Abstract
Background
Primary Sjögren syndrome (pSS) is a chronic inflammatory autoimmune disease that is characterized by lymphocytic infiltration of the exocrine glands and extraglandular organ systems. Interstitial lung disease (ILD) is common in pSS patients and is one of the independent risk factors for a poor prognosis. The previously reported characteristics and potential risks contributing to pSS-associated ILD have been controversial.
Methods
A cohort of 201 newly diagnosed pSS patients were studied over a period of 3 years. Data were from clinical charts. The pSS patients were classified into two groups, namely pSS-ILD or pSS without ILD, according to the lung evaluation.
Results
In total, the prevalence of pSS-associated ILD was 78.6%. The pSS patients associated ILD were more likely to be male, older and smokers in comparison to the pSS patients without ILD. There were no significant differences in multiorgan involvement between the two groups. Nonspecific interstitial pneumonia (NSIP) was the most common radiological pattern (45.5%). pSS with ILD was associated with increasing age [odds ratio (OR) =1.073], smoking (OR =8.544) and antinuclear antibody (ANA) positive (OR =3.286). Over a median follow-up period of 24 months (range, 18–30 months), no patients died, experienced acute exacerbation of ILD, or had newly diagnosed pSS-ILD.
Conclusions
pSS associated ILD were more commonly in males, older patients and smokers. Aging, cigarette smoking, and ANA positivity may be potential risk factors contributing to ILD in pSS patients.
Keywords: Primary Sjögren syndrome (pSS), interstitial lung disease (ILD), smoking, antinuclear antibody (ANA), risk factors
Introduction
Sjögren syndrome (SS) is a chronic inflammatory autoimmune disorder characterized by dry eyes (xerophthalmia) and dry mouth (xerostomia) induced by focal lymphocytic infiltration in the lacrimal and salivary glands (1). SS can occur alone as primary SS (pSS) or in association with other autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosus, which is known as secondary SS (2). pSS is a heterogeneous disease characterized by a wide spectrum of presentations. The extraglandular organ systems, including the lungs, kidneys, small vasculature, and other endocrine glands, are often involved in pSS (2). The presentation of pSS may be significantly influenced by epidemiological characteristics, systemic involvement, or the immunological profile at diagnosis.
Varied pulmonary manifestations were reported as extraglandular complications, depending on the detection methods and patient selection (3-5). High resolution computed tomography (HRCT) is a useful tool to detect lung involvement. HRCT can be used to detect ground-glass attenuation, thin-walled cysts, honeycombing, reticular pattern, small nodules, and enlarged mediastinal lymph nodes in pSS patients (6). The interstitial lung manifestations of pSS include nonspecific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), lymphoid interstitial pneumonia (LIP), organizing pneumonia (OP), and bronchiolitis (7). pSS-associated ILD is one of the independent risk factors for poor prognosis in SS patients (8,9). The potential risks contributing to pSS-associated ILD were not fully clarified because of the small number of patients included in the previous studies and the different classification criteria used.
In this study, we enrolled a cohort of 201 pSS patients and aimed to evaluate the different clinical features of pSS with or without ILD and to explore the risk factors contributing to ILD in pSS patients.
Methods
Patients
A cohort of 201 consecutive inpatients with newly diagnosed pSS were recruited from Beijing Chao-Yang Hospital over a period of 36 months (January 2012 to December 2014) in the prospective study. The diagnosis fulfilled the diagnostic criteria for pSS published by the American-European Consensus Group (10). Clinical data were collected from predesigned clinical charts. The chart contained questions regarding dry cough, dyspnea on exertion, and symptoms and signs of multiorgan involvement, including Raynaud’s phenomenon, arthralgia, phonaesthesia, weight loss, morning stiffness, sicca symptoms, dysphagia, fever of unknown origin, gastroesophageal reflux, rash, oral ulcer, alopecia, and proximal muscle weakness.
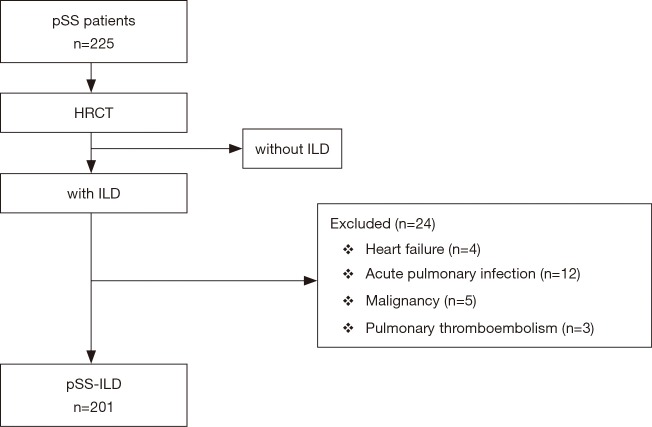
The patients were all receiving their routine medications from their doctors. Twenty-four patients were excluded because they had clinical, radiographic or electrocardiographic signs of heart failure, acute pulmonary infection, malignancy, or pulmonary thromboembolism (Figure 1).
Figure 1.
Flow chart of screening the study population.
The smoking status of all patients was carefully collected, and they were categorized as non-smokers, ex-smokers (had quit smoking ≥12 months previously), and smokers (currently smoking or had quit smoking <12 months previously).
All investigations were conducted in accordance with the ethical standards of Beijing Chao-Yang Hospital and the World Medical Association Declaration of Helsinki. The protocol was approved by the Institutional Review Board (IRB) of Beijing Chao-Yang Hospital. Informed consents were obtained from all patients.
Laboratory testing
Autoimmune serology, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum fibrinogen, and immunoglobulin (including G, A, M, E, and D) measurements were carried out for all patients. Antinuclear antibody (ANA) testing was performed using indirect immunofluorescence, and the result was considered positive when ≥3+. Specific antibody characterization performed in all patients, including tests for anti-Ro/SSA and anti-La/SSB. A rheumatoid factor (RF) result was negative if less than or equal to the upper limit of normal for the laboratory test. RF was analyzed using partial agglutination and the result considered positive when greater than 15.9 IU/mL.
HRCT scans
All 201 patients underwent HRCT scans with a 1-second scanning time with 1-mm sections and 10-mm intervals from the lung apex to base. The scans included both lungs in the field of view. Each HRCT scan was reviewed independently by two experienced thoracic radiologists blinded to the clinical data before the therapeutic interventions. One hundred and fifty-eight (78.6%) patients with radiographic features of interstitial abnormalities were included in the pSS-ILD group, and the remaining patients were classified in the pSS without ILD group. The HRCT patterns were obtained and recorded by experienced thoracic radiologists according to the classification of idiopathic pulmonary interstitial pneumonias (IIPs) (11). The interobserver correlation was good. The kappa value was 0.83.
One hundred and three (65%) pSS-ILD patients underwent percutaneous lung biopsy or bronchoscopy, including bronchoalveolar lavage total cell counts and cell differentials and transbronchial lung biopsy. Five (3%) pSS-ILD patients received surgical lung biopsies. In these patients, the pathological diagnoses were NSIP in three patients and LIP in two patients.
Pulmonary function tests
Pulmonary function tests performed according to the guidelines. For each patient, we recorded the partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2), forced vital capacity (FVC), forced expired volume in the first second (FEV1), residual volume, and total lung capacity (TLC). Moreover, each patient underwent measurements of the maximal expiratory flow after 25% to 75% of the FVC remains to be exhaled (MEF25-75). In addition, we measured the diffusing capacity of the lung for carbon monoxide (DLCO). The DLCO adjusted for alveolar volume using the single-breath method, with the values corrected for the present hemoglobin values. Small airway obstruction on pulmonary function tests represents that MEF25 and MEF50 were lower than 80% predicted, while PEF and MEF75 were at normal range.
Pulmonary hypertension (PH) measurements
PH was defined by a systolic arterial pulmonary pressure ≥50 mmHg, as estimated by the tricuspid regurgitant flow on echocardiography (12). Pulmonary thromboembolism was excluded by computed tomography pulmonary angiography.
Statistical analysis
Patients’ characteristics, clinical symptoms, serum serologic test results and radiographic patterns were reported as the mean ± standard deviation (SD) or as frequency counts and percentages. Comparisons between groups were made using the t-test, χ2 test, or Fisher exact test, as appropriate. All P-values corresponded to two-sided tests, and statistical significance defined by a P value less than 0.05. All analyses were performed with SPSS statistical software (version 17.0). Logistic regressions were used to test the effect of potentially influencing variables on lung involvement. Each outcome was analyzed separately. First, dependent variables were tested individually in simple regressions. Next, variables with P values ≤0.1 were entered into a multiple logistic regression analysis to provide adjusted estimations of the odds ratio (OR). The estimate sample size was calculate that is 20 times as much as the independent variable.
Results
Demographic and clinical characteristics
Of all, the prevalence of pSS with ILD was 78.6% (158/201). The pSS patients with ILD were more likely to be male, older and smokers than the pSS patients without ILD (Table 1). All of the pSS patients with ILD had respiratory syndromes, including dry cough and dyspnea on exertion. There were no differences in the symptoms and signs of multiorgan involvement between the two groups. In addition, no significant difference in PH was found between the two groups.
Table 1. Demographic and cumulative frequency of clinical characteristics in 201 patients with primary Sjögren syndrome.
| Characteristics | pSS-ILD (n=158) | pSS without ILD (n=43) | P value# |
|---|---|---|---|
| Female, n [%] | 134 [85] | 42 [98] | 0.020 |
| Age*, years | 61.6±11.3 | 48.9±14.7 | <0.010 |
| Current smokers, n [%] | 22 [14] | 0 [0] | <0.010 |
| Ex-smokers, n [%] | 15 [9] | 1 [2] | 0.200 |
| Non-smokers, n [%] | 121 [77] | 42 [98] | <0.010 |
| Clinical course†, months | 35.7±59.6 | 38.4±43.1 | 0.840 |
| Signs and symptoms, n [%] | |||
| Cough | 116 [73] | 11 [26] | <0.010 |
| Dyspnea | 87 [55] | 6 [14] | <0.010 |
| Raynaud’s phenomenon | 12 [8] | 4 [9] | 0.750 |
| Arthralgia | 34 [22] | 13 [30] | 0.320 |
| Photaesthesia | 5 [3] | 1 [2] | 1.000 |
| Morning stiffness | 10 [6] | 2 [5] | 1.000 |
| Sicca symptoms | 125 [79] | 36 [84] | 1.000 |
| Dysphagia | 3 [2] | 0 [0] | 1.000 |
| Fever | 1 (1) | 1 [2] | 0.390 |
| Gastro-oesophageal reflux | 3 [2] | 2 [5] | 0.300 |
| Rash | 16 [10] | 5 [12] | 0.790 |
| Oral ulcer | 8 [5] | 5 [12] | 0.170 |
| Alopecia | 1 [1] | 1 [2] | 0.390 |
| Proximal muscle weakness | 1 [1] | 1 [2] | 0.390 |
| Positive lip biopsy#, n [%] | 67 [75] | 10 [83] | 0.740 |
| Pulmonary hypertension&, n [%] | 10 [6.3] | 0 [0] | 0.070 |
Values are given as n (%) or mean ± SD. *, age at the initial diagnosed pSS; †, period from the initial symptoms; #, eighty nine patients in pSS-ILD or 12 patients in pSS without ILD had received lip biopsy respectively; &, 156 (77.6%) pSS patients underwent echocardiography including 115 patients with ILD and 41 patients without ILD; ILD, interstitial lung disease; pSS, primary Sjögren syndrome.
HRCT findings
Diverse pulmonary manifestations were observed in the pSS-ILD patients. The ILD on HRCT manifested linear or reticular abnormalities, ground glass attenuation, consolidation, diffusing cystic shadow, small nodules and/or mosaic sign in pSS patients. According to the ATS/ERS classification of IIPs, the HRCT pattern of pSS-ILD was shown in NSIP, UIP, LIP, OP patterns, unclassifiable interstitial pneumonia and bronchiolitis as well. NSIP was the most common radiological pattern (72, 45.5%). The HRCT scans also indicated the presence of UIP (16, 10.1%), LIP (13, 8.2%), bronchiolitis (12, 7.6%), OP (6, 3.8%) and unclassifiable interstitial pneumonia (39, 24.7%).
Pulmonary function tests
The pulmonary function values for FVC, FEV1, TLC, and DLCO SB were significantly lower in the pSS patients with ILD than in the pSS patients without ILD (Table 2). Small airway obstruction was detect in 76.1% (86/113) of the pSS patients. However, there were no significant differences in the MEF25, MEF50 and MEF75 values between the two groups.
Table 2. Pulmonary function profiles in the patients with primary Sjögren syndrome.
| Variables | pSS-ILD (n=158) | pSS without ILD (n=43) | P value |
|---|---|---|---|
| FVC, % pred | 82.5±20.9 | 109.0±14.2 | 0.006 |
| FEV1, % pred | 79.7±21.4 | 98.0±17.7 | 0.005 |
| FEV1/FVC, % | 80.8±6.9 | 75.4±6.7 | 0.012 |
| TLC, % pred | 75.8±15.8 | 98.4±13.9 | 0.003 |
| RV/TLC, % | 35.6±7.6 | 37.6±5.4 | 0.313 |
| MEF75, % pred | 87.5±25.6 | 98.5±27.3 | 0.164 |
| MEF50, % pred | 65.6±25.5 | 78.5±29.4 | 0.118 |
| MEF25, % pred | 51.7±30.8 | 54.7±18.1 | 0.749 |
| DLCO SB, % pred | 42.9±19.4 | 74.6±17.9 | <0.001 |
Values are given as the mean ± SD. FVC, forced vital capacity; FEV1, forced expired volume in the first second; DLCO SB, diffusion capacity for carbon monoxide of the lung single breath; TLC, total lung capacity; RV, residual volume; MEF25-75, maximal expiratory flow after 25–75% of the FVC has been not exhaled.
Laboratory findings
As shown in Table 3, the pSS patients with ILD had a lower serum IgG level than the pSS patients without ILD. The serum fibrinogen level was approximately in a normal range, although the serum fibrinogen level was higher in pSS-ILD patients than the pSS patients without ILD. In addition, the serum fibrinogen level positively correlated with both ESR (r=0.367, P<0.010) and CRP (r=0.447, P<0.010). ANA positivity was higher in pSS-ILD than in pSS patients without ILD. Moreover, the positive rates of detecting anti-SSA and anti-SSB antibodies were not significantly different between the two groups.
Table 3. Laboratory findings in the patients with primary Sjögren syndrome.
| Findings | pSS-ILD (n=158) | pSS without ILD (n=43) | P value# |
|---|---|---|---|
| RF positive, n (%) | 46 [33] | 16 [41] | 0.350 |
| ESR, mm/h | 26.4±19.6 | 28.3±24.9 | 0.590 |
| CRP, mg/dL | 1.3±2.8 | 1.1±2.8 | 0.580 |
| Fibrinogen, mg/dL | 329.1±82.7 | 295.0±68.3 | 0.010 |
| IgG, mg/dL | 1,523.0±605.7 | 1,947.2±744.2 | <0.010 |
| C3, mg/dL | 97.7±21.2 | 91.6±22.3 | 0.120 |
| C4, mg/dL | 21.4±7.9 | 19.8±6.4 | 0.260 |
| PaO2, mmHg (room air) | 79.7±16.9 | 97.6±23.5 | <0.010 |
| ANA positive, n (%) | 121 [77] | 25 [58] | 0.020 |
| anti-SSA, n (%) | 88 [56] | 30 [70] | 0.120 |
| anti-SSB, n (%) | 26 [17] | 13 [30] | 0.050 |
Values are given as n (%) or mean ± SD. RF, rheumatoid factor; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PaO2, artery pressure of oxygen; pSS, primary Sjögren syndrome.
Analysis of potential risks
Univariate analysis showed that age, sex, cigarettes smoking, fibrinogen, IgG, RF, ANA, SSA and SSB were the key factors related to pSS-ILD. According to the multifactor logistic regression analysis, age, cigarettes smoking and ANA-positive were the potential risk factors for pSS-associated ILD (Tables 4,5).
Table 4. Single factor logistic regression analysis for primary Sjögren syndrome.
| Variables | B | SE | Wals | P value | OR | 95% CI of OR |
|---|---|---|---|---|---|---|
| Age | 0.08 | 0.02 | 25.65 | <0.010 | 1.08 | 1.05–1.12 |
| Sex | 2.02 | 1.04 | 3.80 | 0.050 | 7.52 | 0.99–57.29 |
| Smokers | 2.55 | 1.03 | 6.15 | 0.010 | 12.84 | 1.71–96.53 |
| Fibrinogen | 0.01 | 0.00 | 5.97 | 0.020 | 1.01 | 1.00–1.01 |
| IgG | 0.00 | 0.00 | 11.47 | <0.010 | 1.00 | 0.99–1.00 |
| RF | −0.93 | 0.38 | 6.12 | 0.010 | 0.40 | 0.19–0.83 |
| ANA | 0.86 | 0.36 | 5.61 | 0.020 | 2.36 | 1.16–4.78 |
| SSA | −0.61 | 0.37 | 2.72 | 0.090 | 0.55 | 0.26–1.12 |
| SSB | −0.78 | 0.39 | 3.90 | 0.050 | 0.46 | 0.21–0.99 |
B, regression coefficient; SE, standard error; OR, odds ratio; RF, rheumatoid factor; ANA, antinuclear antibody; SSA, anti-Ro antibody; SSB, anti-La antibody.
Table 5. Multinomial logistic regression model for primary Sjögren syndrome associated interstitial lung disease.
| Variables | B | SE | P value | OR | 95% CI of OR |
|---|---|---|---|---|---|
| Age | 0.07 | 0.02 | <0.010 | 1.07 | 1.04–1.11 |
| Smoking | 2.15 | 1.05 | 0.040 | 8.55 | 1.09–67.19 |
| ANA | 1.19 | 0.47 | 0.010 | 3.29 | 1.30–8.28 |
B, regression coefficient; SE, standard error; OR, odds ratio; ANA, antinuclear antibody.
Therapeutic regimens and follow-up
In total, 48 (23.9%) patients received oral prednisolone alone, 52 (25.9%) patients received immunosuppressants (including cyclophosphamide, azathioprine, colchicine or hydroxychloroquine) alone, 54 (26.9%) patients received oral prednisolone combined with immunosuppressants, and 47 (23.3%) patients received symptomatic treatment only. In pSS-ILD, 133 (84.2%) patients received prednisolone or immunosuppressants or combined of above two. Over a median follow-up period of 24 months (range, 18–30 months), no patients died, experienced acute exacerbation of ILD, or had newly diagnosed pSS-ILD.
Discussion
The prevalence of pSS-ILD was 78.6% in our cohort of patients with pSS. To our knowledge, this study enrolled the largest number of pSS-ILD patients to date to explore the potential risks contributing to pSS-associated ILD. The pSS patients with ILD tended to be male, older, and cigarette smokers in comparison to the pSS patients without ILD. Moreover, there was a higher ANA-positive rate in the pSS-ILD than in the pSS patients without ILD. Our results demonstrate that age, cigarette smoking and ANA-positive status may be potential risk factors contributing to pSS-associated ILD.
pSS is a chronic autoimmune disease, and genetic predisposition may play a role in its pathogenesis (13). A population-based study showed that the annual incidence of pSS was 5.1/100,000 population, and increased with age at pSS diagnosis (18–44 years: 1.8/100,000 vs. ≥75 years: 10.7/100,000) (14). Female patients were more affected (8.7/100,000) than male patients (1.1/100,000) (14). In Greece, the proportion of female patients was high (female to male ratio, 16–22:1), and in Britain and Hungary, the proportion of male patients was higher than usual (female to male ratio, 8:1) (15-18). Our data showed that pSS more commonly affected females who were older, and the female to male ratio of 7:1 was in line with previous reports (19,20).
ILD is commonly observed in pSS patients. According to different studies with varied methodologies, the percentage of pSS patients with ILD has been reported to range from 9% to 75% (14,19,21). In our cohort of patients, the prevalence of pSS-associated ILD was 78.6%. The potential risk factors of pSS-associated ILD are still unclear. Zhang et al. (9) found that pSS patients with ILD were significantly older and more likely to endure a longer course of illness than pSS patients without ILD. pSS patients without prior ILD were found to have a cumulative incidence of ILD of 10% at 1 year after their pSS diagnosis, which increased to 20% at 5 years after the onset of pSS (14). These findings suggest that older pSS patients with a longer course of illness might be more prone to develop ILD. However, ILD can be a preliminary manifestation prior to the diagnosis of pSS. Our data suggest that aging is associated with an increased risk of pSS-associated ILD.
In comparison to the pSS patients without ILD, significantly higher levels of ESR, CRP, fibrinogen, IgG, and C3 and lower levels of albumin were detected in pSS patients with ILD in a study of 87 pSS patients (9). In addition, the development of ILD was associated with a higher frequency of oral ulcers, Reynaud’s phenomenon, positive antineutrophil cytoplasmic antibodies, and galectin-3 findings (9). In a study of 384 pSS patients, ILD was detected in 59 (18.6%) patients, and other signs of lung involvement were detected, including abnormal pulmonary function, PH, multiple lung bullae, and pleural effusion (19). Initial symptoms of parotid enlargement and purpura, anti-La/SSB positivity, and high levels of IgG and IgA were found to be independent variables in pSS patients with lung involvement (19). In contrast with these findings, we found that smoking and ANA-positivity were the potential risks contributing to pSS-associated ILD. Evidence has suggested a causal link between cigarette smoke and the development of alveolar wall fibrosis (22). Cigarette smoking contributes to lung fibrosis by leading to a loss of airspace wall tissue in regions remote from the macroscopic lesions and by causing a net increase in collagen mass (23). Studies revealed mechanistic links between aging and lung fibrosis involving telomere attrition, genomic instability, and epigenetic alterations (24-26). Antihistone antibodies, a type of ANA, are correlated with severe pulmonary fibrosis in systemic sclerosis patients (27). Using immunohistochemistry and immunoprecipitation, Takahashi et al. (28) identified an antibody that precipitated in the cytoplasm of epithelial lung cells from idiopathic pulmonary fibrosis patients, confirming the role of the antibody in the process of lung fibrosis.
The NSIP pattern on CT is the most prevalent pattern (45.5%) of interstitial pneumonia that was observed in our cohort of pSS patients. This result was consistent with previous histological findings in pSS-ILD patients that showed fibrosing or cellular NSIP patterns (1). The clinical entity of NSIP appears to be frequently related to an autoimmune disease. A previous study showed that patients with undifferentiated connective tissue disease (UCTD) were more likely to have an NSIP pattern on biopsy (29). In addition, multifocal cysts were reported on 7–30% of HRCT scans although parenchymal cysts were found less commonly than other radiographic patterns in pSS-ILD (6,7,30,31). In our cohort, ground-glass opacities with multifocal thin-walled cysts, indicating a CT-LIP pattern, were observed in 8.2% of pSS patients. Chest HRCT and histological studies indicated that cystic airspace is a partial airway obstruction caused by peribronchiolar cellular infiltration (32). In a cohort of 60 patients with pSS, the most common CT findings were areas with ground-glass attenuation (92%), which are commonly observed in NSIP or LIP patterns (6). HRCT features appeared to correlate well with the underlying histopathological patterns of ILD with NSIP, UIP, OP, or LIP in pSS patients (7).
The American Thorax Society/European Respiratory Society classification proposed the unclassifiable category of IIP (11). Despite a thorough multidisciplinary evaluation, up to 15% of ILD patients have unclassifiable ILD and cannot be assigned a specific interstitial pneumonia classification (33). Cases that are unclassifiable due to an overlap of histological patterns often indicate related connective tissue diseases. Interstitial pneumonia with follicular bronchiolitis in a patient with pSS may be termed unclassifiable interstitial pneumonia based on the HRCT appearance (11). In our cohort of pSS patients, 24.6% had an unclassifiable HRCT pattern, indicating a diversity of histological lung lesions.
Evidence to guide treatment strategies in pSS-ILD remains limited (34). In one case report, a pSS-LIP patient was treated with corticosteroids, azathioprine, and hydroxychloroquine (35). However, the authors noted other cases with fibrosis progressed despite immunosuppression. Other case series reported symptomatic improvement upon treatment with immunosuppressive therapy or rituximab (7,36). In our cohort of pSS patients, the variety of therapeutic regimens reflected the doctors’ decisions and the patients’ willingness. The therapeutic schemes in pSS-ILD inclined to oral prednisolone, or immunosuppressants, or combined of these two.
The present study had several limitations. Firstly, the selection bias may exist. Our population is not fully representative of the diversity of organ involvements in pSS because our patients were from a single medical center with a reputation for pulmonology. No lymphoma was detect during the study period. All enrolled patients are Chinese Han population, overlooking the potential influence of geolocation on results (37). The patients diagnosed pSS were undertaken chest HRCT to determine whether the lungs was involved or even in the early stage. However, it could exist a potential inclusion bias that might be relevant to assess the validity of study design. Secondly, it is occasionally difficult to discriminate NSIP from UIP and LIP on HRCT without pathologic evaluation. Finally, although we performed a prospective cohort study, the follow-up period was not long enough to observe whether the therapeutic regimens delayed or prevented the onset of ILD in pSS patients.
To conclude, the present study investigated 201 patients with pSS at a single center. The findings indicate that pSS patients had a high prevalence of associated ILD. pSS patients with ILD tended to be male, cigarette smokers, and older. Aging, smoking and ANA positivity may be potential risk factors associated with lung involvement in pSS. Patients with these risk factors should receive follow-ups.
Acknowledgements
We thank all patients and investigators who were involved in this study. We express our thanks to our colleague Y Chen and research assistant M Xu from Beijing No. 2 Middle School International Division.
Funding: The work was supported by National Major Scientific and Technological Special Project for Significant New Drugs Development (2015ZX09J15104) and National Natural Science Foundation of China (81370159).
Ethical Statement: This work was conducted at Beijing Chao-Yang Hospital with approval from the ethics committee of Beijing Chao-Yang Hospital, Capital Medical University (No. 81370159). Informed consent was documented in writing.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ito I, Nagai S, Kitaichi M, et al. Pulmonary manifestations of primary Sjogren's syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med 2005;171:632-8. 10.1164/rccm.200403-417OC [DOI] [PubMed] [Google Scholar]
- 2.Tsuboi H, Asashima H, Takai C, et al. Primary and secondary surveys on epidemiology of Sjogren's syndrome in Japan. Mod Rheumatol 2014;24:464-70. 10.3109/14397595.2013.843765 [DOI] [PubMed] [Google Scholar]
- 3.Kreider M, Highland K. Pulmonary involvement in Sjogren syndrome. Semin Respir Crit Care Med 2014;35:255-64. 10.1055/s-0034-1371529 [DOI] [PubMed] [Google Scholar]
- 4.Palm O, Garen T, Berge Enger T, et al. Clinical pulmonary involvement in primary Sjogren's syndrome: prevalence, quality of life and mortality--a retrospective study based on registry data. Rheumatology (Oxford) 2013;52:173-9. 10.1093/rheumatology/kes311 [DOI] [PubMed] [Google Scholar]
- 5.Reina D, Roig Vilaseca D, Torrente-Segarra V, et al. Sjogren's syndrome-associated interstitial lung disease: A multicenter study. Reumatol Clin 2016;12:201-5. 10.1016/j.reuma.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 6.Koyama M, Johkoh T, Honda O, et al. Pulmonary involvement in primary Sjogren's syndrome: spectrum of pulmonary abnormalities and computed tomography findings in 60 patients. J Thorac Imaging 2001;16:290-6. 10.1097/00005382-200110000-00010 [DOI] [PubMed] [Google Scholar]
- 7.Parambil JG, Myers JL, Lindell RM, et al. Interstitial lung disease in primary Sjogren syndrome. Chest 2006;130:1489-95. 10.1378/chest.130.5.1489 [DOI] [PubMed] [Google Scholar]
- 8.Lin DF, Yan SM, Zhao Y, et al. Clinical and prognostic characteristics of 573 cases of primary Sjogren's syndrome. Chin Med J (Engl) 2010;123:3252-7. [PubMed] [Google Scholar]
- 9.Zhang R, Sun T, Song L, et al. Increased levels of serum galectin-3 in patients with primary Sjogren's syndrome: associated with interstitial lung disease. Cytokine 2014;69:289-93. 10.1016/j.cyto.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 10.Vitali C, Bombardieri S, Jonsson R, et al. European Study Group on Classification Criteria for Sjogren's S: Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002;61:554-8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493-537. 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Zhang K, Chen H, et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjogren's syndrome at 7q11.23. Nat Genet 2013;45:1361-5. 10.1038/ng.2779 [DOI] [PubMed] [Google Scholar]
- 14.Nannini C, Jebakumar AJ, Crowson CS, et al. Primary Sjogren's syndrome 1976-2005 and associated interstitial lung disease: a population-based study of incidence and mortality. BMJ Open 2013;3:e003569. 10.1136/bmjopen-2013-003569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skopouli FN, Dafni U, Ioannidis JP, et al. Clinical evolution, and morbidity and mortality of primary Sjogren's syndrome. Semin Arthritis Rheum 2000;29:296-304. 10.1016/S0049-0172(00)80016-5 [DOI] [PubMed] [Google Scholar]
- 16.Davidson BK, Kelly CA, Griffiths ID. Primary Sjogren's syndrome in the North East of England: a long-term follow-up study. Rheumatology (Oxford) 1999;38:245-53. 10.1093/rheumatology/38.3.245 [DOI] [PubMed] [Google Scholar]
- 17.Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren's syndrome. Arthritis Rheum 2002;46:741-7. 10.1002/art.10221 [DOI] [PubMed] [Google Scholar]
- 18.Horvath IF, Szanto A, Papp G, et al. Clinical course, prognosis, and cause of death in primary Sjogren's syndrome. J Immunol Res 2014;2014:647507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Li Y, Wang L, et al. Primary Sjogren syndrome in Han Chinese: clinical and immunological characteristics of 483 patients. Medicine (Baltimore) 2015;94:e667. 10.1097/MD.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Xu B, Ma Y, et al. Clinical and laboratory profiles of primary Sjogren's syndrome in a Chinese population: A retrospective analysis of 315 patients. Int J Rheum Dis 2015;18:439-46. 10.1111/1756-185X.12583 [DOI] [PubMed] [Google Scholar]
- 21.Shi JH, Liu HR, Xu WB, et al. Pulmonary manifestations of Sjogren's syndrome. Respiration 2009;78:377-86. 10.1159/000214841 [DOI] [PubMed] [Google Scholar]
- 22.Katzenstein AL, Mukhopadhyay S, Zanardi C, et al. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol 2010;41:316-25. 10.1016/j.humpath.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Lang MR, Fiaux GW, Gillooly M, et al. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax 1994;49:319-26. 10.1136/thx.49.4.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armanios M. Telomeres and age-related disease: how telomere biology informs clinical paradigms. J Clin Invest 2013;123:996-1002. 10.1172/JCI66370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricci A, Cherubini E, Ulivieri A, et al. Homeodomain-interacting protein kinase2 in human idiopathic pulmonary fibrosis. J Cell Physiol 2013;228:235-41. 10.1002/jcp.24129 [DOI] [PubMed] [Google Scholar]
- 26.Robinson CM, Neary R, Levendale A, et al. Hypoxia-induced DNA hypermethylation in human pulmonary fibroblasts is associated with Thy-1 promoter methylation and the development of a pro-fibrotic phenotype. Respir Res 2012;13:74. 10.1186/1465-9921-13-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S, Ihn H, Kikuchi K, et al. Antihistone antibodies in systemic sclerosis. Association with pulmonary fibrosis. Arthritis Rheum 1994;37:391-4. 10.1002/art.1780370313 [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Wada I, Ohtsuka Y, et al. Autoantibody to alanyl-tRNA synthetase in patients with idiopathic pulmonary fibrosis. Respirology 2007;12:642-53. 10.1111/j.1440-1843.2007.01140.x [DOI] [PubMed] [Google Scholar]
- 29.Kinder BW, Collard HR, Koth L, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med 2007;176:691-7. 10.1164/rccm.200702-220OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uffmann M, Kiener HP, Bankier AA, et al. Lung manifestation in asymptomatic patients with primary Sjogren syndrome: assessment with high resolution CT and pulmonary function tests. J Thorac Imaging 2001;16:282-9. 10.1097/00005382-200110000-00009 [DOI] [PubMed] [Google Scholar]
- 31.Yazisiz V, Arslan G, Ozbudak IH, et al. Lung involvement in patients with primary Sjogren's syndrome: what are the predictors? Rheumatol Int 2010;30:1317-24. 10.1007/s00296-009-1152-8 [DOI] [PubMed] [Google Scholar]
- 32.Ichikawa Y, Kinoshita M, Koga T, et al. Lung cyst formation in lymphocytic interstitial pneumonia: CT features. J Comput Assist Tomogr 1994;18:745-8. 10.1097/00004728-199409000-00012 [DOI] [PubMed] [Google Scholar]
- 33.Skolnik K, Ryerson CJ. Unclassifiable interstitial lung disease: a review. Respirology 2016;21:51-6. 10.1111/resp.12568 [DOI] [PubMed] [Google Scholar]
- 34.Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest 2013;143:814-24. 10.1378/chest.12-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalvi V, Gonzalez EB, Lovett L. Lymphocytic interstitial pneumonitis (LIP) in Sjogren's syndrome: a case report and a review of the literature. Clin Rheumatol 2007;26:1339-43. 10.1007/s10067-006-0351-x [DOI] [PubMed] [Google Scholar]
- 36.Deheinzelin D, Capelozzi VL, Kairalla RA, et al. Interstitial lung disease in primary Sjogren's syndrome. Clinical-pathological evaluation and response to treatment. Am J Respir Crit Care Med 1996;154:794-9. 10.1164/ajrccm.154.3.8810621 [DOI] [PubMed] [Google Scholar]
- 37.Brito-Zerón P, Acar-Denizli N, Zeher M, et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjögren’s syndrome at diagnosis in 8310 patients: a cross-sectional study from the Big Data Sjögren Project Consortium. Ann Rheum Dis 2017;76:1042-50. 10.1136/annrheumdis-2016-209952 [DOI] [PubMed] [Google Scholar]