Abstract
Background
Heart donor selection criteria have been progressively widened due to increasing donor recipient mismatch. This study evaluates the outcomes of the use marginal donor hearts for orthotopic heart transplantation (OHT) based on a single center experience in China.
Methods
We retrospectively analyzed outcomes of patients undergoing OHT in our hospital between September 2008 and December 2015. All the donor hearts were from voluntary donation of brain-dead patients. The primary outcome was overall survival; secondary outcomes included cardiopulmonary bypass (CPB) time, ventilation time, post-operative mechanical support and medium-term complications.
Results
Overall, 278 patients with OHT were analyzed. Whereas 180 patients (64.7%) underwent OHT utilizing marginal donors (MD group), only 98 patients (35.3%) underwent OHT with standard donors (SD group). Compared to the SD group, the MD group had longer CPB time (P=0.001), ventilation time (P=0.010) and increased mechanical support rate (P=0.011). Survival rates were comparable between the two groups at 30 days, 1 year, 3 years and 5 years (92.2%, 83.3%, 70.6%, 70.6% vs. 95.9%, 91.4%, 80.2%, 80.2% respectively). Multivariate Cox regression analysis revealed that female recipient gender [hazard ratio (HR) =2.632 (1.325–5.227), P=0.006], diagnosis (P=0.014) and abnormal donor heart structure [HR =3.638 (1.005–13.167), P=0.049] were three predictors for 1-year all-cause mortality. The occurrence of complications in the recipients with more than 3-year follow-up did not differ between the two cohorts.
Conclusions
Marginal donor can be reasonably applied to expand the benefits of transplantation. Changing previous MD criteria to include donors with an age greater than 50 years, cold ischemic time less than 6 hours, donor/recipient weight ratio less than 0.8, compatible blood type, hepatitis virus seropositivity and MD used for male recipient will likely offer a good prognosis.
Keywords: Heart transplantation, marginal donors, survival analysis, complications
Introduction
Despite the rapid progress of mechanical circulation assistance, especially ventricular assist devices in heart failure, heart transplantation is still the optimal choice for patients with end-stage heart disease. Till 2014, 120,992 orthotropic heat transplantations have been registered worldwide; only 1,483 of these transplants were performed in mainland China (1).
However, the enormous discrepancy between the growing number of candidates and the limited availability of organs has led to the biggest problem in the transplantation system. More than 30% of the patients die on the waiting list for transplantation (2). A number of options have been proposed to expand the donor pool, including new areas of myocardial protection, advanced systems for organ allocation and social awareness of organ donation (3). Unfortunately, given the stage of development of mainland China, the use of hearts through the liberalization of donor acceptance criteria may be the most realistic method to overcome organ shortage.
Marginal organs are initially turned down by standard transplant recipient lists for left ventricle hypertrophy, structure abnormalities, high inotrope requirements, and positive hepatitis serologies but accepted by patients at risk of imminent death or those at high medical risk who would otherwise not have been offered heart transplantation (4). Nonetheless, different centers have dissimilar criteria (5,6) of marginal donors that focus on their own risk factors and are not in strict accordance with guidelines of the International Society for Heart and Lung Transplantation (ISHLT) (7). As representatives of the highest transplantation volume Chinese center in 2014 and 2015, we will briefly introduce the clinical effect of using marginal donors at our institute.
Methods
This study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (IORG No. IORG0003571) and performed in accordance with the ethical statement of the Declaration of Helsinki and ISHLT.
Study population
Data for all 278 adult (age ≥18) cardiac transplant recipients in our center from September 1, 2008 to December 31, 2015 were collected retrospectively from electronic medical records. All grafts listed in this study were procured from donors after brain death, not from executed prisoners. Patients who underwent cardiac retransplantation or multivisceral transplantation were excluded from this analysis. The recipient population was divided into 2 groups: the marginal donor group (MD, 180) and the standard donor (SD, 98) group.
Combining the experience of Massad (8), Lima et al. (5) and Taghavi et al. (9), donors were considered marginal if they met any of the following criteria: (I) advanced age (≥50 years old and ≤60 years old); (II) long cold ischemic time (>360 min); (III) donor/recipient size mismatch (0.6< donor: recipient weight ratio <0.8); (IV) non-identical but compatible ABO/Rh blood type; (V) hepatitis C seropositivity or hepatitis HBsAg, HBeAb and HBcAb positive simultaneously; (VI) coronary artery disease (CAD, any coronary artery stenosis evident on coronary angiogram or greater than mild calcified plaque) or repairable atrial septal defect (ASD); (VII) requiring high-dose inotropic support defined as the administration of dopamine or dobutamine (≥15 µg/kg/min), epinephrine or norepinephrine (≥0.5 µg/kg/min) (Table 1).
Table 1. Donor characteristics for assignment to the marginal donor group.
| Variables | No. (%) (n=278) |
|---|---|
| Donor | |
| Age ≥50 years old | 25 (8.99) |
| Cold ischemic time >360 min | 102 (36.69) |
| Donor/recipient weight ratio <0.8 | 51 (18.35) |
| Non-identical but compatible ABO/Rh blood type | 44 (15.83) |
| Hepatitis virus seropositivity | 14 (5.04) |
| CAD or ASD | 7 (2.52) |
| High-dose inotropic support | 4 (1.44) |
CAD, coronary artery disease; ASD, atrial septal defect.
Organ preservation and operation technique
A uniform method of preservation was applied to all donor hearts and consisted of 1 L of cold (4 °C) histidine-tryptophan-ketoglutarate (HTK) solution during transport. Additionally, 500 mL of HTK solution was perfused before implantation, and a typical biatrial or bicaval procedure with moderate hypothermia (28 °C) was performed. A total of 5 donor allografts were identified with CAD and subsequently underwent coronary artery bypass graft (CABG). Two additional ASD hearts were repaired using patches. The need for post-transplant mechanical [intra-aortic balloon pump (IABP) or extracorporeal membrane oxygenation (ECMO)] or inotropic support was determined by the surgeon on the basis of intraoperative transesophageal echocardiography (TEE), visualization of the heart and hemodynamic monitoring.
Post-transplantation treatment
Basiliximab (20 mg) was administered intraoperatively and on the 4th day post-operation by intravenous pump for induction immunotherapy. This mediation was followed by a standard triple-drug immunosuppression regimen, including cyclosporine A (CsA)/tacrolimus, mycophenolate mofetil and prednisone. Prophylactic antibiotic therapy was discontinued in patients who exhibited no sign of infection seven days after transplantation. Patients with elevated pulmonary pressure after operation were prescribed iloprost by inhaler and a 3-month course of oral sildenafil (10). Followed by endomyocardial biopsy, acute cellular rejection exceeding grade 2R according to the ISHLT criteria (11) was treated by administering 500 mg of methylprednisolone for three days and increasing the doses of immunosuppressive drugs.
Outcome measures
Demographic and clinical characteristics of all heart transplant donors and recipients were examined. After being discharged from our hospital, all patients were admitted to the outpatient department weekly for the 1st month, biweekly until the 3rd month, monthly from the 4th to 12th month, and twice for one year thereafter.
Immunosuppressant treatment failure resulted in switching to another primary immunosuppressive drug (CsA to tacrolimus or adding sirolimus) due to the obvious adverse effect and the occurrence of acute or chronic rejection. Acute rejection could be noted under surveillance through outpatient review. In contrast, chronic rejection, often with the manifestation of chronic allograft vasculopathy (CAV), which accounts for one-third of all-cause mortality at 5 years (12), might occur in the first half of the postoperative year. CAV was diagnosed if any coronary plaque or stenosis was found on computed tomography angiograph (CTA) image, which was suggested to be conducted annually (13).
The patients who died or received heart retransplantation were included in the main outcome measures. Specifically, we selected 68 recipients from September 1, 2008 to March 1, 2013 with sufficient follow-up time to make a detailed analysis of immune inhibitor-related and unrelated complications.
March 1, 2016 was set as the end point of this study. The mean follow-up duration was 15.54 (6.98–28.73) months, and 269/278 (96.76%) patients completed followed-up.
Statistical analysis
Unless otherwise stated, continuous variables conforming to a normal distribution were expressed as a mean ± standard deviation and analyzed by a 2-sample t-test. Variables fitting a skewed distribution, which were reported as the median [inter-quartile range (IQR)], were analyzed by the Mann-Whitney test. Categorical variables were presented as counts followed by percentages in parentheses and analyzed by the Chi-square test. The time to event analysis was estimated by the Kaplan-Meier method using the log-rank test. Univariate survival and mechanical support analysis were conducted using the Cox proportional hazard model and logistic regression, respectively. Covariates with P<0.05 in univariate analysis were then analyzed by stepwise multivariate regression with a probability of 0.05 and an elimination probability of 0.10. All tests were two-tailed with a 5% significance level. Statistical analysis was performed with SPSS version 21.0 (IBM corporation, Armonk, NY, USA).
Results
Baseline characteristic
Among the total 180 marginal donors listed in Table 1, there were 122 with only one marginal donor criterion, 50 with two marginal donor criteria, 7 with three marginal donor criteria and 1 with four marginal donor criteria. Baseline characteristics are presented in Table 2. The MD group was heavier than the SD group (66.11±14.41 vs. 60.21±9.79 kg, P<0.001) and included more patients with high blood pressure (20.00% vs. 10.20%). Perioperative data are recorded in Table 3. The MD group had longer Intra-operative cardiopulmonary bypass (CPB) time [104.00 (90.00–130.00) vs. 97.00 (86.00–107.00) min, P=0.001] and postoperative ventilation time [28.00 (20.00–42.00) vs. 22.00 (17.75–35.25) min, P=0.010]. The SD group exhibited more blood drainage [385.00 (265.00–505.00) vs. 450.00 (330.00–550.00) mL, P=0.020]. Consistent with preferred use of mechanical support [50 (27.78%) vs. 14 (14.29%), P=0.011 for IABP or ECMO and 46 (25.56%) vs. 12 (12.24%), P=0.009 for IABP usage], dobutamine use rate was higher in the MD group (P<0.001). The number of patients with postoperative complications and treatment rejection [11 (6.11%) vs. 3 (3.06%), P=0.730] did not vary considerably before discharge from our hospital, and hospital mortality did not vary [7 (3.89%) vs. 2 (2.04%), P=0.623].
Table 2. Baseline characteristics of marginal donor group and standard donor group.
| Variables | MD (n=180) | SD (n=98) | P value |
|---|---|---|---|
| Donor | |||
| Gender male (%) | 160 (88.89) | 89 (90.82) | 0.615 |
| Age (years) | 35.30±12.30 | 35.30±8.23 | 1.000 |
| Body weight (kg) | 60.00 (55.00–70.00) | 65.00 (60.00–70.00) | 0.054 |
| Cold ischemic time (min) | 382.00 (156.00–455.00) | 150.00 (123.00–283.50) | <0.001 |
| Donor/recipient weight ratio | 0.92 (0.77–1.14) | 1.05 (0.93–1.17) | <0.001 |
| Recipient | |||
| Gender, male (%) | 144 (80.00) | 78 (79.59) | 0.935 |
| Age (years) | 45.77±12.76 | 46.60±12.40 | 0.604 |
| Body weight (kg) | 66.11±14.41 | 60.21±9.79 | <0.001 |
| BMI (kg/m2) | 23.48±4.03 | 21.51±2.67 | <0.001 |
| Diagnosis | 0.190 | ||
| ICM (%) | 126 (70.00) | 79 (80.61) | |
| CAD (%) | 29 (16.11) | 9 (9.18) | |
| VHD (%) | 8 (4.44) | 5 (5.10) | |
| Others (%) | 17 (9.44) | 5 (5.10) | |
| High blood pressure history (%) | 36 (20.00) | 10 (10.20) | 0.036 |
| Diabetes history (%) | 31 (17.22) | 15 (15.31) | 0.681 |
| Renal impairment (%) | 36/179 (20.11) | 20/97 (20.62) | 0.920 |
| Liver impairment (%) | 46/179 (25.70) | 24/97 (24.74) | 0.862 |
| Neurological impairment (%) | 10/179 (5.59) | 11/97 (11.34) | 0.085 |
| PRA ≥10% (%) | 1 (0.56) | 3 (3.06) | 0.251 |
| LCM (%) | 7.00 (5.00–8.00) | 6.00 (5.00–8.00) | 0.298 |
| Preoperative MPAP (mmHg) | 38.49±13.85 | 38.96±12.84 | 0.796 |
| Preoperative LVEF (%) | 27.00 (21.00–32.00) | 27.00 (21.00–31.00) | 0.584 |
Continuous data of normal distribution expressed as a mean ± standard deviation, and the rest presented as median (IQR 25–75); Categorical data as number (percentage). Renal impairment stands for renal insufficiency, hydronephrosis, hyperuricemia, kidney stone and cyst of kidney. Liver impairment stands for medical history of liver disease including fatty liver, gall-stone, hypohepatia etc. Neurological impairment stands for any neurosurgery, vertebrobasilar insufficiency, lacunar or cerebral infarction, encephalatrophy and epilepsy. BMI, body mass index; ICM, idiopathic cardiomyopathy including dilated cardiomyopathy, hypertrophic cardiomyopathy, restrictive cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; VHD, valvular heart disease; PRA, panel reactive antibody; LCM, lymphocyte cross matching; MPAP, mean pulmonary artery pressure; LVEF, left ventricle ejection fraction.
Table 3. Perioperative data of marginal donor group and standard donor group.
| Variables | MD (n=180) | SD (n=98) | P value |
|---|---|---|---|
| CPB time (min) | 104.00 (90.00–130.00) | 97.00 (86.00–107.00) | 0.001 |
| ICU length of stay (d) | 6.00 (5.00–8.00) | 6.00 (5.00–11.83) | 0.219 |
| Blood drainage (mL) | 385.00 (265.00–505.00) | 450.00 (330.00–550.00) | 0.020 |
| Peak of CVP (mmHg) | 14.53±3.50 | 14.78±4.96 | 0.626 |
| Ventilation time (h) | 28.00 (20.00–42.00) | 22.00 (17.75–35.25) | 0.010 |
| PO mechanical support | |||
| IABP (%) | 46 (25.56) | 12 (12.24) | 0.009 |
| ECMO (%) | 11 (6.11) | 5 (5.10) | 0.730 |
| PO inotropic support | |||
| Using time for epinephrine (d) | 1.00 (0.00–3.00) | 1.00 (0.00–2.00) | 0.143 |
| Epinephrine at 24 h (μg/kg/min) | 0.00 (0.00–2.00) | 0.00 (0.00–1.00) | 0.029 |
| Using time for dopamine (d) | 5.00 (3.00–8.00) | 4.00 (3.00–7.00) | 0.217 |
| Dopamine at 24 h (μg/kg/min) | 5.00 (3.00–6.00) | 4.00 (3.00–5.00) | 0.072 |
| Use of dobutamine (%) | 87 (48.33) | 23 (23.47) | < 0.001 |
| PO LVEF (%) | 66.14±6.12 | 66.24±4.96 | 0.894 |
| Reoperation for bleeding (%) | 6 (3.33) | 2 (2.04) | 0.810 |
| PO ≥2 TI (%) | 17/116 (12.07) | 2/34 (5.88) | 0.289 |
| CRRT (%) | 16 (8.89) | 6 (6.12) | 0.414 |
| PO neurological impairment (%) | 11 (6.11) | 3 (3.06) | 0.410 |
| PO infection (%) | 51 (28.33) | 25 (25.51) | 0.614 |
| PO hospitalization duration (d) | 25.00 (21.00–32.00) | 25.00 (22.00–32.00) | 0.436 |
| PO treated rejection (%) | 11 (6.11) | 5 (5.10) | 0.730 |
| PO mortality (%) | 7 (3.89) | 2 (2.04) | 0.623 |
Continuous data of normal distribution expressed as a mean ± standard deviation, and the rest presented as median (IQR 25–75); Categorical data as number (percentage). CPB, cardiopulmonary bypass; ICU, intensive care unit; CVP, central venous pressure; PO, postoperative; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; TI, tricuspid insufficiency; CRRT, continuous renal replacement therapy.
Given 30% 30-day mortality and 34.6% (14) 1-year morality in recipients with primary graft dysfunction (PGD), logistic regression models were utilized to determine the predictors listed in Tables 2 and 3. After univariate analysis, only cold ischemic time (P=0.012) was found to be independent predictor of hospital mechanical support (Table 4). Of note, times greater than 8 hours [OR =3.617 (1.496–8.750), ≤4 hours as reference, P=0.004] and time between 6 and 8 hours [OR =1.758 (0.894–3.460), ≤4 hours as reference, P=0.102] were both associated with high risk of it. Other donor characteristics, such as compatible but non-identical ABO type, low donor/recipient weight ratio, older donor, donor hepatitis virus seropositive, donor CAD or ASD and high-inotropic support donor did not significantly predict mechanical support.
Table 4. Univariate logistic regression of primary graft dysfunction requiring mechanical support.
| Variables | Odds ratio (95% CI) | P value |
|---|---|---|
| Cold ischemic time | ||
| ≤4 | Ref. | 0.012 |
| [4, 6] | 0.791 (0.315–1.988) | 0.619 |
| [6, 8] | 1.758 (0.894–3.460) | 0.102 |
| >8 | 3.617 (1.496–8.750) | 0.004 |
| ABO type compatible not same (vs. ABO type same) | 0.706 (0.310–1.608) | 0.408 |
| Donor/recipient weight ratio <0.8 (vs. ≥0.8) | 1.056 (0.515–2.165) | 0.882 |
| Donor age ≥50 years old (vs. <50 years old) | 0.975 (0.371–2.560) | 0.959 |
| Donor weight | 0.994 (0.965–1.024) | 0.684 |
| Donor female gender (vs. male gender) | 1.587 (0.684–3.683) | 0.282 |
| Donor infectious status (hepatitis virus seropositive vs. seronegative) | 0.923 (0.249–3.415) | 0.904 |
| Donor CAD or ASD (vs. normal) | 1.348 (0.255–7.121) | 0.725 |
| Donor high-dose inotropic support (vs. normal) | 0.286 (0.040-2.075) | 0.216 |
| Recipient female gender (vs. male gender) | 0.676 (0.320–1.431) | 0.306 |
| Recipient age | 1.006 (0.984–1.029) | 0.615 |
| Recipient weight | 1.013 (0.993–1.035) | 0.207 |
| Recipient BMI | 1.053 (0.976–1.136) | 0.179 |
| Diagnosis | ||
| ICM | Ref. | 0.256 |
| CAD | 1.895 (0.877–4.094) | 0.104 |
| Others | 1.264 (0.533–2.886) | 0.579 |
| High blood pressure (vs. normal blood pressure) | 1.138 (0.539–2.405) | 0.734 |
| Diabetes mellitus (vs. no diabetes mellitus) | 0.784 (0.342–1.794) | 0.564 |
| Renal impairment (vs. no renal impairment) | 1.472 (0.758–2.858) | 0.253 |
| Liver impairment (vs. no liver impairment) | 1.372 (0.735–2.558) | 0.320 |
| Neurological impairment (vs. no neurological impairment) | 1.061 (0.373–3.021) | 0.911 |
CAD, coronary artery disease; ASD, atrial septal defect; ICM, idiopathic cardiomyopathy.
Short and intermediate-term survival rates
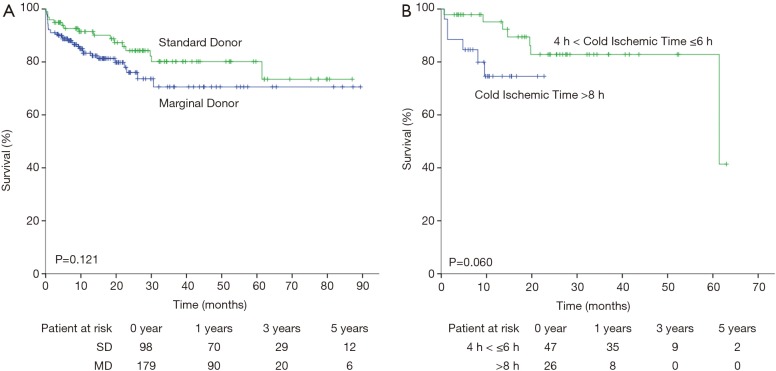
Excluding 1 patient who died on the operating table, the remaining 277 recipients were followed to track survival rates (Figure 1A). The SD group had higher survival rate than the MD group [95.9% vs. 92.2% in 30 days, 91.4% vs. 83.3% in 1 year, 80.2% vs. 70.6% in 3 years and 80.2% vs. 70.6% in 5 years], however, no significant differences in the endpoints were noted (overall P=0.121).
Figure 1.
Kaplan-Meier curve of mortality after heart transplantation for recipients still alive after operation stratified by (A) standard donor (green line) and marginal donor (blue line); (B) cold ischemic time less or equal than 6 hours but above 4 hours (green line) and cold ischemic time above 8 hours (blue line). Log-rank test was used for analysis.
Predictors of survival outcomes
Donor and recipient predictive factors of death or retransplantation are presented in Table 5. According to the univariate analysis, female recipient gender, recipient weight, recipient diagnosis and recipient liver impairment were independent predictors of main outcomes in the cohort. After multivariate analysis, only female recipient gender [HR =2.861 (1.609–5.088), P<0.001] persisted. Kaplan-Meier analysis also revealed that cold ischemic time greater than 8 hours resulted in worse survival outcomes than cold ischemic time between 4 and 6 hours at a marginally significant level (P=0.060, Figure 1B).
Table 5. Univariate and multivariate cox regression analysis for overall survival outcomes.
| Variables | Hazard ratio (95% CI) | P Value |
|---|---|---|
| Univariate | ||
| Cold ischemia time | ||
| ≤4 | Ref. | 0.429 |
| [4, 6] | 0.777 (0.335–1.805) | 0.558 |
| [6, 8] | 1.117 (0.555–2.245) | 0.757 |
| >8 | 1.922 (0.768–4.812) | 0.163 |
| ABO type compatible not same (vs. ABO type same) | 1.226 (0.596–2.523) | 0.580 |
| Donor/recipient weight ratio <0.8 (vs. ≥0.8) | 0.402 (0.145–1.119) | 0.081 |
| Donor age ≥50 years old (vs. <50 years old) | 0.797 (0.246–2.581) | 0.705 |
| Donor weight | 1.018 (0.987–1.050) | 0.265 |
| Donor female gender (vs. male gender) | 1.839 (0.862–3.925) | 0.115 |
| Donor infectious status (hepatitis virus seropositive vs. seronegative) | 1.339 (0.416–4.306) | 0.625 |
| Donor CAD or ASD (vs. normal) | 2.766 (0.857–8.927) | 0.089 |
| Donor high-dose inotropic support (vs. normal) | 1.380 (0.190–10.036) | 0.751 |
| Recipient female gender (vs. male gender) | 2.773 (1.565–4.915) | <0.001 |
| Recipient age | 0.995 (0.972–1.017) | 0.639 |
| Recipient weight | 0.971 (0.949–0.994) | 0.012 |
| Recipient BMI | 0.946 (0.866–1.034) | 0.220 |
| Diagnosis | ||
| ICM | Ref. | 0.037 |
| CAD | 1.276 (0.532–3.061) | 0.585 |
| Others | 2.461 (1.236–4.900) | 0.010 |
| High blood pressure (vs. normal blood pressure) | 0.866 (0.367–2.042) | 0.742 |
| Diabetes mellitus (vs. no diabetes mellitus) | 0.640 (0.254–1.612) | 0.343 |
| Renal impairment (vs. no renal impairment) | 1.608 (0.852–3.037) | 0.143 |
| Liver impairment (vs. no liver impairment) | 1.859 (1.027–3.364) | 0.041 |
| Neurological impairment (vs. no neurological impairment) | 1.800 (0.765–4.235) | 0.178 |
| Multivariate | ||
| Recipient female gender | 2.861 (1.609–5.088) | <0.001 |
CAD, coronary artery disease; ASD, atrial septal defect; ICM, idiopathic cardiomyopathy.
Furthermore, 1-year Cox analysis was also performed to evaluate the predictors for short-term outcomes (Table 6). In addition to female recipient gender, donor CAD or ASD and recipient disease other than idiopathic cardiomyopathy (ICM) and CAD also became risk factors in the final model.
Table 6. Univariate and multivariate cox regression analysis for 1-year survival outcomes.
| Variables | Hazard ratio (95% CI) | P value |
|---|---|---|
| Univariate | ||
| Cold ischemic time | ||
| ≤4 | Ref. | 0.184 |
| [4, 6] | 0.311 (0.072–1.348) | 0.119 |
| [6, 8] | 0.986 (0.439–2.215) | 0.973 |
| >8 | 1.823 (0.718–4.629) | 0.207 |
| ABO type compatible not same (vs. ABO type same) | 0.925 (0.359–2.384) | 0.872 |
| Donor/recipient weight ratio <0.8 (vs. ≥0.8) | 0.416 (0.127–1.361) | 0.147 |
| Donor age ≥50 years old (vs. <50 years old) | 0.951 (0.290–3.116) | 0.934 |
| Donor weight | 1.012 (0.976–1.048) | 0.528 |
| Donor female gender (vs. male gender) | 2.189 (0.956–5.012) | 0.064 |
| Donor infectious status (hepatitis virus seropositive vs. seronegative) | 1.905 (0.583–6.221) | 0.286 |
| Donor CAD or ASD (vs. normal) | 3.413 (1.045–11.148) | 0.042 |
| Donor high-dose inotropic support (vs. normal) | 2.616 (0.353-19.381) | 0.347 |
| Recipient female gender (vs. male gender) | 2.838 (1.443–5.584) | 0.003 |
| Recipient age | 1.005 (0.978–1.032) | 0.737 |
| Recipient weight | 0.959 (0.933–0.987) | 0.004 |
| Recipient BMI | 0.904 (0.815–1.003) | 0.057 |
| Diagnosis | ||
| ICM | Ref. | 0.011 |
| CAD | 1.484 (0.557–3.955) | 0.430 |
| Others | 3.232 (1.508–6.924) | 0.003 |
| High blood pressure (vs. normal blood pressure) | 0.896 (0.348–2.310) | 0.821 |
| Diabetes mellitus (vs. no diabetes mellitus) | 0.965 (0.374–2.487) | 0.941 |
| Renal impairment (vs.. no renal impairment) | 1.897 (0.929–3.873) | 0.079 |
| Liver impairment (vs. no liver impairment) | 2.065 (1.050–4.061) | 0.036 |
| Neurological impairment (vs. no neurological impairment) | 2.017 (0.783–5.201) | 0.146 |
| Multivariate | ||
| Recipient female gender | 2.632 (1.325–5.227) | 0.006 |
| Diagnosis | ||
| ICM | Ref. | 0.014 |
| CAD | 1.383 (0.485–3.947) | 0.544 |
| Others | 3.133 (1.452–6.758) | 0.004 |
| Donor CAD or ASD (vs. normal) | 3.638 (1.005–13.167) | 0.049 |
CAD, coronary artery disease; ASD, atrial septal defect; ICM, idiopathic cardiomyopathy.
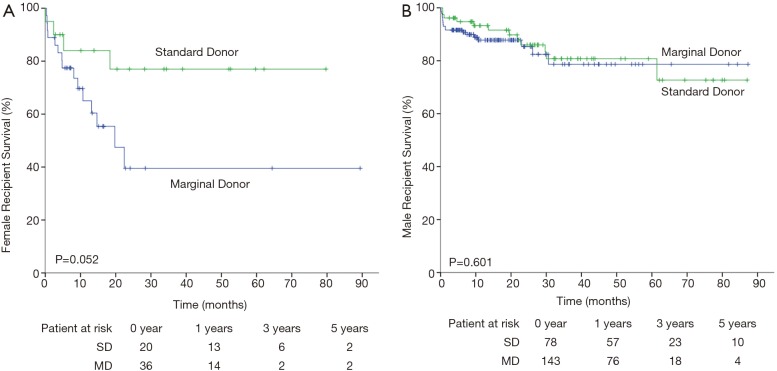
Finally, the risk factors listed above were considered in determining whether marginal donors could be used in these situations. Considering the overall survival curve for female recipients, marginal donors exhibited relatively high mortality compared with standard donors (P=0.052, Figure 2A). No difference was observed for male recipients (P=0.601, Figure 2B). However, there was no significant difference for ICM, CAD recipients utilizing marginal donors (data not shown). The 1- and 3-year survival rates for ICM were 88.1% and 71.5% for the MD group and 92.2% and 82.4% for the SD group, respectively.
Figure 2.
Kaplan-Meier curve of mortality after heart transplantation for (A) female patients and (B) male patients receiving standard (green line) or marginal donor (blue line). Log-rank test was used for analysis.
Intermediate-term complications
From the information obtained through the outpatient department and telephone calls, immunosuppressant usage was recorded for 160 MD recipients and 92 SD recipients. For the typical triple-drug immunosuppression regimen, the SD group had an increased CsA utilization rate (15.22% vs. 3.75%) and immunosuppressant treatment failure (8.70% vs. 3.13%).
Specifically, of the 68 patients who were discharged from the hospital before March 1, 2013 (Table 7), 15 suffered from death or retransplantation and 6 patients were lost to follow-up. Immune inhibitor-related and -unrelated complications were comparable between the groups except that SD patients were more likely to experience ≥ grade 2 tricuspid insufficiency (15,16) (29.73% vs. 6.45%).
Table 7. Medium-term outcomes for 68 recipients.
| Variables | MD (n=31) | SD (n=37) | P value |
|---|---|---|---|
| Immune inhibitors related complications | |||
| Renal insufficiency (%, SCr >133 μmol/L) | 12 (38.71) | 14 (37.84) | 0.941 |
| Hepatic dysfunction (%, ALT >0.68 μkat/L or TB >19.0 μmol/L) | 21 (67.74) | 22 (59.46) | 0.481 |
| New-onset fast blood glucose abnormal (%, FBG >7.0 mmol/L) | 2 (6.45) | 4 (10.81) | 0.840 |
| Tchol abnormal (%, Tchol ≥5.2 mmol/L) | 13 (41.94) | 17 (45.95) | 0.740 |
| LDL-C abnormal (%, LDL-C ≥3.13 mmol/L) | 12 (38.71) | 10 (27.03) | 0.305 |
| New-onset UA abnormal (%, UA ≥420 μmol/L) | 16 (51.61) | 21 (56.76) | 0.671 |
| Pathogenic infection (%) | 9 (29.03) | 9 (24.32) | 0.661 |
| Others (%) | 7 (22.58) | 8 (21.62) | 0.924 |
| Immune inhibitors unrelated complications | |||
| Gastrointestinal symptom (%) | 2 (6.45) | 8 (21.62) | 0.157 |
| ≥ Grade 2 TI (%) | 2 (6.45) | 11 (29.73) | 0.015 |
| Moderate to massive pericardial effusion (%) | 3 (9.68) | 0 (0.00) | 0.090 |
| Pericardial adhesion (%) | 1 (3.23) | 2 (5.41) | 1.000 |
| Arrhythmia (%) | 10 (32.26) | 14 (37.84) | 0.632 |
| CAV (%) | 1 (3.23) | 5 (13.51) | 0.289 |
| LVEF, % | 68.40±4.31 | 65.61±5.76 | 0.108 |
| Blood test | |||
| TB (ìmol/L) | 16.96±9.97 | 18.90±11.16 | 0.563 |
| ALT (ìkat/L) | 0.41±0.26 | 0.35±0.26 | 0.476 |
| BUN (mmol/L) | 6.97 (6.43–9.52) | 8.10 (5.85–9.93) | 0.806 |
| SCr (μmol/L) | 99.60 (82.00–110.30) | 86.50 (72.65–106.00) | 0.145 |
| UA (μmol/L) | 350.52±91.77 | 353.79±90.70 | 0.979 |
| LDL-C (mmol/L) | 2.67±0.86 | 2.15±1.32 | 0.173 |
| Tchol (mmol/L) | 5.09±1.12 | 4.80±1.90 | 0.584 |
| FBG (mmol/L) | 6.31±2.65 | 6.56±1.62 | 0.751 |
Continuous data of normal distribution expressed as a mean ± standard deviation, and the rest presented as median (IQR 25–75); Categorical data as number (percentage). Pathogenic infection stands for positive computed tomography scan or sputum culture. Other disease listed in the table stands for femoral head osteonecrosis, herpes zoster and neoplasm etc. Tchol, total cholesterol; LDL-C, low density lipoprotein cholesterin; UA, uric acid; CAV, cardiac allograft vasculopathy; TB, total bilirubin; ALT, alanine transaminase; BUN, blood urea nitrogen; SCr, serum creatinine; FBG, fasting blood glucose.
Discussion
Since the advent of cardiac transplantation as an effective solution for advanced heart failure in the 1960s, physicians have made considerable efforts to expand donor pool due to the high demand and comparatively low supply of available organs. Numerous modified protocols regarding the suitability of potential cardiac donors have been published over recent decades (17-20). Although common guidelines for donor selection are proposed according to the United Network for Organ Sharing (UNOS) database (7), different institutes should have their own criteria for donor allocation given that higher volume centers have prior experience focusing on complex donor management and higher baseline recipient risk (6). Considering the principles proposed by Duke University Medical Center (5) and University of lllinois (8), including single vessel CAD, higher inotrope requirement, smaller donor, older donor, positive hepatitis serologies, we developed marginal donor criteria at our institute.
Based on the fact that the MD group had a lower weight ratio than the SD group when donor body weight was similar, we concluded that an overweight recipient was the main cause of the donor-recipient size mismatching. The internal relationship between body weight and high blood pressure history might also be explained the fact that more recipients suffered from high blood pressure in the MD group. Unlike the disease distribution of the ISHLT annual report (1), more idiopathic cardiomyopathy (74% vs. 55%) and less CAD (14% vs. 36%) were discovered at our institute. Patients who were designated marginal donors had increased CPB time and mechanical and inotropic support rates, suggesting a sicker status during the post-operation period. The MD group exhibited lower survival rate than the MD group, however, it offered a 70.6% 5-year survival rate to patients who would otherwise be expected to live 1 year. Moreover, intermediate term complications were similar except for ≥ grade 2 tricuspid insufficiency in the SD group, which are worthy of further analysis. Due to the high cost required to diagnose CAV, despite it’s the leading cause of late morbidity and mortality (21), only 14.3% of patients (34.7% recipients received CTA) were diagnosed with CAV in 5 years, compared with 32% when angiography was used in ISHLT data (22).
Assessing 7 marginal donor criteria separately could guide us in determining whether we should follow these criteria in the future. The 2010 guidelines recommended donor younger than 45 years or between 45 and 55 years but with a projected ischemic time ≤4 hours (7). Hong et al. also regarded older age as an independent risk factor for 1-year mortality (23), but the upper limit of the acceptable age continued to increase as time progresses. In our study, age as a continuous variable or categorical variable with a cutoff of 50 years did not affect short and intermediate-term survival rates or the mechanical support incidence rate. There were 25 patients with an average age of 49.3 years who received hearts from donors 50 years or older; the other group consisted of recipients who received hearts from younger donors with an average age of 45.7 years. Matching a younger donor with a young recipient may be basic principle of donor selection.
A 11,700 patients study showed ischemic time between 4 and 6 hours had an odds ratio of 1.4 (1.3–1.6) compared with a time of 2 to 4 hours when concerning 1-year outcomes (23). In our center, 4 to 6 hours resulted in a better outcome, though it made a rapid decline in the 5-year follow-up for lack of enough patients. Meanwhile, cold ischemic time between 6 and 8 hours and greater than 8 hours were predictors for mechanical support and had worse survival outcomes. In conclusion, cold ischemic within 6 hours without other risk factors was acceptable for recipients according to logistic and Cox models at our institute.
With the ability of our experienced physicians to match donor judging from the recipients’ preoperative general condition, infectious status and pulmonary artery pressure, ABO type compatibility, hepatitis virus seropositivity and undersized donors did not influence survival as others stated (9,24,25). Both CAD and repairable ASDs in the donor’s heart undoubtedly lowered the 1-year survival rate. Thus, this metric should not be used, which is further supported by Grauhan et al. (26). There were 4 donors with high-dose inotropic support before heart procurement, and only one of the matched recipient suffered from perioperative death due to acute rejection.
Multivariate analysis combining recipient characteristics could lower the effect of confounding factors and multicollinearity of covariates. Female recipient gender exhibited a higher hazard ratio (HR) in the overall and 1-year Cox regression analysis than male recipient gender. We do not recommend marginal donors for female recipients because female patients may have lower survival rates than those who receive hearts from standard donors. Consistent with Sharven Taghavi’s report of male recipient gender with a 0.882 HR for 1-year mortality (9), the high risk of female recipients in our center would be accounted for by gender mismatching [47/56 (83.9%) mismatching in female recipient group vs. 20/222 (9.0%) in male recipient group], which was thought to increase early mortality (27).
This study is limited by its retrospective design using single center data. Another important limitation is the relatively subjective definition of marginal donors, introducing significant bias in the selection process. Not all marginal donors satisfying the 7 criteria were used considering the general condition of the patients, and donor status should be evaluated with respect to the corresponding recipient, which will cause non-randomized donor selection. In addition, a small number of total cases and low main outcome incidence rates will contribute to fewer covariates in each step of multivariate regression analysis, and this will also make clinically significant data like intermediate term survival rate and predictors for hospital mechanical support be marginally significant.
In conclusion, based on prior experience, standardized clinical pathways and dedicated perioperative staff at our institute, selecting marginal donors using previous criteria may be reasonably applied to expand the benefits of transplantation. Nonetheless the following criteria will likely offer a good prognosis: (I) 50 years old ≤ age ≤60 years old; (II) cold ischemic time <6 hours; (III) 0.6< donor/recipient weight ratio <0.8; (IV) compatible ABO/Rh blood type; (V) hepatitis virus seropositivity; (VI) MD used for male recipient. These findings also suggest the need for developing a national marginal donors mechanism appropriate for Chinese patients to extend the donor pool. However, further studies including data from other centers and the evaluation of long-term outcomes should be performed.
Acknowledgements
This project is attributed to the Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. We are indebted to Dr. Kailun Zhang, Shiliang Xiao, Jiahong Xia and Xinling Du for their generous assistance.
Funding: This work was supported by the Nation High Technology Research and Development Program 863: 2012AA021009.
Ethical Statement: This study was approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (IORG No. IORG0003571) and performed in accordance with the ethical statement of the Declaration of Helsinki and ISHLT.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1244-54. 10.1016/j.healun.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Abouna GM. Organ shortage crisis: problems and possible solutions. Transplant Proc 2008;40:34-8. 10.1016/j.transproceed.2007.11.067 [DOI] [PubMed] [Google Scholar]
- 3.Pierre AF, Sekine Y, Hutcheon MA, et al. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg 2002;123:421-7. 10.1067/mtc.2002.120345 [DOI] [PubMed] [Google Scholar]
- 4.Marelli D, Bresson J, Laks H, et al. Hepatitis C-positive donors in heart transplantation. Am J Transplant 2002;2:443-7. 10.1034/j.1600-6143.2002.20508.x [DOI] [PubMed] [Google Scholar]
- 5.Lima B, Rajagopal K, Petersen RP, et al. Marginal cardiac allografts do not have increased primary graft dysfunction in alternate list transplantation. Circulation 2006;114:I27-32. 10.1161/CIRCULATIONAHA.105.000737 [DOI] [PubMed] [Google Scholar]
- 6.Kilic A, Weiss ES, Allen JG, et al. Should orthotopic heart transplantation using marginal donors be limited to higher volume centers? Ann Thorac Surg 2012;94:695-702. 10.1016/j.athoracsur.2012.03.069 [DOI] [PubMed] [Google Scholar]
- 7.Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914-56. 10.1016/j.healun.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 8.Massad MG. Current trends in heart transplantation. Cardiology 2004;101:79-92. 10.1159/000075988 [DOI] [PubMed] [Google Scholar]
- 9.Taghavi S, Jayarajan SN, Wilson LM, et al. Cardiac transplantation with ABO-compatible donors has equivalent long-term survival. Surgery 2013;154:274-81. 10.1016/j.surg.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 10.Torres Macho J, Delgado Jimenez JF, Sanz Salvo J, et al. Effect of different pharmacologic agents to reverse severe pulmonary hypertension among end-stage heart failure patients. Transplant Proc 2009;41:2477-9. 10.1016/j.transproceed.2009.06.054 [DOI] [PubMed] [Google Scholar]
- 11.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710-20. 10.1016/j.healun.2005.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Trulock EP, Christie JD, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant 2007;26:782-95. 10.1016/j.healun.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 13.Gregory SA, Ferencik M, Achenbach S, et al. Comparison of sixty-four-slice multidetector computed tomographic coronary angiography to coronary angiography with intravascular ultrasound for the detection of transplant vasculopathy. Am J Cardiol 2006;98:877-84. 10.1016/j.amjcard.2006.04.027 [DOI] [PubMed] [Google Scholar]
- 14.Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant 2014;33:327-40. 10.1016/j.healun.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Vilchez F, Zarauza J, Vazquez de Prada JA, et al. Assessment of tricuspid regurgitation by Doppler color flow imaging: angiographic correlation. Int J Cardiol 1994;44:275-83. 10.1016/0167-5273(94)90292-5 [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. 10.1016/S0894-7317(03)00335-3 [DOI] [PubMed] [Google Scholar]
- 17.Hunt SA, Baldwin J, Baumgartner W, et al. Cardiovascular management of a potential heart donor: a statement from the Transplantation Committee of the American College of Cardiology. Crit Care Med 1996;24:1599-601. 10.1097/00003246-199609000-00026 [DOI] [PubMed] [Google Scholar]
- 18.Groeneveld AB, Jansen EK, Verheij J. Mechanisms of pulmonary dysfunction after on-pump and off-pump cardiac surgery: a prospective cohort study. J Cardiothorac Surg 2007;2:11. 10.1186/1749-8090-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khasati N, Barnard J, Bittar MN, et al. Donor heart selection: Wythenshawe experience. Transplant Proc 2005;37:1331-2. 10.1016/j.transproceed.2004.12.208 [DOI] [PubMed] [Google Scholar]
- 20.Wittwer T, Wahlers T. Marginal donor grafts in heart transplantation: lessons learned from 25 years of experience. Transpl Int 2008;21:113-25. 10.1111/j.1432-2277.2007.00603.x [DOI] [PubMed] [Google Scholar]
- 21.Costanzo MR, Naftel DC, Pritzker MR, et al. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant 1998;17:744-53. [PubMed] [Google Scholar]
- 22.Trulock EP, Edwards LB, Taylor DO, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-third official adult lung and heart-lung transplantation report--2006. J Heart Lung Transplant 2006;25:880-92. 10.1016/j.healun.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 23.Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg 2011;92:520-7. 10.1016/j.athoracsur.2011.02.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huprikar S, Danziger-Isakov L, Ahn J, et al. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant 2015;15:1162-72. 10.1111/ajt.13187 [DOI] [PubMed] [Google Scholar]
- 25.Patel ND, Weiss ES, Nwakanma LU, et al. Impact of donor-to-recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation. 2008;118:S83-8. 10.1161/CIRCULATIONAHA.107.756866 [DOI] [PubMed] [Google Scholar]
- 26.Grauhan O, Siniawski H, Dandel M, et al. Coronary atherosclerosis of the donor heart--impact on early graft failure. Eur J Cardiothorac Surg 2007;32:634-8. 10.1016/j.ejcts.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 27.Michel S, Meiser B, Kaczmarek I. Impact of donor and recipient sex on outcome. Curr Opin Organ Transplant 2011;16:543-7. 10.1097/MOT.0b013e32834a9869 [DOI] [PubMed] [Google Scholar]