Abstract
Objectives
Mucoepidermoid carcinoma (MEC) is an uncommon malignancy that most commonly occurs in the parotid gland followed by the minor salivary glands of the upper aerodigestive tract, most notably in the oral cavity (OC) and oropharynx (OP). Because of its rarity, few studies have been performed that are specific to MEC within the OC and OP. The objective of this study is to describe the tumor characteristics and prognostic features for MEC of the OC and OP.
Materials and Methods
The National Cancer Database (NCDB) was used for this study. The primary outcome measure was 5-year overall survival (OS). The secondary outcome measure was occult nodal disease. Fischer’s exact tests, chi-square tests, log-rank tests and Cox proportional hazards analyses were performed.
Results
We identified 3005 patients with MEC of the OC/OP. The 5-year overall survival for MEC of the OC and OP was 87%. Increasing age, male sex, Charlson/Deyo comorbidity score of 2+, clinical T3-4 tumors, nodal + disease, high grade tumors and positive margins were independently associated with decreased 5-year OS. Occult nodal disease occurred in 14.1% and 17.3% of high grade and clinical T3-T4 tumors respectively.
Conclusion
MEC of the OC/OP has an excellent survival as the majority of these patients have low/intermediate grade and early stage disease. Negative prognosticators include increasing age, male sex, Charlson/Deyo comorbidity score of 2+, clinical T3-4 tumors, nodal+ disease, high grade tumors and positive margins. Our findings justify strong consideration of prophylactic neck dissection for high grade and clinical T3-4 tumors.
Keywords: Mucoepidermoid carcinoma, Minor salivary glands, Oral cavity, Oropharynx, Overall survival, Occult nodal disease, National cancer database
Introduction
Salivary gland malignancies are rare and account for only 3% of head and neck cancers [1]. Despite their rarity, these neoplasms are heterogeneous and are classified into 24 different histologic subtypes by the World Health Organization [2]. Mucoepidermoid carcinoma (MEC) is the most common salivary gland malignancy and is most often located in the parotid gland followed by the minor salivary glands throughout the upper aero-digestive tract, most notably in the oral cavity (OC) and oropharynx (OP) [3,4].
Clinicopathologic characteristics, oncologic outcomes, and prognostic factors for MEC of the parotid gland have been well defined [5,6]. However, because of their scarcity, few studies have been performed that are specific to MEC of the OC and OP [7]. Instead, previous publications have grouped MEC of the OC/OP with MEC of the major salivary glands [8,9]. Other studies have focused on minor salivary gland malignancies as a conglomerate thereby grouping MEC with other histologic subtypes [10–13]. Therefore, there is limited data specific to MEC of the OC and OP and a head and neck surgical oncologist must counsel patients and make treatment decisions with evidence inferred from studies containing heterogeneous tumor subsites and/or histologies.
In the following study, we utilize the National Caner Database (NCDB), the world’s largest tumor registry, to describe the clinicopathologic characteristics, survival and prognostic factors specific to MEC of the OC and OP.
Methods
Data source and study population
The NCDB is a hospital-based registry that is the result of a joint effort between the Commission on Cancer (COC) of the American College of Surgeons and the American Cancer Society. It captures 70% of all cancer cases in the United States and collects data from more than 1500 COC-accredited programs. The Medical University of South Carolina Institutional Review Board deemed this study exempt from review.
We reviewed the NCDB from 2004 to 2013. We selected cases using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histologic code “8430” and topography codes for all histologically confirmed MEC of the OC and OP. A schematic illustrating the inclusion/exclusion criteria for this study is illustrated in Fig. 1. We identified 3312 patients with MEC of the minor salivary glands in the upper aerodigestive tract (UADT), the majority of which (3005) occurred within the OC/OP. Given the rarity of non OC/OP MEC and our desire to analyze a clinically homogenous data set, we decided to exclude non OC/OP MEC from analysis. Because of the challenge in distinguishing MEC of the OC from those of the OP (e.g. oral vs. base of tongue and hard vs. soft palate), we decided to analyze both OC and OP MEC.
Fig. 1.
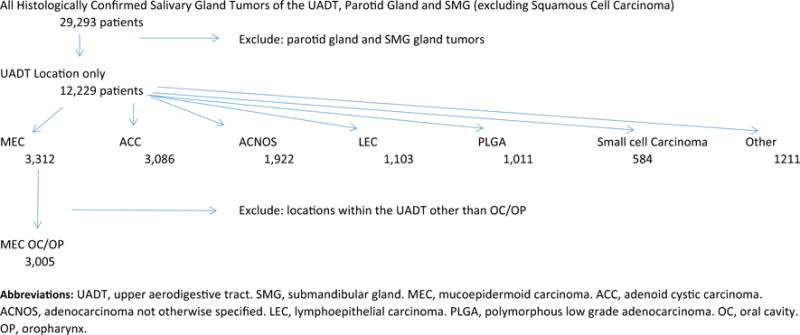
Definition of study cohort.
Outcome measures
The primary outcome measure was 5-year overall survival (OS). OS was defined as the time period from the date of diagnosis to death from any cause. Neither patterns of failure nor disease-specific survival are available in the NCDB. The secondary outcome measure was rate of occult nodal disease, which was defined as the number of clinical N0 patients who were pathologic N+ divided by all clinical N0 patients with available pathologic N staging.
Study variables
Relevant demographic and clinical variables were extracted for analysis including age at diagnosis, gender, race, Charlson/Deyo comorbidity score, tumor grade, clinical (c) and pathologic (p) Tumor-Node-Metastasis stage in accordance with the American Joint Committee on Cancer classification (AJCC), treatment modality, and overall survival (OS). Comorbidity is categorized in the NCDB as 0, 1, or ≥ 2. Tumor grade was classified as low, intermediate and high grade as described previously using the NCDB [5,14].
Statistical analysis
The study variables listed above were imported into the SPSS, version 23 (IBM Corp., Armonk, NY) for analysis. Patients missing overall survival data were included for clinicopathologic characteristics and occult nodal disease calculations but were excluded (307 patients) from survival analysis. Clinical and pathologic information were summarized by means of summary statistics. Comparisons were made with Fisher’s exact test and chi-square test where appropriate. 5-year OS data was tabulated using the life-tables function and compared using the log-rank test. Univariate and multivariate Cox proportional hazards analyses were performed to determine factors associated with 5-year OS. Patients with known metastatic disease or unknown metastatic coding were excluded from our proportional hazard model (102 patients). Log minus log plots were performed for each categorical variable to confirm that the proportional hazards assumption was satisfied. Variables deemed clinically relevant and/or statistically significant on univariate Cox regression analysis were included in our multivariate analysis. Variables included in our multivariate analysis were age, sex, comorbidity score, tumor subsite, clinical tumor stage, clinical nodal disease, grade and surgical margins. A backwards stepwise entry method with p > 0.1 as exclusion criteria was used for our multivariate analysis; subsite was dropped out of the final multivariate model. All statistical tests were two-sided and a p value < 0.01 was considered significant for all tests given the large sample size.
Results
Clinical, pathologic and treatment characteristics
We identified 12,229 patients with histologically confirmed salivary gland malignancies within the UADT. MEC was the most common histology (3312, 27.1%) followed by adenoid cystic (3086, 25.3%) and adenocarcinoma not otherwise specified (1922, 15.7%). Among the 3312 MEC within the upper aero-digestive tract, 3005 (90.7%) were in the OC/OP (1813 in the OC, 699 in the OP and 493 in the OC/OP NOS). Of the 3005 MEC within the OC/OP, 1276 occurred on the palate (42.5%). Clinical, pathologic and treatment characteristics are presented in Table 1. Regarding treatment modality, 2193 patients (75.7%) received surgery alone, 422 (14.6%) received surgery and radiotherapy, 44 (1.5%) received surgery and chemoradiotherapy, 88 (3.0%) received radiotherapy alone, 48 (1.7%) received other, and 101 (3.5%) received none.
Table 1.
Clinical, pathologic and treatment characteristics.
| N | % | ||
|---|---|---|---|
| Total | 3005 | 100 | |
| Age at diganosis | |||
| Median | 52 | ||
| Range | 6 to 90 | ||
| Sex | |||
| Female | 1771 | 58.9 | |
| Male | 1234 | 41.1 | |
| Race | |||
| White | 2416 | 80.4 | |
| Black | 376 | 12.5 | |
| Other | 213 | 7.1 | |
| Comorbidity score | |||
| 0 | 2622 | 87.3 | |
| 1 | 314 | 10.4 | |
| 2+ | 69 | 2.3 | |
| Subsite | |||
| OC | 1813 | 60.3 | |
| OP | 699 | 23.2 | |
| OC/OP NOS | 493 | 16.4 | |
| Clinical T-stage | |||
| T1-2 | 1807 | 86.3 | |
| T3-4 | 286 | 13.7 | |
| Unknown | 912 | ||
| Clinical N-stage | |||
| N0 | 2056 | 89.8 | |
| N1 | 94 | 4.1 | |
| N2+ | 140 | 6.1 | |
| Unknown | 715 | ||
| Pathologic N-stage | |||
| N0 | 1120 | 83.4 | |
| N1 | 70 | 5.2 | |
| N2+ | 153 | 11.4 | |
| Unknown | 1662 | ||
| Distant metastasis | |||
| No | 2837 | 98.8 | |
| Yes | 35 | 1.2 | |
| Unknown | 133 | ||
| AJCC Clinical stage | |||
| I-II | 1682 | 79.9 | |
| III-IV | 423 | 20.1 | |
| Unknown | 900 | ||
| Grade | |||
| Low | 1174 | 45.8 | |
| Intermediate | 1057 | 41.2 | |
| High | 333 | 13.0 | |
| Unknown | 441 | ||
| Positive margins | |||
| No | 2312 | 87.2 | |
| Yes | 339 | 12.8 | |
| Unknown | 354 | ||
| Treatment modality | |||
| Surgery | 2193 | 75.7 | |
| Surgery + Radiotherapy | 422 | 14.6 | |
| Surgery + Chemoradiotherapy | 44 | 1.5 | |
| Radiotherapy | 88 | 3.0 | |
| Other | 48 | 1.7 | |
| None | 101 | 3.5 | |
| Unknown | 109 |
Abbreviations: OC, oral cavity. OP, oropharynx. NOS, not otherwised specified. T, tumor. N, nodal. AJCC, American Joint Committee on Cancer.
Occult nodal disease
Because elective management of the neck remains a controversial topic both in oral cavity malignancies and major salivary gland malignancies we sought to characterize the incidence of occult nodal disease and factors associated with increased risk of occult nodal disease [14,15]. Overall, occult nodal disease was present in 4.3% (23/530) of OC MEC and 8.2% (13/159) of OP MEC. The risk of occult nodal disease was related to grade and cT stage, but not subsite (Table 2) [15]. For the entire cohort of patients with MEC of the OC/OP, patients with high grade disease had a nearly 6-fold increased risk relative to patients with low grade disease (OR 5.58, 99% CI 1.67–18.7). When analyzed by cT stage, patients with cT3-4 tumors had an 17.3% risk of occult nodal disease (14/81), which is a 6-fold increased risk relative to patients with cT1-2 tumors (OR 6.14, 99% CI 2.38–15.85).
Table 2.
Occult nodal disease incidence.
| Variable | N = 798 | % | OR (99% CI) | P Value | |
|---|---|---|---|---|---|
| Grade | |||||
| Low | */315* | <3* | 1 (Ref) | ||
| Intermediate | 17/303 | 5.6 | 2.02 (0.68–5.97) | 0.094 | |
| High | 11/78 | 14.1 | 5.58 (1.67–18.7) | <0.001 | |
| Clinical T-stage | |||||
| T1-2 | 21/638 | 3.3 | 1 (Ref) | ||
| T3-4 | 14/81 | 17.3 | 6.14 (2.38–15.85) | <0.001 | |
| Subsite | |||||
| OC | 23/530 | 4.3 | 1 (Ref) | ||
| OP | 13/159 | 8.2 | 1.96 (0.78–4.96) | 0.061 |
Abbreviations: OR, odds ratio. T, tumor. OC, oral cavity. OP, oropharynx.
Certain rows/columns may not sum to the total in cases in which one of the categorical variables had a cell size < 10 to protect patient identity as per National Cancer Data Base policy.
Cell suppressed because n < 10 per NCDB data use agreement rules.
Survival
The 5-year overall survival for the entire cohort was 87%. Significant clinical and pathologic variables influencing OS are listed in Table 3. Of note, patients with cN2+ disease (34% 5-year OS) and M+(9% 5-year OS) had the worst prognosis.
Table 3.
Overall survival by clinical and pathologic variables.
| Variable | N | 5 year OS | P value | |
|---|---|---|---|---|
| Overall | 2698 | 87% | ||
| Subsite | <0.001 | |||
| OC | 1639 | 92% | ||
| OP | 631 | 77% | ||
| OC/OP NOS | 428 | 85% | ||
| Clinical T-stage | <0.001 | |||
| T1-2 | 1601 | 90% | ||
| T3-4 | 264 | 53% | ||
| Unknown | 833 | |||
| Clinical N-stage | <0.001 | |||
| N0 | 1810 | 91% | ||
| N1 | 85 | 68% | ||
| N2+ | 129 | 34% | ||
| Unknown | 674 | |||
| AJCC Clinical stage | <0.001 | |||
| I-II | 1481 | 93% | ||
| III-IV | 390 | 55% | ||
| Unknown | 827 | |||
| Grade | <0.001 | |||
| Low | 1071 | 94% | ||
| Intermediate | 935 | 91% | ||
| High | 308 | 56% | ||
| Unknown | 384 | |||
| Positive margins | <0.001 | |||
| No | 2068 | 93% | ||
| Yes | 311 | 81% | ||
| Unknown | 319 | |||
| Distant metastasis | <0.001 | |||
| No | 2562 | 88% | ||
| Yes | 32 | 9% | ||
| Unknown | 104 |
Abbreviations: OS, overall survival. OC, oral cavity. OP, oropharynx. NOS, not otherwised specified. T, tumor. N, nodal. AJCC, American Joint Committee on Cancer.
Prognostic factors
The results of the univariate and multivariate Cox regression analysis are summarized in Table 4. On multivariate Cox regression analysis, we found increasing age, male sex, a comorbidity score of 2+, cT3-4 tumor stage, cN+ disease, high grade tumors and positive margins to be independently associated with decreased 5-year overall survival. There was no statistically significant difference in overall survival between MEC of the OC and MEC of the OP and thus it was dropped from the multivariate model.
Table 4.
Univariate and multivariate regression analysis.
| Univariate | Unadjusted HR (99% CI) | P Value | Multivariate | Adjusted HR (99% CI) | P Value | ||
|---|---|---|---|---|---|---|---|
| Age, continuous | 1.06 (1.05–1.07) | <0.001 | Age, continuous | 1.06 (1.05–1.08) | <0.001 | ||
| Sex | Sex | ||||||
| Female | 1 (Ref) | Female | 1 (Ref) | ||||
| Male | 1.78(1.34–2.35) | <0.001 | Male | 1.65 (1.14–2.39) | <0.001 | ||
| Comorbidity score | Comorbidity score | ||||||
| 0 | 1 (Ref) | 0 | 1 (Ref) | ||||
| 1 | 1.55 (1.03–2.34) | 0.006 | 1 | 1.35 (0.81–2.24) | 0.126 | ||
| 2+ | 4.63 (2.67–8.01) | <0.001 | 2+ | 2.52 (1.15–5.50) | 0.002 | ||
| Subsite | Clinical T-stage | ||||||
| OC | 1 (Ref) | T1-2 | 1 (Ref) | ||||
| OP | 2.56(1.88–3.47) | <0.001 | T3-4 | 1.73 (1.03–2.92) | 0.007 | ||
| OC/OP NOS | 1.62(1.09–2.40) | 0.002 | Clinical nodal Disease | ||||
| Clinical T-stage | N0 | 1 (Ref) | |||||
| T1-2 | 1 (Ref) | N+ | 4.04 (2.40–6.81) | <0.001 | |||
| T3-4 | 5.46 (3.86–7.72) | <0.001 | Grade | ||||
| Clinical nodal disease | Low | 1 (Ref) | |||||
| N0 | 1 (Ref) | Intermediate | 1.39 (0.86–2.25) | 0.075 | |||
| N+ | 7.29 (5.23–10.18) | <0.001 | High | 3.20 (1.95–5.26) | <0.001 | ||
| Grade | Positive surgical margins | ||||||
| Low | 1 (Ref) | No | 1 (Ref) | ||||
| Intermediate | 1.52 (1.01–2.29) | 0.008 | Yes | 1.75 (1.15–2.68) | <0.001 | ||
| High | 7.84 (5.36–11.49) | <0.001 | |||||
| Positive surgical margins | |||||||
| No | 1 (Ref) | ||||||
| Yes | 2.63 (1.76–3.94) | <0.001 |
Abbreviations: HR, hazard ratio. OC, oral cavity. OP, oropharynx. NOS, not otherwised specified. T, tumor, N, nodal.
Discussion
To date prognostic factors associated with MEC of the OC and OP have not been well characterized. In the current study, the largest series of MEC of the OC and OP is presented in an effort to identify the clinicopathologic variables that correlate with overall survival. We found MEC to be the most common minor salivary gland tumor within the UADT followed by adenoid cystic carcinoma and adenocarcinoma not otherwise specified. Of MEC tumors occurring within the UADT, the vast majority occurred within the OC and OP (90.7%). MEC of the OC and OP has an excellent 5-year OS of 87% which is secondary to it usually being low/intermediate grade (87%), low stage (79.9% Stage I-II) and being highly amendable to surgical resection with negative margins (87.2%). Despite the generally favorable prognosis, we identified seven independent variables portending worse overall survival for patients with MEC of the OC and OP: increasing age, male sex, 2 + comorbidity score, cT3-4 staging, cN + disease, high grade tumors and positive surgical margins.
TNM stage has previously been shown to be the strongest prognostic factor of survival in all minor salivary gland tumors [4,12,16]. We found advanced cT stage (T3-4) and the presence of clinical nodal disease (N+) to confer a 1.73 and 4.04-fold increase of death at 5 years respectively. Additionally, as anticipated the presence of metastatic disease was shown to be associated with a grave prognosis (9%, 5-year OS). Because of the low incidence of metastatic disease, patients with metastatic disease were excluded from our Cox proportional hazard regression analyses in order to provide a more accurate model. Our percentage of positive margins (12.8%) is lower than previous studies analyzing MEC of the parotid (46.9% positive margins) and minor salivary tumors throughout the UADT (23% positive margins) [6,12]. This is likely secondary to the relative amenability to surgery within the OC and OP compared to other UADT locations and the parotid gland due to the facial nerve frequently abutting the deep surgical margin.
We also found patients with high grade MEC tumors have significantly worse survival than patients with low and intermediate grade tumors on both univariate and multivariate analyses. Other studies have identified grade as an important prognosticator with a clear distinction between low-grade and high-grade tumors; however, there is controversy regarding the significance of intermediate grade tumors [5,6,17]. Intermediate grade tumors did slightly worse than low grade (94% vs. 91% 5-year overall survival, p < 0.001), yet this finding did not remain on our multivariate model (p = 0.075) and is most likely not clinically significant. Additionally, we noticed significant differences regarding tumor grade between our cohort and prior series addressing MEC of the major salivary glands [5,6]. Direct comparison of our cohort compared to a SEER study limited to MEC of the parotid revealed MEC of the OC/OP to be associated with lower grade (45.8% low grade and 13.0% high grade) compared to MEC of the parotid (21.8% low grade and 30.9% high grade) [5]. Future studies comparing MEC of the minor salivary glands to the major salivary glands are warranted.
Rates of occult nodal disease for MEC of the OC and OP are previously unreported to our knowledge. Xiao et al. reported an occult nodal disease rate of 9.3% for MEC of the parotid, a rate which was found to be dependent on tumor size and grade. [14] In our study, we found an occult nodal disease rate of < 6%. The risk of occult nodal disease was associated with clinical tumor size and grade. Subsequent analysis revealed an occult nodal disease rate of < 3%, 5.6% and 14.1% for low, intermediate and high grades, respectively and 3.3% and 17.3% for cT1-2 and cT3-4 tumors, respectively (p < 0.001). Our findings justify strong consideration of prophylactic neck dissection for high grade and T3-4 tumors with a more conservative approach indicated for low-intermediate grade and T1-2 tumors. However, the appropriate management of the clinically negative neck in minor salivary gland tumors remains controversial and requires future study.
Limitations to this study are important to note. Our findings may overestimate or underestimate the true incidence of occult nodal disease. We defined occult nodal disease incidence as cN0 patients who were staged pN+ divided by all clinically negative patients who underwent some form of neck dissection, the extent of which we cannot verify. We also may have not included patients who were diagnosed as cN0 but underwent resection of the primary tumor without neck dissection but received therapeutic radiotherapy to the neck. The only perfect measurement of occult nodal disease would be to perform elective neck dissection on all salivary gland tumors regardless of risk as has been reported at certain institutions [18]. In our analysis, the low incidence of occult nodal disease precluded us from creating a multivariate model to determine independent predictors of occult nodal disease. Per the NCDB data use agreement, we were required to suppress reporting of small cell sizes (n < 10) which limited a more detailed reporting of occult nodal disease incidence. Additionally, NCDB lacks certain pathologic details including perineural invasion, which has been shown to be common and associated with worse outcomes in MEC of the major salivary glands [6]. Finally, we did not compare the effect of different treatment modalities on survival (e.g. surgery alone versus adjuvant radiotherapy versus chemoradiotherapy) because of the treatment selection biases inherent within a retrospective database such as NCDB.
Conclusion
MEC of the OC/OP has an excellent 5-year overall survival. Negative independent prognosticators include increasing age, male sex, 2+ Charlson/Deyo comorbidity score, clinical T3-4 tumors, clinical nodal disease, high grade tumors and positive margins. Overall, occult nodal disease is rare but is more common in high grade and clinical T3-4 tumors. Our findings justify strong consideration of prophylactic neck dissection for high grade and clinical T3-4 tumors.
Acknowledgments
The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
Disclosures
The authors have no financial disclosures at this time. No funding was received to assist with the creation of this manuscript.
Conflict of interest
Non declared.
References
- 1.Spitz Margaret R, Batsakis John G. Major salivary gland carcinoma: descriptive epidemiology and survival of 498 patients. Archives Otolaryngology. 1984;110(1):45–9. doi: 10.1001/archotol.1984.00800270049013. [DOI] [PubMed] [Google Scholar]
- 2.Barnes Leon, et al. Tumours of the salivary glands. World Health Organization Classification of Tumours Pathology Genetics Head and Neck Tumours. 2005:209–81. [Google Scholar]
- 3.McHugh Jonathan B, Visscher Daniel W, Barnes Leon E. Update on selected salivary gland neoplasms. Arch Pathol Lab Med. 2009;133(11):1763–74. doi: 10.5858/133.11.1763. [DOI] [PubMed] [Google Scholar]
- 4.Vander Poorten Vincent, et al. Recent trends in the management of minor salivary gland carcinoma. Head Neck. 2014;36(3):444–55. doi: 10.1002/hed.23249. [DOI] [PubMed] [Google Scholar]
- 5.Chen MM, Roman SA, Sosa JA, Judson BL. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head Neck. 2014;36(2):158–63. doi: 10.1002/hed.23256. [DOI] [PubMed] [Google Scholar]
- 6.McHugh CH, Roberts DB, El-Naggar AK, Hanna EY, Garden AS, Kies MS, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer. 2012;118(16):3928–36. doi: 10.1002/cncr.26697. [DOI] [PubMed] [Google Scholar]
- 7.Olsen KD, Devine KD, Weiland LH. Mucoepidermoid carcinoma of the oral cavity. Otolaryngology–Head Neck Surgery. 1981;89(5):783–91. doi: 10.1177/019459988108900518. [DOI] [PubMed] [Google Scholar]
- 8.Marco Guzzo, et al. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002;9(7):688–95. doi: 10.1007/BF02574486. [DOI] [PubMed] [Google Scholar]
- 9.Kokemueller Horst, et al. Mucoepidermoid carcinoma of the salivary glands—clinical review of 42 cases. Oral Oncol. 2005;41(1):3–10. doi: 10.1016/j.oraloncology.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Baddour HM, Fedewa SA, Chen AY. Five-and 10-Year cause-specific survival rates in carcinoma of the minor salivary gland. JAMA Otolaryngology-Head Neck Surgery. 2016;142(1):67–73. doi: 10.1001/jamaoto.2015.2805. [DOI] [PubMed] [Google Scholar]
- 11.Kakarala Kiran, Bhattacharyya Neil. Survival in oral cavity minor salivary gland carcinoma. Otolaryngology-Head Neck Surgery. 2010;143(1):122–6. doi: 10.1016/j.otohns.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Poorten Vander, Vincent LM, et al. Stage as major long term outcome predictor in minor salivary gland carcinoma. Cancer. 2000;89(6):1195–204. doi: 10.1002/1097-0142(20000915)89:6<1195::aid-cncr2>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Carrillo JF, Maldonado F, Carrillo LC, Ramirez-Ortega MC, Pizano JGG, Melo C, et al. Prognostic factors in patients with minor salivary gland carcinoma of the oral cavity and oropharynx. Head Neck. 2011;33(10):1406–12. doi: 10.1002/hed.21641. [DOI] [PubMed] [Google Scholar]
- 14.D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373(6):521–9. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 15.Xiao CC, Zhan KY, White-Gilbertson SJ, Day TA. Predictors of nodal metastasis in parotid malignancies a national cancer data base study of 22,653 Patients. Otolaryngology–Head and Neck Surgery. 2015:0194599815607449. doi: 10.1177/0194599815607449. [DOI] [PubMed] [Google Scholar]
- 16.Spiro RH, Thaler HT, Hicks WF, Kher UA, Huvos AH, Strong EW. The importance of clinical staging of minor salivary gland carcinoma. Am J Surgery. 1991;162(4):330–6. doi: 10.1016/0002-9610(91)90142-z. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd S, James BY, Ross DA, Wilson LD, Decker RH. A prognostic index for predicting lymph node metastasis in minor salivary gland cancer. Internat J Radiat Oncology* Biology* Phys. 2010;76(1):169–75. doi: 10.1016/j.ijrobp.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Stennert E, Kisner D, Jungehuelsing M, Guntinas-Lichius O, Schröder U, Eckel HE, et al. High incidence of lymph node metastasis in major salivary gland cancer. Arch Otolaryngol-Head Neck Surgery. 2003;129(7):720–3. doi: 10.1001/archotol.129.7.720. [DOI] [PubMed] [Google Scholar]


