Abstract
A tetra-manganese cluster in the photosystem II (PSII) pigment-protein complex plays a critical role in the photosynthetic oxygen evolution process. PsbY, a small membrane-spanning polypeptide, has recently been suggested to provide a ligand for manganese in PSII (A.E. Gau, H.H. Thole, A. Sokolenko, L. Altschmied, R.G. Herrmann, E.K. Pistorius [1998] Mol Gen Genet 260: 56–68). We have constructed a mutant strain of the cyanobacterium Synechocystis sp. PCC 6803 with an inactivated psbY gene (sml0007). Southern-blot and polymerase chain reaction analysis showed that the mutant had completely segregated. However, the ΔpsbY mutant cells grew normally under photoautotrophic conditions. Moreover, growth of the wild-type and mutant cells were similar under high-light photoinhibition conditions, as well as in media without any added manganese, calcium, or chloride, three required inorganic cofactors for the oxygen-evolving complex of PSII. Analysis of steady-state and flash-induced oxygen evolution, fluorescence induction, and decay kinetics, and thermoluminescence profiles demonstrated that the ΔpsbY mutant cells have normal photosynthetic activities. We conclude that the PsbY protein in Synechocystis 6803 is not essential for oxygenic photosynthesis and does not provide an important binding site for manganese in the oxygen-evolving complex of PSII.
During oxygenic photosynthesis in plants, algae, and cyanobacteria, two reaction-center-containing integral membrane protein complexes, photosystem I (PSI) and photosystem II (PSII), are involved in the initial steps of the conversion of solar energy into usable chemical energy. Among them, PSII, a large-pigment protein, mediates electron transfer from water to plastoquinones, with simultaneous evolution of molecular oxygen. The process of water oxidation takes place in the lumen of thylakoid and is catalyzed by the oxygen-evolving complex (OEC) of PSII. Three inorganic ions, manganese, calcium, and chloride, are the known cofactors of the OEC. However, their locations in PSII and the polypeptides that coordinate these ions remain unclear (Hankamer and Barber, 1997).
Isolated photoactive PSII complexes may contain as many as 22 polypeptides (Debus, 1992; Hankamer and Barber, 1997). Many of them have been suggested to constitute the OEC. The most probable candidate is the PSII reaction center protein D1 (Boerner et al., 1992; Nixon and Diner, 1992; Nixon et al., 1992; Chu et al., 1994a, 1994b, 1995; Whitelegge et al., 1995). Recent studies in spinach and tobacco have raised the possibility that the product of the newly identified psbY gene provides a binding site for the manganese cofactor (the manganese cluster) (Gau et al., 1995, 1998). The PsbY protein, also known as an L-Arg-metabolizing enzyme, was first isolated from a calcium-chloride-washed BBY (Berthold et al., 1981) PSII-enriched membrane preparation from spinach (Gau et al., 1995). This protein has also been shown to contain a redox-active group and to require manganese for its Arg-metabolizing activity (Gau et al., 1995).
In two higher plants, spinach (Gau et al., 1998) and Arabidopsis (Mant and Robinson, 1998), the nuclear-encoded PsbY protein is initially synthesized as an approximately 20-kD polyprotein precursor, and subsequently undergoes a number of cleavages that result in a heterodimeric form of two small polypeptides, PsbY-A1 and PsbY-A2, embedded in the thylakoid membrane. Each of these polypeptides has one membrane-spanning domain, with its N terminus exposed in the lumen, and C terminus in the stroma. It has been proposed that two Trp residues, one on each of the PsbY subunits, form an o-quinoid complex that provide ligands for the manganese cluster in PSII (Gau et al., 1998). Comparison of amino acid sequences has revealed the presence of psbY homologs in cyanelles (orf8), plastomes of red algae and diatoms (ycf32), and in the cyanobacterium Synechocystis sp. PCC 6803 (sml0007) (Gau et al., 1998).
The sequence of the PsbY protein in Synechocystis 6803 (sml0007 gene product) shares 23% identity and 46% similarity with that of the PsbY-A1 protein from spinach (Gau et al., 1998). In view of the proposed important role of the PsbY protein in the form and function of the manganese cluster in PSII, in this study we generated a targeted interruption mutation in the sml0007 open reading frame (ORF) in Synechocystis 6803. Our data showed that the mutant strain containing the disrupted gene had completely segregated. However, using various characterization methods, we could not detect any significant difference in growth or photosynthetic properties of the mutant and the wild-type strains. Our conclusion is that the PsbY protein does not play an essential role in the photosynthetic water oxidation process catalyzed by PSII.
MATERIALS AND METHODS
Bacterial Strains and Culture Conditions
The following Synechocystis strains were used: the Glc-tolerant wild-type PCC 6803, ΔpsbY (this study) and ΔCK (Bartsevich and Pakrasi, 1996). Cyanobacterial cells were grown in the BG11 medium (Allen, 1968) at 30°C and under 50 μmol m−2 s−1 white fluorescent light. Unless indicated otherwise, all media were supplemented with 5 mm Glc. The medium for the ΔpsbY mutant was also supplemented with 10 μg/mL gentamycin, and that for the ΔCK mutant with 20 μg/mL kanamycin. The BG11 medium without manganese was prepared as described by Bartsevich and Pakrasi (1996). For growth of cyanobacteria under starvation conditions for manganese, calcium, and chloride ions, BG11 medium was prepared by omitting manganese chloride, calcium chloride, and ferric ammonium citrate, and adding 10 μm ferric nitrate and 20 μm citric acid. Growth of Synechocystis cells was quantified by light scattering at 730 nm on a spectophotometer (model DW2000, SLM-Aminco, Urbana, IL).
The Escherichia coli strain XL1-Blue (F'::Tn10 proA+B+ lacIq Δ(lacZ)M15/recA1 endA1 gyrA96 (Nalr) thi hsdR17 (rk−mk+) supE44 relA1 lac) and the plasmid pCR2.1 (Invitrogen, Carlsbad, CA) were used in all genetic cloning experiments. E. coli cells were grown at 37°C in the Luria-Bertani medium (Sambrook et al., 1989) supplemented with 100 μg/mL ampicillin or 30 μg/mL gentamycin when necessary.
DNA Manipulation and Genetic Transformation
Basic DNA manipulation and Southern-blot analysis were performed according to the method of Sambrook et al. (1989). Enzymes used for recombinant DNA techniques were from New England Biolabs (Beverly, MA). KlenTaq polymerase used for PCR amplification was obtained from W. Barnes (Washington University School of Medicine, St. Louis). Oligonucleotides were synthesized by Life Technologies (Cleveland). [α-32P]dCTP and GeneScreen Plus nylon membrane for Southern hybridization were from New England Nuclear (Boston). Isolation of chromosomal DNA from Synechocystis 6803 cells and transformation of Synechocystis 6803 were performed essentially as described by Williams (1988).
Measurement of Oxygen Evolution
A Clark-type oxygen electrode was used to determine the rates of photosynthetic electron transport as described elsewhere (Mannan and Pakrasi, 1993). Synechocystis cells were harvested during the mid- to late-exponential growth phases and resuspended in fresh BG11 medium. Samples were adjusted to a final chlorophyll a concentration of 5 μg/mL, as measured in methanol extracts (Lichtenthaler, 1987). Whole-chain electron transport rates were measured in the presence of 1 mm sodium bicarbonate, whereas PSII-mediated rates were measured in the presence of 0.5 mm 2,6-dichloro-p-benzoquinone (Eastman-Kodak, Rochester, NY) and 1 mm K3FeCN6 (Sigma, St. Louis).
Flash-induced oxygen yield was measured at room temperature on a home-built, bare platinum, Joliot-type electrode, and recorded on a Gateway 2000 computer (Gateway, North Sioux City, SD). The harvested cells were resuspended in HN buffer (10 mm HEPES, pH 7.1, and 30 mm NaCl) at a 6 μg/mL chlorophyll concentration, measured in intact cells as described by Arnon et al. (1974), and 1-mL aliquots were centrifuged to form uniform layers of cell pellet on the electrode surface. After 5 min of dark incubation, cells were exposed to a series of 25-μs saturating flashes applied at 4 Hz.
Measurement of Chlorophyll Fluorescence and Thermoluminescence
Time-based fluorescence measurements were performed on a dual-modulation kinetic fluorometer (model FL-100, Photon Systems Instruments, Brno, Czech Republic) interfaced with a Gateway 2000 computer. In all experiments, the duration of the measuring flashes was 3 μs and the measurements were performed at room temperature. For sample preparation, harvested cells were resuspended in fresh BG11 medium and adjusted to 2 μg chlorophyll/mL. In the fluorescence induction experiment, the duration of each actinic flash was 5 μs and the light intensity used was one-fifth of the saturating amount.
To determine the kinetics of charge recombination between QA− and P680+, cells were dark-incubated for 10 min in the presence of 0.3 mm phenyl-p-benzoquinone (Sigma) and 1 mm K3Fe(CN)6 to fully oxidize QA, followed by 1 min of incubation in the presence of 40 μm dichlorophenyl-dimethylurea (DCMU) (Sigma), and the time course of emitted fluorescence was determined following a single saturating actinic flash. To measure their relative PSII contents, cyanobacterial cells were dark-incubated for 10 min in the presence of 0.3 mm phenyl-p-benzoquinone and 1 mm K3Fe(CN)6 and for 1 min in 40 μm DCMU (Chu et al., 1994a). Twenty-micromolar hydroxylamine was then added and fluorescence was recorded within 20 s.
Measurements of thermoluminescence from intact cyanobacterial cells were performed as described in Tal et al. (1999).
RESULTS
Insertional Inactivation of the psbY Gene of Synechocystis 6803
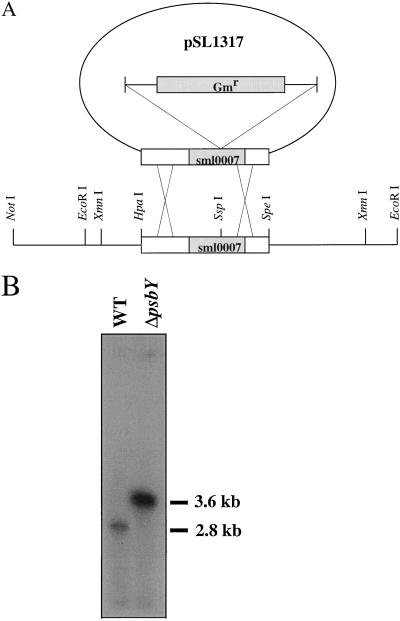
As described earlier, the sml0007 ORF in Synechocystis 6803 corresponds to the psbY gene in higher plants (Gau et al., 1998). To inactivate this gene, we first used two synthetic oligonucleotides (5′-AGGCCGCAATGGAAGACATA-3′ and 5′-ATTCGGCCAAA ATCTCCGTC-3′) for PCR amplification from Synechocystis 6803 chromosomal DNA of an 871-bp fragment that included the sml0007 ORF. An HpaI-SpeI fragment of this PCR product was then cloned into the pCR2.1 plasmid digested with EcoRV and SpeI enzymes (Fig. 1A). The donor plasmid (pSL1317) for insertional inactivation of the psbY gene was generated by inserting an 850-bp SmaI fragment containing a gentamycin-resistance gene cassette (Schweizer, 1993) at a SspI site in the middle of the sml0007 ORF. Wild-type Synechocystis 6803 strain was transformed with pSL1317 and the desired mutant was selected on the basis of gentamycin resistance.
Figure 1.
Construction of a ΔpsbY mutant strain of Synechocystis 6803. A, Scheme for the insertional inactivation of the psbY gene. A 0.85-kb gentamycin-resistance cassette was inserted at a SspI site in the middle of the sml0007 ORF. Wild-type (WT) cells were transformed with this construct to generate the ΔpsbY strain. B, Southern-blot analysis of XmnI-digested chromosomal DNA from wild-type and ΔpsbY cells probed with a 32P-labeled PCR product corresponding to the sml0007 ORF.
Disruption of the sml0007 ORF and segregation of the mutant strain were confirmed by PCR (data not shown) and Southern-blot analysis (Fig. 1B). On the Southern blot, a single hybridizing 2.8-kb band was observed with DNA from the wild-type cells. In contrast, only a 3.6-kb band was seen with DNA from the ΔpsbY mutant strain, indicating that the ΔpsbY mutant had completely segregated.
Growth and Photosynthetic Properties of the ΔpsbY Mutant Strain
As shown in Table I, under 50 μmol m−2 s−1 light intensity at 30°C, both photoautotrophic and photoheterotrophic growth rates of the ΔpsbY mutant strain were not significantly different from that of the wild-type cells. Moreover, under high-light conditions (200 μmol m−2 s−1) or in media depleted of manganese, calcium, and chloride ions, this mutant strain grew at rates similar to those of the wild-type cells (data not shown). Thus, the absence of the PsbY protein did not affect photosynthetic growth of Synechocystis 6803 cells under “normal” and various “stressful” conditions.
Table I.
Growth and photosynthetic properties of wild-type and ΔpsbY strains
| Wild Type | ΔpsbY | |
|---|---|---|
| Doubling time (h) | ||
| In BG11 | 20 ± 2 | 18 ± 3 |
| In BG11 + Glc | 13 ± 1 | 11 ± 2 |
| Electron transfer ratesa (μmol O2 mg−1 chlorophyll h−1) | ||
| Whole chain (H2O–CO2) | 280 ± 40 | 260 ± 60 |
| PSII (H2O–DCBQb/FeCNc) | 660 ± 160 | 560 ± 60 |
| Relative PSII contentd | 100% | 82% |
Each value is the mean ± sd of at least three independent measurements.
Determined by polarographic measurements of rates of oxygen evolution.
DCBQ, 2,6-Dichloro-p-benzoquinone.
FeCN, K3Fe(CN)6.
Determined as the maximum flash-induced fluorescence yield normalized to the initial level of fluorescence, (Fmax − F0)/F0, in the presence of 20 mm hydroxylamine and 40 μm DCMU.
Measurements of steady-state oxygen evolution demonstrated that the rates of whole-chain or PSII-mediated light-induced electron-transfer reactions were not significantly different between wild-type and ΔpsbY mutant cells (Table I). In addition, the relative amount of active PSII centers in the ΔpsbY mutant was only slightly lower than that in the wild-type strain (Table I). These data demonstrated that the disruption of the psbY gene did not significantly affect the function of PSII in Synechocystis 6803.
Kinetics of Fluorescence Induction
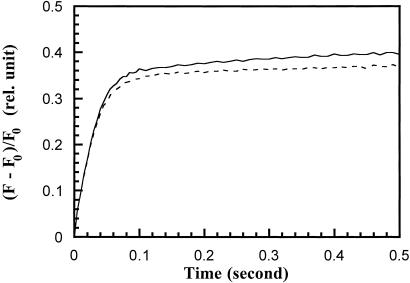
Measurements of chlorophyll fluorescence kinetics provide a sensitive and noninvasive assay to monitor photosynthetic activities in vivo. In the absence of other fluorescence quenching species, the amount of such emitted fluorescence is largely proportional to the level of QA− accumulation (Diner, 1998). The kinetics of fluorescence induction, therefore, reflect the ability of PSII to catalyze electron transfer from water to QA. As shown in Figure 2, there was no detectable difference in the kinetics of fluorescence induction between wild-type and ΔpsbY strains. We conclude that there is no significant change in the electron-transfer reactions on the donor and acceptor sides of PSII as a consequence of the inactivation of the psbY gene in Synechocystis 6803.
Figure 2.
Kinetics of QA− formation in wild-type (----) and ΔpsbY (——) cells. Cells were dark-incubated for 5 min before the application of nonsaturating light pulses at 200 Hz. The amounts of emitted fluorescence (F) were normalized to the initial fluorescence level (F0).
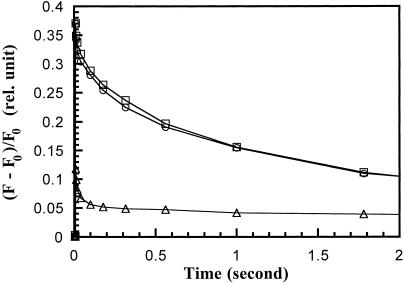
Because the PsbY protein in higher plants has been suggested to play an important role in providing ligands for the manganese cluster of PSII (Gau et al., 1998), we performed additional experiments to specifically examine the functions of the donor side of PSII. In particular, we measured the kinetics of fluorescence decay in the presence of 40 μm DCMU. Because DCMU blocks electron transfer from QA to QB, the predominant path for the reoxidation of QA− is by charge recombination with the donor side (Diner, 1998). It is known that in mutants with an impaired PSII donor side, the decay of fluorescence under these conditions is significantly faster. As shown in Figure 3, both wild-type and ΔpsbY cells exhibited nearly identical kinetics of charge recombination following a saturating flash, confirming the previous result that the disruption of the psbY gene did not influence the activity of the manganese cluster in donating electrons to P680. In contrast, the ΔCK mutant strain, grown in the absence of added manganese, exhibited a 10-fold increase in the rate of this decay kinetics. These results are expected for this mutant since it has a nonfunctional ABC transporter for manganese (Bartsevich and Pakrasi, 1996). When grown in a manganese-deficient medium, the ΔCK cells are depleted of manganese, and the assembly of the manganese cluster in the PSII complexes is presumably affected.
Figure 3.
Kinetics of charge recombination between QA− and P680+ for wild-type (○), ΔpsbY (□), and ΔCK (▵) cells. Cells were dark-incubated for 10 min in the presence of 0.3 mm phenyl-p-benzoquinone and 1 mm K3Fe(CN)6. Then, 40 μm DCMU was added 1 min prior to the application of a single saturating flash. The decay of fluorescence (F) was measured and normalized to the initial fluorescence level (F0). The wild-type and ΔpsbY cells were grown in BG11 medium, whereas the ΔCK cells were grown in BG11 without any added manganese.
Measurements of Flash-Induced Oxygen Evolution
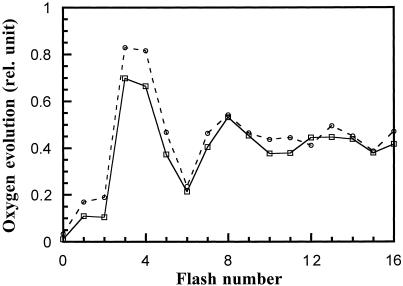
The activities of the donor side of PSII can also be assayed by examining the oscillation pattern of flash-induced oxygen evolution from intact cyanobacterial cells (Burnap et al., 1992). As shown in Figure 4, the pattern of flash-induced oxygen evolution from the ΔpsbY mutant cells had a period of four, and was almost identical to that from the wild-type cells. Therefore, we conclude that the PSII donor side in the ΔpsbY mutant remained intact.
Figure 4.
Flash-induced oxygen evolution from wild-type (dashed line) and ΔpsbY (solid line) cells. Cells were dark-incubated for 5 min and then illuminated with saturating single-turnover flashes at 4 Hz. Oxygen concentration was measured on a bare platinum electrode, and the maximum level of oxygen following each flash was recorded.
Analysis of Thermoluminescence
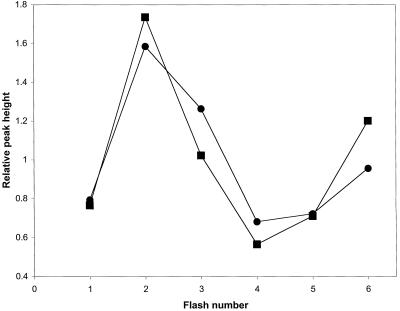
The thermoluminescence emission profile is a function of the activation energy for back electron transfer in PSII (Tal et al., 1999, and refs. therein). The peak of S2,3/QB− thermoluminescence emission from the wild-type cells was at 27.5°C ± 1°C, whereas it was at 29.7°C ± 1°C from the ΔpsbY mutant cells. In the presence of DCMU, the thermoluminescence emission resulting from recombination of the S2/QA− states was downshifted to 10°C in both strains. The oscillation of the peak height with the number of flashes (Fig. 5) was similar in the two strains, implying that the ratio of S0/S1 states after dark adaptation and the transition between the S states were not altered in the ΔpsbY mutant strain.
Figure 5.
Oscillation pattern of thermoluminescence B-band from wild-type (●) and ΔpsbY (▪) cells. One-hundred-microliter samples containing 20 μg of chlorophyll were dark-adapted for 3 min at 30°C prior to cooling. During the cooling of the samples to −25°C, groups of one to six saturating single-turnover flashes were applied at 0°C. Thermoluminescence emission was measured while warming the samples at a constant rate of 0.7°C/s. The B-band emission peaks for both wild-type and ΔpsbY cells were at 30°C. x axis, Number of excitation flashes; y axis, relative B-band peak intensity calculated as YX/Σ(1–6)Y.
DISCUSSION
Light-induced evolution of dioxygen from water is a unique reaction catalyzed by PSII. However, in the absence of a crystal structure of this large integral membrane protein complex, considerable uncertainty remains about the identity of the ligands for the tetra-manganese cluster, as well as the calcium and chloride ions in the OEC of PSII. Based on binding data for 14C-labeled amines, Barry and coworkers have recently suggested that a quinoid-type redox group may be involved in coordinating manganese in the catalytic site for water oxidation in PSII (Oulette et al., 1998). Since the PsbY protein has been shown to require manganese for its L-Arg oxidation activity (Gau et al., 1995), Pistorius and coworkers have recently postulated that a Trp residue in the membrane-spanning domain of this protein is a key component of an o-quinoid type structure that acts as a ligand for one or more manganese atoms in the manganese cluster in OEC. To investigate such an important role of the PsbY protein in PSII function, we engineered a targeted inactivation mutant strain ΔpsbY of Synechocystis 6803.
The data presented herein, however, conclusively demonstrate that the PsbY protein does not play a vital role in photosynthetic growth and PSII activity of Synechocystis 6803. Both steady-state (Table I) and flash-induced oxygen evolution (Fig. 4) assays showed that in the absence of the PsbY protein, PSII complex assembles in this cyanobacterium and can mediate light-induced oxidation of water. Moreover, measurements of room-temperature fluorescence induction (Fig. 2) and decay of flash-induced fluorescence emission (Fig. 3) indicated that both forward and backward reactions through PSII in the mutant cells had kinetics similar to those in the wild-type cells. Thermoluminescence measurements (Fig. 5) also demonstrated that the ΔpsbY mutation did not affect donor- or acceptor-side functions of PSII. It is noteworthy that the PsbY protein is also not essential for growth of Synechocystis 6803 cells under high light intensity or under nutritional limitations for manganese, calcium, and chloride ions.
The data presented in this manuscript raise three possibilities regarding the functional role of the PsbY protein. First, it may be a component of the photosynthetic apparatus, but either has a nonessential function or an important function under abnormal growth conditions not used in this study. Second, because the psbY gene in Synechocystis 6803 was identified by sequence homology with the spinach and Arabidopsis genes encoding the PsbY protein, it is possible that the cyanobacterial gene is not a true ortholog of the psbY gene in higher plants. Moreover, there are certain distinct differences in the form and function of PSII in cyanobacteria and higher plants (Pakrasi, 1995). For example, the psbO gene product, a thylakoid-lumen-localized protein, is essential for PSII function in chloroplasts, whereas it is dispensable for the function of this protein complex in cyanobacteria (Burnap et al., 1992). Thus, the PsbY protein in higher plants may have some important functional role in PSII. Third, it remains a distinct possibility that the PsbY protein is not a component of PSII in vivo, even though the isolated PsbY protein from spinach may appear to interact with the PSII complex in vitro (Gau et al., 1998). In fact, this possibility cannot be ruled out for a number of other low-molecular-weight polypeptides of unknown functions (e.g. PsbI, PsbM, PsbN, PsbT, PsbW, and PsbU) (Hankamer and Barber, 1997). The presence and function of such polypeptides in PSII will be better understood when a detailed three-dimensional structure of PSII becomes available.
ACKNOWLEDGMENTS
We thank Dr. V.V. Bartsevich for the ΔCK mutant strain, and Wing-on Ng for collegial discussions.
Footnotes
This work was supported by grants from the National Institutes of Health (to H.B.P.) and the International Human Frontier Science Program (to H.B.P. and I.O.). H.B.P.'s visit to Jerusalem was supported by a Lady Davis Visiting Professorship at the Hebrew University. M.M. was partially supported by a Development and Promotion of Science and Technology Talented Students scholarship from the government of Thailand and by a summer research fellowship from the Howard Hughes Medical Institute.
LITERATURE CITED
- Allen MM. Simple conditions for growth of unicellular blue-green algae on plates. J Phycol. 1968;4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974;357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Bartsevich VV, Pakrasi HB. Manganese transport in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1996;271:26057–26061. doi: 10.1074/jbc.271.42.26057. [DOI] [PubMed] [Google Scholar]
- Berthold DA, Babcock GT, Yocum CF. A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett. 1981;134:231–234. [Google Scholar]
- Boerner RJ, Nguyen AP, Barry BA, Debus RJ. Evidence from directed mutagenesis that aspartate 170 of the D1 polypeptide influences the assembly and/or stability of the manganese cluster in the photosynthetic water-splitting complex. Biochemistry. 1992;31:6660–6672. doi: 10.1021/bi00144a005. [DOI] [PubMed] [Google Scholar]
- Burnap RL, Shen JR, Jursinic PA, Inoue Y, Sherman LA. Oxygen yield and thermoluminescence characteristics of a cyanobacterium lacking the manganese-stabilizing protein of photosystem II. Biochemistry. 1992;31:7404–7410. doi: 10.1021/bi00147a027. [DOI] [PubMed] [Google Scholar]
- Chu HA, Nguyen AP, Debus RJ. Site-directed photosystem II mutants with perturbed oxygen-evolving properties. 1. Instability or inefficient assembly of the manganese cluster in vivo. Biochemistry. 1994a;33:6137–6149. doi: 10.1021/bi00186a013. [DOI] [PubMed] [Google Scholar]
- Chu HA, Nguyen AP, Debus RJ. Site-directed photosystem II mutants with perturbed oxygen-evolving properties. 2. Increased binding or photooxidation of manganese in the absence of the extrinsic 33-kDa polypeptide in vivo. Biochemistry. 1994b;33:6150–6157. doi: 10.1021/bi00186a014. [DOI] [PubMed] [Google Scholar]
- Chu HA, Nguyen AP, Debus RJ. Amino acid residues that influence the binding of manganese or calcium to photosystem II. 1. The lumenal interhelical domains of the D1 polypeptide. Biochemistry. 1995;34:5839–5858. doi: 10.1021/bi00017a016. [DOI] [PubMed] [Google Scholar]
- Debus RJ. The manganese and calcium ions of photosynthetic oxygen evolution. Biochim Biophys Acta. 1992;1102:269–352. doi: 10.1016/0005-2728(92)90133-m. [DOI] [PubMed] [Google Scholar]
- Diner BA. Application of spectroscopic techniques to the study of photosystem II mutations engineered in Synechocystis and Chlamydomonas. Methods Enzymol. 1998;297:337–360. [Google Scholar]
- Gau AE, Thole HH, Pistorius EK. Isolation and partial characterization of a manganese requiring l-arginine metabolizing enzyme being present in photosystem II complexes of spinach and tobacco. Z Naturforsch. 1995;50c:638–651. [Google Scholar]
- Gau AE, Thole HH, Sokolenko A, Altschmied L, Herrmann RG, Pistorius EK. PsbY, a novel manganese-binding, low-molecular-mass protein associated with photosystem II. Mol Gen Genet. 1998;260:56–68. doi: 10.1007/s004380050870. [DOI] [PubMed] [Google Scholar]
- Hankamer B, Barber J. Structure and membrane organization of photosystem II in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:641–671. doi: 10.1146/annurev.arplant.48.1.641. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–383. [Google Scholar]
- Mannan RM, Pakrasi HB. Dark heterotrophic growth conditions result in an increase in the content of photosystem II units in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Plant Physiol. 1993;103:971–977. doi: 10.1104/pp.103.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant A, Robinson C. An Arabidopsis cDNA encodes an apparent polyprotein of two non-identical thylakoid membrane proteins that are associated with photosystem II and homologous to algal ycf32 open reading frames. FEBS Lett. 1998;423:183–188. doi: 10.1016/s0014-5793(98)00089-1. [DOI] [PubMed] [Google Scholar]
- Nixon PJ, Diner BA. Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry. 1992;31:942–948. doi: 10.1021/bi00118a041. [DOI] [PubMed] [Google Scholar]
- Nixon PJ, Trost JT, Diner BA. Role of the carboxy terminus of polypeptide D1 in the assembly of a functional water-oxidizing manganese cluster in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: assembly requires a free carboxyl group at C-terminal position 344. Biochemistry. 1992;31:10859–10871. doi: 10.1021/bi00159a029. [DOI] [PubMed] [Google Scholar]
- Ouellette AJA, Anderson LB, Barry BA. Amine binding and oxidation at the catalytic site for photosynthetic water oxidation. Proc Natl Acad Sci USA. 1998;95:2204–2209. doi: 10.1073/pnas.95.5.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi HB. Genetic analysis of the form and function of photosystem I and photosystem II. Annu Rev Genet. 1995;29:755–776. doi: 10.1146/annurev.ge.29.120195.003543. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schweizer HP. Small broad-host-range gentamycin resistance gene cassettes for site- specific insertion and deletion mutagenesis. Biotechniques. 1993;15:831–834. [PubMed] [Google Scholar]
- Tal S, Keren N, Hirschberg Y, Ohad I. Photosystem II activity and turnover of the D1 protein are impaired in the psbA Y112L mutant of Synechocystis sp. PCC 6803. J Photochem Photobiol B Biol. 1999;48:120–126. doi: 10.1016/s1011-1344(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Whitelegge JP, Koo D, Diner BA, Domian I, Erickson JM. Assembly of the photosystem II oxygen-evolving complex is inhibited in psbA site-directed mutants of Chlamydomonas reinhardtii: aspartate 170 of the D1 polypeptide. J Biol Chem. 1995;270:225–235. doi: 10.1074/jbc.270.1.225. [DOI] [PubMed] [Google Scholar]
- Williams JGK. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988;167:766–778. [Google Scholar]