Abstract
Objectives
To investigate the relationship between insomnia (INS) combined with chronic musculoskeletal pain (MSP) in postmenopausal women and its characteristics regarding MSP, menopausal and mood symptoms, sleep and quality of life (QOL).
Methods
A cross-sectional control study in 4 groups of postmenopausal women: control (n = 15), MSP (n = 15), INS (n =15) and INS + MSP (n = 17). Sixty-two participants completed questionnaires and had blood collected, and 43 underwent polysomnography.
Results
INS was associated with increased anxiety (P = 0.04) and sleep fragmentation (P = 0.02); worse MSP severity (P = 0.00), MSP interference with daily function (P = 0.00), higher pain intensity at midday (P = 0.02) and menopausal symptoms (P = 0.00); and reduced QOL (P = 0.00). MSP was associated with increased anxiety (P = 0.02) and menopausal symptoms (P = 0.00), and reduced QOL (P = 0.05). In the whole sample, depression symptoms were higher but no statistical differences were found between groups (P = 0.47). Worse QOL was associated with both higher depressive symptoms (P = 0.01) and worse pain interference (P = 0.02)
Conclusions
INS + MSP was related to higher menopausal and anxiety symptoms, more sleep fragmentation and complaints of MSP severity and interference, more pain sites and worse QOL. The presence of INS was associated to more MSP. Sleep management is essential in women who have developed chronic MSP.
Keywords: Insomnia, Menopausal Syndrome, Musculoskeletal pain, Postmenopause, Quality of life, Sleep
Introduction
Menopause is the permanent cessation of the menstrual cycles following the loss of ovarian reproductive function and is a natural physiological process related to aging.1 The postmenopausal period is clinically and retrospectively diagnosed with the following amenorrhea for more than 12 months.
There is evidence of a worsening quality of life (QOL) after menopause, especially in relation to the presence of climacteric symptoms, their frequency and intensity.1 Among the climacteric symptoms that affect QOL are insomnia (INS) and musculoskeletal pain (MSP).
Epidemiological investigations focusing on the postmenopause stage worldwide show an alarming prevalence of MSP2 and INS,3 although the different methods used to evaluate INS and the frequent lack of a clinical diagnosis hamper the comparison among the studies. As women age, both sleep disturbances and pain complaints tend to increase.
INS seems to influence the onset and level of MSP in different populations,4 although most studies focus on both sexes and different age ranges, rather than specifically in postmenopausal women. The co-occurrence of poor sleep quality, pain, and mood symptoms, such as anxiety and depression is evidenced in the literature and presented as a cluster of symptoms in women.5 However, there is no evidence in the literature about the association between chronic MSP complaints and INS in postmenopausal women and its characteristics regarding MSP, menopausal and mood symptoms, sleep and and QOL. We also aimed to examine the predictor factors for menopausal symptoms and QOL.
Materials and Methods
1. Ethical aspects
The present study was approved by the Ethics Committee of the Universidade Federal de São Paulo (CEP/UNIFESP #786.299/2014). Recruitment of general women took place between February 2015 and October 2016.
2. Recruitment criteria
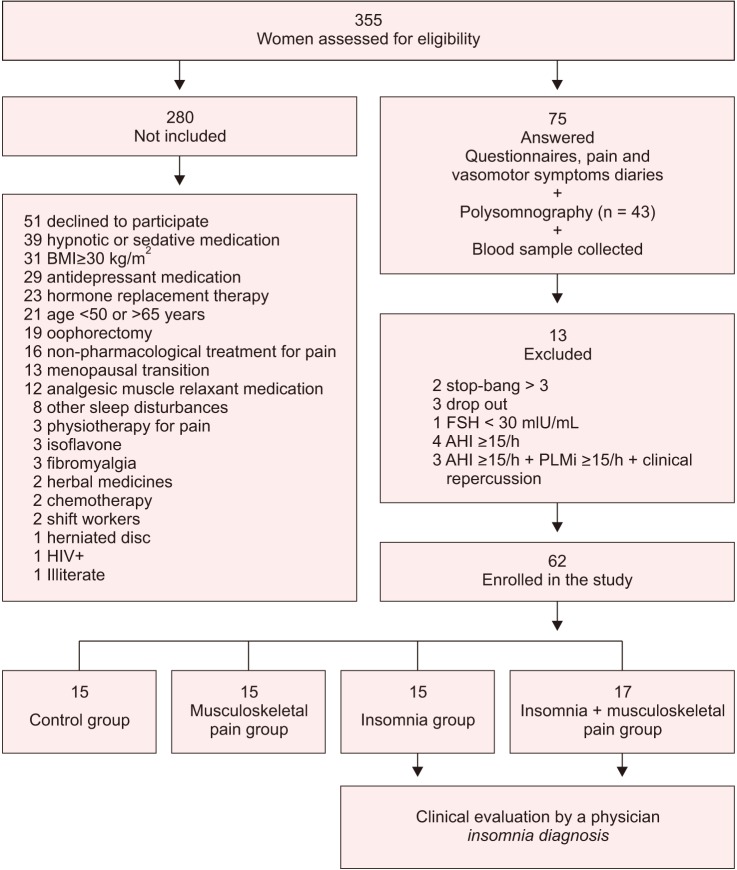
A total of 355 women were assessed for eligibility to take part in the study, with 62 being selected after the evaluation of the inclusion and exclusion criteria (Fig. 1). The inclusion criteria consisted of being women aged 50 to 65 years; present at least 1 year of amenorrhea; follicle-stimulating hormone concentrations ≥30 mIU/mL; undergo a clinical consultation for confirmation of menopause diagnosis; to not have taken hormonal therapy in the previous 6 months; and to not be obese (body mass index [BMI] <30 kg/m2). As exclusion criteria, we considered: high risk for sleep disordered breathing, assessed by the Stop-Bang questionnaire;6 self-report of uncontrolled clinical; major neurologic, orthopedic and rheumatologic diseases, autoimune disorders or conditions that could influence pain; psychiatric disorders; use of psychoactive drugs such as hypnotic, antidepressants, anxiolytics, benzodiazepines and central nervous system stimulants; attending psychotherapy, physiotherapy or treatments for INS or pain; shift work; illiteracy; and the presence of other sleep disorders diagnosed by polysomnography (PSG) exam (narcolepsy, obstructive sleep apnea evidenced by an apnea-hypopnea index (AHI) ≥15/hr, periodic limb movements (PLM) index ≥15/hr and parasomnias).
Fig. 1. The consolidated standards of reporting trials flow chart. BMI: body mass index, HIV+: human immunodeficiency virus-positive, FSH: follicle-stimulant hormone, AHI: apnea-hypopnea index, PLMi: periodic limb movements index.
3. Sociodemographic and clinical assessment
Participants provided sociodemographic data and reported any comorbidities on their first visit. Weight and height were assessed in the first visit, allowing for the calculation of BMI (Kg/m2). All volunteers completed questionnaires upon enrollment. On their second visit, volunteers underwent a laboratory PSG exam on a scheduled night followed by a blood sample collection in the following morning. On the third visit, volunteers with an INS Severity Index ≥157 had a clinical consultation to confirm their INS diagnosis.
4. Postmenopausal assessment
The postmenopausal stage was classified according to Stages of Reproductive Aging Workshop (STRAW) criteria into early, which covers the first 8 years since menopause; and late, which covers the subsequent period.8
5. Questionnaires
Brief Pain Inventory - MSP severity and MSP interference with daily function.
This questionnaire was used to evaluate the severity and interference of MSP.9 The answers are distributed on a numerical scale from 0–10, with 0 representing “does not interfere” and 10 representing “interferes completely”.
6. Pain visual analog scale (VAS) - pain intensity
Participants completed 3 times a day over 10 days a pain diary, composed of an average pain rating on a VAS (0 “no pain”, 10 “worst pain imaginable”).10 VAS scores were averaged across days for each period to assess the intensity of chronic MSP during the day.
7. Menopausal symptomatology
The Menopause Rating Scale accessed menopausal symptoms in 3 domains: somatic, psychological and urogenital.11
8. Quantification of vasomotor symptoms
Participants reported the number of hot flashes and night sweats before going to bed of the previous night daily during 10 days. The number of vasomotor symptoms was averaged across days.
9. QOL
The World Health Organization QOL Questionnaire, brief form (WHOQOL-BREF) assesses the individual's perception in a general index, independently of its 4 domains: physical, psychological, social relations and environment. The score ranges from 0 to 100, the closer to 100, the better is QOL perception.12
10. Mood symptoms
The Beck Anxiety Inventory investigates common symptoms of anxiety, and results range from 0 to 63: 0 to 7 is interpreted as a “minimal level of anxiety”; 8 to 15 as “mild"; 16 to 25 as “moderate”; and 26 to 63 as “severe anxiety symptoms”.13
The Beck Depression Inventory examines episodes of depression. It ranges from 0 to 63; 0 to 9 is interpreted as a “no depression symptoms or minimal”; 10 to 18 as “mildmoderate”; 19 to 29 as “moderate to severe”; 30 to 63 as “severe depression symptoms”.14
11. Sleep quality and daytime sleepiness
The Pittsburgh Sleep Quality Index (PSQI) is a self-rated questionnaire that assesses sleep quality and disturbances over a 1-month period. A PSQI ≤ 5 indicates good sleep quality, while > 5 is associated with poor sleep quality, and > 10 indicates sleep disturbances.15
Epworth Sleepiness Scale was used to evaluate excessive daytime sleepiness. The score ranges from 0 to 24, and 0 to 9 indicates “no sleepiness symptoms”, while > 9 may be “suggestive of daytime sleepiness”.16
12. PSG
We used PSG for assessment of objective sleep patterns and to exclude volunteers with other comorbid sleep disorders from the study. All participants underwent a basal PSG, performed using a digital system (EMBLA® N7000; Embla Systems Inc., Broomfield, CO, USA) during their usual sleep time. The following physiological variables were evaluated: electroencephalogram (EEG), electrooculogram (bilateral), electromyogram, electrocardiogram (derivation D2 modified), airflow detection by a thermocouple and by nasal pressure, respiratory effort using thoracic and abdominal x-trace belts, snoring and body position by EMBLA sensors, percutaneous oxygen saturation (SpO2) and pulse rate by an EMBLA oximeter. All PSG were visually scored by a registered and trained PSG technologist, blinded to group allocation. All sleep stages, EEG arousals, leg movements and respiratory events were scored according to the guidelines of the American Academy of Sleep Medicine.17
13. Definition of groups
Participants were categorized into 4 groups: control, postmenopausal healthy controls without MSP and INS; MSP, volunteers presenting chronic MSP pain complaints without INS, assessed using the Nordic Musculoskeletal Questionnaire; 18 INS, participants presenting an INS disorder diagnosis without MSP (ISI ≥ 15 had a clinical consultation to confirm the INS diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria19), and INS + MSP, volunteers having both conditions.
14. Sample size calculation
The sample size was calculated for 80% power, an effect size of 0.72 with Cohens' d and an α error of 0.05. For the subjective parameters (pain VAS as outcome), the required sample size was 12 per group and a total of 48 volunteers. The sample size calculation was made using G * Power software version 3.1.9.2 (Franz Faul; Universitat Kiel, Kiel, Germany).
15. Statistical analysis
Continuous variables were analyzed through a two-way analysis of variance (ANOVA). The distributions were evaluated for normality and homogeneity of variances by Shapiro-Wilk and Levene's test, respectively. Based on this assessment, the following variables were square root transformed, to satisfy the models' normality assumptions of distributed residuals: time since menopause, pain interference, sleep latency, rapid eye movement (REM) sleep latency, wake after sleep onset (WASO), sleep efficiency, non-REM (NREM; NREM stage N1 sleep, NREM stage N2 sleep, arousal index, respiratory disturbance index, AHI, PLM and number of vasomotor symptoms. Pearson's χ2 test was used to verify associations between categorical variables. Generalized linear models with Gamma or Tweedie distributions were used to assess the associated factors for menopausal symptomatology and QOL continuous variables, and was followed by Sidak post hoc analysis when necessary. All analyses were conducted using SPSS version 18 software (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P ≤ 0.05
Results
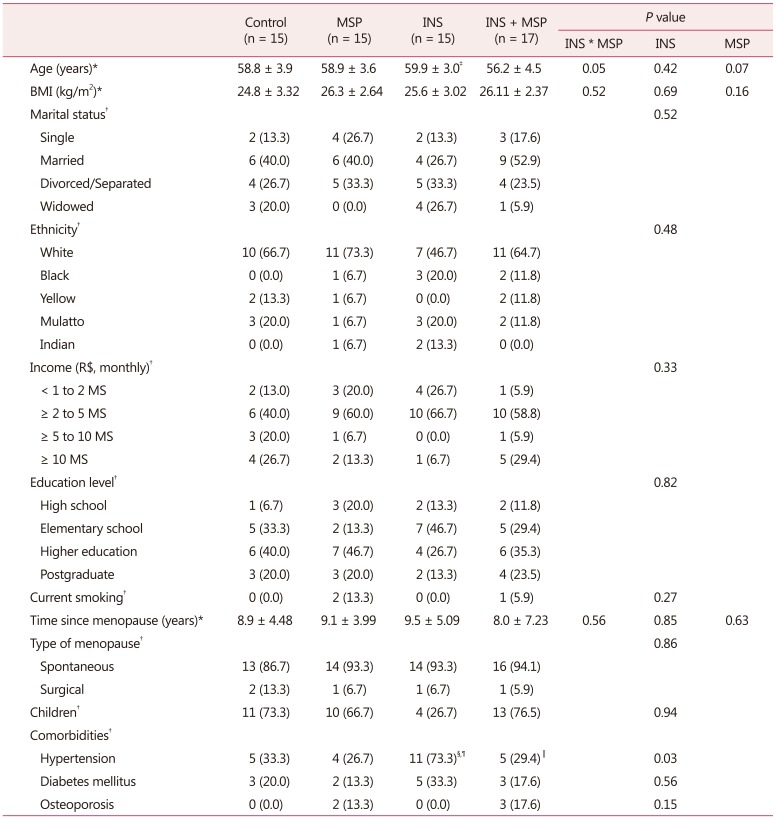
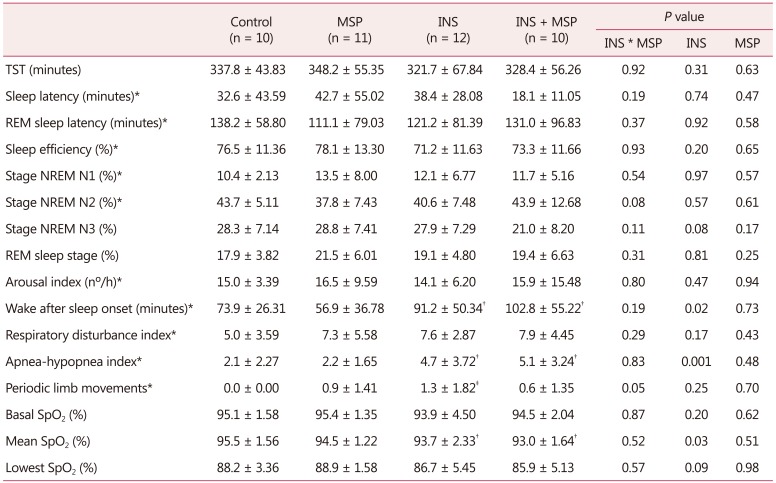
From a total of 75 women who entered the protocol, 62 were eligible and were allocated into 4 groups: control, MSP, INS, and INS + MSP (Fig. 1). The sample was homogeneous as demonstrated in Table 1, except for age, with the INS group being older than all other groups (F = 3.88, df = 1, P = 0.05), and the presence of hypertension in the INS group was significantly higher compared to the other groups (χ2 = 9.1; df = 1;P = 0.03)
Table 1. Mean ± standard deviation or frequency of sociodemographic and clinical characteristics of control, musculoskeletal pain, insomnia, and insomnia + musculoskeletal pain groups (n = 62).
The data is presented as mean ± standard deviation or n (%). All volunteers stated they had not used alcohol in the previous 3 months
*Two-way analysis of variance
†Pearson's Χ2 test
‡P < 0.05 compared to other groups
§P < 0.05 compared to control
∥P < 0.05 compared to INS
¶Ps < 0.05 compared to MSP
BMI: body mass index, MS: minimal salary in Brazilian currency (Reais), MSP: musculoskeletal pain, INS: insomnia
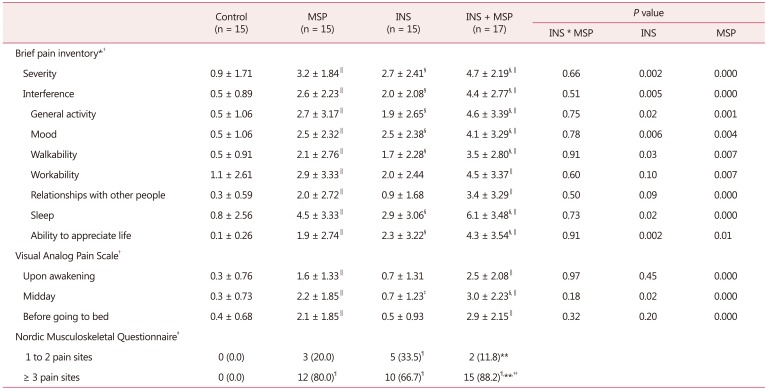
INS and MSP were individually and independently associated with higher MSP severity (F = 10.31, df = 1, P = 0.002; F = 15.27, df = 1, P = 0.000; respectively, Table 2). A similar pattern was observed for the interference of pain with daily function (F = 10.53, df = 1, P = 0.005; F = 26.96, df = 1, P = 0.000) and its domains, such as general activity (F = 5.4, df = 1, P = 0.02; F = 12.01, df = 1, P = 0.001), mood (F = 8.23, df = 1, P = 0.006; F = 8.86, df = 1, P = 0.004), walking ability (F = 5.03, df = P1, = 0.03; F = 7.85, df = 1, P = 0.007), sleep (F = 5.41, df = 1, P = 0.02; F = 18.72, df = 1, P = 0.00) and ability to appreciate life (F = 10.89, df = 1, P = 0.002; F = 6.99, df = 1, P = 0.01). For the working ability and relationships with other people domains, we observed an association with MSP only, worsening these domains (F = 7.86, df = 1, P = 0.007; F = 12.71, df = 1, P = 0.001; respectively, Table 2).
Table 2. Mean ± standard deviation of pain-related characteristics among control, musculoskeletal pain, insomnia, and insomnia + musculoskeletal pain groups (n = 62).
*1–4: mild pain, 5–6: moderate pain, 7–10: severe pain
†Two-way analysis of variance
‡Pearson's Χ2 test
§P < 0.05 compared to INS-free groups
∥P < 0.05 compared to MSP-free groups
¶P < 0.05 compared to control
**P < 0.05 compared to INS
††P < 0.05 compared to MSP
MSP: musculoskeletal pain, INS: insomnia
Regarding the intensity of pain recorded in a 10-day pain diary, as expected the presence of MSP, independently of INS, was associated with increased pain intensity in all 3 periods evaluated: upon waking (F = 20.33, df = 1, P = 0.000), at midday (F = 20.13, df = 1, P = 0.000) and before going to bed (F = 27.24, df = 1, P = 0.000). At midday, the INS groups presented increased pain compared to non-INS groups (F = 5.22, df = 1, P = 0.02; Table 2). Additionally, the INS + MSP group presented a higher frequency of women reporting 3 or more pain sites (88.2%) compared to all the other groups (Table 2). MSP was a result of the following disorders: plantar fasciitis, elbow and shoulder tendinitis, hip and shoulder bursitis, carpal tunnel syndrome, low back pain, cervical spine pain, ankylosing spondylitis, temporomandibular disorder, and hand, shoulder, arm, back, knee, hip, leg and foot pain.
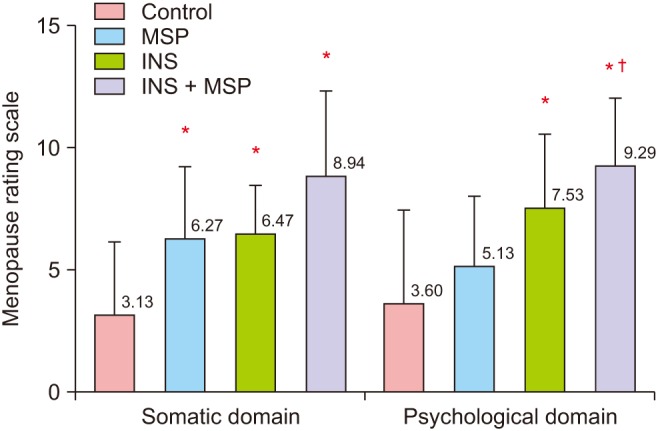
INS with MSP and INS alone were associated with higher menopausal symptoms (F = 10.76, df = 3, P = 0.000; Fig. 2). In relation to the Menopause Rating Scale domains, the somatic (F = 10.52, df = 3, P = 0.000), and the psychologic (F = 9.98, df = 3, P = 0.000) domains presented the same pattern (Fig. 3). We found no significant statistical difference among groups in the urogenital domain (F = 1.19, df = 3 , P = 0.32)
Fig. 2. Mean ± standard deviation of the menopausal symptoms derived from the Menopause Rating Scale among the control, musculoskeletal pain (MSP), insomnia (INS), and INS + MSP groups. Data analyzed through analysis of variance. *P < 0.05 compared to control; †P < 0.05 compared to MSP. The Menopause Rating Scale score is classified as 0 to 4: asymptomatic or scarce menopausal symptoms, 5 to 8: mild symptoms, 9 to 15: moderate symptoms, and 16 to 44: severe menopausal symptoms.
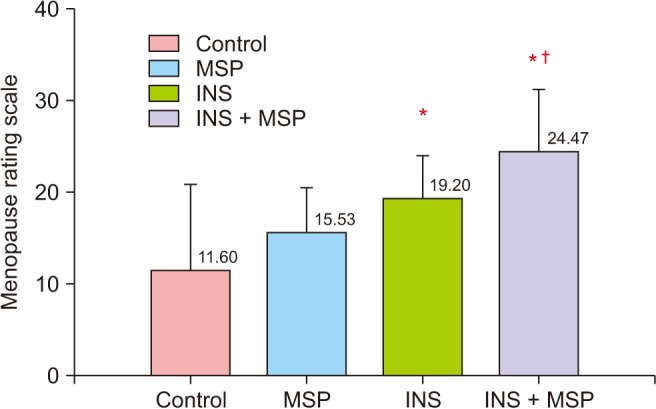
Fig. 3. Mean ± standard deviation of the somatic and psychological domains derived from the Menopause Rating Scale among control, musculoskeletal pain (MSP), insomnia (INS), and INS + MSP groups. Data analyzed through analysis of variance. *P < 0.05 compared to control; †P < 0.05 compared to MSP. The Menopause Rating Scale score is classified as 0 to 4: asymptomatic or scarce, 5 to 8: mild symptoms, 9 to 15: moderate symptoms, and 16 to 44: severe symptoms.
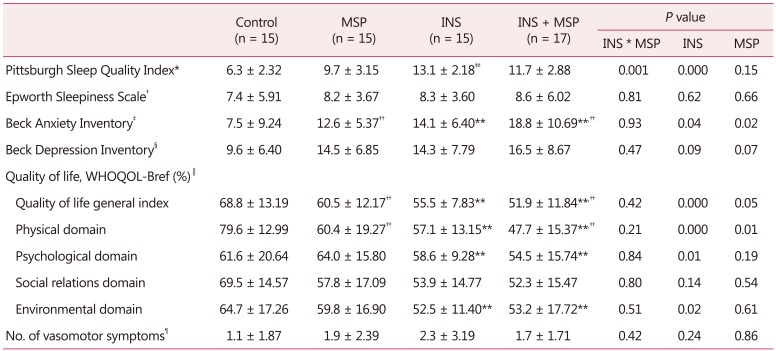
In relation to mood symptoms, INS alone (F = 9.20, df = 1, P = 0.04) and MSP alone increase anxiety symptoms (F = 5.33, df = 1, P = 0.02). Regarding depression symptoms, no statistical differences were observed among the groups (F = 0.53, df = 1, P = 0.47; Table 3).
Table 3. Mean ± standard deviation of insomnia severity index, sleep quality, sleepiness, symptoms of anxiety and depression, habitual physical activity, quality of life, and vasomotor symptoms among control, musculoskeletal pain, insomnia, and insomnia + musculoskeletal pain groups (n = 62).
Two-way analysis of variance
*0–4: good sleep, 5–10: bad sleep, ≥10: requires medical assistance
†0–9: no sleepiness symptoms, ≥10: suggestive of daytime sleepiness and requires medical assistance
‡0–7: minimum degree of anxiety, 8–15: mild anxiety, 16–25: moderate anxiety, 26–63: severe anxiety
§0–9: no depression symptoms, 10–15: mild depression symptoms, 16–19: mild to moderate depression symptoms, 20–30: moderate to severe depression symptoms, >30: severe depression symptoms
∥Score range from 0 to 100, and closer to 100, better quality of life
¶Mean of 10 days recording of day and night vasomotor symptoms
**P < 0.05 compared to INS-free groups
††P < 0.05 compared to MSP-free groups
‡‡P < 0.05 compared to all other groups
WHOQOL-Bref: brief form of the World Health Oragnization Quality of Life, MSP: musculoskeletal pain, INS: insomnia
No significant differences were observed among the groups regarding vasomotor symptoms (F = 0.67, df = 1, P = 0.42; Table 3).
In relation to sleep, a significant interaction between INS and MSP was also observed for sleep quality (F = 83.06, df = 1, P = 0.001), as the INS group presented worse sleep quality compared to all other groups. No statistical differences were found for daytime sleepiness among the groups (F = 0.06, df = 1, P = 0.81; Table 3)
A significant interaction effect between INS and MSP was observed for PLM (F = 4.20, df = 1, P = 0.05), showing that the INS group had the highest PLM index compared to other groups. The presence of INS was associated with increased WASO and AHI, and decreased mean SpO2, independent of chronic MSP (F = 5.61, df =1, P = 0.02; F = 7.16, df = 1, P = 0.001; F = 4.85, df = 1, P = 0.03; respectively Table 4).
Table 4. Mean ± standard deviation of objective sleep pattern among control, musculoskeletal pain, insomnia, and insomnia + musculoskeletal pain groups (n = 43).
Two-way analysis of variance. Reference values of polysomnography exam: TST (variable within person); sleep efficiency, the ratio of TST to the total amount of time spent in bed in percentage (>85% of TST); sleep latency, the length of time in minutes it takes to transits from wake to sleep (<30 minutes); REM sleep latency, the length of time in minutes to enter REM sleep stage (90–120 min); NREM stage N1 sleep (up to 5% of TST); NREM stage N2 sleep (45–55% of TST); NREM stage N3 sleep (slow wave sleep or delta sleep - up to 23% of TST); REM sleep stage (20–25% of TST); wake after sleep onset, the amount of time in minutes spent awake after sleep has been initiated (sleep fragmentation - up to 30 min); wake index, the number of awakenings per hour; periodic limb movements index, number per hour of involuntary movement of limbs during sleep (<15/hr); respiratory disturbance index, the index of respiratory disorders during sleep; apneahypopnea index, indicates the mean number of obstructive apneas and hypopneas per hour of sleep (<5/hr); and SpO2 ≥ 90%
*Non-parametric data, square root normalization of data
†P < 0.05 compared to INS-free groups
‡P < 0.05 compared to other groups
TST: total sleep time, REM: rapid eye movement, NREM: non-rapid eye movement, SpO2: percutaneous oxygen saturation, MSP: musculoskeletal pain, INS: insomnia
Regarding QOL, the results showed that INS alone (F =14.08, df = 1, P = 0.00) and an MSP aonle (F = 4.18, df = 1, P = 0.05) worsened QOL general index. The physical domain of QOL followed the same pattern as INS (F = 20.13, df = 1, P = 0.00) and MSP (F = 13.22, df = 1, P = 0.01) were independently associated with worse QOL. The presence of INS alone was associated with decrease in both psychological and environmental domains of QOL (F = 8.01, df = 1, P = 0.01; F = 5.28, df = 1, P = 0.02; respectively, Table 3).
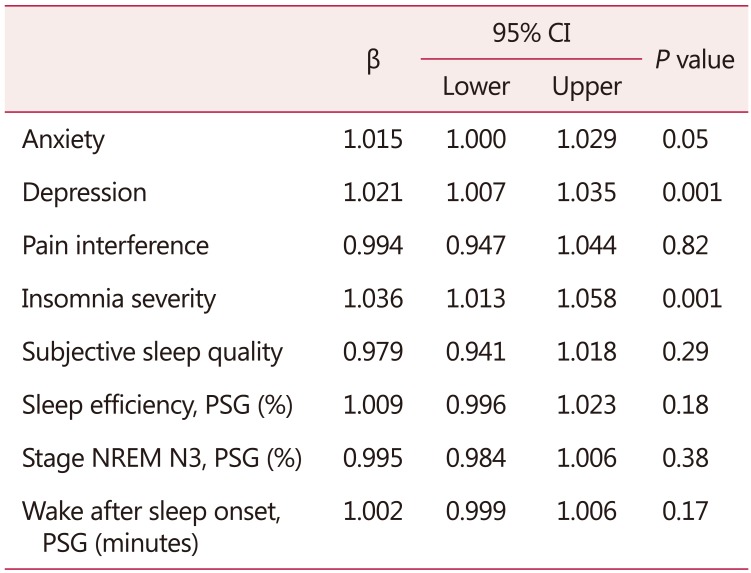
As shown in Table 5, the generalized linear model showed increased symptoms of anxiety and depression, as well as INS severity, were positively associated with higher menopausal symptoms. Each 1-unit increase in anxiety symptoms score was associated with a 50% addition in the menopausal symptoms score. Each 1-unit increase in depressive symptoms score and INS severity score were associated with a 2.1-fold and 3.6-fold increase of menopausal symptoms score, respectively.
Table 5. Generalized linear mixed model considering menopausal symptoms as dependent variable and symptoms of anxiety, depression, and insomnia severity as independent variables after controlling for age and hypertension (n = 62).
Generalized linear model, Tweedie regression
PSG: polysomnography exam, NREM: non-rapid eye movement, CI: confidence interval
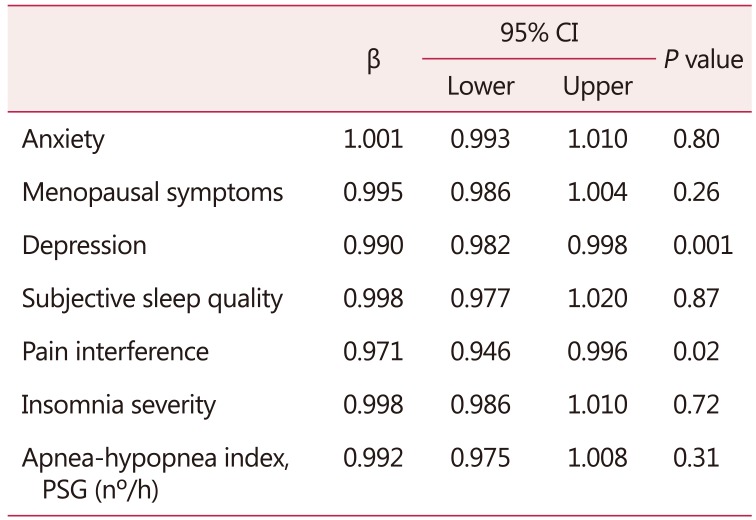
Regarding the predictor factors of QOL in the whole sample of women included in the current study, the results showed that each 1-unit increase of depressive symptoms score was associated with a 10% decrease in QOL. Additionally, each 1-unit increase in pain interference score was associated with a 30% decrease in QOL (Table 6).
Table 6. Generalized linear mixed model considering the quality of life as dependent variable and symptoms of anxiety, depression, menopausal symptoms, subjective sleep quality, pain interference, insomnia severity, and apnea-hypopnea index as independent variables after controlling for age and hypertension(n = 62).
Generalized linear model, Tweedie regression
PSG: polysomnography exam, CI: confidence interval
Discussion
This is the first study, to the best of our knowledge, to show the characteristics of INS + MSP combined on increased anxiety, worse MSP severity and higher interference with daily function, more MSP intensity at midday, increased number of MSP sites, more sleep fragmentation (WASO) and worse QOL in postmenopausal women.
There is strong evidence about the sleep-pain interaction and its bidirectionality in the general population. INS, independently of MSP, has been shown to be associated with MSP severity and MSP interference in most of its domains. Chronic MSP conditions have been linked to disturbed sleep10,11 and short sleep duration (<360 min).4
INS alone was associated with increased pain intensity at midday. A study by Tang and colleagues,20 in the general population, showed that sleep helped to reduce pain intensity. A possible hypothesis for this is that sleep deprivation increases plasma levels of cortisol which is responsible for increased pain sensitivity. Cortisol concentrations start to decline from midday and reach their lowest concentrations in the late evening and during the early part of the sleep period.21 In addition, the presence of restorative sleep seems to reduce chronic generalized pain22 and in our sample, this restorative sleep “acted” only until midday, as our INS + MSP group complained of worse MSP intensity at midday.
Another hypothesis is related to the midday body temperature decline. The body temperature is a biologic rhythm closely associated with the circadian rhythm of sleepiness.23 The thermoregulatory mechanism that lowers body temperature and the decline in cortisol concentrations can promote sleep. We speculate that both could have influenced the increased perception of MSP at midday, so the relief of pain generated by sleep was evident only until the first half of the day. Moreover, our sample did not present daytime sleepiness.
Both INS and MSP are core symptoms of menopause. INS influences the perception of other menopausal symptoms24 such as MSP in our investigation. This finding, especially in relation to the somatic and psychological domains, is corroborated by another study that found INS symptoms as more correlated with psychological than with somatic symptoms.25 Vasomotor symptoms, considered the hallmark indicator of menopause, represent a possible sleep interference. No statistical differences among groups in vasomotor symptoms were found in our sample.
Although no statistical difference was found in relation to depressive symptoms, they were, together with anxiety symptoms, predictive factors for menopausal symptomatology. Anxious people, in general, are more likely to have INS symptoms and individuals with INS appear to have a 17-fold increased chance of experiencing anxiety.26
The INS groups presented higher sleep fragmentation (WASO). Sleep fragmentation was the most common alteration of sleep in chronic pain patients.27 We observed increased AHI and decreased mean SpO2 in the sample of women with INS, but despite the statistical significance of these findings, they do not have clinical relevance, being within the normal range of reference parameters. MSP did not influence objective sleep parameters in our investigation.
Our postmenopausal women had impaired sleep with a short sleep duration of < 360 minutes on the PSG exam; poor sleep efficiency below 85% indicating non-restorative sleep; increased N1 sleep stage, indicating more superficial sleep; and increased WASO, evidence of sleep fragmentation. Lowest SpO2 was under the reference values in 90% of our sample.
INS alone worsened QOL in our sample. INS may produce conditions that impair QOL such as fatigue, physical tiredness, mental exhaustion, irritability28 and also MSP.20 MSP alone was associated with lower QOL in the physical domain in our investigation. We found depression and pain interference as predictor factors for worse QOL. Menopausal symptoms have been shown to affect QOL in other studies, particularly in the physical domain, the highest rates of complaints being muscle and joint pain.29 Sleep is an essential component of good QOL.30
There are some limitations in this study that should be considered. First, this was a cross-sectional design, which does not allow the establishment of causality between factors. Second, the MSP was assessed through self-reported data while combined subjective and objective data may be more suitable for research (e.g., Quantitative Sensory Testing). Third, the information about comorbidities was based on the participants' self-reports. Lastly, there was no night of adaptation for the PSG exam, w hich may decrease overall the sleep quality of the participants from all groups.
The present results demonstrate that INS + MSP combined was related to higher menopausal and anxiety symptoms, more sleep fragmentation, more complaints of MSP severity and interference, more pain sites and worse QOL. The presence of INS was associated to more MSP intensity and interference on daily function. However, the presence of MSP was not associated with sleep. This indicates that in the bidirectional association between INS and MSP in postmenopausal women, INS seems to play a major role, but investigations of the direction are warranted. Sleep management is essential in women who have developed chronic MSP to improve QOL and reduce the possible development of comorbidities, such as anxiety, depression and INS.
Acknowledgement
The authors would like to thank Associação Fundo Incentivo à Pesquisa - AFIP, and São Paulo Research Foundation - FAPESP (#2014/18722-5 to CF). We are immensely grateful to Gianni Santos for her help with statistical analysis and to Paul Ferri with his help with manuscript edition.
Footnotes
This work was supported by Associação Fundo de Incentivo à Pesquisa (AFIP); Conselho Nacional de Desenvolvimento Científico e Tecnológico (MLA and HH are recipients of CNPq fellowship); and São Paulo Research Foundation (FAPESP #2014/15259-2 to CH and #2014/18722-5 to CF). Teh sponsors had no role in the design or conduct of this research.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Davis SR, Lambrinoudaki I, Lumsden M, Mishra GD, Pal L, Rees M, et al. Menopause. Nat Rev Dis Primers. 2015;1:15004. doi: 10.1038/nrdp.2015.4. [DOI] [PubMed] [Google Scholar]
- 2.Neslihan Carda S, Bilge SA, Ozturk TN, Oya G, Ece O, Hamiyet B. The menopausal age, related factors and climacteric symptoms in Turkish women. Maturitas. 1998;30:37–40. doi: 10.1016/s0378-5122(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 3.Monterrosa-Castro A, Marrugo-Flórez M, Romero-Pérez I, Chedraui P, Fernández-Alonso AM, Pérez-López FR. Prevalence of insomnia and related factors in a large midaged female Colombian sample. Maturitas. 2013;74:346–351. doi: 10.1016/j.maturitas.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Generaal E, Vogelzangs N, Penninx BW, Dekker J. Insomnia, sleep duration, depressive symptoms, and the onset of chronic multisite musculoskeletal pain. Sleep. 2017;40 doi: 10.1093/sleep/zsw030. [DOI] [PubMed] [Google Scholar]
- 5.Woods NF, Hohensee C, Carpenter JS, Cohen L, Ensrud K, Freeman EW, et al. Symptom clusters among MsFLASH clinical trial participants. Menopause. 2016;23:158–165. doi: 10.1097/GME.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 7.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 8.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celik EC, Yalcinkaya EY, Atamaz F, Karatas M, Ones K, Sezer T, et al. Validity and reliability of a Turkish Brief Pain Inventory Short Form when used to evaluate musculoskeletal pain. J Back Musculoskelet Rehabil. 2017;30:229–233. doi: 10.3233/BMR-160738. [DOI] [PubMed] [Google Scholar]
- 10.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 11.Heinemann LA, Potthoff P, Schneider HP. International versions of the Menopause Rating Scale (MRS) Health Qual Life Outcomes. 2003;1:28. doi: 10.1186/1477-7525-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skevington SM, Lotfy M, O'Connell KA. The World Health Organization's WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Biering-Sørensen F, Andersson G, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon. 1987;18:233–237. doi: 10.1016/0003-6870(87)90010-x. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 20.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35:675–687a. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Cauter E, Tasali E. Endocrine physiology in relation to sleep and sleep disturbances. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6th ed. Philadelphia, PA: Elsevier; 2017. pp. 202–219. [Google Scholar]
- 22.Roehrs T, Carskadon MA, Dement WC, Roth T. Daytime sleepiness and alertness. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6th ed. Philadelphia, PA: Elsevier; 2017. pp. 39–48. [Google Scholar]
- 23.Davies KA, Macfarlane GJ, Nicholl BI, Dickens C, Morriss R, Ray D, et al. Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford) 2008;47:1809–1813. doi: 10.1093/rheumatology/ken389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Terauchi M, Hiramitsu S, Akiyoshi M, Owa Y, Kato K, Obayashi S, et al. Associations between anxiety, depression and insomnia in peri- and post-menopausal women. Maturitas. 2012;72:61–65. doi: 10.1016/j.maturitas.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 27.Bjurstrom MF, Irwin MR. Polysomnographic characteristics in nonmalignant chronic pain populations: A review of controlled studies. Sleep Med Rev. 2016;26:74–86. doi: 10.1016/j.smrv.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampio L, Saaresranta T, Polo O, Polo-Kantola P. Subjective sleep in premenopausal and postmenopausal women during workdays and leisure days: a sleep diary study. Menopause. 2013;20:655–660. doi: 10.1097/gme.0b013e31827ae954. [DOI] [PubMed] [Google Scholar]
- 29.Mirhaghjou SN, Niknami M, Moridi M, Pakseresht S, Kazemnejad E. Quality of life and its determinants in postmenopausal women: a population-based study. Appl Nurs Res. 2016;30:252–256. doi: 10.1016/j.apnr.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 30.The WHOQOL Group. The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. 1998;46:1569–1585. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]