Abstract
Purpose
To evaluate the efficacy of minimally invasive spinal fusion in comparison to open fusion for adult lumbar spondylolisthesis or spondylosis.
Materials and Methods
The present study was conducted as a meta-analysis of all estimates from studies that were selected after comprehensive literature search by two independent reviewers.
Results
Of 745 articles, nine prospective cohort studies were identifed. The quality of evidence was downgraded because of study design, inconsistency, imprecision, and publication bias. Greater Oswestry Disability Index score improvement [weighted mean difference (WMD), 3.2; 95% confdence interval (CI), 1.5 to 5.0; p=0.0003] and a lower infection rate (odds ratio, 0.3; 95% CI, 0.1 to 0.9; p=0.02) were observed in the minimally invasive group (low-quality evidence). The minimally invasive group had less blood loss (WMD, 269.5 mL; 95% CI, 246.2 to 292.9 mL; p<0.0001), a shorter hospital stay (WMD, 1.3 days; 95% CI, 1.1 to 1.5 days, p<0.0001), and longer operation time (WMD, 21.0 minutes; 95% CI, 15.9 to 26.2 minutes; p<0.0001) and radiation exposure time(WMD, 25.4 seconds; 95% CI, 22.0 to 28.8 seconds, p<0.0001) than the open group (low-quality evidence). There were no significant differences in pain improvement, fusion rate, complications, or subsequent surgeries between the two treatment groups (low-quality evidence).
Conclusion
Although present findings are limited by insufficient evidence and there is a lack of adequately powered high-quality randomized controlled trials to address this gap in evidence, our results support that minimally invasive lumbar fusion is more effective than open fusion for adult spondylolisthesis and other spondylosis in terms of functional improvement, reducing infection rate, and decreasing blood loss and hospital stay.
Keywords: Minimally invasive, percutaneous pedicle screw, spinal fusion, lumbar spine, efficacy, meta-analysis
INTRODUCTION
Pedicle screw instrumented fusion is used as a safe and effective treatment for adult lumbar spondylolisthesis and other spondylosis.1,2 However, it is associated with extensive blood loss, a lengthy hospital stay, signifcant cost, and high reoperation rates.3,4 Standard instrumented fusion requires extensive tissue dissection to expose entry points that provide the lateral-to-medial orientation for optimal screw trajectory. Extensive injury to the back muscles during surgery has been shown to correlate with poor long-term outcomes.5,6
To overcome these problems, minimally invasive instrumented fusion through small, separate wounds without extensive tissue dissection has been introduced.7 This technique significantly reduces back muscle injury and blood loss, which leads to better trunk muscle performance and faster recovery and rehabilitation.8 However, the potential benefts of minimized tissue disruption, reduced blood loss, and shorter hospital stay must be weighed against the increased rate of neurological complications associated with this technique.9 Moreover, hardware-related complications and pseudarthrosis have been reported in recent studies.10,11
Evidence regarding the efficacy of minimally invasive lumbar spinal fusion employing percutaneous pedicle screws exclusively for posterior augmentation has not been synthesized, while plenty of meta-analyses have explored mixed data from studies that utilized conventional pedicle screws for miniopen instrumentation as an alternative to percutaneous pedicle screws. The primary purpose of the current study was to investigate the efficacy of minimally invasive instrumented fusion for adult lumbar spondylolisthesis and other spondylosis. We compared minimally invasive and open pedicle screw instrumented lumbar fusion, especially with respect to 1) pain and functional improvements and fusion rate, 2) complications and subsequent surgeries, and 3) perioperative outcomes (blood loss, hospital stay, operation time, and radiation exposure time).
MATERIALS AND METHODS
We conducted a thorough and comprehensive review of the literature according to the guidelines for performance and reporting of systematic reviews and meta-analyses outlined in the Meta-analysis of Observational Studies in Epidemiology (MOOSE)12 and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).13 This study was exempt from Institutional Review Board review. We searched the literature comparing minimally invasive lumbar spinal fusion with open fusion, including transforaminal lumbar interbody fusion (TLIF), posterior lumbar interbody fusion (PLIF), or posterolateral fusion (PLF), for the treatment of spondylolisthesis and other spondylosis. The literature searches were restricted to randomized controlled trials (RCTs), controlled clinical trials (or quasi-RCTs), and prospective cohort studies published in English. The searches were also limited to studies in which percutaneous pedicle screws were exclusively utilized for posterior spinal fixation in the intervention group. Studies with instrumented conventional pedicle screws instead of percutaneous pedicle screws and a mini-open approach were excluded. We also identified articles with overlapping populations and sought to determine the extent of overlap. In the case of substantial overlap (patients in one article were a subset of those in a larger study), the smaller study was excluded. Our detailed eligibility criteria are listed in Table 1.
Table 1. Inclusion and Exclusion Criteria.
| Study components | Inclusion | Exclusion |
|---|---|---|
| Participants | Adults | Children (age ≤18 years) |
| Pathology | A pathology of spondylolisthesis, spondylosis (degenerative disease) | A pathology of deformity, trauma, infection, inflammatory disease, or tumor |
| Interventions | Posterior lumbar/lumbosacral spinal fusion (including transforaminal/posterior lumbar interbody fusion and posterolateral fusion) utilizing percutaneous pedicle screw fixation | Decompression only without fusion |
| Fusions extended to cervical and thoracic spine | ||
| Stand-alone anterior or posterior fusion | ||
| Presacral (axial) anterior fusion | ||
| Posterior instrumentation with facet screws or interspinous process devices | ||
| Unilateral instrumentation | ||
| Robot-assisted instrumentation | ||
| Mini-open instrumentation* | ||
| Mixed instrumentation | ||
| Comparator | Conventional open pedicle screw instrumented fusion | |
| Study outcomes | Clinical outcomes for pain and function, fusion rate, subsequent surgery, complications, and perioperative surgical data | Other radiographic measures (excluding fusion): alignment, range of motion, etc. |
| Nonclinical outcomes | ||
| Study design | Randomized controlled trials | Retrospective cohort studies |
| Controlled clinical trials | Case-control studies | |
| Prospective cohort studies | Case series | |
| Case reports | ||
| Nonclinical studies | ||
| Publication | Studies published in English in peer-reviewed journals | Abstracts, editorials, letters |
| Duplicate publications of the same study that do not report on different outcomes | ||
| Single-center reports from multicenter trials | ||
| Studies reporting on the technical aspects of the surgery | ||
| White papers or narrative reviews | ||
| Articles identified as preliminary reports when results are published in later versions |
*Conventional pedicle screws were instrumented through a mini-open approach without use of percutaneous pedicle screw systems.
For inclusion in the present analysis, studies must have reported the following primary outcomes: postoperative back pain and leg pain improvement measured via a visual analogue scale (VAS); functional improvement measured via Oswestry Disability Index (ODI) score; fusion rate; complications (neurological, hardware-related, and surgical-site complications); subsequent surgeries (revision, removal, reoperation, and supplemental fixation); and perioperative outcomes (blood loss, length of hospital stay, operation time, and radiation exposure time)
Literature search and study selection
Two authors independently performed a comprehensive literature search of PubMed, Embase, and the Cochrane Library database for relevant studies published up to December 2017 using derivatives of the following Medical Subject Headings (MeSH): percutaneous pedicle screw, minimally invasive fusion, minimally invasive arthrodesis, mini-open fusion, miniopen arthrodesis, minimal access fusion, and minimal access arthrodesis. The detailed search strategy is illustrated in Supplementary Table 1 (only online). The reference lists of included articles were also systematically checked to identify additional eligible articles.
One reviewer (SOS) screened titles and abstracts to determine potential inclusion, with a 10% random sample of records independently screened by a second reviewer (SBL). Articles were double blind coded. Inclusion was subsequently confirmed by a team of three reviewers (SOS, SBL, and JWH) who independently checked the full text of all retrieved articles. Uncertainties and disagreements were resolved through team discussion and/or contact with study authors.
Data extraction and analysis
The study reviewers then used a custom data extraction form to extract relevant study data in duplicate. Data elements extracted included methodology data to confirm study eligibility, study design, patient demographics, performed interventions, outcomes of interest, statistical methods, and study results. One reviewer (SOS) then entered extracted data into a spreadsheet (Microsoft Excel 2013, Microsoft Corp., Redmond, WA, USA) with the accuracy of data entry confirmed by the second reviewer (SBL).
We pooled data from each included study and performed meta-analyses (both fixed-effect and random-effects methods) using Comprehensive Meta-Analysis software package Version 2 (Biostat, Englewood, NJ, USA) and STATA Version 14.0 (Stata Corp., College Station, TX, USA). The odds ratio (OR) for the intervention group and the accompanying 95% confidence interval (CI) were calculated for dichotomous outcomes, and the weighted mean difference (WMD) and 95% CI were calculated for continuous outcomes. We reported outcome measures according to the length of follow up: short (<1 year), intermediate (1 to 5 years), and long-term (≥5 years). Pain and functional improvements were analyzed using data from baseline to last follow-up. Fusion rate, complications, and subsequent surgeries were analyzed using data from the last follow-up visit.
The overall quality of evidence for each outcome was categorized as high, moderate, low, or very low according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) protocol.12,13 Five specific domains were used for grading study quality: risk of bias, inconsistency, indirectness, imprecision, and publication bias. We downgraded the evidence by 1 point when fewer than three domains were judged “serious or unclear” or when the study design was not an RCT. We downgraded the evidence by 2 points when four or more domains were judged “serious or unclear.”
Risk of bias
Two independent authors assessed the risk of bias and other major methodological flaws in the included studies using the checklist for RCTs12,13 or the checklist for cohort studies by Cowley.14 We defned high-quality studies as those that fulfilled ≥6 of the 12 criteria for RCTs or ≥9 of the 17 criteria of Cowley. We downgraded the quality of evidence by 1 point when risk of bias was serious or when major methodological flaws were noted. Disagreements were resolved by discussion.
Inconsistency
We evaluated statistical heterogeneity with the Q-test and I2 value. We defined substantial statistical heterogeneity as a Q-test with a p-value lower than 0.1 or an I2 value greater than 75%.15,16 We downgraded the quality of evidence by 1 point when heterogeneity was substantial.
Indirectness
We assessed whether the question being addressed in this meta-analysis varied from the available evidence with regard to population, intervention, comparators, or outcomes.
Imprecision
Results were considered imprecise when trials included relatively few patients and few events and thus had wide CIs around the estimate of the effect.
Publication bias
We downgraded the quality of evidence by 1 point when a funnel plot suggested publication bias. The possibility of publication bias was not evaluated for statistical signifcance if a small number (<10) of studies was assessed.13,17
RESULTS
We identifed 745 potentially relevant citations from the electronic database and reference searches after duplicates were eliminated. Sixty-seven studies were selected for full-text assessment after the initial title and abstract screening. Forty articles were excluded because of the study design (27 retrospective, 12 case series, and 1 unclear), and nine (including 1 RCT18) were excluded because open pedicle screws were used in the intervention group instead of percutaneous screws.
A total of 11 studies19,20,21,22,23,24,25,26,27,28,29 met the inclusion criteria and two20,28 were removed because of overlapping populations: 1) a study population published in 2009 by Peng, et al.28 was determined to be a subset of those in another study published in 2012 by Lee, et al.22 The prior study by Peng, et al.28 overlapped their case-enroll period with the later larger one by Lee, et al.22 Therefore, the study by Peng, et al.28 was excluded. 2) A subset of patients in the study published in 2014 by Wang, et al.20 were judged to be overlapped with those of two other studies published in 201026 and 201125 by the same authors. The other two studies25,26 did not have overlapping populations with each other. Consequently, the study published in 2014 by Wang, et al.20 was excluded.
Finally, nine studies19,21,22,23,24,25,26,27,29 were selected for analysis (Fig. 1). Characteristics of all included studies are summarized in Table 2 and Supplementary Table 2 (only online). A total of 707 participants (363 in the minimally invasive group and 344 in the open group) were included in the nine prospective cohort studies. Mean duration of follow-up was 22.2±6.8 months in the minimally invasive group and 24.1±7.6 months in the open group. Detailed demographic and surgical data at baseline are illustrated in Table 3. The baseline data were similar between the two groups (all p>0.05).
Fig. 1. Flow diagram demonstrating the individual steps in the literature-selection process.
Table 2. Characteristics of All Included Comparative Observational Studies.
| Studies | Study design, year published, year enrollment, intervention | Comparison group | N | Age (yr) | Gender Male (%) | Fusion level | Diagnoses (number of patients in percutaneous group, open group) | Instrumentations including cage and pedicle screw | Bone graft | Follow-up (month) |
|---|---|---|---|---|---|---|---|---|---|---|
| Parker, et al.19 | Prospective cohort study, 2014, NR, Single-level TLIF | Minimally invasive group | 50 | 53.5±12.5 | 16 (32) | L3–4; 4 | Degenerative spondylolisthesis grade I (50, 50) | A single PEEK interbody cage and percutaneous pedicle screw system (implants NR) | Local autogenous bone with or without bone extensors (i.e., DBM) | 24, % followed: NR |
| L4–5; 32 | ||||||||||
| L5–S1; 14 | ||||||||||
| Open group | 50 | 52.6±11.6 | 18 (36) | L3–4; 3 | A single PEEK interbody cage and conventional pedicle screw system (implants NR) | Local autogenous bone with or without bone extensors (i.e., DBM) | 24, % followed: NR | |||
| L4–5; 30 | ||||||||||
| L5–S1; 17 | ||||||||||
| Gu, et al.21 | Prospective cohort study, 2013, 2010–2011, Two-level TLIF | Minimally invasive group | 44 | 66.4±6.7 | 19 (43.2) | L3–5; 13 | Degenerative disc disease (15, 11) Spinal stenosis (18, 14) Spinal stenosis with segmental instability (11, 13) |
A single PEEK interbody cage (Capstone; Medtronic, Memphis, TN, USA) and percutaneous pedicle screw system (Sextant; Medtronic) | Local autologous bone | 20.6±4.5, % followed: NR |
| L4–S1; 31 | ||||||||||
| Open group | 38 | 64.1±7.8 | 15 (39.5) | L3–5; 14 | NR | NR | 20.0±3.3, % followed: NR | |||
| L4–S1; 24 | ||||||||||
| Lee, et al.22 | Prospective cohort study, 2012, 2002–2008, Single-level TLIF | Minimally invasive group | 72 | 52.2±13.8 | 20 (27.8) | L3–4; 6 | Spondylolisthesis (Grade 1 and 2) Recurrent disc herniation Spinal stenosis requiring resection of more than 50% of either facet joint |
A single PEEK interbody cage (Capstone; Medtronic) and percutaneous pedicle screw system (Sextant; Medtronic) | Local autogenous bone with DBM (Osteofil; Medtronic) | 24, 95.8% (69/72) followed for 24 months |
| L4–5; 49 | ||||||||||
| L5–S1; 17 | ||||||||||
| Open group | 72 | 56.6±14.6 | 22 (30.6) | L3–4; 4 | Degenerated collapsed disc requiring disc-space height restoration (Specific number; NR) | A single PEEK interbody cage (Capstone; Medtronic) and conventional pedicle screw system (implants NR) | Local autogenous bone with DBM (Osteofil; Medtronic) and one case with rhBMP-2 (Infuse; Medtronic) | 24, 91.7% (66/72) followed for 24 months | ||
| L4–5; 54 | ||||||||||
| L5–S1; 14 | ||||||||||
| Mobbs, et al.23 | Prospective cohort study, 2011, 2006–2010, Single- or multi-level PLIF | Minimally invasive group | 37 | 68.56±12.99 | 19 (51.4) | T11–12; 0 | Isthmic spondylolisthesis (4, 9) Degenerative spondylolisthesis (18, 9) Degenerative scoliosis (1, 4) Degenerative disc disease with foraminal stenosis (14, 8) |
A single rotatable interbody cage (implants NR) and percutaneous pedicle screw systems (Denali/ Serengeti system; K2M, Leesburg, VA, USA and MANTIS; Stryker, Kalamazoo, MI, USA) | Local autogenous bone with or without synthetic bone | 11.5 (5.4–20.1), % followed: NR |
| L2–3; 1 | ||||||||||
| L3–4; 2 | ||||||||||
| L4–5; 20 | ||||||||||
| L5–S1; 6 | ||||||||||
| Multi-level; 8 | ||||||||||
| Open group | 30 | 67.48±13.19 | 16 (53.3) | T11–12; 1 | A single rotatable interbody cage and conventional pedicle screw system (implants NR) | Local autogenous bone with or without synthetic bone | 18.7 (8.1–40.0), % followed: NR | |||
| L2–3; 0 | ||||||||||
| L3–4; 0 | ||||||||||
| L4–5; 15 | ||||||||||
| L5–S1; 9 | ||||||||||
| Multi-level; 5 | ||||||||||
| Kotani, et al.24 | Prospective cohort study, 2011, 2005–NR, Single-level PLF | Minimally invasive group | 43 | 63±9 | 14 (32.6) | L3–4; 4 | Degenerative spondylolisthesis (43, 37) | Percutaneous pedicle screw system (Sextant; Medtronic) | Autogenous posterior iliac crest bone | 32 (24–49), % followed: NR |
| Open group | 37 | 66±9 | 12 (32.4) | L4–5; 76 (no specific declaration between groups) | Conventional polyaxial pedicle screw and rod system (implants NR) | Autogenous posterior iliac crest bone | 40 (24–60), % followed: NR | |||
| Wang, et al.25 | Prospective cohort study, 2011, 2006–2008, Single- or two-level TLIF | Minimally invasive group | 25 | 54.8±10.9 | 13 (52.0) | L3–4; 2 | Recurrent disc herniation (7, 8) Postsurgical foraminal stenosis (10, 9) Postsurgical segmental instability (5, 7) Postsurgical spondylolisthesis grade 1 (3, 3) |
A single PEEK interbody cage (OIC; Stryker) and percutaneous pedicle screw system (Sextant; Medtronic) | Local autogenous bone with or without autogenous iliac crest bone | Overall, 27.5 (12–38), % followed: NR |
| L4–5; 11 | ||||||||||
| L5–S1; 9 | ||||||||||
| Two-level; 3 | ||||||||||
| Open group | 27 | 56.2±13.6 | 15 (55.6) | L3–4; 2 | A single PEEK interbody cage (OIC; Stryker) and conventional pedicle screw system (implants NR) | NR | ||||
| L4–5; 11 | ||||||||||
| L5–S1; 10 | ||||||||||
| Two-level; 4 | ||||||||||
| Wang, et al.26 | Prospective cohort study, 2010, 2006–2008, Single-level TLIF | Minimally invasive group | 42 | 47.9±8.5 | 13 (30.1) | L3–4; 3 | Degenerative spondylolisthesis (24, 22) | A single PEEK interbody cage (OIC, Stryker) and percutaneous pedicle screw system (Sextant; Medtronic) | Local autogenous bone | Overall, 26.3 (13–35), % followed: NR |
| L4–5; 21 | ||||||||||
| L5–S1; 18 | ||||||||||
| Open group | 43 | 53.2±10.6 | 16 (37.2) | L3–4; 3 | Isthmic spondylolisthesis (18, 21) | NR | NR | |||
| L4–5; 23 | ||||||||||
| L5–S1; 17 | ||||||||||
| Schizas, et al.27 | Prospective cohort study, 2008, NR, Single-level TLIF | Minimally invasive group | 18 | 45.5±NR | NR | L5–S1; 12 | Isthmic spondylolisthesis (15, 6) Degenerative disc disease with foraminal stenosis (2, 12) Iatrogenic spondylolysis (1, 0) |
A single PEEK interbody cage (Medtronic) and percutaneous pedicle screw system [Sextant (11 cases); Medtronic and Viper (7 cases); DePuy Spine, USA] | Local autologous bone with autogenous iliac crest bone | 22, % followed: NR |
| Other level (specific level;NR); 6 | ||||||||||
| Open group | 18 | 48.1±NR | NR | L5–S1; 11 | NR | Local autologous bone with autogenous iliac crest bone | 24, % followed: NR | |||
| Other level (specific level; NR); 7 | ||||||||||
| Park and Ha29 | Prospective cohort study, 2007, 2003–2004, Single-level PLIF | Minimally invasive group | 32 | 62.1±9.6 | 8 (25) | L3–4; 2 | Isthmic spondylolisthesis (6, 7) Degenerative spondylolisthesis (7, 5) Lumbar disc herniation (1, 3) |
A single PEEK interbody cage (Telamon; Medtronic) and percutaneous pedicle screw system (Sextant; Medtronic) | Local autogenous bone | 12, % followed: NR |
| L4–5; 23 | ||||||||||
| L5–S1; 7 | ||||||||||
| Open group | 29 | 59.0±12.2 | 13 (44.8) | L3–4; 3 | Spinal stenosis with segmental instability (18, 14) | A single PEEK interbody cage (Telamon; Medtronic) and conventional pedicle screw system (implants NR) | Local autogenous bone | 12, % followed: NR | ||
| L4–5; 18 | ||||||||||
| L5–S1; 8 |
TLIF, transforaminal lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; PLF, posterolateral fusion; PEEK, polyetheretherketone; NR, not reported; DBM, demineralized bone matrix, and rhBMP-2, recombinant human bone morphogenetic protein-2.
Table 3. Demographic and Surgical Data between the Two Surgical Groups.
| Overall | Minimally invasive group | Open group |
|---|---|---|
| Number of patients | 363 | 344 |
| Age (yr) | 57.1±8.2 | 58.1±6.6 |
| Gender, male (%) | 122 (33.6) | 127 (36.9) |
| Diagnosis | ||
| Spondylolisthesis (low-grade) | 188 | 169 |
| Degenerative | 142 | 123 |
| sthmic | 43 | 43 |
| Postsurgical | 3 | 3 |
| Other spondylosis | 103 | 103 |
| Degenerative disc disease | 15 | 11 |
| Lumbar disc herniation | 1 | 3 |
| Spinal stenosis | 18 | 14 |
| Foraminal stenosis | 16 | 20 |
| Spinal stenosis with segmental instability | 29 | 27 |
| Recurrent lumbar disc herniation | 7 | 8 |
| Postsurgical foraminal stenosis | 10 | 9 |
| Postsurgical segmental instability | 5 | 7 |
| Degenerative scoliosis | 1 | 4 |
| Iatrogenic spondylolysis | 1 | 0 |
| Number of each separate diagnosis NR | 72 | 72 |
| Fusion modalities | ||
| TLIF | 251 | 248 |
| Single-level | 204 | 206 |
| Two-level | 47 | 42 |
| PLIF | 69 | 59 |
| Single-level | 61 | 54 |
| Multi-level | 8 | 5 |
| PLF | ||
| Single-level | 43 | 37 |
| Fusion level | ||
| Single-level | 308 | 297 |
| T11–12 | 0 | 1 |
| L2–3 | 1 | 0 |
| L3–4 | 19 | 15 |
| L4–5 | 156 | 151 |
| L5–S1 | 83 | 86 |
| Level NR | 49 | 44 |
| Two-level | 47 | 42 |
| L3–L5 | 13 | 14 |
| L4–S1 | 31 | 24 |
| Level NR | 3 | 4 |
| Multi-level | ||
| Level NR | 8 | 5 |
| Follow-up periods | 22.2±6.8 | 24.1±7.6 |
TLIF, transforaminal lumbar interbody fusion; PLIF, posterior lumbar interbody fusion; PLF, posterolateral fusion; NR, not reported.
The differences of all baseline data were not significant. p>0.05.
Quality assessment
All studies had a Cowley score of 9 or more, and we judged these studies to have a low risk of bias (Table 4). Serious inconsistency was noted in all perioperative outcome measures (blood loss, hospital stay, operation time, and radiation exposure time) with substantial heterogeneity (I2>75%, p<0.1). Serious imprecision was noted in the primary outcome measures for pain and functional improvement, as well as in all perioperative outcome measures (effect size of mean difference crosses 0.5). Publication bias was judged to be unclear because it could not be quantifed due to the small number of studies analyzed.
Table 4. Risk of Bias Assessment of Included Comparative Observational Studies.
| Parker, et al.19 | Gu, et al.21 | Lee , et al.22 | Mobbs, et al.23 | Kotani, et al.24 | Wang, et al.25 | Wang, et al.26 | Schizas, et al.27 | Park and Ha29 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 Method of selection of patients identified and appropriateness | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2 Number of patients deceased or lost to follow-up reported or included in appropriate statistical analysis | No | No | Yes | No | No | No | No | No | No |
| 3 Follow-up period range and mean given (minimum=n) | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| 4 Prosthesis models specified | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 Clearly defined criteria for measuring outcomes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6 Valid statistical analysis undertaken | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7 Data given for deceased patients (information) | No | No | Yes | No | No | No | No | No | No |
| 8 Age range and mean age reported | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 9 Numbers of males and females given | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 10 Weight range and mean weight given | No | No | No | No | No | No | No | No | Yes |
| 11 Preoperative diagnoses with percentages of patients given | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 12 Clinical evaluation independent of operating surgeon | Yes | Unclear | Yes | Unclear | Unclear | No | No | Unclear | Unclear |
| 13 Radiological evaluation independent and blinded to clinical results | No | Yes | Yes | Yes | Unclear | Yes | Yes | Unclear | Unclear |
| 14 Results given for specific models | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 15 Quantification of outcomes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 16 Follow-up data compared with preoperative data (mean and range) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| 17 Independence of investigators (no vested interest) stated | No | Yes | No | Unclear | Yes | Yes | Yes | No | Yes |
| Scores | 9 | 13 | 14 | 12 | 12 | 13 | 13 | 9 | 11 |
A positive answer (Yes) to any question counts as 1 point.
Based on the GRADE protocol, all studies suffered from methodological flaws (limitations in the design and implementation), leading us to downgrade their quality by 1 point (Table 5). We downgraded the evidence for primary outcomes of back pain, leg pain, and functional improvement by 2 points (low-quality evidence) because of study design and because two domains (imprecision and publication bias) were judged “serious or unclear.” The evidence for primary outcomes of fusion rate, complications, and subsequent surgeries was also downgraded by 2 points (low-quality evidence) because of the design and because one domain (publication bias) was judged “serious or unclear.” Moreover, the evidence for perioperative outcomes (blood loss, hospital stay, operation time, and radiation exposure time) was downgraded by 2 points (low quality evidence) because of the design and because three domains (inconsistency, imprecision, and publication bias) were judged “serious or unclear.”
Table 5. The Quality Assessment of Evidence for Each Outcome.
| Number of studies | Study design | Risk of bias* | Inconsistency | Indirectness | Imprecision | Publication bias‡‡ | Quality |
|---|---|---|---|---|---|---|---|
| Back pain improvement: 5 | No RCTs | No serious | No serious† | No serious | Serious** | Unclear | Low |
| Leg pain improvement: 2 | No RCTs | No serious | No serious† | No serious | Serious** | Unclear | Low |
| Functional improvement: 5 | No RCTs | No serious | No serious† | No serious | Serious** | Unclear | Low |
| Fusion rate: 8 | No RCTs | No serious | No serious† | No serious | No serious†† | Unclear | Low |
| Neurological complications: 7 | No RCTs | No serious | No serious† | No serious | No serious†† | Unclear | Low |
| Harware complications: 6 | No RCTs | No serious | No serious† | No serious | No serious†† | Unclear | Low |
| Surgical-site complications: 7 | No RCTs | No serious | No serious† | No serious | No serious†† | Unclear | Low |
| Subsequent surgeries: 6 | No RCTs | No serious | No serious† | No serious | No serious†† | Unclear | Low |
| Blood loss: 6 | No RCTs | No serious | Serious‡ | No serious | Serious** | Unclear | Low |
| Hospital stay: 6 | No RCTs | No serious | Serious§ | No serious | Serious** | Unclear | Low |
| Operation time: 7 | No RCTs | No serious | Serious∥ | No serious | Serious** | Unclear | Low |
| Radiation exposure time: 4 | No RCTs | No serious | Serious¶ | No serious | Serious** | Unclear | Low |
RCTs, randomized controlled trials; df, degrees of freedom.
*All studies fulfilled 9 or more criteria of checklist by Cowley and these studies were judged at low risk of bias, †Heterogeneity: I2=0%, ‡Heterogeneity: χ2=89.096, df=5 (p<0.0001); I2=94.4%, §Heterogeneity: χ2=91.483, df=5 (p<0.0001); I2=94.5%, ∥Heterogeneity: χ2=42.123, df=6 (p<0.0001); I2=85.8%, ¶Heterogeneity: χ2=44.986, df=3 (p<0.0001); I2=93.3%, **Weighted mean difference effect size crosses 0.5, ††Odds ratio effect size did not cross 2.5, ‡‡Publication bias was not calculated due to the small number of studies analyzed.
Pain and functional improvements
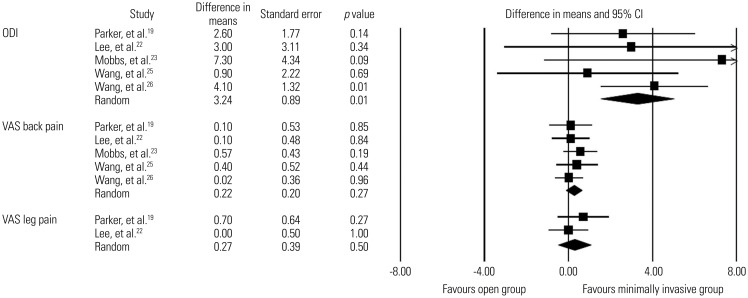
Low-quality evidence from five studies19,22,23,25,26 revealed that improvement in VAS back pain score was not signifcantly different between the minimally invasive spinal fusion and open fusion groups (WMD, 0.2; 95% CI, −0.2–0.6; p=0.3; mean follow-up, 20.9±7.2 months). Likewise, low-quality evidence from two studies19,22 revealed that improvement in VAS leg pain score did not differ significantly between the two groups (WMD, 0.3; 95% CI, −0.5–1.0; p=0.5; mean follow-up, 25.2±2.0 months). In contrast, there was low-quality evidence from five studies19,22,23,25,26 in which the minimally invasive group had significantly greater improvement in ODI score than the open group (WMD, 3.2; 95% CI, 1.5–5.0; p=0.0003; mean follow-up, 24.2±4.8 months)(Fig. 2). The detailed clinical outcome scores of all included studies are summarized in Supplementary Tables 3 and 4 (only online).
Fig. 2. Comparisons of ODI scores for functional improvement and VAS scores for back pain and leg pain between minimally invasive and open lumbar spinal fusion. Heterogeneity: ODI score [τ2=0.000; χ2=2.549, df=4 (p=0.636); I2=0.0%], VAS back pain [τ2=0.000; χ2=1.192, df=4 (p=0.879); I2=0.0%], and VAS leg pain [τ2=0.000; χ2=0.748, df=1 (p=0.387); I2=0.0%]. ODI, Oswestry Disability Index; VAS, visual analogue scale; df, degrees of freedom; CI, confidence interval.
Fusion rate
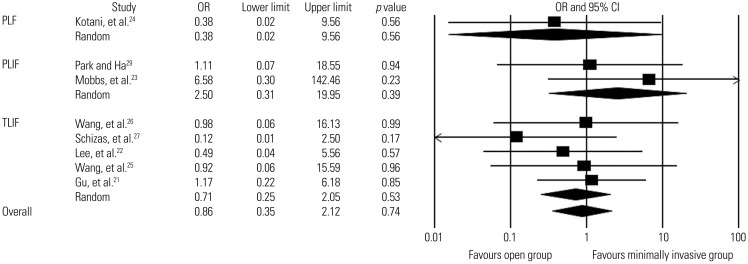
Fusion rates were reported in all studies, with overall rates of 96.7% in the minimally invasive group (351/363 patients; mean follow-up, 22.2±6.8 months) and 97.4% in the open group (335/344 patients; mean follow-up, 24.1±7.6 months) (Supplementary Tables 5 and 6, only online). Eight of nine studies were eligible for the meta-analysis; one study was not eligible because the fusion rate was 100% in both groups.19 We found low-quality evidence from eight studies in which there was no statistically signifcant difference in the overall fusion rate between the two groups (OR, 0.9; 95% CI, 0.3–2.1; p=0.7). Low-quality evidence was obtained from subgroup analyses according to the fusion method (5 studies for TLIF,21,22,25,26,27 two studies for PLIF,23,29 and a single study for PLF24), which revealed no significant difference in the fusion rate between the two groups (Fig. 3).
Fig. 3. Comparison of fusion rates between minimally invasive and open lumbar spinal fusion. Heterogeneity: fusion rates for PLF [τ2=0.000; χ2=0.000, df=0 (p=1.000); I2=0.0%], fusion rates for PLIF [τ2=0.000; χ2=0.701, df=1 (p=0.402); I2=0.0%], fusion rates for TLIF [τ2=0.000; χ2=1.836, df=4 (p=0.766); I2=0.0%], and overall fusion rates [τ2=0.000; χ2=3.925, df=7 (p=0.788); I2=0.0%]. PLF, posterolateral fusion; PLIF, posterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion; df, degrees of freedom; OR, odds ratio; CI, confidence interval.
Complications
The detailed complications of all included studies are summarized in Supplementary Table 7 (only online).
Neurological complications
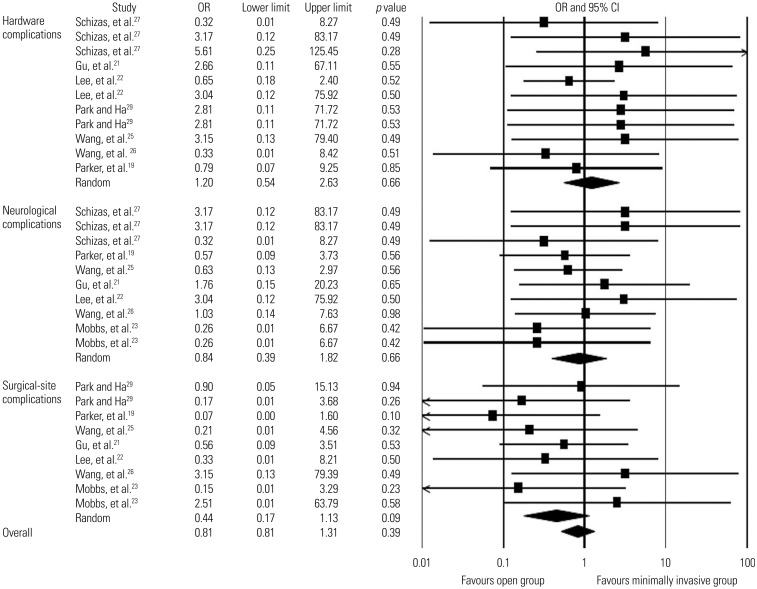
Seven studies19,21,22,23,25,26,27 addressed neurological complications, including dural tear, CSF leakage, nerve root injury, and postoperative radiculopathy (Table 6). The overall rates of neurological complications were 4.5% in the minimally invasive group (13/288 patients; mean follow-up, 22.3±5.3 months) and 4.7% in the open group (13/278 patients; mean follow-up, 23.5±3.2 months). We found low-quality evidence from these studies that there was no statistically significant difference in the overall rate of neurological complications between the two groups (OR, 0.8; 95% CI, 0.4–1.8; p=0.7) (Figure 4).
Table 6. Complications and Subsequent Surgeries between the Two Surgical Groups.
| Outcomes | N of studies | Minimally invasive group | Open group | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| N of OR events (%) | Total N of patients | N of events (%) | Total N of patients | |||
| Surgical-site complications | ||||||
| Overall | 7 | 5 (1.7) | 302 | 13 (4.5) | 289 | 0.4 (0.2 to 1.1) |
| Infection | 6 | 3 (1.2) | 260 | 13 (5.3) | 246 | 0.3 (0.1 to 0.9)* |
| Surgical-site infection | 3 | 0 (0.0) | 159 | 5 (3.3) | 152 | 0.2 (0.02 to 0.9)† |
| Superficial wound infection | 3 | 2 (2.0) | 101 | 7 (7.4) | 94 | 0.3 (0.02 to 1.4) |
| Deep wound infection | 1 | 1 (3.1) | 32 | 1 (3.4) | 29 | 0.9 (0.05 to 15.1) |
| Hematoma | 2 | 2 (2.5) | 79 | 0 (0.0) | 73 | 2.8 (0.3 to 27.6) |
| Neurological complications | ||||||
| Overall | 7 | 13 (4.5) | 288 | 13 (4.7) | 278 | 0.8 (0.4 to 1.8) |
| Dural tear/CSF leak | 7 | 12 (4.2) | 288 | 11 (4.0) | 278 | 0.9 (0.4 to 2.1) |
| Nerve root injury (L5 root paresis) | 1 | 1 (5.6) | 18 | 0 (0.0) | 18 | 3.2 (0.1 to 83.2) |
| Postoperative radiculopathy (transient L3 radicular pain) | 2 | 0 (0.0) | 55 | 2 (4.2) | 48 | 0.3 (0.03 to 2.9) |
| Hardware complications | ||||||
| Overall | 6 | 14 (5.4) | 258 | 9 (3.6) | 250 | 1.2 (0.5 to 2.6) |
| Screw-related complications | 6 | 8 (3.1) | 258 | 2 (0.8) | 250 | 1.8 (0.6 to 5.8) |
| Screw malposition | 4 | 4 (2.0) | 196 | 2 (1.0) | 194 | 1.1 (0.3 to 5.1) |
| Screw loosening | 1 | 2 (11.1) | 18 | 0 (0.0) | 18 | 5.6 (0.3 to 125.4) |
| Screw breakage | 1 | 1 (5.6) | 18 | 0 (0.0) | 18 | 3.2 (0.1 to 83.2) |
| Overlong screw | 1 | 1 (2.3) | 44 | 0 (0.0) | 38 | 2.7 (0.1 to 67.1) |
| Cage-related complications | 3 | 5 (4.1) | 122 | 7 (5.9) | 119 | 0.7 (0.2 to 2.2) |
| Cage migration | 2 | 5 (4.8) | 104 | 6 (5.9) | 101 | 0.8 (0.2 to 2.7) |
| Cage fracture during insertion | 1 | 0 (0.0) | 18 | 1 (5.6) | 18 | 0.3 (0.01 to 8.3) |
| Graft dislodgement | 1 | 1 (2.4) | 42 | 0 (0.0) | 43 | 3.1 (0.1 to 79.4) |
| Pseudarthrosis | 9 | 12 (3.3) | 363 | 9 (2.6) | 344 | 1.2 (0.5 to 2.9) |
| Other complications | ||||||
| Overall | 3 | 3 (2.4) | 127 | 7 (5.8) | 120 | 0.6 (0.2 to 1.9) |
| Deep vein thrombosis | 1 | 0 (0.0) | 37 | 1 (3.3) | 30 | 0.3 (0.01 to 6.7) |
| Myocardial infarction | 1 | 0 (0.0) | 72 | 1 (1.4) | 72 | 0.3 (0.01 to 8.2) |
| Pneumonia | 1 | 1 (1.4) | 72 | 1 (1.4) | 72 | 1.0 (0.06 to 16.3) |
| Paralytic ileus | 1 | 0 (0.0) | 37 | 3 (10) | 30 | 0.1 (0.005 to 2.1) |
| Urinary tract infection | 1 | 1 (2.7) | 37 | 0 (0.0) | 30 | 2.5 (0.1 to 63.8) |
| Postoperative anemia | 1 | 0 (0.0) | 72 | 1 (1.4) | 72 | 0.3 (0.01 to 8.2) |
| Brachial plexus injury due to positioning | 1 | 1 (5.6) | 18 | 0 (0.0) | 18 | 3.2 (0.1 to 83.2) |
| Subsequent surgeries‡ | ||||||
| Overall | 6 | 11 (4.4) | 251 | 9 (3.7) | 242 | 0.9 (0.4 to 2.3) |
| Revision | 4 | 5 (2.6) | 196 | 2 (1.0) | 194 | 1.1 (0.3 to 4.5) |
| Revision for malpositioned screw | 4 | 4 (2.0) | 196 | 2 (1.0) | 194 | |
| Revision for migrated cage | 1 | 1 (3.1) | 32 | 0 (0.0) | 29 | |
| Removal for pseudarthrosis | 3 | 3 (3.4) | 87 | 3 (3.9) | 77 | 1.3 (0.2 to 7.9) |
| Reoperation | 4 | 3 (1.5) | 196 | 4 (2.1) | 194 | 0.6 (0.1 to 2.8) |
| Reoperation for graft dislodgement | 1 | 1 (2.4) | 42 | 0 (0.0) | 43 | |
| Reoperation for surgical-site infection | 2 | 0 (0.0) | 122 | 3 (2.5) | 122 | |
| Reoperation for deep wound infection | 1 | 1 (3.1) | 32 | 1 (3.4) | 29 | |
| Reoperation for hematoma | 1 | 1 (2.4) | 42 | 0 (0.0) | 43 | |
| Supplemental fixation | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | |
N, number; OR, odds ratio; CI, confidence interval; CSF, cerebrospinal fluid.
*p=0.02, †p=0.04, ‡Complications lead to a subsequent surgical intervention. Subsequent surgical intervention was categorized as follows: a revision is a procedure that adjusts or in any way modifies or removes part of the original implant configuration, with or without replacement of a component; a revision may also include adjusting the position of the original configuration (revision for migrated cage, removal of screws, etc.). A removal is a procedure where all of the original system configuration are removed with or without replacement (removal for pain at operative site but after fusion, for pseudarthrosis, etc.). A reoperation is any surgical procedure at the involved level(s) that does not removal, modification, or addition of any components to the system. A supplemental fixation is a procedure in which additional instrumentation not under study in the protocol is implanted.
Fig. 4. Comparison of complications rates (hardware-related, neurological, and surgical-site complications) between minimally invasive and open lumbar spinal fusion. Heterogeneity: hardware-related complications [τ2=0.000; χ2=4.922, df=10 (p=0.896); I2=0.0%], neurological complications [τ2=0.000; χ2=3.909, df=9 (p=0.917); I2=0.0%], surgical-site complications [τ2=0.000; χ2=5.232, df=8 (p=0.732); I2=0.0%], and overall complication rates [τ2=0.000; χ2=16.605, df=29 (p=0.968); I2=0.0%]. df, degrees of freedom; OR, odds ratio; CI, confidence interval.
Hardware complications
Six studies19,21,22,26,27,29 described hardware-related complications, including screw malposition, screw loosening, screw breakage, overlong screw, cage migration, cage fracture, and graft dislodgement (Table 6). The overall rates of hardware complications were 5.4% in the minimally invasive group (14/258 patients; mean follow-up, 21.8±5.4 months) and 3.6% in the open group (9/250 patients; mean follow-up, 22.3±5.2 months). There was low-quality evidence that the overall rate of hardware complications did not differ significantly between the two groups (OR, 1.2; 95% CI, 0.5–2.6; p=0.7) (Fig. 4).
Surgical-site complications
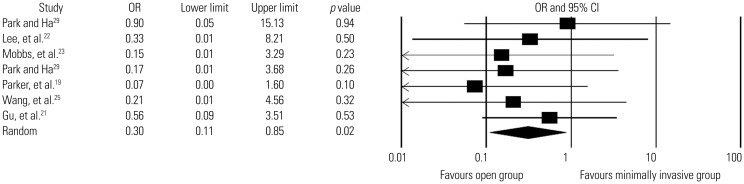
Seven studies19,21,22,23,25,26,29 reported surgical-site complications, including surgical-site infection, superficial infection, deep infection, and hematoma (Table 6). The overall rates of surgical-site complications were 1.7% in the minimally invasive group (5/302 patients; mean follow-up, 20.9±6.9 months) and 4.5% in the open group (13/289 patients; mean follow-up, 22.6±4.0 months). Low-quality evidence from these seven studies revealed that the overall rate of surgical-site complications did not differ signifcantly between the two groups (OR, 0.4; 95% CI, 0.2–1.1; p=0.1) (Fig. 4). The overall rates of infection (including surgical-site infection, superfcial infection, and deep wound infection) were 1.2% in the minimally invasive group (3/260 patients; mean follow-up, 19.9±6.7 months) and 5.3% in the open group (13/246 patients; mean follow-up, 21.0±5.4 months). There was low-quality evidence from six studies19,21,22,23,25,29 in which the minimally invasive group had a significantly lower rate of infection than the open group (OR, 0.3; 95% CI, 0.1–0.9; p=0.02) (Fig. 5).
Fig. 5. Comparison of infection rates between minimally invasive and open lumbar spinal fusion. Heterogeneity: τ2=0.000; χ2=2.197, df=6 (p=0.901); I2=0.0%. df, degrees of freedom; CI, confidence interval; OR, odds ratio.
Subsequent surgeries
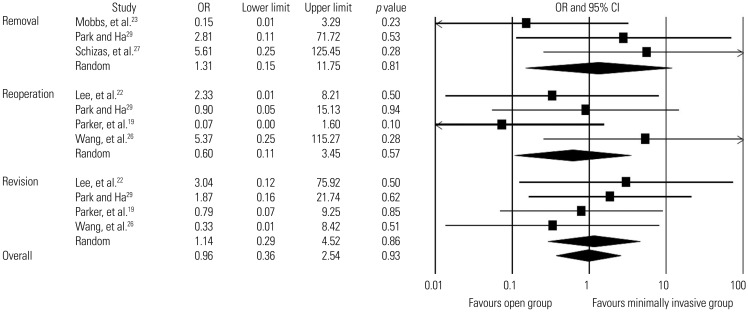
Six studies included information about subsequent surgeries,19,22,23,26,27,29 and a total of 493 patients (251 in the minimally invasive group and 242 in the open group) were analyzed (Table 6). We found low-quality evidence suggesting that the overall rate of subsequent surgeries did not differ significantly between the two groups (4.4% in the percutaneous group and 3.7% in the open group; OR, 0.9; 95% CI, 0.4–2.3; p=0.9; mean follow-up, 20.2±6.8 months). Revisions, removals, and reoperations were also analyzed in four studies,19,22,26,29 three studies,23,27,29 and four studies,19,22,26,29 respectively. The overall rates of revisions were 2.6% in the minimally invasive group (5/196 patients; mean follow-up, 21.9±6.8 months) and 1.0% in the open group (2/194 patients; mean follow-up, 21.9±6.8 months). Overall, 3.4% underwent removals in the minimally invasive group (3/87 patients; mean follow-up, 15.2±5.9 months) and 3.9% in the open group (3/77 patients; mean follow-up, 18.2±6.0 months). The overall rates of reoperations were 1.5% in the minimally invasive group (3/196 patients; mean follow-up, 21.9±6.8 months) and 2.1% in the open group (4/194 patients; mean follow-up, 21.9±6.8 months). Low-quality evidence revealed that the two groups did not differ significantly in rates of revision (OR, 1.1; 95% CI, 0.3–4.5; p=0.9), removal (OR, 1.3; 95% CI, 0.2–11.8; p=0.8), and reoperation (OR, 0.6; 95% CI, 0.1–3.5; p=0.6) (Fig. 6)
Fig. 6. Comparison of subsequent surgery rates between minimally invasive and open lumbar spinal fusion. Heterogeneity: Removal [τ2=1.204; χ2=2.938, df=2 (p=0.230); I2=31.9%], reoperation [τ2=0.774; χ2=3.965, df=3 (p=0.265); I2=24.3%], revision [τ2=0.000; χ2=1.154, df=3 (p=0.764); I2=0.0%], overall subsequent surgery rates [τ2=0.000; χ2=8.565, df=10 (p=0.574); I2=0.0%]. df, degrees of freedom; OR, odds ratio; CI, confidence interval.
Perioperative outcomes
The detailed perioperative outcome data are summarized in Supplementary Tables 8, 9, and 10 (only online).
Blood loss
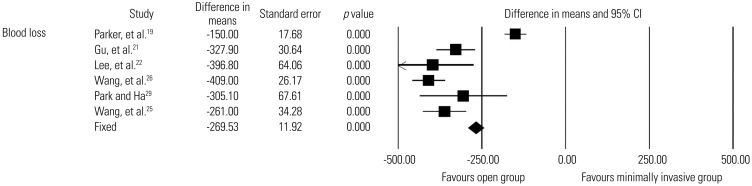
Six studies19,21,22,25,26,29 reported estimated blood loss during surgery, with 265 patients in the minimally invasive group and 259 in the open group. Low-quality evidence indicated that the minimally invasive group had significantly less blood loss than the open group (WMD, 269.5 mL; 95% CI, 246.2–292.9 mL; p<0.0001) (Fig. 7).
Fig. 7. Comparison of blood loss between minimally invasive and open lumbar spinal fusion. Heterogeneity: τ2=0.747; χ2=57.666, df=5 (p<0.0001); I2=91.3%. df, degrees of freedom; CI, confidence interval.
Hospital stay
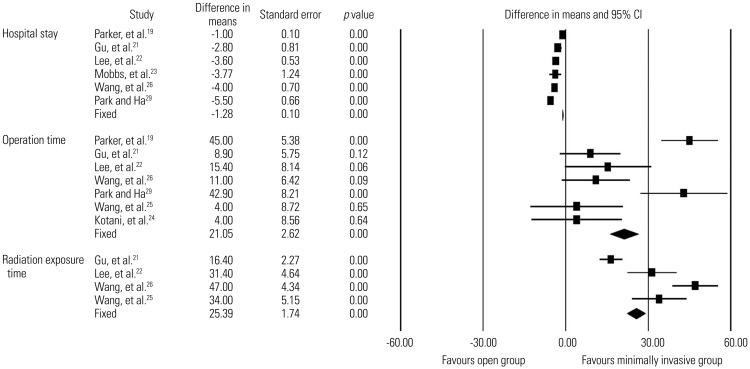
Six studies19,21,22,23,26,29 reported length of hospital stay, with 277 patients in the minimally invasive group and 262 in the open group. Low-quality evidence suggested that the minimally invasive group had a significantly shorter hospital stay than the open group (WMD, 1.3 days; 95% CI, 1.1–1.5 days; p<0.0001)(Fig. 8).
Fig. 8. Comparison of hospital stay, operation time, and radiation exposure time between minimally invasive and open lumbar spinal fusion. Heterogeneity: hospital stay [τ2=0.236; χ2=26.011, df=5 (p<0.0001); I2=80.8%], operation time [τ2=0.297; χ2=40.069, df=6 (p<0.0001); I2=85.0%], and radiation exposure time [τ2=0.240; χ2=14.309, df=3 (p=0.003); I2=79.0%]. df, degrees of freedom; CI, confidence interval.
Operation time
Seven studies19,21,22,24,25,26,29 reported operation time, with 302 patients in the minimally invasive group and 289 in the open group. There was low-quality evidence indicating that the minimally invasive group had a significantly longer operation time than the open group (WMD, 21.0 minutes; 95% CI, 15.9–26.2 minutes; p<0.0001) (Fig. 8).
Radiation exposure time
Four studies21,22,25,26 reported radiation exposure time, with 183 patients in the minimally invasive group and 180 in the open group. Low-quality evidence suggested that the minimally invasive group had a significantly longer radiation exposure time than the open group (WMD, 25.4 seconds; 95% CI, 22.0–28.8 seconds; p<0.0001) (Fig. 8).
DISCUSSION
This meta-analysis highlights low-quality evidence that indicates minimally invasive lumbar spinal fusion for the treatment of adult spondylolisthesis and spondylosis is more effective than open fusion with regard to functional improvement and reduced infection rate in the intermediate term. The two fusion methods showed similar results in terms of pain relief, fusion rate, complications, and subsequent surgeries, although the evidence was low quality. In addition, we noted low-quality evidence indicating that minimally invasive fusion is associated with decreased blood loss and length of hospital stay, but was less advantageous in terms of operation time and radiation exposure time than open fusion.
Although screw- or cage-related nerve root injuries and secondary radiculopathies were reported, most studies indicated resolution of any neurological deficits and pain with subsequent surgery. These complications also showed an equivalent incidence with open fusion. There were no reports of visceral or vascular injury associated with percutaneous pedicle screw instrumentation. Significant reduction of paraspinal muscle injury via minimally invasive surgery and subsequent preservation of trunk muscle performance are thought to result in greater functional improvement, a lower risk of infection, and better perioperative surgical outcomes (less blood loss, quicker recovery, and shorter hospital stay).
To our knowledge, this study is the first systematic review evaluating the efficacy of minimally invasive lumbar spinal fusion exclusively employing percutaneous pedicle screw instrumentation. The strengths of our study include the exhaustive search strategy, reproducible protocols, and strict adherence to systematic review methodology. The use of standardized and validated data collection and extraction tools limited bias and increased inter-rater reliability.
The major limitations of the present study are the lack of RCTs and the small number of included articles. These weaknesses prevented synthesis of higher-quality evidence. In particular, there were very few studies comparing multi-level instrumented fusion in adult spondylosis patients. Overall, the quality of the evidence was “low” in our comparison of primary outcomes between minimally invasive fusion and open fusion. The magnitude of the effect sizes was small. All studies had attrition bias with no reported number of dropouts, and the mean follow-up duration was less than two years.
Another weakness was variation in the type of arthrodesis (e.g., TLIF, PLIF, or PLF) and bone graft material (e.g., local autogenous bone, autogenous iliac crest bone, or synthetic bone extensor). Variation in preoperative diagnosis (e.g., spondylolisthesis, other spondylosis, and mixed diagnoses) and fusion assessment methods was another drawback of this analysis. Computed tomography (CT) to evaluate screw placement was not routinely performed in all studies. The scarcity of CT imaging data may have led to underestimation of screw malposition, implant loosening, implant breakage, and pseudarthrosis in the reviewed studies.
On intermediate-term follow-up, our results showed low-quality evidence that percutaneous pedicle screw instrumented fusion is more effective at improving ODI score, reducing infection rate, and decreasing blood loss and hospital stay, but less effective at reducing operation and radiation exposure time than open fusion. Furthermore, the two methods were comparable with regard to pain relief, fusion rate, complications, and subsequent surgeries based on low-quality evidence. Several methodological flaws and weaknesses limited the reported results. In particular, there were no well-designed RCTs from which to synthesize high-quality evidence.
The ambiguity in these findings could lead to major alterations of the results derived from our analyses and highlights the need for adequately powered RCTs that will assess the long-term efficacy of minimally invasive lumbar spinal fusion. Future studies should compare subgroups based on fusion modality (e.g., TLIF, PLIF, PLF), spine disorder (e.g., spondylolisthesis, other spondylosis, deformity, trauma), and surgery level (e.g., single or multi-level fusion).
Although the findings are limited by insufficient evidence and lack of adequately powered high-quality RCTs to address this gap in evidence, our results support that minimally invasive lumbar spinal fusion is more effective than open fusion for adult spondylolisthesis and other spondylosis in terms of functional improvement, reducing infection rate, and decreasing blood loss and hospital stay.
ACKNOWLEDGEMENTS
The authors thank Hyun-Sun Lim, MS, PhD for her great support of statistical expertise in the data analyses.
Footnotes
The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIALS
Search Strategies for PubMed, Embase, and Cochrane Library
Characteristics of all prospective comparative studies*
Clinical outcome scores in the prospective comparative studies*
Clinical outcome scores in the prospective comparative studies*
Fusion rate in the prospective comparative studies*
Subgroup analysis of fusion rate*
Complications in the prospective comparative studies
Perioperative surgical data in the prospective comparative studies*
Perioperative surgical data
Subgroup analysis of perioperative surgical data according to the fusion methods*
References
- 1.Gaines RW., Jr The use of pedicle-screw internal fixation for the operative treatment of spinal disorders. J Bone Joint Surg Am. 2000;82-A:1458–1476. doi: 10.2106/00004623-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Yuan HA, Garfin SR, Dickman CA, Mardjetko SM. A historical cohort study of pedicle screw fixation in thoracic, lumbar, and sacral spinal fusions. Spine (Phila Pa 1976) 1994;19(20 Suppl):2279S–2296S. doi: 10.1097/00007632-199410151-00005. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen K, Christensen FB, Eiskjaer SP, Hansen ES, Fruensgaard S, Bünger CE. 1997 Volvo Award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine (Phila Pa 1976) 1997;22:2813–2822. doi: 10.1097/00007632-199712150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bjarke Christensen F, Stender Hansen E, Laursen M, Thomsen K, Bünger CE. Long-term functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion: randomized clinical study with a 5-year follow-up. Spine (Phila Pa 1976) 2002;27:1269–1277. doi: 10.1097/00007632-200206150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Sihvonen T, Herno A, Paljärvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine (Phila Pa 1976) 1993;18:575–581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Rantanen J, Hurme M, Falck B, Alaranta H, Nykvist F, Lehto M, et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine (Phila Pa 1976) 1993;18:568–574. doi: 10.1097/00007632-199304000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Foley KT, Gupta SK. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97(1 Suppl):7–12. doi: 10.3171/spi.2002.97.1.0007. [DOI] [PubMed] [Google Scholar]
- 8.Kim DY, Lee SH, Chung SK, Lee HY. Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976) 2005;30:123–129. [PubMed] [Google Scholar]
- 9.Villavicencio AT, Burneikiene S, Roeca CM, Nelson EL, Mason A. Minimally invasive versus open transforaminal lumbar interbody fusion. Surg Neurol Int. 2010;1:12. doi: 10.4103/2152-7806.63905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sclafani JA, Kim CW. Complications associated with the initial learning curve of minimally invasive spine surgery: a systematic review. Clin Orthop Relat Res. 2014;472:1711–1717. doi: 10.1007/s11999-014-3495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y, Ha JW, Lee YT, Sung NY. Minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis and degenerative spondylosis: 5-year results. Clin Orthop Relat Res. 2014;472:1813–1823. doi: 10.1007/s11999-013-3241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Cowley DE. Prostheses for primary total hip replacement. A critical appraisal of the literature. Int J Technol Assess Health Care. 1995;11:770–778. doi: 10.1017/s026646230000920x. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbord RM, Harris RJ, Sterne JA. Updated tests for small-study effects in meta-analyses. Stata J. 2009;9:197–210. [Google Scholar]
- 18.Wang HL, Lü FZ, Jiang JY, Ma X, Xia XL, Wang LX. Minimally invasive lumbar interbody fusion via MAST Quadrant retractor versus open surgery: a prospective randomized clinical trial. Chin Med J (Engl) 2011;124:3868–3874. [PubMed] [Google Scholar]
- 19.Parker SL, Mendenhall SK, Shau DN, Zuckerman SL, Godil SS, Cheng JS, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost-utility analysis. World Neurosurg. 2014;82:230–238. doi: 10.1016/j.wneu.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Zhou Y, Feng Zhang Z, Qing Li C, Jie Zheng W, Liu J. Comparison of the clinical outcome in overweight or obese patients after minimally invasive versus open transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2014;27:202–206. doi: 10.1097/BSD.0b013e31825d68ac. [DOI] [PubMed] [Google Scholar]
- 21.Gu G, Zhang H, Fan G, He S, Cai X, Shen X, et al. Comparison of minimally invasive versus open transforaminal lumbar interbody fusion in two-level degenerative lumbar disease. Int Orthop. 2014;38:817–824. doi: 10.1007/s00264-013-2169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KH, Yue WM, Yeo W, Soeharno H, Tan SB. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21:2265–2270. doi: 10.1007/s00586-012-2281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobbs RJ, Sivabalan P, Li J. Minimally invasive surgery compared to open spinal fusion for the treatment of degenerative lumbar spine pathologies. J Clin Neurosci. 2012;19:829–835. doi: 10.1016/j.jocn.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur Spine J. 2012;21:1171–1177. doi: 10.1007/s00586-011-2114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Minimally invasive or open transforaminal lumbar interbody fusion as revision surgery for patients previously treated by open discectomy and decompression of the lumbar spine. Eur Spine J. 2011;20:623–628. doi: 10.1007/s00586-010-1578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19:1780–1784. doi: 10.1007/s00586-010-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schizas C, Tzinieris N, Tsiridis E, Kosmopoulos V. Minimally invasive versus open transforaminal lumbar interbody fusion: evaluating initial experience. Int Orthop. 2009;33:1683–1688. doi: 10.1007/s00264-008-0687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2009;34:1385–1389. doi: 10.1097/BRS.0b013e3181a4e3be. [DOI] [PubMed] [Google Scholar]
- 29.Park Y, Ha JW. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976) 2007;32:537–543. doi: 10.1097/01.brs.0000256473.49791.f4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search Strategies for PubMed, Embase, and Cochrane Library
Characteristics of all prospective comparative studies*
Clinical outcome scores in the prospective comparative studies*
Clinical outcome scores in the prospective comparative studies*
Fusion rate in the prospective comparative studies*
Subgroup analysis of fusion rate*
Complications in the prospective comparative studies
Perioperative surgical data in the prospective comparative studies*
Perioperative surgical data
Subgroup analysis of perioperative surgical data according to the fusion methods*