | - |
| Household indoor residual spraying, n (%) | 9 (100) | 9 (100) | - |
| Fever on presentation, n (%) | 2 (22) | 1 (11) | 0.66 |
| Anti-malarial use within prior 1 mo., n (%) | 2(22) | 1 (11) | 0.66 |
| Parasite density at enrollment, μL−1, median (IQR) | 9,920 (1,640–18,480) | 17,040 (3,640–32,360) | 0.21 |
| Time to clearance, h, mean (SD) | 26 (13) | 35 (11) | 0.10 |
| Day 7 lumefantrine concentration, ng/ml, mean (SD) | 181 (158) | 235 (276) | 0.64 |
P values were estimated from chi-squared or Student’s t test. For parasite density at enrollment, the log-transformed data were used for statistical testing.
SD, standard deviation. IQR, interquartile range.
4. Discussion
We developed a method for the semi-quantitation of the antimalarial drug LF from dried capillary whole blood collected onto untreated filter paper, and demonstrated its utility via exploratory PK-PD analyses of malaria parasite clearance. The method has relevance to retrospective field-based studies of LF where DBS were collected but chemical pretreatment of filter paper to facilitate drug elution was not done.
Day 7 LF concentrations from untreated filter paper were slightly lower (median: 111 ng/ml) than published results by others using DBS collected onto pretreated filter paper in similar populations (216 and 304 ng/ml) [5, 6]. This may be due to true variations in drug exposure among the different sample populations, or may represent inherent limitations of the assay, which we hypothesize is contributed to by compound degradation beyond 3 months and poor ability to elute the drug from untreated filter paper. We sought to mitigate these challenges by employing specially-prepared calibrators and validators and bypassing liquid- and solid-phase extraction; and by convention used a deuterated internal standard which adequately compensated for matrix effect (Table 4).
Calibration standards and validation samples were prepared using untreated DBS stored for 3 months prior to assay in order to mimic field specimens. Previous methods have used liquid- or solid-phase extraction [10, 11], which increase sample purity but at the expense of diminishing yield. We opted to forego this step in the interest of preserving yield. Our method which bypassed either liquid- or solid-phase extraction achieved a similar yield (~30%) compared to previously published methods incorporating one or the other (~30–50%) [10, 11] while retaining sensitivity to a lower limit of quantitation of 100 ng/ml. However, the samples used in recovery experiments were 6 days old whereas standard, control, and patient samples were ≥ 3 months, therefore the recovery experiments may have overestimated extractability. We observed rapid decrease in recovery over several days, similar to previous findings by others which further found that extractability plateaued at 6–7 days and remained constant out to 120 days [10]. Reassuringly, we detected no association between time in storage and LF concentration among the clinical specimens (Table 5), suggesting that after an initial period of decline in recovery, the amount of remaining intact compound able to be eluted remains nearly constant for at least 24 months, the age of our oldest specimens. We did not conduct a systematic assessment of drying time or temperature effects, although a previous study demonstrated stability at ambient temperature for a few months beyond which time freezing is recommended [10].
With these specifications, validation results across the range of standard concentrations (100–20,000 ng/ml) and quality controls were slightly below those required to satisfy regulatory guidelines due to unreliable accuracy at high concentrations. Accuracy of high concentration quality controls fell short of FDA criteria for acceptance, which recommend accuracy and precision within 15% (20% for the lower limit of quantitation) [13]. This limitation, in addition to low drug recovery from the untreated filter paper, leads us to characterize this method as semi-quantitative. The assay exhibited good precision and accuracy at low to intermediate drug concentrations (100–5,000 ng/ml). This range excludes typical peak concentrations (Cmax) of LF seen in children with malaria treated with AL (4,000–9,000 ng/ml) [5, 9, 14, 15], indicating the assay may be less informative for PK and PK-PD estimations that rely on Cmax values.
As expected, there are limitations to the assay arising from difficulty eluting the drug from the untreated paper and compound degradation, but these limitations did not entirely preclude the utility of the assay in PK-PD studies. We found that LF concentrations measured using this method correlated in a biologically consistent manner with parasite clearance. Greater LF exposure, approximated by post-treatment day 7 PK measurements, was associated with faster parasite clearance; and stratification by high and low baseline parasite burden showed an expected attenuation of drug effect at higher parasite densities (Fig. 2). We did not find a significant association between LF exposure and the prevalence of recurrent parasitemia in the nested case-control study, though the small sample size (n=9 per study group) provided low statistical power.
5. Conclusions
Despite inherent limitations stemming from a suboptimal sample and matrix, this method of semi-quantitation of LF from untreated DBS demonstrated applicability to field-based PK-PD studies. Ideally, calibration standards and quality controls should be prepared and stored contemporaneously with clinical specimens in order to account for decrements in recovery over time. The implication is that DBS collected and stored during clinical studies of LF in malarious regions may be adjunctively assessed in retrospective PK-PD analyses even when pre-acidification of collection paper was not done, albeit with caveat. Measured concentrations are less reliable at higher drug exposures (e.g. Cmax), and may be overall slightly lower than those measured from chemically pretreated DBS, rendering results perhaps most useful as relative values rather than absolute values. Nonetheless, when applied to exploratory PK-PD analyses of malaria parasite clearance, LF measurements obtained using this method produced tenable results.
Highlights.
Field studies of antimalarial drugs often involve collection of blood samples as dried blood spots (DBS) on filter paper for subsequent analysis.
Lumefantrine (LF) is among the most widely deployed antimalarial drugs.
Methods for its quantitation in DBS have been previously described but rely on pre-acidification of filter paper to facilitate drug elution.
We report a method that permits semi-quantitation of LF in DBS in the absence of pre-acidification of the collection paper.
In exploratory pharmacokinetic-pharmacokinetic analyses of parasite clearance and reinfection we demonstrate the method’s applicability.
Acknowledgments
This research was supported by the National Institutes of Health (R01HD068174) and by the Johns Hopkins Malaria Research Institute. M.M.I. receives funding support from the National Institute of General Medical Sciences (T32GM066691) and additional support from the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases, Division of Infectious Diseases of the Johns Hopkins University School of Medicine. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Fisher Center or Johns Hopkins University School of Medicine.
Footnotes
Competing interests
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. World Malaria Report. WHO Global Malaria Programme; Geneva, Switzerland: 2017. [Google Scholar]
- 2.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CL, Smith DL, Hay SI, Cibulskis RE, Gething PW. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–11. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph D, Kabanywanyi AM, Hulser R, Premji Z, Minzi OM, Mugittu K. Exploration of in vivo efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria in under fives in Tabora region. Tanzania, Malar J. 2013;12:60. doi: 10.1186/1475-2875-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, Nair S, Nash D, Singhasivanon P, Anderson TJ, Krishna S, White NJ, Nosten F. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42(11):1570–7. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchaparian E, Sambol NC, Arinaitwe E, McCormack SA, Bigira V, Wanzira H, Muhindo M, Creek DJ, Sukumar N, Blessborn D, Tappero JW, Kakuru A, Bergqvist Y, Aweeka FT, Parikh S. Population pharmacokinetics and pharmacodynamics of lumefantrine in young Ugandan children treated with artemether-lumefantrine for uncomplicated malaria. J Infect Dis. 2016;214(8):1243–51. doi: 10.1093/infdis/jiw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngasala BE, Malmberg M, Carlsson AM, Ferreira PE, Petzold MG, Blessborn D, Bergqvist Y, Gil JP, Premji Z, Bjorkman A, Martensson A. Efficacy and effectiveness of artemether-lumefantrine after initial and repeated treatment in children <5 years of age with acute uncomplicated Plasmodium falciparum malaria in rural Tanzania: a randomized trial. Clin Infect Dis. 2011;52(7):873–82. doi: 10.1093/cid/cir066. [DOI] [PubMed] [Google Scholar]
- 7.Minzi OM, Marealle IA, Shekalaghe S, Juma O, Ngaimisi E, Chemba M, Rutaihwa M, Abdulla S, Sasi P. Comparison of bioavailability between the most available generic tablet formulation containing artemether and lumefantrine on the Tanzanian market and the innovator’s product. Malar J. 2013;12:174. doi: 10.1186/1475-2875-12-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. Br J Clin Pharmacol. 1998;46(6):553–61. doi: 10.1046/j.1365-2125.1998.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother. 2000;44(3):697–704. doi: 10.1128/aac.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blessborn D, Romsing S, Annerberg A, Sundquist D, Bjorkman A, Lindegardh N, Bergqvist Y. Development and validation of an automated solid-phase extraction and liquid chromatographic method for determination of lumefantrine in capillary blood on sampling paper. J Pharm Biomed Anal. 2007;45(2):282–7. doi: 10.1016/j.jpba.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Ntale M, Ogwal-Okeng JW, Mahindi M, Gustafsson LL, Beck O. A field-adapted sampling and HPLC quantification method for lumefantrine and its desbutyl metabolite in whole blood spotted on filter paper. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876(2):261–5. doi: 10.1016/j.jchromb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Li X, Marzan F, Lizak PS, Aweeka FT. Determination of lumefantrine in small-volume human plasma by LC-MS/MS: using a deuterated lumefantrine to overcome matrix effect and ionization saturation. Bioanalysis. 2012;4(2):157–66. doi: 10.4155/bio.11.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Drug Administration; U.S. Department of Health and Human Services, editor. Guidance for industry: bioanalytical method validation (draft guidance) Rockville, Maryland: 2013. [Google Scholar]
- 14.Djimde AA, Tekete M, Abdulla S, Lyimo J, Bassat Q, Mandomando I, Lefevre G, Borrmann S, Group BS. Pharmacokinetic and pharmacodynamic characteristics of a new pediatric formulation of artemether-lumefantrine in African children with uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother. 2011;55(9):3994–9. doi: 10.1128/AAC.01115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwesigwa J, Parikh S, McGee B, German P, Drysdale T, Kalyango JN, Clark TD, Dorsey G, Lindegardh N, Annerberg A, Rosenthal PJ, Kamya MR, Aweeka F. Pharmacokinetics of artemether-lumefantrine and artesunate-amodiaquine in children in Kampala, Uganda. Antimicrob Agents Chemother. 2010;54(1):52–9. doi: 10.1128/AAC.00679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]