Abstract
Background
This study aimed to evaluate the prognostic difference between limited resection and lobectomy among elderly patients with small size lung adenocarcinoma.
Methods
A total of 666 patients >65 years old with stage I lung adenocarcinoma and tumor size ≤2 cm were included. The patient survival was evaluated by disease-free survival (DFS) and overall survival (OS). Results: No DFS or OS advantage was found between the lobectomy and wedge resection groups when tumor sizes were ≤1 cm (DFS, P=0.112; OS, P=0.294). The wedge resection group had a significantly worse OS (P=0.041) than that in the lobectomy group when tumor sizes were >1 cm and ≤2 cm.
Conclusions
We conclude that wedge resection may be a reasonable surgical choice for elderly patients with tumor sizes ≤1 cm.
Keywords: Invasive lung adenocarcinoma, stage I, resection, lobectomy, wedge resection
Introduction
Lung adenocarcinoma continues to be life-threatening, especially in developing countries (1). Recently, the incidence of early-stage, non-small cell lung cancer (NSCLC) was dramatically higher than before in China (2). Histologically, the most common pathologic type was adenocarcinoma, which presents heterogeneous histological patterns in 80–90% of tumor specimens (3). Radiographically, a single, small-size tumor nodule always presents in the lung periphery (4,5). Clinically, according to the National Comprehensive Cancer Network (NCCN) guidelines, the standard treatment for early-stage NSCLC patients is curative-intent surgical lobectomy plus mediastinal lymph node dissection or systematic sampling (6). However, several previous publications have demonstrated that limited resection, including anatomic segmentectomy and nonanatomic wedge resection, will lead to equivalent survival outcomes compared with lobectomy, particularly among elderly patients (7-10). Although the epidemic of lung cancer shows a trend of younger patients, there remains an urgent need to develop effective treatment methods for older patients. Limited resection may play an import role in the surgical strategy for elderly patients for the advantage of preserving pulmonary function and vital lung parenchyma (11).
The International Association Study of Lung Cancer (IASLC), the American Thoracic Society (ATS) and the European Respiratory Society (ERS) proposed a new edition of the lung adenocarcinoma classification system in 2011 (12). It has been effectively used to predict the prognosis of lung adenocarcinoma patients. The classification was established on the most predominant subtype presented: invasive adenocarcinoma with solid (SOL) or micropapillary (MIP) carcinoma, which is associated with a poor survival (13-17). Both adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) have nearly 100% postsurgical survival, and limited resection is appropriate for those patients. However, to date, whether limited resection is adequate for early-stage invasive lung adenocarcinoma patients, especially for patients who are older than 65, has not been thoroughly studied (8). In this study, a large cohort investigation was carried out to determine the correlation between surgical procedures and postoperative survival among elderly lung adenocarcinoma patients with tumor sizes ≤2 cm. Our study may help to design personalized surgical strategies for older patients.
Methods
Patient cohort
The retrospective study was permitted by the Institutional Review Board of Shanghai Chest Hospital at Shanghai Jiao Tong University and was performed in compliance with the guidelines of clinical research [ethic approval ID: KS(P)1802].
We retrospectively reviewed patients who underwent surgical resection at our hospital between January 2009 and March 2015. The inclusion criteria were patients 65 years old or older, presentation of a single lung nodule, the postsurgical pathological specimen examination validated that the tumor size was less than or equal to 2 cm, there was no lymph node invasion, and there was no distant organ metastasis. The exclusion criteria included patients who had a history of malignancy, had multiple nodules, received neoadjuvant chemotherapy AIS or MIA. Invasive adenocarcinoma variants (such as mucinous, enteric or fetal morphologies) were also excluded for low incidence. Finally, this study included a total of 666 patients.
In our clinical practice, patients with the following characteristics were treated with limited resection: tumor size no larger than 2 cm (in most cases, the tumor size was not larger than 1 cm), radiological features included peripheral pure ground nodular ground-glass opacity (GGO), <1 cm part-solid GGO presented on CT, or poor pulmonary function which could not tolerate lobectomy, especially in elderly patients. We usually perform lobectomy in patients with tumor sizes >2 cm, ≥1 cm part-solid GGO or solid nodule with any size. The final surgical procedure was performed with the agreement of patient.
Clinicopathological evaluation
We reevaluated the histological classification of resected specimens that were obtained before the new IASLC/ATS/ERS classification method for lung adenocarcinoma was conducted in the department of pathology of our institution. According to the new IASLC/ATS/ERS classification criteria, invasive adenocarcinomas were divided into five major subtypes including lepidic (LEP), papillary (PAP), acinar (ACN), SOL and MIP predominant subgroups.
All the patients were respectively staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging manual (18). The clinicopathological features including age, sex, tumor size, surgical procedure, history of adjuvant therapy, visceral pleural invasion (VPI), lymphovascular invasion (LVI) and survival status were gathered from patients’ medical records.
Surveillance protocol
Disease-free survival (DFS) was defined as the time from surgery to the date of recurrence or metastasis detection. We defined overall survival (OS) as the time between the first surgery and the date of death resulting from any cause. At the end of the follow-up, patients without any events were censored. DFS and OS status were obtained from either clinical medical records or by telephone.
The routine preoperative examination included a head and chest computed tomography (CT) scan, upper abdominal sonography to exclude multiple nodules and distant metastasis, pulmonary function testing, and heart sonography. Positron emission tomography (PET) scans were recommended for patients with suspicious hilum or mediastinal lymph node enlargement. The postsurgical surveillance was performed as previously described (19,20): chest CT and neck and upper abdominal ultrasound examinations were performed every 3 months for the first year after surgery and at 6-month intervals thereafter. Brain magnetic resonance imaging (MRI) and whole-body bone scanning were performed each year. Additional examinations were included if patients incurred any other symptoms regardless of the routine follow-up schedule. For those patients who attended regular follow-up appointments after surgery at a different, local hospital, we conducted follow-up telephone calls to determine the survival status.
Statistical methods
Chi-square (χ2) tests were used to compare categorical and continuous variables between the lobectomy and limited resection groups. The Cochran-Mantel-Haenszel test was used to estimate the associations among the different surgical procedure groups and covariates. The log-rank test was applied to evaluate the differences in DFS and OS between various surgical treatments for univariable analysis. Multivariable Cox models were stratified by trial. They were adjusted for VPI, tumor size, histologic subtype, gender, adjuvant chemotherapy, surgical procedure, and LVI. The predictive value of these variables on patient survival was also measured. The value of statistical significance was set to 0.05 (pooled analysis). Statistical analyses were performed via SPSS software (version 19; SAS Institute, Cary, NC, USA) and GraphPad (Prism 5).
Results
Out of the 666 patients, 558 (83.8%) patients were older than 65 but less than or equal to 75 years old, while 108 (16.2%) individuals were over 75 years old. There were 290 (43.5%) males and 376 (56.5%) females. In total, 104 (15.6%) patients had tumor sizes less than or equal to 1 cm, while most patients (562, 84.4%) had tumor sizes greater than 1 cm but less than or equal to 2 cm. A total of 120 (18.0%) patients presented VPI, while 25 (3.8%) patients had LVI. The majority patients (442, 66.4%) underwent a lobectomy, and 224 (33.6%) patients underwent a limited resection, including 166 cases of wedge resection and 58 cases of segmentectomy. Only 69 (10.4%) patients received adjuvant chemotherapy after surgery. According to the predominant tumor pattern, there were 74 (11.1%) LEP, 332 (49.8%) ACN, 234 (35.1%) PAP, 3 (0.5%) MIP and 23 (3.5%) solid patterns. There were 546 (82.0%) and 120 (18.0%) patients diagnosed with stage T1a and T2a, respectively. The demographic characteristics of all patients are summarized in Table 1.
Table 1. Baseline characteristics of lung adenocarcinoma patients with tumor size less than or equal to 2 cm.
| Characteristic | Total, N (%) | Lobectomy, N (%) | Limited resection | P value* | |
|---|---|---|---|---|---|
| Segmentectomy, N (%) | Wedge, N (%) | ||||
| Sex | 0.247 | ||||
| Male | 290 (43.5) | 185 (41.9) | 18 (31.0) | 87 (52.4) | |
| Female | 376 (56.5) | 257 (58.1) | 40 (69.0) | 79 (47.6) | |
| Age, years | 0.000 | ||||
| ≤75 | 558 (83.8) | 391 (88.5) | 54 (93.1) | 113 (68.1) | |
| >75 | 108 (16.2) | 51 (11.5) | 4 (6.9) | 53 (31.9) | |
| Tumor size | 0.054 | ||||
| ≤1 cm | 104 (15.6) | 60 (13.6) | 12 (20.7) | 32 (19.3) | |
| 1–2 cm | 562 (84.4) | 382 (86.4) | 46 (79.3) | 134 (80.7) | |
| Visceral pleural invasion | 0.088 | ||||
| Yes | 120 (18.0) | 88 (19.9) | 3 (5.2) | 29 (17.5) | |
| No | 546 (82.0) | 354 (80.1) | 55 (94.8) | 137 (82.5) | |
| Lymphovascular invasion | 0.195 | ||||
| Yes | 25 (3.8) | 20 (4.5) | 1 (1.7) | 4 (2.4) | |
| No | 641 (96.2) | 422 (95.5) | 57 (98.3) | 162 (97.6) | |
| Adjuvant chemotherapy | 0.284 | ||||
| Yes | 69 (10.4) | 50 (11.3) | 2 (3.4) | 17 (10.2) | |
| No | 597 (89.6) | 392 (88.7) | 56 (96.6) | 149 (89.8) | |
| Adenocarcinoma subtype | 0.627 | ||||
| Lepidic | 74 (11.1) | 46 (10.4) | 10 (17.2) | 18 (10.8) | |
| Acinar | 332 (49.8) | 223 (50.5) | 32 (55.2) | 77 (46.4) | |
| Papillary | 234 (35.1) | 158 (35.7) | 16 (27.6) | 60 (36.1) | |
| Micropapillary | 3 (0.5) | 1 (0.2) | 0 (0) | 2 (1.2) | |
| Solid | 23 (3.5) | 14 (3.2) | 0 (0) | 9 (5.4) | |
*, χ2 test was calculated from logistic regression model stratified by trail. P value is for the comparison between lobectomy and limited resection.
Survival analysis
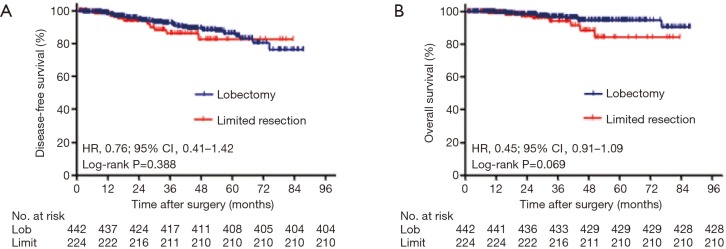
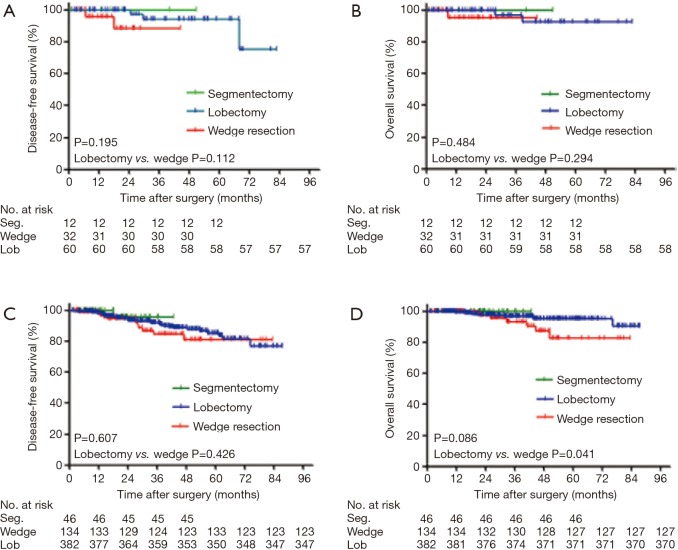
At the end of the follow-up period, 53 (8.0%) patients experienced recurrence, and 21 died of lung cancer among the 22 (3.3%) patients who were gone. The median follow-up period was 36.2 months (range, 6.0–87.4 months). When the segmentectomy group and the wedge resection group were merged as a limited resection group, the univariable analysis showed that no significant prognostic difference was observed between lobectomy and limited resection for DFS [hazard ratio (HR), 0.76; 95% CI, 0.41–1.42; P=0.388) or OS (HR, 0.45; 95% CI, 0.19–1.09; P=0.069; Figure 1A,B]. To identify appropriate patients for limited surgery, we classified all patients into two groups according to tumor size: ≤1 cm or 1–2 cm (containing 2 cm). For patients with tumor sizes less than or equal to 1 cm, there were no significant differences in DFS (P=0.195) and OS (P=0.484) among the three surgical procedures. Wedge resection was equivalent to lobectomy for the nonsignificant DFS (HR, 1.12; 95% CI, 0.02–1.51, P=0.112) and OS (HR, 0.26; 95% CI, 0.02–3.51; P=0.294; Figure 2A,B). For patients with tumor sizes larger than 1 cm but less than or equal to 2 cm, the survival analysis showed that DFS (P=0.607) and OS (P=0.086) were not influenced by the type of surgical procedure. However, wedge resection was not equivalent to lobectomy, showing a nonsignificant DFS (HR, 0.76; 95% CI, 0.39–1.50; P=0.426) but a significant OS (HR, 0.39; 95% CI, 0.15–1.00; P=0.041; Figure 2C,D).
Figure 1.
Survival cures for DFS (A) and OS (B) according to surgical procedures in stage I lung adenocarcinoma patients with tumor size ≤2 cm. P values from log-rank test. DFS, disease-free survival; OS, overall survival; HR, hazard ratio; Lob, lobectomy.
Figure 2.
Survival cures for DFS (A,C) and OS (B,D) according to surgical procedures in stage I lung adenocarcinoma patients with tumor size ≤2 cm. (A,B) Survival cures for patients with tumor size ≤1 cm; (C,D) survival cures for patients with tumor size >1 but ≤2 cm. P values from log-rank test. DFS, disease-free survival; OS, overall survival; Seg., segmentectomy; Lob, lobectomy.
Risk factors for DFS and OS
To identify the risk factors for recurrence or death after surgery, we performed a multivariable analysis using multivariable Cox models. The multivariable survival analysis was adjusted for age, gender, tumor size, surgery type, VPI, histology, adjuvant chemotherapy and LVI. For patients with tumor sizes less than or equal to 1 cm, the multivariable survival analysis showed that there was no significant risk factor for survival (Table 2). For patients with tumor size lager than 1 cm but less than or equal to 2 cm, the multivariable survival analysis showed that wedge resection was related to worse OS (HR, 0.33; 95% CI, 0.12–0.90; P=0.030) but not DFS (HR, 0.85; 95% CI, 0.41–1.77; P=0.663) than lobectomy. Independent risk factors for DFS included VPI (HR, 2.32; 95% CI, 1.22–4.41; P=0.010) and LVI (HR, 3.06; 95% CI, 1.31–7.16; P=0.010). Other independent risk factors for OS included LVI (HR, 4.33; 95% CI, 1.15–16.32; P=0.030) and SOL or MIP predominant subtype (HR, 4.55; 95% CI, 1.26–16.42; P=0.021; Table 3).
Table 2. Multivariate analysis of DFS and OS among patients with tumor size ≤1 cm.
| Predictor | DFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex (female vs. male) | 2.93 | 0.36–23.90 | 0.316 | 3.52 | 0.26–47.59 | 0.343 | |
| Age (>75 vs. ≤75 years) | 0.78 | 0.06–9.46 | 0.843 | 0 | – | 0.989 | |
| Visceral pleural invasion (yes vs. no) | 7.74 | 0.61–98.12 | 0.114 | 12.22 | 0.83–180.80 | 0.069 | |
| Lymphovascular invasion (yes vs. no) | 4.09 | – | 0.999 | 0.38 | – | 0.999 | |
| Adjuvant chemotherapy (yes vs. no) | 0 | – | 0.980 | 0 | – | 0.973 | |
| Histology (SOL/MIP vs. LEP/ACN/PAP) | 0 | – | 0.997 | 0 | – | 0.998 | |
| Surgical procedure | 0.393 | 0.656 | |||||
| Segmentectomy vs. wedge resection | 0 | – | 0.991 | 0.27 | 0.02–4.34 | 0.359 | |
| Lobectomy vs. wedge resection | 0.23 | 0.03–1.90 | 0.172 | 0 | – | 0.989 | |
HR, hazard ratio; CI, confidence interval; ACT, adjuvant chemotherapy; LEP, lepidic; ACN, acinar; PAP, papillary; MIP, micropapillary; SOL, solid; DFS, disease-free survival; OS, overall survival.
Table 3. Multivariate analysis of DFS and OS among patients with tumor size >1 but ≤2 cm.
| Predictor | DFS | OS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex (female vs. male) | 1.40 | 0.77–2.53 | 0.270 | 1.20 | 0.48–3.04 | 0.697 | |
| Age (>75 vs. ≤75 years) | 1.23 | 0.57–2.66 | 0.604 | 0.53 | 0.15–1.89 | 0.327 | |
| Visceral pleural invasion (yes vs. no) | 2.32 | 1.22–4.41 | 0.010 | 2.41 | 0.91–6.42 | 0.078 | |
| Lymphovascular invasion (yes vs. no) | 3.06 | 1.31–7.16 | 0.010 | 4.33 | 1.15–16.32 | 0.030 | |
| Adjuvant chemotherapy (yes vs. no) | 1.29 | 0.62–2.70 | 0.502 | 0.41 | 0.09–1.93 | 0.261 | |
| Histology (SOL/MIP vs. LEP/ACN/PAP) | 1.80 | 0.63–5.14 | 0.273 | 4.55 | 1.26–16.42 | 0.021 | |
| Surgical procedure | 0.900 | 0.094 | |||||
| Segmentectomy vs. wedge resection | 0.76 | 0.09–6.17 | 0.795 | 0 | – | 0.983 | |
| Lobectomy vs. wedge resection | 0.85 | 0.41–1.77 | 0.663 | 0.33 | 0.12–0.90 | 0.030 | |
HR, hazard ratio; CI, confidence interval; ACT, adjuvant chemotherapy; LEP, lepidic; ACN, acinar; PAP, papillary; MIP, micropapillary; SOL, solid; DFS, disease-free survival; OS, overall survival.
Discussion
Elderly patients with invasive lung adenocarcinoma may be associated with poor pulmonary function and cardiac insufficiency (11,21), so there was an increased risk for those patients to experience peri- or post-operative complications when they underwent lobectomy (7,13,22). Limited resection has the advantages of being less invasive and allowing a faster recovery; however, the survival outcomes in the long run remain uncertain. We conducted a large-scale cohort study among pathologic stage I lung adenocarcinoma patients with tumor sizes less than 2 cm, our research revealed that for patients aged ≥65 years old, wedge resection was equivalent to lobectomy in terms of oncologic outcomes when tumor size ≤1 cm. However, wedge resection could not achieve comparable oncologic outcomes with lobectomy and revealed a significantly worse OS when tumor size was larger than 1 cm but less than or equal to 2 cm. Our conclusions will help to select optimal surgical treatment for eligible lung adenocarcinoma patients.
The novel IASLC/ATS/ERS classification categorizes adenocarcinoma into various histological subtypes, which effectively distinguish a patient’s prognosis (3,23). SOL and MIP components present aggressive biological behavior and are associated with an unfavorable prognosis (16,24). In this study, a multivariable analysis validated that SOL or MIP tumor subtypes were independent risk factors for poor OS. The univariable analysis showed that in our cohort, patient who underwent segmentectomy had a favorable DFS and OS, partly because there was no patient with SOL or MIP predominant subtype in this group, while these two histological patterns possessed a similar percentage in the wedge resection and lobectomy groups. In addition, the number of patients in the segmentectomy group was inadequate and only accounted for 8.7% of all patients. Therefore, in this study, we mainly focus on the survival outcomes compared between the lobectomy and wedge resection groups.
The major differences between wedge resection and lobectomy are resection margin and the number of lymph nodes examined. In our center, the margin of wedge resection was two times larger than the diameter of the tumor. However, lymph node sampling was not routinely performed. For patients who underwent lobectomy, we performed a complete lymph node dissection for accurate staging, which included stations 2, 4, 7, 8, 9, 10, 11, and 12 on the right and stations 5, 6, 7, 8, 9, 10, 11, and 12 on the left.
To date, there remains a lack of standard criteria of surgical procedure selection for early-stage NSCLC patients. The factors that influence the surgical choice for elderly patients with stage I lung adenocarcinoma include tumor size, tumor density on CT, pulmonary function and so on (25). AIS or MIA usually presented with GGO nodules, and patients with AIS or MIA had a 5-year survival of approximately 100%; therefore, patients with GGO nodules may be optimal candidates for limited resection. Tumor size is another important factor in the choice of surgical approach. In our cohort, only elderly patients with tumor sizes less than or equal 2 cm were included in the study. Previous reports revealed that in our hospital, the percentage of lymph node involvement for tumor size ≤1.0 cm, tumor size >1.0 but ≤2.0 cm, and tumor size >2.0 but ≤3.0 cm were 3.2%, 14.5% and 31.1%, respectively (26). Limited resection is not adequate for patients with lymph node involvement. Similar to our results, Veluswamy and colleagues (27) identified stage IA invasive adenocarcinoma and squamous cell carcinoma with tumor size ≤2 cm among patients older than 65 years. They indicated that limited resection, particularly wedge resection, did not lead to equivalent outcomes as lobectomy. Segmentectomy was equivalent to lobectomy only in patients with invasive adenocarcinoma. However, according to the International Association for the Study Lung Cancer staging project, the 8th TNM classification for lung cancer demonstrated that each divided centimeter of a tumor can predict a significantly different prognosis. Consistent with several previous publications (28,29), our study found that for patients with tumor sizes ≤1.0 cm, wedge resection was equivalent to lobectomy, but wedge resection predicted a significantly worse OS for patients with tumor sizes >1.0 but ≤2.0 cm, which indicated that wedge resection was insufficient for those patients.
There are several limitations in this article. First, all patients were selected from one medical center, so the inherent bias was inevitable, and the follow-up time was inadequate. Next, this was a nonrandomized, retrospective study, and the surgical procedure selection bias was inevitable.
Conclusions
In summary, our study revealed that for elderly patients, wedge resection provides an equivalent outcome to lobectomy only for patients with tumor sizes no larger than 1 cm. For those patients whose tumor size is larger than 1 cm, limited resection should be carefully considered by balancing the potential short- and long-term risks.
Acknowledgements
Funding: This study was funded by the National Science Foundation of China (No. 81301996), Wu Jieping Medical Foundation (No. 320.6750.17525) and Open Fund of Zhejiang Provincial Top Key Discipline of Pharmacology (No. YKFJ2-001).
Ethical Statement: The retrospective study was permitted by the Institutional Review Board of Shanghai Chest Hospital at Shanghai Jiao Tong University [ethic approval ID: KS(P)1802] and was performed in compliance with the guidelines of clinical research. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. 10.3322/caac.21166 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Tsao MS, Marguet S, Le Teuff G, et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J Clin Oncol 2015;33:3439-46. 10.1200/JCO.2014.58.8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. 10.1093/jnci/djt166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Wang R, Zhang Y, et al. Precise Diagnosis of Intraoperative Frozen Section Is an Effective Method to Guide Resection Strategy for Peripheral Small-Sized Lung Adenocarcinoma. J Clin Oncol 2016;34:307-13. 10.1200/JCO.2015.63.4907 [DOI] [PubMed] [Google Scholar]
- 6.Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. 10.6004/jnccn.2015.0071 [DOI] [PubMed] [Google Scholar]
- 7.Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. 10.1097/JTO.0b013e3181ae285d [DOI] [PubMed] [Google Scholar]
- 8.Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. 10.1097/SLA.0b013e3181c0e5f3 [DOI] [PubMed] [Google Scholar]
- 9.Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8. [DOI] [PubMed]
- 10.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 11.Keenan RJ, Landreneau RJ, Maley RH, Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. 10.1016/j.athoracsur.2004.01.024 [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056. 10.1016/j.athoracsur.2005.06.071 [DOI] [PubMed] [Google Scholar]
- 14.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. 10.1038/modpathol.2010.232 [DOI] [PubMed] [Google Scholar]
- 15.Yanagawa N, Shiono S, Abiko M, et al. The Clinical Impact of Solid and Micropapillary Patterns in Resected Lung Adenocarcinoma. J Thorac Oncol 2016;11:1976-83. 10.1016/j.jtho.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Li J, Wang R, et al. The prognostic and predictive value of solid subtype in invasive lung adenocarcinoma. Sci Rep 2014;4:7163. 10.1038/srep07163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Wang R, Shen X, et al. Minor Components of Micropapillary and Solid Subtypes in Lung Adenocarcinoma are Predictors of Lymph Node Metastasis and Poor Prognosis. Ann Surg Oncol 2016;23:2099-105. 10.1245/s10434-015-5043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. 10.1097/JTO.0b013e31812f3c1a [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Huang Q, Wang R, et al. Prognostic and predictive value of the novel classification of lung adenocarcinoma in patients with stage IB. J Cancer Res Clin Oncol 2016;142:2031-40. 10.1007/s00432-016-2192-6 [DOI] [PubMed] [Google Scholar]
- 20.Luo J, Wang R, Han B, et al. Analysis of the clinicopathologic characteristics and prognostic of stage I invasive mucinous adenocarcinoma. J Cancer Res Clin Oncol 2016;142:1837-45. 10.1007/s00432-016-2201-9 [DOI] [PubMed] [Google Scholar]
- 21.Donington J, Ferguson M, Mazzone P, et al. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest 2012;142:1620-35. 10.1378/chest.12-0790 [DOI] [PubMed] [Google Scholar]
- 22.Sigel K, Bonomi M, Packer S, et al. Effect of age on survival of clinical stage I non-small-cell lung cancer. Ann Surg Oncol 2009;16:1912-7. 10.1245/s10434-009-0475-8 [DOI] [PubMed] [Google Scholar]
- 23.Hung JJ, Wu YC, Chou TY, et al. Adjuvant Chemotherapy Improves the Probability of Freedom From Recurrence in Patients With Resected Stage IB Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1346-53. 10.1016/j.athoracsur.2015.10.075 [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Zhu LZ, Jiang MJ, et al. Clinical impacts of a micropapillary pattern in lung adenocarcinoma: a review. Onco Targets Ther 2015;9:149-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsutani Y, Miyata Y, Nakayama H, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest 2014;145:66-71. 10.1378/chest.13-1094 [DOI] [PubMed] [Google Scholar]
- 26.Yu Y, Jian H, Shen L, et al. Lymph node involvement influenced by lung adenocarcinoma subtypes in tumor size ≤3 cm disease: A study of 2268 cases. Eur J Surg Oncol 2016;42:1714-9. 10.1016/j.ejso.2016.02.247 [DOI] [PubMed] [Google Scholar]
- 27.Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. 10.1200/JCO.2014.60.6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawabata N. Pulmonary wedge resection for Stage I non-small-cell lung cancer: possible alternative strategy to lobectomy. Eur J Cardiothorac Surg 2017;53:484. 10.1093/ejcts/ezx370 [DOI] [PubMed] [Google Scholar]
- 29.Tsutani Y, Mimura T, Kai Y, et al. Outcomes after lobar versus sublobar resection for clinical stage I non-small cell lung cancer in patients with interstitial lung disease. J Thorac Cardiovasc Surg 2017;154:1089-96.e1. 10.1016/j.jtcvs.2017.03.116 [DOI] [PubMed] [Google Scholar]