Abstract
18F-AV-1451 is a tau PET ligand that has high affinity for paired helical filament tau. However, various off-target bindings unrelated to tau have also been reported. Herein, we report a case of 83-year-old woman, who showed abnormal uptake of AV-1451 that was shown to be subacute infarction. Clinicians should recognize that increased uptake of AV-1451 may be related to stroke.
Keywords: 18F-AV-1451 PET, subacute infarction, tau, blood-brain-barrier
INTRODUCTION
18F-AV-1451 is a recently developed tau PET ligand that has high affinity for paired helical filaments of hyperphosphorylated tau in neurofibrillary tangles. Previous studies showed that 18F-AV-1451 uptake was correlated with pathological tau staging.1,2 However, high AV-1451 retention has also been observed in other conditions unrelated to tau.3,4,5 This ‘off-target binding’ includes the substantia nigra, basal ganglia, and choroid plexus. A recent report also showed incidental AV-1451 retention in the regions of meningiomas, vascular malformations, and remote infarcts.3 Nevertheless, there is little knowledge regarding 18F-AV-1451 PET findings in acute or subacute stage of ischemic stroke patients. Herein, we report a case of abnormally increased uptake of AV-1451 which was shown to be a subacute infarction.
CASE REPORT
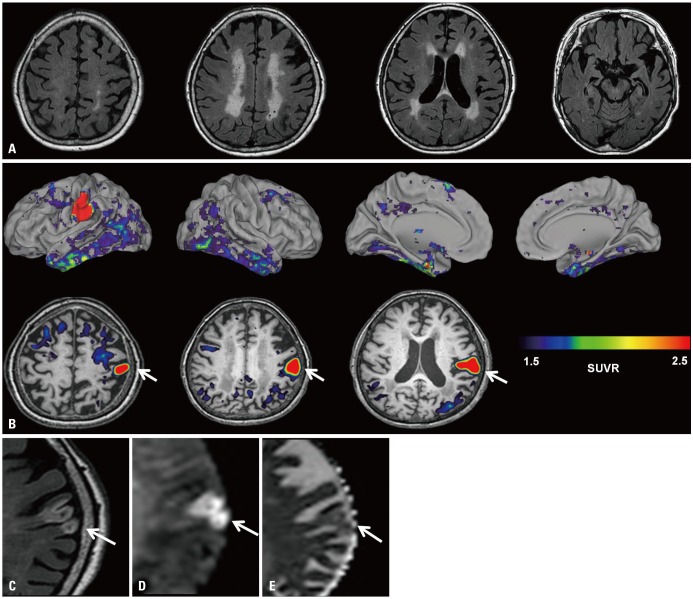
An 83-year-old woman who experienced three years of progressive cognitive decline and apathy visited the Memory Clinic at Samsung Medical Center, Seoul, Korea. She had dyslipidemia and had 9 years of formal education. On neurologic exam, she was alert and showed no focal neurological signs including asymmetric weakness or sensory change. She scored 13 out of 30 on the Mini-Mental State Examination. Neuropsychological testing6 revealed cognitive impairment in multiple domains(language, visuospatial, memory, and frontal-executive dysfunction) which limited her activities of daily living. Brain MRI showed confluent white matter hyperintensities and diffuseatrophy (Fig. 1A). Amyloid PET, assessed with 18F-florbetaben radiotracer, was positive and no asymmetric focal high uptake was observed. Apolipoprotein E genotype was e2/e3. Based on her clinical and imaging findings, we assumed that her dementia was due to mixed etiology: Alzheimer's disease and small vessel disease.
Fig. 1. Brain images of an 83-year old woman. (A) FLAIR showed subcortical white matter hyperintensities and no cortical lesions. (B) 18F-AV-1451 PET, taken 20 days after initial MRI, showed a focal hot uptake of AV-1451 in the left sensory cortex (arrows). SUVR was measured using cerebellar grey matter as the reference region. The patient was re-evaluated with brain MRI 30 days after 18F-AV-1451 PET. The left sensory cortical lesion was revealed to be a subacute infarction. (C) Axial FLAIR image shows geographic hyperintensity involving the left primary sensory cortical region (arrow).(D) Diffusion weighted imaging shows restricted diffusion with high signal on the b600 image (arrow) and (E) isointense signal intensity on the apparent diffusion coefficient map (arrow). FLAIR, fluid-attenuated inversion recovery; SUVR, standardized uptake value ratio.
She further underwent 18F-AV-1451 PET to search for distribution of paired helical filament tau. Using cerebellar gray matter as a reference region, we created AV-1451 standardized uptake value ratio images based on mean uptake over 90-110 min post-injection. AV-1451 uptake was observed in the bilateral medial temporal, lateral temporal, and inferior parietal areas, with the overall pattern looking similar to that of Alzheimer's disease. 7,8 However, a focal hot uptake in the left primary sensory cortex was very unusual (Fig. 1B). This finding was unexpected because there were no relevant symptoms or signs and no structural lesions in that area on brain MRI, taken 20 days before the 18F-AV-1451 PET (Fig. 1A). We re-evaluated the brain MRI 30 days after 18F-AV-1451 PET and found a subacute infarction in the left primary sensory cortex (Fig. 1C, D, and E) with no hemorrhagic transformation.
We obtained written informed consent from the patient. This study was approved by the Institutional Review Board of Samsung Medical Center.
DISCUSSION
We report a case of focal high uptake of AV-1451 which was shown to be a subacute infarction. Although 18F-AV-1451 is a tau PET ligand that has high affinity for paired helical filament tau, various off-target bindings unrelated to tau have been reported. 3,4,5,9 The underlying substrate of AV-1451 off-target binding in the substantia nigra, basal ganglia, and choroid plexus are not well understood. Previous studies suggested that AV-1451 binds to neuromelanin containing neurons in the substantia nigra.4 Elevated in vivo AV-1451 uptake in the basal ganglia was observed in elderly individuals, which might be due to iron component or different kinetic profile of the tracer in the basal ganglia.5 Another AV-1451 off-target binding is the choroid plexus and the underlying substrate of tracer's uptake is suggested to be leptomeningeal melanocyte or calcifications. 4,5 AV-1451 uptake in the regions of meningiomas, vascular malformations, and remote infarcts3 has recently been reported, and the authors suggested that increased vascular permeability was responsible for tracer retention.
The reason why AV-1451 uptake was increased in ischemic stroke can be explained in several ways. AV-1451 might bind to tau in the area of the ischemic insult as a result of ischemic neuronal damage, apoptosis, or gliosis, which are reported to be associated with tau hyperphosphorylation.10 Alternatively, increased uptake of AV-1451 might be due to increased tracer leakage related to blood-brain-barrier dysfunction11 as it becomes permeable immediately after hypoxia-ischemia.12
Our present case is in line with a recent report showing two cases of elevated AV-1451 uptake in chronic infarction regions,3 and the author suggested that vascular permeability might have contributed to AV-1451 retention. Similarly, a study of Pittsburgh compound B (PiB) PET showed focal PiB retention in subacute ischemic stroke regions, and the authors suggested leakage of PiB across the blood-brain barrier damaged by the ischemic process and reperfusion injury as a possible mechanism.13,14 However, controversies exist. In a larger study of subacute ischemic stroke patients, there was no significant increase of PiB uptake in the infarct area.14
There are several limitations in this case report. First, the interval between AV-1451 and brain MRI was quite long and we might have missed the acute stage of infarction. Second, we lacked pathology data to confirm the exact etiology of the AV-1451 uptake. Therefore, our findings need to be further examined in a group of patients with ischemic stroke along with pathology data.
Nevertheless, we believe that this case is noteworthy because this is the first report to show increased AV-1451 uptake in a recent infarction area. We suggest that one should be cautious in the interpretation of increased AV-1451 uptake and that ischemic stroke should be considered as a possible cause.
ACKNOWLEDGEMENTS
This research was supported by National Research Foundation of Korea (NRF) grant funded by the Korea government(MSIP) (NRF-2015R1C1A2A01053281); the Original Technology Research Program for Brain Science through the NRF funded by the Korean government (MSIP) (2014M3C7A1064752); an NRF grant funded by the Korea government (MSIP) (NRF-2017R1A2B2005081); Research of Korea Centers for Disease Control and Prevention (2017-ER6203-00); and the Korea Ministry of Environment (MOE) as “the Environmental Health Action Program” (Grant Number: 1485014533).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Cho H, Choi JY, Hwang MS, Kim YJ, Lee HM, Lee HS, et al. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann Neurol. 2016;80:247–258. doi: 10.1002/ana.24711. [DOI] [PubMed] [Google Scholar]
- 2.Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia C, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J Alzheimers Dis. 2014;38:171–184. doi: 10.3233/JAD-130098. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart SN, Ayakta N, Winer JR, La Joie R, Rabinovici GD, Jagust WJ. Elevated 18F-AV-1451 PET tracer uptake detected in incidental imaging findings. Neurology. 2017;88:1095–1097. doi: 10.1212/WNL.0000000000003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. doi: 10.1186/s40478-016-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquié M, Verwer EE, Meltzer AC, Kim SJW, Agüero C, Gonzalez J, et al. Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson's case. Acta Neuropathol Commun. 2017;5:75. doi: 10.1186/s40478-017-0482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25:1071–1076. doi: 10.3346/jkms.2010.25.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139(Pt 5):1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain. 2015;138(Pt 5):1370–1381. doi: 10.1093/brain/awv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen Y, Yang S, Liu R, Simpkins JW. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain. 2004;1022:30–38. doi: 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Muñoz Maniega S, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 12.Petito CK. Early and late mechanisms of increased vascular permeability following experimental cerebral infarction. J Neuropathol Exp Neurol. 1979;38:222–234. doi: 10.1097/00005072-197905000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ly JV, Rowe CC, Villemagne VL, Zavala JA, Ma H, Sahathevan R, et al. Subacute ischemic stroke is associated with focal 11C PiB positron emission tomography retention but not with global neocortical Aβ deposition. Stroke. 2012;43:1341–1346. doi: 10.1161/STROKEAHA.111.636266. [DOI] [PubMed] [Google Scholar]
- 14.Sahathevan R, Linden T, Villemagne VL, Churilov L, Ly JV, Rowe C, et al. Positron emission tomographic imaging in stroke: crosssectional and follow-up assessment of amyloid in ischemic stroke. Stroke. 2016;47:113–119. doi: 10.1161/STROKEAHA.115.010528. [DOI] [PMC free article] [PubMed] [Google Scholar]