Abstract
Emphysema causes significant morbidity and mortality, incurring both financial and psychosocial costs. Alternatives to medical therapy and surgical lung volume reduction surgery (LVRS) have increased interest in bronchoscopic techniques. Bronchoscopic lung volume reduction (BLVR) is still in its infancy and additional trials and follow-up are critical. However, several new randomized clinical trials (RCTs) have demonstrated improvement in lung function, quality of life and exercise capacity in select patients receiving endobronchial valves and coil therapy. This article highlights recent data regarding bronchoscopic treatment of emphysema.
Keywords: Bronchoscopic lung volume reduction surgery (BLVR surgery), endobronchial valves, endobronchial coils, endobronchial vapor therapy, emphysema
Introduction
Chronic obstructive pulmonary disease (COPD) is the third leading cause of mortality in the world (1). Medical therapy includes short- and long-acting bronchodilators, inhaled and oral corticosteroids, and other medications coupled with oxygen support, pulmonary rehabilitation and smoking cessation, among other therapies. With advanced disease, response to treatment wanes and few options exist. Lung volume reduction surgery (LVRS) improves pulmonary function, exercise capacity and quality of life in a subset of patients, but due to perceived risks and costs is not commonly offered (2). Bronchoscopic lung volume reduction (BLVR) offers a possible alternative and much interest has been generated in the techniques. This review offers emerging evidence regarding the efficacy of BLVR.
Expert panel recommendation update 2017
Based on the recently published randomized clinical trials (RCTs), treatment decisions of a previously outlined original 2016 Expert Panel Report were recently updated (3). Furthermore, although BLVR has been incorporated into the 2017 update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, it stated that additional data are needed to define techniques and their long-term durability of improvement relative to side effects (4).
The expert panel focuses on patient selection and discussed possible endoscopic techniques. Patients should receive optimal medical therapy as defined by GOLD, have completed or are undergoing pulmonary rehabilitation or a structured physical therapy program, and have definitively quit smoking. Evaluation includes a full medical assessment, complete lung function measurements with use of body plethysmography, computed chest tomography (CT, specifications below) and a 6-minute walk test (6MWT). Patients with very severe airflow obstruction (GOLD stages 3 and 4, FEV1 20–45%) who are highly symptomatic may be considered with accompanying data, such as Cognitive Ability Test ≥10, modified Medical Research Council (mMRC) scores ≥2, hyperinflation [residual volume (RV) ≥175% or RV/TLC (total lung capacity) ≥0.58], and reduced 6MWT with a distance 100–500. Severe pulmonary hypertension should be excluded and other abnormalities detected on imaging should be addressed, when applicable, including malacia, interstitial disease or nodules (3).
The radiographic assessment includes a non-contrast volume acquisition CT on a multidetector scanner platform with thin (0.6–1.25 mm) series and some overlap. The quantification of emphysema is usually expressed as the proportion of pixels being less than −910 (thick slice, >3 mm) or −950 Hounsfield units (HU) on 1 mm scans. Although no clear definition exists for defining heterogeneity, a >25% difference in ipsilateral lobes in the proportion of pixels less than -910 HU or >15% difference in the proportion of pixels of less than −950 HU has been used in most trials. Greater software sophistication that evaluates fissure completeness of more than 85% on all three axes (sagittal, axial and coronal) may improve analysis of fissure integrity over the subjectivity and inconsistency of doing so with human eyes. Additional dedicated lung quantitative imaging software has been developed to determine quantitative CT (QCT) predictors of outcome with the goal of selecting likely responders. Fissure integrity (P<0.0001), low attenuation clusters in the treated lobe (P=0.01) and vascular volumetric percentage of patient’s detected smallest vessels (P=0.02) were the primary QCT indicators of endoscopic lung volume reduction and the expert panel recommends use of QCT analysis if available (3).
With regards to devices, the expert panel noted that only LVRS and endobronchial valves reached the evidence level to be used outside of clinical trials, yet recommended registries for both. They recommended the use of biological lung volume reduction with the sealant system (AeriSeal) only in clinical trials as the initial product was withdrawn from clinical use based on significant adverse effects. Although bronchoscopic thermal vapor ablation (BTVA, Uptake Medical Corporation, Seattle, Washington, USA) shows promise when restricted to targeted upper lobe heterogeneous diseased patients, again the panel recommends use only in the context of clinical trials.
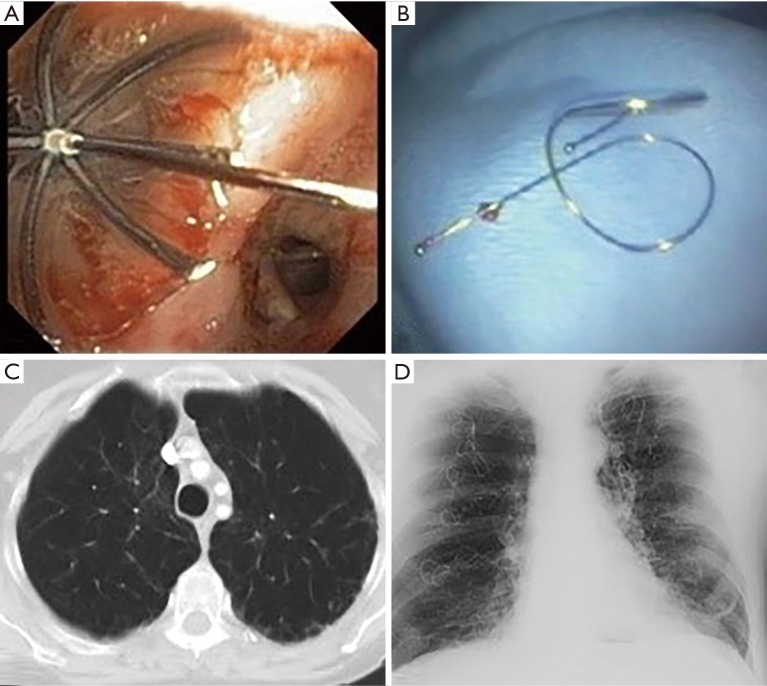
Techniques (Figures 1 and 2)
Figure 1.
Bronchoscopic lung volume reduction techniques include (A) intrabronchial valves and (B) coils. A patient with emphysema (C) subsequently had coils placed (D).
Figure 2.
Key points regarding endobronchial valves, coils, vapor and sealant therapy for severe emphysema. BLVR, bronchoscopic lung volume reduction.
Endobronchial valves
Valves include endobronchial (EBV, Zephyr, Pulmonx, Inc., Neuchatel, Switzerland) and intrabronchial (IBV, Spiration, Olympus, Tokyo, Japan) types. Valves induce lobar atelectasis but depend on the absence of collateral ventilation. They allow air to escape the lung during expiration but prevent it from entering during inspiration. They are placed bronchoscopically using a flexible delivery catheter after measuring the airway diameter.
Recently published RCTs using endobronchial valves were included in the most recent Cochrane Database review (5) and a comprehensive meta-analysis (6). These included IMPACT (7), BeLieVeR-HIFi (8), European VENT (9), USA VENT (10), STELVIO (11) and VENT 2014 (12) (not included in Cochrane). The VENT studies assessed the presence of intact fissures in post-hoc analysis. Tools used to evaluate lung function, exercise capacity and quality of life typically included forced expiratory volume in 1 s (FEV1), 6MWT, St George’s Respiratory Questionnaire (SGRQ) and the mMRC dyspnea scale. The minimal clinically important difference (MCID) is the smallest change in an outcome that a patient would benefit and was determined to be ∆FEV1 ≥10%, ∆6MWT ≥26 meters, ∆SGRQ ≥4 points and ∆mMRC ≥1 point (6). Notably, this definition was slightly different in individual studies [such as FEV1 ≥15% (5)].
Noting difference in time to follow-up, distribution of emphysema (heterogenous vs. homogenous), identifying presence of collateral ventilation and ability to achieve complete lobar occlusion, the above RCTs favored the experimental (valve) group over control group. The risk ratio (RR) and [95% confidence interval (CI)] for EBV in the meta-analysis was 2.96 (1.49–5.87) for BLVR effect on FEV1, 2.90 (1.24–6.79) for effect on 6MWT, 1.53 (1.22–1.92) for effect on SGRQ and 2.53 (1.71–3.76) for effect on mMRC (6). In the studies of EBV vs. conventional group with complete fissures or low collateral ventilation, the weighted mean difference was ∆FEV1 of 17.50% (11.86–23.13), ∆6MWT of 50.17 m (25.04–75.29 m) and ∆SGRQ points of −8.55 (−12.83 to −4.26), all of which were statistically significant. These changes were not as marked when subsets with incomplete fissures were included.
Additional improvements in lung function were found, including a decrease in RV, improvement in TLC, reduction of RV/TLC and improvement of diffusion capacity of lungs for carbon monoxide (DLCO), although these end points were not routinely reported (5).
In a research letter denoting the experience of control patients in the BeLieVeR-HIFi study who subsequently underwent open label valve treatment, and those in the treatment arm who did not have collateral ventilation, the authors observed that valve treatment resulted in clinically significant improvements in lung function, exercise capacity and quality of life in the majority of patients when appropriately selected. However, one patient died of pneumothorax at their home 4 days after treatment. The authors noted that 70% of pneumothoraces occurred within 72 hours of treatment, implying inpatient hospitalization for 4 days after therapy could be prudent (13).
Complications of the EBV group compared to conventional therapy included COPD exacerbation with hospitalization (RR 2.01, 95% CI: 1.19–3.40), hemoptysis (RR 6.42, 95% CI: 1.21–34.01) and pneumothorax (RR 9.65, 95% CI: 3.04–30.60). Pneumonia had a RR 2.17 (95% CI: 0.86–5.49) that was not statistically significant (P=0.10). Mortality was not different between the two groups [RR 1.56 (95% CI: 0.47–5.18); P=0.47].
Intrabronchial valves (IBV)
The efficacy and safety profile of IBV for treatment of emphysema is limited by the small number of RCTs (14,15). Wood et al. (15) reported a decrease in FEV1 of −2.11% for IBV vs. 0.04% for control, a significant difference favoring the control group. Ninane et al. (14) did not report a difference between the intervention group and control for FEV1. Using both studies, the weighted mean difference for ∆6MWT favored the control group [−18.77 m (−35.27 to −2.28)]. There was no significant difference in ∆SGRQ [2.30 (−1.50 to 6.11)] or in ∆mMRC [−0.08 (−0.29 to 0.13)] between groups. Notably, in these IBV studies, patients with upper lobe predominant emphysema were evaluated and the studies did not aim to cause complete lobar occlusion (6).
A separate study evaluated complete unilateral vs partial bilateral BLVR (16). There was a significant increase in FEV1 for the unilateral group (21.4%, SD 10.7%) but not the bilateral group (−3.1%, SD 15.0%), an increase in 6MWT by 48.9 m in the unilateral but not bilateral group, a decrease in total score of SGRQ (−11.8 units, SD 10.6) for the unilateral but not bilateral group, and similar scores on mMRC (5). More complications occurred in the bilateral group and the authors concluded that unilateral IBV placement appeared superior to bilateral partial occlusion (16).
Changes in RV favored control, there was no difference in TLC and Ninane et al. reported a change in RV/TLC favoring control (14). There was no difference in DLCO (5). Whereas in one study the number of adverse events was higher for participants in the intervention group [OR 3.41 (1.48–7.84)] (5), including COPD exacerbations, respiratory failure, pneumonia and bronchospasm, the Ninane study (14) showed no significant difference in adverse events. There was no difference in mortality between the groups [OR 4.95 (0.85–28.94)] (5).
CT fissure analysis and Chartis collateral ventilation
The Chartis® system is a proprietary assessment tool to determine which patients are most likely to respond to IBV placement. During bronchoscopy, the balloon catheter is advanced through the working channel of the bronchoscope into the target lobe. The system measures expiratory air flow, resistance and pressure to identify which patients have minimal to no air flow. Whether CT fissure analysis can replace the Chartis system is the focus of a recent study which included 12 papers for analysis. The results suggest that in patients with a CT-fissure integrity ≥95%, valves could be directly implanted without using Chartis. In patients with a fissure integrity 75–90%, Chartis could provide additional data to dictate which patients are most likely to respond to valve placements. In patients with a fissure integrity <75%, the negative predictive value is 100%, suggesting this population will not benefit from valves (17).
Degree of emphysema
Although the initial studies focused on heterogenous emphysema, STELVIO 2015 and IMPACT 2016 trials included patients with homogenous emphysema, suggesting possible effectiveness of EBV in heterogenous and homogenous emphysema (18). The recently published TRANSFORM study (19) demonstrated patients with heterogeneous emphysema without collateral ventilation treated with EBV had clinically meaningful benefits in lung function, dyspnea and quality of life.
Computational analysis of structure-function relationships
Mondonedo and Suki (20) performed a computational analysis to predict the structure-function relations and survival after lung volume reduction for emphysema. To do so, they simulated emphysema progression by removal of elastic elements in the network and tracked changes in compliance and structure. The study suggested that macroscopic functional improvements in network compliance were related to microscopic changes in force heterogeneity. Denoting effects on the local mechanical environment, changes that would be impossible to detect by imaging or functional studies, the analysis demonstrated that BLVR could be effective for advanced emphysema. In this case, elevated forces in close proximity to reduced areas may promote local tissue destruction. Their study suggested that bronchoscopic treatments that aim at removal of pure RV allow for expansion of more normal regions. This suggests a possible benefit of coupling computational analysis with CT imaging in the future. Given the non-invasiveness, this approach may be uniquely suited to infer outcomes prior to treatment and to aid in clinical decision-making. Interestingly, the authors note in their limitations that incomplete fissures are not captured by their computations but that this may be similar to the actions of coils and sealants that function independent of collateral pathways.
Coils
Endobronchial coil therapy (PneumRx, Inc. Mountain View, California, USA) is a non-blocking partially irreversible treatment that is independent of collateral ventilation. The mechanism of action is thought to be parenchymal compression with resultant improvement of elastic recoil. Coil implantation is performed using bronchoscopy, fluoroscopy and 3 sizes of coils. After measuring airway length, the coils are advanced through the delivery catheter into the appropriate area and, when the catheter is pulled back, the coil assumes its shape.
Three randomized control trials were included in the most recent Cochrane Database review (5) and in a recently published meta-analysis (6). These included RENEW (21), RESET (22,23) and REVOLENS (24). Tools used to evaluate lung function were similar to those for valves including FEV1, 6MWT, SGRQ and mMRC.
Noting difference in time to follow-up, the above RCTs favored the experimental (coils) group over control group. The RR and (95% CI) for endobronchial coils in the meta-analysis was 2.37 (1.61–3.48) for BLVR effect on FEV1, 2.05 (1.18–3.53) for effect on 6MWT and 2.32 (1.77–3.03) for effect on SGRQ (6).
In the studies of coils vs conventional group, the weighted mean difference of ∆FEV1 was 7.31% (4.65–9.97), ∆6MWT was 31.72 m (4.95–58.49), ∆SGRQ points of −9.16 (−11.64 to −6.68) and ∆mMRC points of −0.36 (−0.69 to −0.03), all of which were statistically significant. Within the coils group, weighted mean differences between heterogeneous and homogenous emphysema were not statistically significant for FEV1, 6MWT or SGRQ scores.
Additional improvements in lung function were found, including a decrease in RV, improvement in FVC and reduction of RV/TLC ratio, although these end points were less commonly reported (5). No significant difference in TLC was found, and DLCO values were not reported.
Complications in the coil group compared to conventional therapy were more common (OR 2.14, 95% CI: 1.41–3.23) (5) and included pneumonia (RR 4.42, 95% CI: 2.20–8.88) and pneumothorax (RR 8.17, 95% CI: 2.22–30.03). There was no statistically significant difference in the rates of COPD exacerbation with hospitalization (RR 1.29, 95% CI: 0.81–2.05; P=0.28) or hemoptysis (RR 5.98, 95% CI: 0.73–49.25; P=0.10). There was also no difference in mortality between the two groups (RR 1.27, 95% CI: 0.59–2.72; P=0.54) (6).
A few other studies have recently been reported that provide additional insight into coil therapy. Makris et al. (25) studied the changes in lung mechanics due to coils from baseline to 6 months in a prospective study involving 22 patients. Compared to usual care, the coil group exhibited an improvement in FEV1 [median ∆FEV1 2% (IQR, −0.5% to +4.0%); P=0.01] and FVC, with a decrease in static lung volumes and dynamic hyperinflation [∆RV −49.5% (IQR, −62.9% to −6.9%); P=0.048, ∆FRC −27% (IQR, −40.5% to −16.0%); P=0.03, ∆PEEPi −10.5% (IQR, −17.3% to −6.5%); P=0.09, ∆EELV dyn% −1.08% (IQR, −2.9% to −0.70%); P=0.02]. This was associated with a decrease in lung compliance [median ∆Cldyn −20% (IQR, −24.3% to −6.9%); P=0.01], increase in peak endurance time [+12.4% (IQR, +9.4% to +15.5%); P=0.01] and improvement in inspiratory capacity at rest and exercise. Overall, patients had improved expiratory airflow and decreased dynamic collapse of small airways.
Lador et al. (26) studied pulmonary perfusion prospectively in 17 patients treated with coils, and showed the potential benefit of coils in improving ventilation-perfusion matching. Contrast-enhanced dual-energy computed tomography (DECT) scans were utilized before and after coil treatment, and pulmonary perfusion was noted to significantly improve in areas next to coil placement and in coil-free areas within the same lung, with a mean perfusion increase of 65% and 61% perfusion respectively. This correlated with an improvement in 6MWD, although there was no significant change in contralateral perfusion.
Kontogianni et al. (27) retrospectively analyzed the efficacy of endobronchial coils in 86 patients. There was significant improvement in FEV1, TLC, 6MWD and mMRC at 90 days post-coil placement. However, by the 180-day follow-up, FEV1 had decreased. At 365 days, the initial improvements in VC, RV, 6MWD and mMRC were not sustained. There were also several adverse events, including four fatalities.
Previous studies have also similarly suggested a gradual waning benefit of coils after 1 year (23,28), leading Hartman et al. (29) to perform a pilot study in 8 patients to evaluate the efficacy of re-treatment with coils in patients who initially received coils at least 2 years previously. Each patient received 10–15 coils during one procedure, and were followed for a period of 6 months. While the authors demonstrated the safety and feasibility of retreatment, there was no significant change demonstrated in patients’ quality of life, PFTs and exercise capacity.
Gulsen et al. (30) retrospectively analyzed the benefit of coils up to 6 months following treatment in 40 patients. In all patients, FEV1 increased (+150 mL, +6.5%; P<0.001), RV decreased (−82 mL, −14.5%; P<0.001), 6MWD improved (+48 m; P<0.001), pO2 increased (+12.6 mmHg; P<0.001) and quality of life improved as evidenced by change in SGRQ scores by −10.4 points (P<0.001). However, there was no significant improvement in pCO2 or pulmonary arterial pressure. COPD exacerbation (41.4%), cough (41%) and pneumonia (17%) were the most frequent potential adverse events observed in patients, with Pseudomonas colonization noted in 8.1% of patients during the procedure. It is unclear if patients with Pseudomonas colonization or infection should be excluded in future.
Simon et al. (31) performed a retrospective observational study in 10 patients to determine the safety and efficacy of bilateral coil placement in patients with hypercapnic respiratory failure. These patients were on long term O2 therapy, and 70% of them used non-invasive ventilation intermittently. There was a significant decrease in paCO2 by 8.6% [from 53±5 to 48±4 mmHg (P=0.03)], associated with an increase in FEV1 by 19.1% (P=0.004) and decrease in RV by 8.5% (P=0.02). The main adverse event was hemoptysis, which was mild and self-limiting in most patients, except for one patient who required bronchial artery embolization.
Common adverse events reported in most studies include COPD exacerbations (with or without hospitalizations), pneumonia with some patients developing sepsis, coil-related inflammation/opacities, hemoptysis, pneumothorax and chest pain (5,6,27,30). Debray et al. (32) recently reported a case of new bronchiectasis developing at the coil insertion site, with clinical worsening at the 18-month follow-up visit. The authors described possible theories such as a reaction to the coil, subsegmental airway closure distal to the coil, local ischemia due to bronchial artery distortion or tension-induced inflammation. However, this did not lead to increased expectoration or pneumonia.
Simon et al. (33) retrospectively analyzed the risk of hemoptysis in 62 patients after the placement of coils. Of these, 65.3% of patients developed early, mild hemoptysis, which resolved without intervention in 98.5% of cases. One patient required bronchial artery embolization due to persistent hemoptysis despite vasoconstrictive medications via bronchoscopy. Risk factors for development of hemoptysis appeared to be upper lobe treatment (P=0.008) and concurrent aspirin ingestion (P=0.005), although discontinuation of aspirin was not felt to be related or helpful in a few of the patients. Three patients developed “late” hemoptysis (after initial hospitalization), requiring antibiotic treatment for infection (1 patient) and bronchial artery embolization (2 patients) for resolution. There was no association between hemoptysis and mild to moderate pulmonary artery hypertension. Pre-procedural discontinuation of antiplatelet agents was suggested as a potential preventative strategy, although further studies are required for clarification.
Pleuritic chest wall pain has been reported as a complication of coil therapy, and can occur with distal, subpleural placement of coils. Dutau et al. (34) reported successful removal of 2 very distal coils 10 months after initial placement. There was no hemoptysis or pneumothorax following removal, and pain completely resolved.
In summary, coils have been shown to significantly improve lung volumes and elastic recoil of the lungs, minimizing hyperinflation and dynamic small airway collapse. Coils are unique in being effective in both heterogeneous and homogenous emphysema, independent of collateral ventilation. They can therefore be considered preferentially to valves in the patient refractory to standard medical therapy with homogenous emphysema and significant hyperinflation or with heterogeneous emphysema and collateral ventilation (35). They are non-blocking devices (36) and can be used as destination therapy or as a bridge to transplantation (37). However, despite their advantages, utilization is tempered by serious adverse events, and appropriate candidate selection is important. Results of additional studies are awaited to further our understanding of the mechanism and patient response to coils (REACTION, RECOIL, Improvement of Sleep Quality).
BTVA
BTVA (Uptake Medical Corporation, Seattle, Washington, USA) is a nonblocking technique used in patients with heterogeneous upper lobe predominant emphysema. It is not included in GOLD 2017 guidelines. Heated vapor is instilled in the target area, inciting a local inflammatory reaction that ultimately evokes fibrosis, scarring and shrinkage of the target. This may be used at a segmental rather than lobar level. The target and vapor dose depend on the density and volume of the targeted lung tissue as determined by dedicated software. A catheter with balloon at its distal tip is advanced, the balloon inflated and the heated water vapor is delivered. It is independent of collateral ventilation.
Side effects of therapy include the localized inflammatory reaction accompanied by a symptomatic systemic response. Symptoms include cough, fever, dyspnea and hemoptysis. Prophylactic antibiotics are recommended for 2 weeks after the procedure, and close monitoring is required (18).
A recent multinational, multicenter randomized controlled trial [Sequential Staged Treatment of Emphysema with Upper Lobe Predominance (STEP-UP)] evaluated patients with upper lobe predominant emphysema. The inclusion and exclusion criteria were well defined (38). One segment was targeted during the first treatment and up to 2 segments during the second session, performed 13 weeks later. The primary outcomes were changes in FEV1 and SGRQ-C. The average difference at 6 months was 24.7% in FEV1 (7.8–21.5, P<0.0001) and −9.7 points for SGRQ-C (−15.7 to −3.7, P=0.0021). Adverse events included COPD exacerbation (n=11, 24%), pneumonia or pneumonitis (n=8, 18%), and hemoptysis (n=1). There was one late pneumothorax (after 30 days) that did not require treatment. There was one death (day 84 post treatment). This study was the first RCT demonstrating that reduction of more diseased segments leads to meaningful improvement in pulmonary function and quality of life. The authors suggested the staged approach showed a favorable safety profile.
A sub study of the STEP-UP study evaluated patients with collateral ventilation (39). A post-hoc fissure analysis of all treated and control patients was performed and patients with collateral ventilation were identified if adjacent fissures were <90% complete. There was a mean difference in FEV1 of 14.6% (P=0.0137) when comparing improvement in the treatment arm (Improved 9.2%) and the decrease in the control group (5.4%) at 12 months. The investigators also noted an improvement in SGRQ-C in the treatment arm (8.4 points; P=0.0712). The investigators concluded that patients with incomplete fissures improved with vapor ablation and that the strategy of reducing the most diseased segments and preservation of less diseased segments was safe and improved lung function and quality of life.
Polymeric lung volume reduction
Similar to heated vapor ablation, bronchoscopy is used to deliver a biologic sealant or synthetic polymer in a wedged portion of the emphysematous lung. This induces local inflammation resulting in remodeling, scar formation and hence, lung volume reduction. The only trial [AeriSeal System for Hyperinflation Reduction in Emphysema (ASPIRE)] demonstrated improvements in FEV1, mMRC and SGRQ but was accompanied by inflammatory response, COPD exacerbation, pneumonia and respiratory failure. A significant proportion of patients experienced complications, necessitating pre-clinical re-examination of polymer type, dosage and techniques (18).
Patients undergoing BLVR with biologic sealants or synthetic polymers have radiographic changes following therapy. Lieberman et al. (40) evaluated 4 patients treated with biologic sealants (fibrinogen, thrombin) and 7 with synthetic polymers (Aeris Therapeutics, Woburn, MA, USA). Most abnormalities, such as nodules or consolidation, resolved after short term follow-up in patients who underwent biologic sealants. Patients who underwent synthetic polymers had abnormalities in each treated lobe, including nodules, masses and consolidations; Most nodules or masses were cavitary. Most slowly decreased in size although a minority grew and demonstrated higher than baseline PET avidity. The investigators suggested that radiologists and treating physicians should be aware of these potential radiographic changes after sealant or polymer therapy as they may be confused for possible malignancy.
Summary
Patients undergoing consideration of bronchoscopic lung volume reduction may include those with advanced emphysema who have failed maximal medical therapy, undergone pulmonary rehabilitation and are not smoking. Chest CT imaging to assess collateral ventilation, and the use of the Chartis system, may further determine in this group who is most likely to respond to therapy. There is now some evidence for treatment of both heterogeneous and homogenous emphysema. For those with collateral ventilation, surgical LVRS may be best. In patients with no collateral ventilation and who have intact fissures, endobronchial valves may be considered, especially since they are reversible. IBV might be efficacious when placed unilaterally. In patients with marked hyperinflation (RV >225%) and centrilobular emphysema, or those who lack fissure integrity and have collateral ventilation, coils may be considered. Finally, when emphysema is heterogeneous within an upper lobe, segmental sclerosing therapy might have a place. Multidisciplinary teams may help select the best candidates. Additional studies monitoring side effects, costs and long-term outcomes are needed.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Agteren JE, Carson KV, Tiong LU, et al. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst Rev 2016;10:CD001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herth FJF, Slebos DJ, Criner GJ, et al. Endoscopic Lung Volume Reduction: An Expert Panel Recommendation - Update 2017. Respiration 2017;94:380-8. 10.1159/000479379 [DOI] [PubMed] [Google Scholar]
- 4.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J 2017;49(3). [DOI] [PubMed] [Google Scholar]
- 5.van Agteren JE, Hnin K, Grosser D, et al. Bronchoscopic lung volume reduction procedures for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;2:CD012158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Lai TW, Xu F, et al. Efficacy and safety of bronchoscopic lung volume reduction therapy in patients with severe emphysema: a meta-analysis of randomized controlled trials. Oncotarget 2017;8:78031-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. 10.1164/rccm.201607-1383OC [DOI] [PubMed] [Google Scholar]
- 8.Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. 10.1016/S0140-6736(15)60001-0 [DOI] [PubMed] [Google Scholar]
- 9.Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J 2012;39:1334-42. 10.1183/09031936.00161611 [DOI] [PubMed] [Google Scholar]
- 10.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. 10.1056/NEJMoa0900928 [DOI] [PubMed] [Google Scholar]
- 11.Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. 10.1056/NEJMoa1507807 [DOI] [PubMed] [Google Scholar]
- 12.Valipour A, Herth FJ, Burghuber OC, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J 2014;43:387-96. 10.1183/09031936.00133012 [DOI] [PubMed] [Google Scholar]
- 13.Zoumot Z, Davey C, Jordan S, et al. Endobronchial valves for patients with heterogeneous emphysema and without interlobar collateral ventilation: open label treatment following the BeLieVeR-HIFi study. Thorax 2017;72:277-9. 10.1136/thoraxjnl-2016-208865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012;39:1319-25. 10.1183/09031936.00019711 [DOI] [PubMed] [Google Scholar]
- 15.Wood DE, Nader DA, Springmeyer SC, et al. The IBV Valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol 2014;21:288-97. 10.1097/LBR.0000000000000110 [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt R, Gompelmann D, Schuhmann M, et al. Complete unilateral vs partial bilateral endoscopic lung volume reduction in patients with bilateral lung emphysema. Chest 2012;142:900-8. 10.1378/chest.11-2886 [DOI] [PubMed] [Google Scholar]
- 17.Fiorelli A, Santini M, Shah P. When can computed tomography-fissure analysis replace Chartis collateral ventilation assessment in the prediction of patients with emphysema who might benefit from endobronchial valve therapy? Interact Cardiovasc Thorac Surg 2018;26:313-8. 10.1093/icvts/ivx272 [DOI] [PubMed] [Google Scholar]
- 18.Gompelmann D, Sarmand N, Herth FJ. Interventional pulmonology in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2017;23:261-8. 10.1097/MCP.0000000000000373 [DOI] [PubMed] [Google Scholar]
- 19.Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. 10.1164/rccm.201707-1327OC [DOI] [PubMed] [Google Scholar]
- 20.Mondonedo JR, Suki B. Predicting Structure-Function Relations and Survival following Surgical and Bronchoscopic Lung Volume Reduction Treatment of Emphysema. PLoS Comput Biol 2017;13:e1005282. 10.1371/journal.pcbi.1005282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sciurba FC, Criner GJ, Strange C, et al. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA 2016;315:2178-89. 10.1001/jama.2016.6261 [DOI] [PubMed] [Google Scholar]
- 22.Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233-40. 10.1016/S2213-2600(13)70047-X [DOI] [PubMed] [Google Scholar]
- 23.Zoumot Z, Kemp SV, Singh S, et al. Endobronchial coils for severe emphysema are effective up to 12 months following treatment: medium term and cross-over results from a randomised controlled trial. PLoS One 2015;10:e0122656. 10.1371/journal.pone.0122656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deslee G, Mal H, Dutau H, et al. Lung Volume Reduction Coil Treatment vs Usual Care in Patients With Severe Emphysema: The REVOLENS Randomized Clinical Trial. JAMA 2016;315:175-84. 10.1001/jama.2015.17821 [DOI] [PubMed] [Google Scholar]
- 25.Makris D, Leroy S, Pradelli J, et al. Changes in dynamic lung mechanics after lung volume reduction coil treatment of severe emphysema. Thorax 2017. [Epub ahead of print]. 10.1136/thoraxjnl-2017-210118 [DOI] [PubMed] [Google Scholar]
- 26.Lador F, Hachulla AL, Hohn O, et al. Pulmonary Perfusion Changes as Assessed by Contrast-Enhanced Dual-Energy Computed Tomography after Endoscopic Lung Volume Reduction by Coils. Respiration 2016;92:404-13. 10.1159/000452477 [DOI] [PubMed] [Google Scholar]
- 27.Kontogianni K, Gerovasili V, Gompelmann D, et al. Coil therapy for patients with severe emphysema and bilateral incomplete fissures - effectiveness and complications after 1-year follow-up: a single-center experience. Int J Chron Obstruct Pulmon Dis 2017;12:383-94. 10.2147/COPD.S117655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015;20:319-26. 10.1111/resp.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartman JE, Klooster K, Ten Hacken NH, et al. The Safety and Feasibility of Re-treating Patients with Severe Emphysema with Endobronchial Coils: A Pilot Study. COPD 2017;14:339-43. 10.1080/15412555.2017.1318842 [DOI] [PubMed] [Google Scholar]
- 30.Gulsen A, Sever F, Girgin P, et al. Evaluation of bronchoscopic lung volume reduction coil treatment results in patients with severe emphysema. Clin Respir J 2017;11:585-92. 10.1111/crj.12387 [DOI] [PubMed] [Google Scholar]
- 31.Simon M, Harbaum L, Oqueka T, et al. Endoscopic lung volume reduction coil treatment in patients with chronic hypercapnic respiratory failure: an observational study. Ther Adv Respir Dis 2017;11:9-19. 10.1177/1753465816676222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debray MP, Marceau A, Dombret MC, et al. Bronchiectasis Complicating Lung Volume Reduction Coil Treatment. Chest 2017;152:e57-60. 10.1016/j.chest.2017.04.189 [DOI] [PubMed] [Google Scholar]
- 33.Simon M, Ittrich H, Harbaum L, et al. Bleeding Complications After Endoscopic Lung Volume Reduction Coil Treatment: A Retrospective Observational Study. Arch Bronconeumol 2016;52:590-5. [DOI] [PubMed] [Google Scholar]
- 34.Dutau H, Bourru D, Guinde J, et al. Successful Late Removal of Endobronchial Coils. Chest 2016;150:e143-5. 10.1016/j.chest.2016.03.026 [DOI] [PubMed] [Google Scholar]
- 35.Shah PL, Herth FJ, van Geffen WH, et al. Lung volume reduction for emphysema. Lancet Respir Med 2017;5:147-56. 10.1016/S2213-2600(16)30221-1 [DOI] [PubMed] [Google Scholar]
- 36.Aggelou K, Siafakas N. Medical lung volume reduction for severe emphysema: A review. Respir Med 2017;131:141-7. 10.1016/j.rmed.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 37.Connolly TA. Lung Volume Reduction Coils as a Novel Bronchoscopic Treatment for Emphysema. Methodist Debakey Cardiovasc J 2016;12:17. 10.14797/mdcj-12-4s1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185-93. 10.1016/S2213-2600(16)00045-X [DOI] [PubMed] [Google Scholar]
- 39.Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung Volume Reduction with Vapor Ablation in the Presence of Incomplete Fissures: 12-Month Results from the STEP-UP Randomized Controlled Study. Respiration 2016;92:397-403. 10.1159/000452424 [DOI] [PubMed] [Google Scholar]
- 40.Lieberman S, Shulimzon TR, Davidson T, et al. Long-term Imaging of the Lungs After Sealant Bronchoscopic Lung Volume Reduction. J Thorac Imaging 2016;31:391-7. 10.1097/RTI.0000000000000233 [DOI] [PubMed] [Google Scholar]