Abstract
During postnatal refractive development, an emmetropization mechanism uses refractive error to modulate the growth rate of the eye. Hyperopia (image focused behind the retina) produces what has been described as “GO” signaling that increases growth. Myopia (image focused in front of the retina) produces “STOP” signaling that slows growth. The interaction between GO and STOP conditions is non-linear; brief daily exposure to STOP counteracts long periods of GO. In young tree shrews, long-wavelength (red) light, presented 14 h per day, also appears to produce STOP signals. We asked if red light also shows temporal non-linearity; does brief exposure slow the normal decrease in hyperopia in infant animals? At 11 days after eye opening (DVE), infant tree shrews (n=5/group) began 13 days of daily treatment (red LEDs, 624±10 or 636±10nm half peak intensity bandwidth) at durations of 0 h (normal animals, n=7) or 1, 2, 4, or 7 h. Following each daily red period, colony lighting resumed. A 14 h red group had no colony lights. Refractive state was measured daily; ocular component dimensions at the end of the 13-day red-light period. Even 1 h of red light exposure produced some hyperopia. The average hyperopic shift from normal rose exponentially with duration (time constant 2.5 h). Vitreous chamber depth decreased non-linearly with duration (time constant, 3.3 h). After red treatment was discontinued, refractions in colony lighting recovered toward normal; the initial rate was linearly related to the amount of hyperopia. The red light may produce STOP signaling similar to myopic refractive error.
Keywords: Emmetropization, Hyperopia, Animal models, Retinal signaling, Emmetropization, Growth control
1. INTRODUCTION
Refractive error occurs when there is a mismatch between the location of the focal plane, produced by the cornea and the crystalline lens, and the location of the retina, which is controlled by the axial length of the eye. In the early postnatal period, refractive error is common in both humans and animals. Most eyes initially are hyperopic (focal plane behind the retina) (Bradley, Fernandes, Lynn, Tigges & Boothe, 1999, Cook & Glasscock, 1951, Mutti, Mitchell, Jones, Friedman, Frane, Lin, Moeschberger & Zadnik, 2005, Norton & McBrien, 1992b, Wallman, Adams & Trachtman, 1981). An emmetropization feedback mechanism uses the hyperopic refractive error to modulate the axial growth rate of the eye, increasing it so that the retina comes to lie very near the focal plane in the unaccommodated eye (near emmetropia, but typically slightly hyperopic) (Norton, 1999, Norton, Siegwart & Amedo, 2006, Schaeffel & Howland, 1988, Wallman & Winawer, 2004, Wildsoet, 1997). This process seems to involve retinal neurons (likely, amacrine cells) (Bitzer & Schaeffel, 2006, Feldkaemper, Burkhardt & Schaeffel, 2004, Fischer, Miethke, Morgan & Stell, 1998, Fischer, Morgan & Stell, 1999, Fischer, Seltner & Stell, 1997, Mathis & Schaeffel, 2007, Seltner & Stell, 1995, Stell, Tao, Karkhanis, Siegwart & Norton, 2004, Vessey, Lencses, Rushforth, Hruby & Stell, 2005a, Vessey, Rushforth & Stell, 2005b, Zhong, Ge, Smith & Stell, 2004) that detect the refractive error and generate what have been described as GO signals (Rohrer & Stell, 1994). There is evidence that these travel in a signaling cascade through a direct, spatially local pathway (retinal pigment epithelium, choroid) to the sclera where they produce altered mRNA levels leading to biochemical and biomechanical remodeling that increases the axial elongation rate. (Guo, Frost, He, Siegwart & Norton, 2013, Guo, Frost, Siegwart & Norton, 2014, He, Frost, Siegwart & Norton, 2014a, He, Frost, Siegwart & Norton, 2018, He, Frost, Siegwart & Norton, 2014b, Norton, Essinger & McBrien, 1994, Schaeffel, Troilo, Wallman & Howland, 1990, Siegwart & Norton, 1999). Note that GO signaling is a conceptualization that summarizes the effect of visual stimuli on the axial elongation rate of the growing eye. In tree shrews, as in other species, a minus-power lens (ML), held continuously in front of an eye, shifts the focal plane away from the cornea and behind the retina, creating hyperopic refractive error. This stimulates the emmetropization mechanism to produce GO signaling that raises the axial elongation rate and, consequently, decreases the induced hyperopia. As the retina approaches the shifted focal plane, the GO signaling gradually diminishes (Guo et al., 2013, He et al., 2014a); the eye stabilizes when it is approximately emmetropic while wearing the lens. Form deprivation (FD), produced by holding a translucent diffuser in front of an eye, prevents clear images from forming on the retina and also produces increased axial elongation and myopia that increase with continued FD. If ML wear or FD is then discontinued, the elongated eye is myopic.
A refractive myopia also can be produced by placing a plus-lens (PL) on an emmetropic eye. In response, the emmetropization mechanism generates what has been described as “STOP” signaling (Rohrer & Stell, 1994) that slows the axial elongation rate (Guo, Frost, Siegwart & Norton, 2012, Guo et al., 2014, He et al., 2014a, Rohrer & Stell, 1994). The continued maturation of the cornea and lens shifts the focal plane toward the retina so the eye compensates for the induced myopia, becoming nearly emmetropic while wearing the lens and hyperopic when the lens is removed (Howlett & McFadden, 2009, Irving, Sivak & Callender, 1992a, Schaeffel, Glasser & Howland, 1988a). PL wear in infantile tree shrews, but not juveniles, also produces slowed axial elongation (Siegwart & Norton, 2010).
The interaction between GO and STOP signaling produced with induced hyperopia (ML wear) or myopia (PL wear or recovery from ML wear or FD) has been extensively studied and has been found to be temporally non-linear (Kee, Hung, Qiao-Grider, Ramamirtham, Winawer, Wallman & Smith, 2007, Lan, Feldkaemper & Schaeffel, 2014, Leotta, Bowrey, Zeng & McFadden, 2013, Winawer & Wallman, 2002, Zhu, 2013, Zhu & Wallman, 2009). Brief daily periods of STOP conditions (myopia or removal of hyperopia) counteract much longer exposure to GO conditions (hyperopia or FD). For instance, if animals with ML wear or FD have the lens or diffuser removed for 1 – 2 h daily, the myopia that normally would develop is dramatically reduced. The rapid rise in the effectiveness of the STOP signaling with its daily duration is very similar in chicks, guinea pigs, tree shrews, and monkeys (Kee et al., 2007, Leotta et al., 2013, Napper, Brennan, Barrington, Squires, Vessey & Vingrys, 1995, Schmid & Wildsoet, 1996, Shaikh, Siegwart & Norton, 1999, Smith, Hung, Kee & Qiao, 2002).
Recent studies in tree shrews have identified another visual condition that appears to produce STOP signaling (Gawne, Siegwart, Ward & Norton, 2017a). Placing infant, juvenile or adolescent tree shrews in cages illuminated with narrow-band long-wavelength (red) light for 14 h per day (dark the remaining 10 h) produced slowed axial elongation and hyperopia. Infant monkeys raised wearing filters that only transmitted red wavelengths also developed hyperopia (Smith, Hung, Arumugam, Holden, Neitz & Neitz, 2015). The purpose of the present study was to examine, in tree shrews, if this red light exhibits temporal non-linearity similar to that of removal of ML wear or FD. If so, the hyperopiagenic effect of short periods of the red light should rise non-linearly as the duration of the daily red exposure increased. We tested this hypothesis on infantile tree shrews (a diurnal mammal closely related to primates) by exposing them to varying durations of red light. Normally, the hyperopia decreases rapidly at this age. We measured the extent to which daily periods of red light exposure would prevent this decrease, producing hyperopic eyes that are shorter than normal at the end of red treatment.
2. MATERIALS AND METHODS
2.1 Subjects
All procedures complied with the Statement for the Use of Animals in Ophthalmic and Visual Research of the Association for Research in Vision and Ophthalmology and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (UAB) and were in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). As in previous studies from this laboratory (Guo et al., 2013, He et al., 2014b, McBrien & Norton, 1992), tree shrews (Tupaia glis belangeri) were raised in our breeding colony by their mothers. Tree shrews are small mammals (dichromats) with good vision that are closely related to primates (Luckett, 1980). They are born with their eyes closed. The first day both eyes are open, which occurs about three weeks after birth, is considered to be the first day of visual experience (DVE). At eye opening, tree shrews are approximately 25 D hyperopic (Norton & McBrien, 1992b). The hyperopia decreases rapidly over the next two weeks as the eyes elongate. We began treatment at an age (11 days after eye opening) when the hyperopia has decreased to 5 – 7 D, but continues, in normal animals, to decrease rapidly towards emmetropia. This decrease is driven by the hyperopia; continuous PL wear at this age produces slowed axial (vitreous chamber) elongation and prevents the continued decrease in refraction (Siegwart & Norton, 2010).
2.2 Stimulus Conditions
Colony lighting was provided by fluorescent bulbs (type F34CW RS WM ECO) in the ceiling of the colony rooms on a 14 h light/10 h dark cycle. The colony illuminance on the floor of the cages housing the animals during the study was 100 – 300 lux as measured with a LX1330B digital illuminance meter (Hisgadget, Inc.). A gray PVC tube (10 cm diameter) with one open end on the floor of the cage served as a nest that the animals could enter and leave at will.
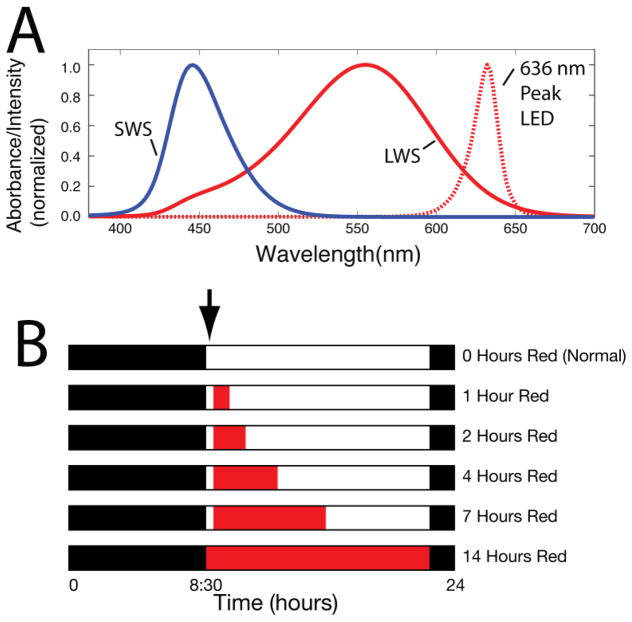
The narrow-band ambient red light was provided by LEDs (peak wavelength of either 624 ± 10 nm or 636 ± 10 nm half peak intensity bandwidth) (Fig. 1A) obtained from American Superlite, San Fernando, CA as described previously (Gawne, Ward & Norton, 2017b). The LEDs were affixed in a uniform array to a white-painted 60 cm by 60 cm piece of plywood that was then placed on top of the cage, which was a cube 60 cm on a side. The mean illuminance of the red light at the cage floor ranged from 527 to 749 human lux. A white resting board 28 cm above the cage floor was often used by the animals. Light intensity on the shelf was approximately 40 percent greater than on the cage floor. These illuminance levels were greater than that of the colony lighting. However, a previous study (Gawne et al. 2017b) with the same red and colony illuminances found that the red-light effect was due to the restricted wavelength, not to the difference in illuminance.
Fig. 1.
(A) Normalized absorption profile of tree shrew short wavelength sensitive (SWS) and long wavelength sensitive (LWS) cones (solid lines) and profile of the intensity emitted by the 636 ± 10 nm (half peak intensity bandwidth) red LED (dotted line). The absorbance of the ocular media was taken into account in calculating the cone absorbance profiles. (B) Red light duration groups. Red bars indicate periods in which animals were exposed to red light each day. White bars indicate daily periods when the colony lights were on. The black bars indicate the 10-h daily period when the colony lights were off. The arrow denotes that refractive and axial dimension measures were measured in the morning before red treatment began for the 1, 2, 4, and 7 h groups and just after red began in the 14 h group.
Tree shrew retinas contain long wavelength sensitive (LWS “red”, peak absorption at 555 ± 6 nm spectral width at half absorbance) and short wavelength sensitive (SWS “blue”, peak absorption 428 ± 15 nm spectral width at half absorbance) cones (Petry and Harosi 1990). As shown in Fig. 1A, the light provided by the LEDs was far removed from the SWS-cone absorption peak (Petry and Harosi 1990). The calculated absorption of the emitted wavelengths was between 6 and 7 log units below the SWS cone peak. Thus, the red light effectively activated only the LWS cones and not the SWS cones.
2.3 Experimental Groups
The experimental groups are illustrated in Fig. 1B. A normal group (n=7) received colony lighting (0 h red) throughout the entire experimental period (11 to 50 DVE) as reported previously (Gawne et al., 2017b). For the five red treated groups (n = 5 per group) red treatment began at 11 DVE (plus or minus one day) and continued for 13 days (until 24 DVE). During the red treatment period, four groups were first exposed to colony lighting for approximately 30 – 45 min each day and then exposed to red light for a duration of 1, 2, 4, or 7 h/day. The animals in these four groups were prevented from entering their nest boxes during red treatment to ensure full exposure to the red light. Each day, the red light was then turned off just as the colony lights were turned on for the remainder of the 14 h light-on period. The red array was removed from atop the cage to provide standard colony illuminance. The 14 h group was exposed to red light for 14 h and dark for 10 h with no colony light.
After 13 days of red light treatment, animals in the red-treatment groups were returned to standard fluorescent colony lighting for an additional 27-day “recovery” period. This allowed us to assess if animals that had developed a refractive state different from normal would return to an age-normal refractive state when exposed to colony lighting.
2.4 Pedestal Installation
In order to efficiently and painlessly align the animals for refractive and axial component measures, a dental acrylic pedestal was installed on the skull of all animals at 10 DVE (plus or minus one day) following procedures described previously (Siegwart & Norton, 1994). In brief, the animals were anesthetized with 100 mg/kg ketamine, 7 mg/kg xylazine, i.m. Anesthesia was supplemented with 0.5 – 2.0% isoflurane as needed. While anesthetized, atropine i.p. 0.27 mg/kg, buprenorphine i.m. 0.02 mg/kg, and carprofen s.q. 5 mg/kg were administered. After recovery from anesthesia, the animals were weaned and, in the red-treated groups, housed in colony lighting for the rest of the lights-on period before wavelength treatment began the next morning.
2.5 Measures of Refractive State and Axial Component Dimensions
Awake, non-cycloplegic refractive measures were taken on each animal with a Nidek ARK-700A infrared auto-refractor (Marco Ophthalmic, Jacksonville, FL) (Norton, Wu & Siegwart, 2003) calibrated periodically with refractive standards provided by the manufacturer. Since atropine may interfere with the development of myopia, cycloplegic refractive measures were not performed (McKanna & Casagrande, 1981). However, previous studies have shown that non-cycloplegic measures provide a valid estimate of the refractive state, and of induced refractive error, in tree shrews (Amedo & Norton, 2003, Norton, Siegwart, German, Robertson & Wu, 2000, Norton et al., 2003). All refractive values were measured at the corneal plane and were corrected for the small eye artifact (Glickstein & Millodot, 1970) previously shown to be approximately +4 D in tree shrews (Norton et al., 2003).
To provide a pre-treatment measure, refractive state was measured in awake animals on 11 DVE (day 1) just before the first red-light treatment began. Daily measurements continued through the 13-day red-treatment period. On day 14, red treatment was discontinued; the animals remained in colony lighting. Refractive measures continued during the recovery period until 50 DVE.
During red treatment, measurements were made approximately 30 minutes after the colony lights were turned on, before each day’s “dose” of red light, except for the 14 h group which was only in red light. Alignment with the autorefractor was facilitated by dim background light. For animals in the 14 h red-treatment group red LEDs were used as background light. Animals in the other groups were measured with dim fluorescent background light.
Axial component dimensions were measured with a Lenstar LS-900 optical biometer (Haag-Streit USA, Mason, OH) (Gawne et al., 2017a, Ward, Siegwart, Frost & Norton, 2016, Ward, Siegwart, Frost & Norton, 2017). A pre-treatment measure was made on day 1 (11 DVE) to insure there were no significant differences across groups; an end-of treatment was made 13 days later on day 14 (24 DVE). The Lenstar provides stored waveforms with peaks corresponding to the front and back of the cornea, anterior and posterior lens surfaces, retinal and choroidal thickness. Off-line, cursors were moved to each peak to provide measures of corneal thickness, anterior chamber depth, lens thickness, vitreous chamber depth, retinal and choroidal thickness.
2.6 Data Analysis
Because the treatment was binocular and because the refractions of the two eyes were similar throughout the study as previously shown (Gawne et al., 2017a), the refractive and axial component measurement of the right and left eyes were averaged. Refractive measures across days for each animal were examined in Excel spreadsheets.
Statistics were performed using the SAS software (Cary, NC). Repeated measures ANOVA was used to examine the change in refraction over time between normal animals and those exposed to red light. An interaction term was added to the model to detect possible differential changes over time between groups. Separate models were used for each duration of red light exposure. A Kruskal-Wallis test was used to compare mean refraction (treated vs. normal) at each time point. The analysis was repeated stratified by sex. As a secondary analysis, the repeated measures ANOVA was used to examine changes in refraction over time between those exposed to 1, 2, 4, and 7 hours of red light compared to those exposed to 14 hours of red light. Significance was set at P < 0.05.
Because we did not have daily measures of axial dimensions, we only performed a one-way ANOVA on the end-of-treatment axial component dimensions. If this was significant, we performed a Kruskal-Wallis test to determine the difference between each duration of red light treatment and normals.
We used the MATLAB (Mathworks, Natick, MA) “fit” function to fit a three-parameter exponential curve to both the average hyperopic shift as a function of red duration and previous data on the effect of the duration of daily removal of a minus lens (Shaikh et al., 1999). From this we calculated the time constant for the non-linear effect of the red light: the duration at which the non-linear effect reaches approximately 63% of its final (asymptotic) value. A time constant also was calculated for the non-linear effect of removing a minus-lens.
3. RESULTS
3.1 Refractive Development
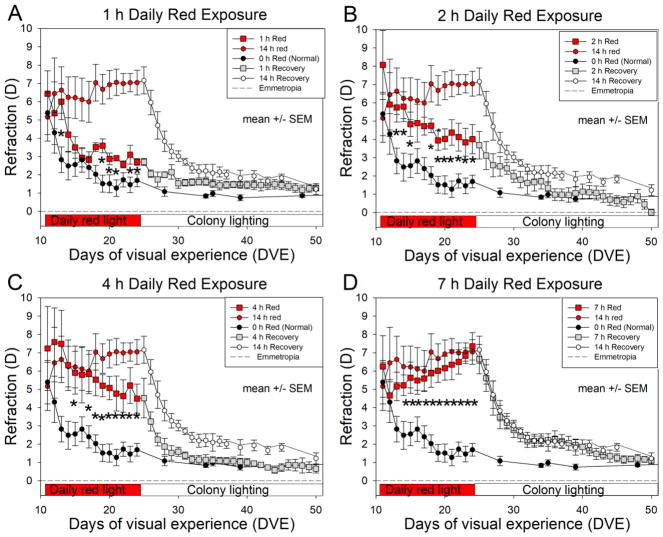
Fig. 2 shows the effect of red-light duration on refractive development, compared with the normal (0-h red) and 14 h red groups. As was previously shown (Gawne et al., 2017b), the hyperopic refractions of the normal animals decreased (showed a negative slope) from an initial hyperopia (5.4 ± 1.2 D SEM, Standard Error of the Mean) toward emmetropia (1.7 ± 0.3 D SEM), whereas the eyes of the 14 h red light group remained hyperopic throughout the 13-day treatment period. By treatment day 4 (after 3 days of red treatment) the eyes of the 14 h red group were significantly hyperopic compared with normal and remained significantly hyperopic throughout the rest of the 13-day red treatment period. The refractive state for these, and the other groups, at the end of the red treatment period are shown in Fig. 3A.
Fig. 2. Effect of daily red light duration on refractive development.
The red light duration groups (square symbols) are compared with the 0 h (normal) group (black symbols) and with the 14 h group (red circles). Asterisks (*) indicate days on which the red duration groups were significantly different from the normal group (Kruskal-Wallis, P < 0.05). Data from the 14 h group are from Gawne et al. (2017a). Error bars are SEM.
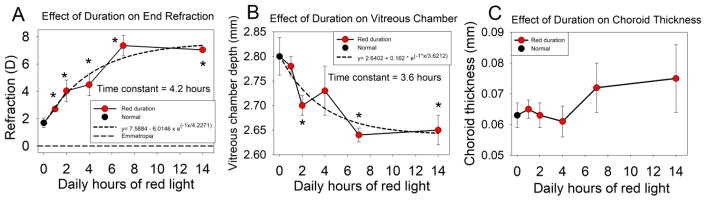
Fig. 3. Effect of red light duration on end-of-treatment refractive state, vitreous chamber depth and choroid thickness.
(A) As red-light duration increased the induced hyperopia increased non-linearly (time constant 4.2 h). (B) vitreous chamber depth was shorter than normal at the end of treatment at longer red durations (time constant 3.6 h). (C) Choroid thickness values did not vary significantly but were slightly greater in the 7 h and 14 h groups. In A and B the dashed curves are a three-parameter exponential curve fit to the data with the MATLAB (Mathworks, Natick, MA) “fit” function. Asterisks (*) indicate durations at which the values of groups were significantly different from the normal group (Kruskal-Wallis, P < 0.05). Error bars are SEM.
A repeated measures ANOVA showed that all of the red light groups had significantly higher refractions than the normal animals over time (1 h: P = 0.0453, 2 h: P = 0.0210, 4 h: P = 0.0234, 7 h: P = 0.0073, 14 h: P = 0.0072). When we compared the slopes of the refraction over time, neither the 1 h, 2 h, nor 4 h groups differed significantly from normals. The 7 h group did show a significantly different slope of refraction over time compared to normal (P for interaction = 0.0080). The 14 h group also showed a different slope of refraction over time compared to normals, but it was not statistically significant (P for interaction = 0.059). The pattern of results was similar for males and females.
For the secondary analysis, the mean refraction of those exposed to 1 hour of red light was significantly different from those exposed to 14 hours of red light (P = 0.0171). Additionally, the amount of change over time differed between these two groups (P for interaction = 0.048). The mean refraction of those exposed to 2 h, 4 h, and 7 h groups did not differ significantly from the 14 h group.
3.2 Ocular Components
Vitreous chamber depth was the only ocular component that differed significantly as a function of red-light duration. As shown in Fig. 3B, the vitreous chamber depth at the end of red treatment was similar to normal at the shortest red duration and decreased non-linearly as red duration increased. Corneal thickness, anterior chamber depth, lens thickness, retinal thickness and choroid thickness showed no significant difference with red-treatment duration (ANOVA, P > 0.05). However, as shown in Fig. 3C, for red light durations of 7 h and 14 h, choroid thickness was greater than normal, although not significantly different from normal.
3.3 Overall Effect of Red-treatment Duration on Refraction
To estimate the effect of the daily duration of red-light treatment across the red treatment period, we measured, for each group on treatment days 2–14, the difference between the group refractive state and the refraction of the normal animals. The difference (red group – normal) was summed across days and then was divided by the number of treatment days (13) to provide an average difference (in diopter-days) between each groups’ refraction and normal refractions. The data from day 1 were excluded because it was a pre-treatment measurement.
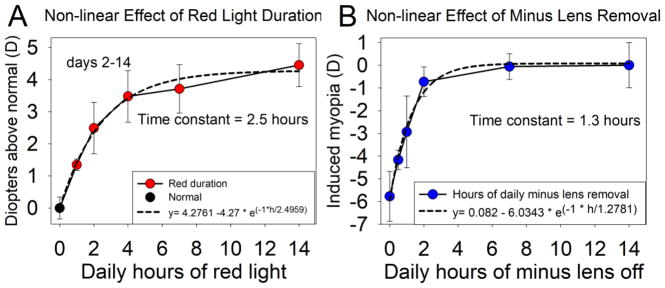
Fig. 4A plots the hyperopic shift from normal vs. the duration of the daily red-light treatment. As was the case with the end-of-treatment refractive state, we found that the overall hyperopic shift produced by the red treatment increased rapidly with small increases in the duration of daily red treatment. As the daily red duration increased from 0 to 4 h, the hyperopic shift increased rapidly. With further increases in red duration to 7 h and 14 h, the hyperopic shift increased more slowly. This non-linear increase in hyperopia with increasing red duration was well fit with an exponential equation (Fig. 4A) with a time constant of 2.5 h that accounted for 98.6% of the variance in the data.
Fig. 4. Comparison of two STOP conditions: (A) the nonlinear hyperopic shift produced by red light duration and (B) the nonlinear reduction in myopia produced by minus lens removal.
(A) The calculated diopter-days across the 13 days of red light treatment for each group is plotted against the daily red light duration. Along with an exponential equation fit to the data with a time constant of 2.5 h./the curve explains 98.6% of the variance. (B) The effect of interrupted ML wear was well fit by an exponential curve with a time constant of 1.3 h. We used the MATLAB (Mathworks, Natick, MA) “fit” function to fit a three-parameter exponential curve to both previous data from (Shaikh et al 1999) and the data of the present study. Error bars are SEM.
Fig. 4B shows a similar non-linear increase in the effect of another condition that generates STOP signaling, interrupted minus-lens wear in juvenile tree shrews. As the length of time each day that a −5 D lens was removed increased, the amount of myopia at the end of treatment decreased non-linearly (Shaikh et al., 1999). The non-linear effect of removing the minus lens appeared to be stronger than the effect of the red light exposure duration. From the fitted curve, 63% of the total effect occurred with 1.3 h of lens removal vs. 2.5 h for red light exposure duration.
3.4 Recovery
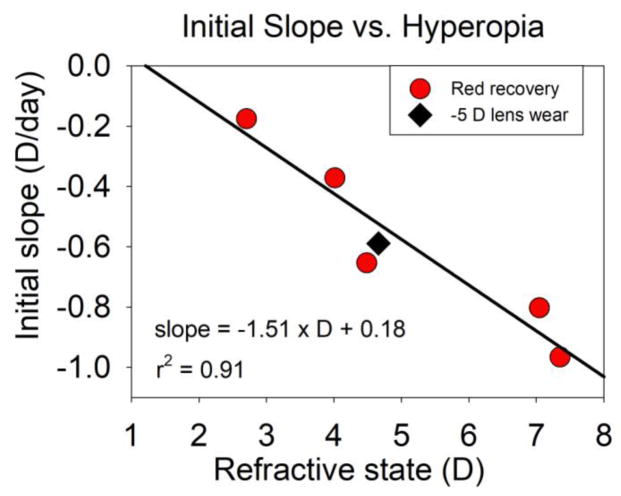
When red light treatment ceased (24 DVE), the hyperopic refractions of the red-treatment groups decreased toward emmetropia (Fig. 2), rapidly at first and then more slowly, and did not differ significantly from normal at the end of the recovery period. The rate of recovery was strongly related to the amount of hyperopia at the end of treatment (Fig. 5). During the first 4 days, the initial the rate of recovery was highest (−0.97 D/day) in the 7 h group, which was 7.4 ± 0.7 SEM D hyperopic at the start of recovery. The initial rate of recovery was lower in the groups that were less hyperopic. The 1 h group showed a nearly identical rate (−0.18 D/day) to the normal group (−0.15 D/day) that was still slowly progressing toward emmetropia.
Fig. 5. Initial recovery slope as a function of hyperopia.
The circles show the rate of recovery (decrease in hyperopia, diopters per day) for the red-treatment groups during the first 4 days of recovery plotted against each group’s average hyperopia at the end of treatment. The diamond plots the initial slope of decrease in hyperopia in age-matched animals in response to a −5 D lens vs. the lens-induced hyperopia, measured with the lens in place (Norton et al. 2010). The line is the regression for the red groups.
In another study, when age-matched tree shrews began to wear a −5 D lens (Norton et al. 2010), the lens initially produced a hyperopic shift of approximately 5 D in the eye’s refractive state. In response, the eyes elongated, reducing the hyperopia, and becoming myopic when measured without the lens. The initial 4-day rate of this decrease (−0.67 D/day, diamond symbol), was very close to the regression for the recovery from red-induced hyperopia.
4. DISCUSSION
4.1 Red light STOP signaling
The main finding of this study is that the narrow-band long wavelength light we used exhibits the same type of non-linear increase in effectiveness with duration as do other visual conditions (removal of ML wear, PL wear) that reduce the response to myopiagenic stimuli (ML wear and FD). As is well established, the emmetropization mechanism responds to hyperopia, producing increased axial elongation that reduces the hyperopia (Wallman & Winawer, 2004). In tree shrews, Siegwart and Norton (Siegwart & Norton, 2010) found that the decrease in hyperopia, as seen here in the normal (0 h red) animals, was interrupted by PL wear. Binocular PL wear, starting at the same age in this study, quickly arrested the normal decrease in refractive state and slowed the axial elongation of the eyes, indicating that the normal decrease was driven by the refractive hyperopia.
As also shown previously (Gawne et al., 2017a), exposure to 14 h red (no colony light, Fig. 2) prevented the normal decrease in hyperopia at this age, suggesting that this produced STOP signaling similar to PL wear. The results of the present study showed another similarity to other stimuli that produce STOP signaling: as the daily red-light duration increased from 1 h, the red-produced STOP signaling rapidly increased in effectiveness. The time constant of this non-linearity varied somewhat, depending on if one measured refraction across the entire treatment period (2.5 h) or at the end of treatment (4.2 h), or examined the effect on slowing vitreous chamber elongation (3.6 h).
The effect on vitreous chamber depth was consistent with other animal studies that show that vitreous chamber depth is the primary structural change associated with induced refractive error (Hung, Crawford & Smith, 1995, Irving, Callender & Sivak, 1991, Irving, Sivak & Callender, 1992b, McBrien & Norton, 1992, Schaeffel, Glasser & Howland, 1988b).
The red-light STOP signaling in the present study, as measured by the time constants, was less effective (about by half) than was the effect of interrupted ML wear in tree shrews (Shaikh et al. 1999). However, this may not accurately reflect the relative effectiveness of these two conditions. Treatment in the interrupted ML wear study (Shaikh et al., 1999) began at 24 DVE at a point when the refractive state had become relatively stable (Fig. 2). The animals in the present study were younger and their refractive state was rapidly decreasing. A more accurate comparison of red light vs. interrupting ML wear might be achieved if short-duration red treatment had been examined in older tree shrews at the same age as used in the Shaikh et al. (1999) study.
The magnitude of the time constant is less important than the fact that both conditions showed a non-linear relationship between duration and the effect on refractive state. The non-linear interaction between refractively-produced GO and STOP signaling is well established. Exposure to even very brief periods of myopic defocus (STOP) can counteract the effects of hyperopic defocus (GO) (Zhu, 2013). When both are presented simultaneously, as when chicks or guinea pigs wear dual focus lenses that simultaneously produce myopia (STOP) and hyperopia (GO), eyes primarily respond to the STOP signaling (McFadden, Tse, Bowrey, Leotta, Lam, Wildsoet & To, 2014, Tse, Lam, Guggenheim, Lam, Li, Liu & To, 2007). The results of the present study suggest that the STOP signaling produced by the red light in tree shrews may similarly be a powerful tool to slow axial elongation in the presence of competing hyperopia.
In many studies, choroid has been found to respond with an increase in thickness in response to stimuli that slow the axial elongation rate (See review by (Nickla & Wallman, 2010)). Consistent with this, significant thickening of the choroid was found with 14 h red exposure (no colony light) (Gawne et al., 2017b). In the present study, there was a suggestion that choroid thickness may have been affected, but only at the longer red treatment durations. (7 h and 14 h). The effect in choroid was small (~12 μm) compared with the effect on the vitreous chamber (~150 μm) and could only account for <0.5 D of the hyperopic shift (Norton & McBrien, 1992a).
The timing of the choroid measurement in this study may have reduced the impact of red treatment. The animals in the 1, 2, 4, and 7 h red duration groups were exposed to colony fluorescent light for approximately 30 - 45 min each morning, including the day the ocular component measures were made. Because choroid has been found to respond rapidly to stimuli that slow axial elongation in chicks and humans (Chakraborty, Read & Collins, 2012, Nickla & Wallman, 2010), it is possible that even this brief exposure to colony light may have affected choroidal thickness. If measurements had been made instead at the end of the red treatment periods, an increase in choroid thickness might have been evident in the shorter-duration red-treatment groups.
4.2 STOP and GO Signaling
As used here, STOP and GO signaling, are operational constructs that summarize events that lead to increased axial elongation rate (GO) or slowed axial elongation rate (STOP), resulting in changes in refractive state. These signals start in the retina, where the sign of defocus is detected. The details of how the emmetropization mechanism extracts this information from the visual scene are still only vaguely understood (Bitzer & Schaeffel, 2006, Feldkaemper et al., 2004, Fischer et al., 1998, Fischer et al., 1999, Fischer et al., 1997, Mathis & Schaeffel, 2007, Seltner & Stell, 1995, Stell et al., 2004, Vessey et al., 2005a, Vessey et al., 2005b, Zhong et al., 2004).
GO and STOP signaling also include events that occur in the signaling cascade through the RPE and choroid to the sclera that produce biochemical and biomechanical remodeling that alters the axial elongation rate (Frost & Norton, 2012, Guo et al., 2013, Guo et al., 2014, Harper, Wang, Moiseyev, Ma & Summers, 2016, He et al., 2014a, He et al., 2014b). Potentially, GO and STOP signaling could also include signaling through central visual pathway that increase or decrease accommodation, such as creating a lag of accommodation that increases hyperopic defocus and may participate in myopia development (Gwiazda, Thorn, Bauer & Held, 1993, Smith, 2013, Smith, Hung, Huang & Arumugam, 2013).
It is not yet known how LWS cone stimulation without SWS cone stimulation, the condition during red-light exposure, initiates STOP signaling. It must involve many retinal neurons that are different from the ones involved in STOP signaling produced by other stimuli (myopia, clear images). Similarly, hyperopia, form deprivation and, in tree shrews, exposure to continuous darkness, that all initiate GO signaling must involve many differing retinal neurons (Choh, Lew, Nadel & Wildsoet, 2006, Kee, Marzani & Wallman, 2001, Nickla & Schroedl, 2012, Schaeffel, Hagel, Bartmann, Kohler & Zrenner, 1994). How, and where, these various visual conditions converge to produce decreases (STOP), or increases (GO) in axial elongation is an important, unsolved issue in understanding the function of the emmetropization mechanism. Having an additional stimulus, narrow-band red light, that produces a type of STOP signaling may be of use in unraveling this conundrum.
4.4 Response to Hyperopia after Red- light Treatment
In all groups, when red light treatment was discontinued, the hyperopic refractive state, induced by the red light, dissipated; refractions decreased until they matched those of the normal group (Fig. 2). As shown in Fig. 4, the initial (first 4 day) slope of recovery was linearly related to the amount of hyperopia that was present at the end of red treatment. Moreover, the rate was very similar to the rate of response in age-matched normal animals in which hyperopia was produced with ML wear (Norton, Amedo & Siegwart, 2010). The recovery toward emmetropia provides reassurance that red treatment does not have a lasting effect on the functioning of the emmetropization mechanism; when red treatment ends, the response to this hyperopia is the same that occurs when hyperopia is produced by wearing a minus-power lens.
In addition, these data may also provide insight into the question of whether the emmetropization mechanism uses only defocus (i.e., all or nothing), or if it responds proportionally to the magnitude of defocus (i.e., respond faster with larger refractive errors than with smaller ones) (Schaeffel et al., 1988b). If the emmetropization mechanism only used the presence or absence of hyperopia to generate GO signaling, one would expect that the initial slope would have been the same across groups because all experienced hyperopia. Such a relationship was found in chick choroid by Hammond et al.(Hammond, Wallman & Wildsoet, 2013). In the present study, the linear relationship found here between the amount of hyperopia and the initial slope in refractive change suggests that the emmetropization mechanism can use the amount of refractive error to guide eyes toward emmetropia.
4.3 Limitations and Strengths
The ability of narrow-band long wavelength light to produce STOP signaling in tree shrews and monkeys is recent and much remains to be explored. For instance, we only have used two very similar wavelengths (624 +/− 10 nm or 636 +/− 10 nm half peak intensity bandwidth). We noted no difference between their effectiveness, nor did we find a difference in effect on male and female tree shrews. Much shorter (blue) wavelengths produce very different effects in tree shrews (Gawne et al., 2017a, Gawne, Ward & Norton, 2017c), but there has, as yet, been no systematic evaluation of the effects of progressively shorter narrow-band wavelengths or of broader-band stimuli with the same peaks used here.
It also is unclear why inconsistent results using long-wavelength light have been experienced across species, including fish (Kröger & Wagner, 1996), chicks (Rohrer, Schaeffel & Zrenner, 1992) (Rucker & Wallman, 2009) and monkeys (Liu, Hu, He, Zhou, Dai, Qu, Liu & Chu, 2014). As noted previously (Smith et al., 2015), there are many differences in the precise wavelengths used, the retinal illuminance levels, age of the animals, whether the lights are steady or flickering, the length of treatment, the species of animals and, across species, the number of cone types and the wavelengths at which their cones experience peak absorbance. How, or if, these differences have combined to produce the differing results either must await studies that use the same stimulus conditions, ages and treatment durations across species, or ones that provide a more systematic exploration of these parameters within a species. What is established is that, in tree shrews, the specific wavelengths used here consistently produce STOP signaling (Gawne et al., 2017a, Gawne et al., 2017b).
Although the results are clear-cut and consistent in dichromatic tree shrews, it is not yet known if these stimuli will produce the same result in humans. However, trichromacy in mammals is a relative recent evolutionary development and dichromacy has been suggested to be the baseline state of color vision for all mammals including primates (Jacobs, 1993). As demonstrated by successful emmetropization in dichromatic animals and humans (Qian, Chu, He, Sun, Zhou, Zhao, Hu, Hoffman, Dai, Qu & Pao, 2009) trichromacy is not necessary for the emmetropization mechanism to function.
An underlying aim of studying animal models of refractive development is to discover principles that can be applied to slowing myopia development in children. An important discovery involving the interaction between GO and STOP signaling is that STOP signaling exhibits non-linear interaction with GO and is “stronger” than GO signaling (see review by Zhu (2013)). This discovery has led to the development of optical interventions, such as bifocal contact lenses, intended to create myopic defocus to counteract the myopiagenic effect of peripheral hyperopia in myopic children. The red light, at least in tree shrews, is a novel way to stimulate similar STOP signaling.
A potentially important difference between red light STOP signaling and that produced by refractive stimuli is that red light is effective in juvenile and adolescent tree shrews, ages when PL wear is ineffective. Siegwart and Norton (2010) and Guo et al. (Guo et al., 2012) found that PL wear in tree shrews that began at 24 DVE or 35 DVE did not produce slowed axial elongation and refractive hyperopia. It is not known if a similar loss of response to PL wear occurs with age in other species. The ability of the wavelengths used in tree shrews to counteract hyperopia-driven GO signaling, and the non-linear summation with red duration found in the present study, raise the possibility that extended treatment with brief daily periods of narrow-band long-wavelength light may be a robust source of STOP signaling at older ages with potential to be developed as a treatment for progressing myopia.
Acknowledgments
Supported by NIH/NEI grants R21EYE025254, and P30 EY003909 (core), a UAB Faculty Development Grant, and the UAB Comprehensive Neuroscience Center (CNC). The authors acknowledge the assistance of colleagues Dr. John Siegwart, Jr. and Dr. Lawrence Sincich, and the technical assistance of, Ms Regina Rab, Dr. Alex Zotov, and Mr. Eric Worthington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amedo AO, Norton TT. Comparison of Infrared photoretinoscope and autorefractor in tree shrews with and without induced myopia. Optometry and Vision Science. 2003;80(suppl):120. [Google Scholar]
- Bitzer M, Schaeffel F. ZENK expression of retinal glucagon amacrine cells in chicks: the effect of defocus presented in vivo, in vitro and under anesthesia. Vision Res. 2006;46(6–7):848–859. doi: 10.1016/j.visres.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40(1):214–229. [PubMed] [Google Scholar]
- Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012;103:47–54. doi: 10.1016/j.exer.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Choh V, Lew MY, Nadel MW, Wildsoet CF. Effects of interchanging hyperopic defocus and form deprivation stimuli in normal and optic nerve-sectioned chicks. Vision Res. 2006;46(6–7):1070–1079. doi: 10.1016/j.visres.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. American Journal of Ophthalmology. 1951;34:1407–1413. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Burkhardt E, Schaeffel F. Localization and regulation of glucagon receptors in the chick eye and preproglucagon and glucagon receptor expression in the mouse eye. Exp Eye Res. 2004;79(3):321–329. doi: 10.1016/j.exer.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Miethke P, Morgan IG, Stell WK. Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form-deprivation myopia. Brain Res. 1998;794(1):48–60. doi: 10.1016/s0006-8993(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Research. 1999;39(4):685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RP, Stell WK. N-methyl-d-aspartate-induced excitotoxicity causes myopia in hatched chicks. Canadian Journal of Ophthalmology - Journal Canadien d Ophtalmologie. 1997;32(6):373–377. [PubMed] [Google Scholar]
- Gawne TJ, Siegwart JT, Jr, Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017a;155:75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017b;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ, Ward AH, Norton TT. Wavelength cues are essential to maintain emmetropia in tree shrews. Invest Ophthalmol Vis Sci. 2017c ARVO E-abstract 2744. [Google Scholar]
- Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Guo L, Frost MR, He L, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to three myopiagenic conditions. Invest Ophthalmol Vis Sci. 2013;54(10):6806–6819. doi: 10.1167/iovs.13-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew sclera in response to two STOP visual conditions: recovery from minus-lens wear, and plus-lens wear. Invest Ophthalmol Vis Sci. 2012;53 ARVO E-Abstract 3455. [Google Scholar]
- Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Scleral gene expression during recovery from myopia compared with expression during myopia development in tree shrew. Mol Vis. 2014;20:1643–1659. [PMC free article] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34(3):690–694. [PubMed] [Google Scholar]
- Hammond DS, Wallman J, Wildsoet CF. Dynamics of active emmetropisation in young chicks--influence of sign and magnitude of imposed defocus. Ophthalmic Physiol Opt. 2013;33(3):215–226. doi: 10.1111/opo.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res. 2014a;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JT, Jr, Norton TT. Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear. Exp Eye Res. 2018;168:77–88. doi: 10.1016/j.exer.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Frost MR, Siegwart JT, Norton TT. Gene expression signatures in tree shrew choroid in response to three myopiagenic conditions. Vision Research. 2014b;102:52–63. doi: 10.1016/j.visres.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49(2):219–227. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Irving EL, Callender MG, Sivak JG. Inducing myopia, hyperopia, and astigmatism in chicks. Optometry and Vision Science. 1991;68:364–368. doi: 10.1097/00006324-199105000-00007. [DOI] [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992a;12(4):448–456. [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic and Physiological Optics. 1992b;12:448–456. [PubMed] [Google Scholar]
- Jacobs GH. The distribution and nature of colour vision among the mammals. Biol Rev Camb Philos Soc. 1993;68(3):413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao-Grider Y, Ramamirtham R, Winawer J, Wallman J, Smith EL., III Temporal constraints on experimental emmetropization in infant monkeys. Invest Ophthalmol Vis Sci. 2007;48(3):957–962. doi: 10.1167/iovs.06-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42(3):575–583. [PubMed] [Google Scholar]
- Kröger RHH, Wagner H-J. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. Journal of Comparative Physiology [A] 1996;179(6):837–842. doi: 10.1007/BF00207362. [DOI] [PubMed] [Google Scholar]
- Lan W, Feldkaemper M, Schaeffel F. Intermittent episodes of bright light suppress myopia in the chicken more than continuous bright light. PLoS ONE. 2014;9(10):e110906. doi: 10.1371/journal.pone.0110906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotta AJ, Bowrey HE, Zeng G, McFadden SA. Temporal properties of the myopic response to defocus in the guinea pig. Ophthalmic Physiol Opt. 2013;33(3):227–244. doi: 10.1111/opo.12062. [DOI] [PubMed] [Google Scholar]
- Liu R, Hu M, He JC, Zhou XT, Dai JH, Qu XM, Liu H, Chu RY. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci. 2014;55(3):1901–1909. doi: 10.1167/iovs.13-12276. [DOI] [PubMed] [Google Scholar]
- Luckett WP. Comparative Biology and Evolutionary Relationships of Tree Shrews. New York: Plenum Press; 1980. [Google Scholar]
- Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol. 2007;245(2):267–275. doi: 10.1007/s00417-006-0282-x. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Research. 1992;32(5):843–852. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- McFadden SA, Tse DY, Bowrey HE, Leotta AJ, Lam CS, Wildsoet CF, To CH. Integration of defocus by dual power Fresnel lenses inhibits myopia in the mammalian eye. Invest Ophthalmol Vis Sci. 2014;55(2):908–917. doi: 10.1167/iovs.13-11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna JA, Casagrande VA. Atropine affects lid-suture myopia development. Doc Ophthalmol Proc Ser. 1981;28:187–192. [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46(9):3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Napper GA, Brennan NA, Barrington M, Squires MA, Vessey GA, Vingrys AJ. The duration of normal visual exposure necessary to prevent form deprivation myopia in chicks. Vision Research. 1995;35(9):1337–1344. doi: 10.1016/0042-6989(94)00226-c. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Schroedl F. Parasympathetic influences on emmetropization in chicks: evidence for different mechanisms in form deprivation vs negative lens-induced myopia. Exp Eye Res. 2012;102:93–03. doi: 10.1016/j.exer.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. ILAR J. 1999;40(2):59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50(6):564–576. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Visual Neuroscience. 1994;11(1):143–153. doi: 10.1017/s0952523800011184. [DOI] [PubMed] [Google Scholar]
- Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992a;32(5):833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Research. 1992b;32(5):833–842. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Siegwart JT, German AJ, Robertson J, Wu WW. Comparison of cycloplegic streak retinoscopy with autorefractor measures in tree shrew eyes with, and without, induced myopia. Invest Ophthalmol Vis Sci. 2000;41 ARVO Abstract 563. [Google Scholar]
- Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47(11):4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80(9):623–631. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YS, Chu RY, He JC, Sun XH, Zhou XT, Zhao NQ, Hu DN, Hoffman MR, Dai JH, Qu XM, Pao KE. Incidence of myopia in high school students with and without red-green color vision deficiency. Invest Ophthalmol Vis Sci. 2009;50(4):1598–1605. doi: 10.1167/iovs.07-1362. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Schaeffel F, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol. 1992;449:363–376. doi: 10.1113/jphysiol.1992.sp019090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-b) act as stop and go signals to modulate postnatal ocular growth in the chick. Experimental Eye Research. 1994;58(5):553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- Rucker FJ, Wallman J. Chick eyes compensate for chromatic simulations of hyperopic and myopic defocus: evidence that the eye uses longitudinal chromatic aberration to guide eye-growth. Vision Res. 2009;49(14):1775–1783. doi: 10.1016/j.visres.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988a;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Research. 1988b;28(5):639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Research. 1994;34(2):143–149. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Howland HC. Mathematical model of emmetropization in the chicken. Journal of the Optical Society of America. 1988;5(12):2080–2086. doi: 10.1364/josaa.5.002080. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Troilo D, Wallman J, Howland HC. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis Neurosci. 1990;4(2):177–183. doi: 10.1017/s0952523800002327. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Research. 1996;36(7):1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Seltner RL, Stell WK. The effect of vasoactive intestinal peptide on development of form deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vision Res. 1995;35(9):1265–1270. doi: 10.1016/0042-6989(94)00244-g. [DOI] [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76(5):308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39(2):387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Exp Eye Res. 2010;91:660–669. doi: 10.1016/j.exer.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Laboratory Animal Science. 1994;44:292–294. [PubMed] [Google Scholar]
- Smith EL., 3rd Optical treatment strategies to slow myopia progression: effects of the visual extent of the optical treatment zone. Exp Eye Res. 2013;114:77–88. doi: 10.1016/j.exer.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015;56(11):6490–6500. doi: 10.1167/iovs.15-17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Huang J, Arumugam B. Effects of local myopic defocus on refractive development in monkeys. Optom Vis Sci. 2013;90(11):1176–1186. doi: 10.1097/OPX.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Kee CS, Qiao Y. Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Invest Ophthalmol Vis Sci. 2002;43(2):291–299. [PubMed] [Google Scholar]
- Stell WK, Tao J, Karkhanis A, Siegwart JT, Jr, Norton TT. Amacrine cells responsive to optical conditions regulating eye growth in the tree shrew, (Tupaia glis belangeri) Invest Ophthalmol Vis Sci. 2004;45 ARVO E-abstract 1159. [Google Scholar]
- Tse DY, Lam CS, Guggenheim JA, Lam C, Li KK, Liu Q, To CH. Simultaneous defocus integration during refractive development. Invest Ophthalmol Vis Sci. 2007;48(12):5352–5359. doi: 10.1167/iovs.07-0383. [DOI] [PubMed] [Google Scholar]
- Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005a;46(11):3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2005b;46(11):3932–3942. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- Wallman J, Adams JI, Trachtman JN. The eyes of young chickens grow toward emmetropia. Invest Ophthalmol Vis Sci. 1981;20(4):557–561. [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ward AH, Siegwart JT, Jr, Frost MR, Norton TT. The effect of intravitreal injection of vehicle solutions on form deprivation myopia in tree shrews. Exp Eye Res. 2016;145:289–296. doi: 10.1016/j.exer.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AH, Siegwart JT, Jr, Frost MR, Norton TT. Intravitreally-adminstered dopamine D2-like (and D4), but not D1-like, receptor agonists reduce form-deprivation myopia in tree shrews. Visual Neuroscience. 2017;34 doi: 10.1017/S0952523816000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthalmic & Physiological Optics. 1997;17(4):279–290. [PubMed] [Google Scholar]
- Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42(24):2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- Zhong X, Ge J, Smith EL, III, Stell WK. Image defocus modulates activity of bipolar and amacrine cells in macaque retina. Invest Ophthalmol Vis Sci. 2004;45(7):2065–2074. doi: 10.1167/iovs.03-1046. [DOI] [PubMed] [Google Scholar]
- Zhu X. Temporal integration of visual signals in lens compensation (a review) Exp Eye Res. 2013;114:69–76. doi: 10.1016/j.exer.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wallman J. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50(1):37–46. doi: 10.1167/iovs.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]