Abstract
Cardiac radiotherapy is rarely used in clinical practice because of concern of adverse effects on the heart. We present a case of a 64-year-old man with advanced small cell lung cancer (SCLC) treated with chemo-radiotherapy who attained partial remission initially but had disease progression to bulky cardiac metastasis and significant pericardial effusion. Severe heart failure with hepatic failure was found. Chemotherapy and pericardiocentesis were contraindicated because of the associated high risk and bleeding tendency. Emergent palliative cardiac radiotherapy resulted in rapid improvements of dyspnea, liver function, and urine output. Pericardiocentesis was performed 5 days later and effusion cytology confirmed metastatic SCLC. To our knowledge, this is the first case of effective cardiac radiotherapy for SCLC with life-threatening cardiac metastasis. Palliative cardiac radiotherapy may be an effective alternative treatment for radiosensitive malignancy with cardiac metastasis in cases of multiple organ dysfunction and unsuitability for chemotherapy and pericardiocentesis.
Keywords: Cardiac tumor, pericardial effusion, radiotherapy, small cell lung cancer (SCLC)
Introduction
Cardiac radiotherapy for radiation-sensitive cardiac tumor seems a reasonable treatment but is rarely used in clinical practice. Only few reports have described experiences in cardiac radiotherapy for cardiac tumors. Cardiac radiotherapy is far from a standard treatment in oncology. We report the first case of small cell lung cancer (SCLC) with life-threatening cardiac metastasis, complicated with both severe heart failure and hepatic failure, and were successfully relieved with cardiac radiotherapy.
Case presentation
A 64-year-old man had left upper lobe SCLC and neck lymph node metastasis, cT1N3M1b, stage IV. He received chemotherapy with etoposide and platinum for six cycles and palliative radiotherapy for better symptomatic control, and achieved partial remission. Two months after completion of six courses of chemotherapy, he developed productive cough, exertional dyspnea, orthopnea, and right upper quadrant pain. On physical examination, the vital signs were stable, and both legs were edematous. Laboratory examination revealed the following results: white blood cell count, 9,550/µL; hemoglobin level, 12.5g/dL; and platelet count, 57,000/µL; elevated serum aminotransferase (AST/ALT) level of 1,174/1,892 U/L; elevated total bilirubin level, 6.6 mg/dL; international normalized ratio, 1.63; elevated lactate dehydrogenase level, 1,271 U/L; blood urea nitrogen level, 55 mg/dL; creatinine level, 1.28 mg/dL; sodium, 118 mEq/L; potassium, 5.5 mEq/L; negative hepatitis A, B, and C markers; creatine kinase level, 112 U/L; troponin I level, 0.25 ng/mL; and B-type natriuretic peptide level, 781.4 pg/mL.
Abdominal sonography revealed no significant liver or biliary lesion. Chest CT revealed pericardial effusion with lobulated epicardial tumor, and progressive tumors in the mediastinum and left pulmonary hilum (Figure 1). Echocardiography revealed moderate pericardial effusion, with epicardial mass encasement all over the heart, and suspected myocardial invasion. The left ventricle ejection fraction was 63%, with constrictive effect on cardiac filling motion (Figure 2).
Figure 1.

Chest CT image showing lobulated cardiac mass (arrows) with moderate pericardial effusion (asterisks), and mild bilateral pleural effusions.
Figure 2.

Echocardiogram showing significant pericardial effusion (asterisks) and epicardial mass (arrows) encasement all over the heart, with suspected myocardial invasion, especially around the right ventricle.
Severe heart failure due to cardiac metastasis and pericardial effusion, followed by hepatic failure and pre-renal azotemia, was impressed. However, pericardiocentesis was contraindicated because of the high procedural risk, coagulopathy, and initially moderate amount effusion. Chemotherapy was also hampered because of poor liver function and performance status. Therefore, emergent palliative cardiac radiotherapy was given (24 Gy in 8 fractions, Figure 3). His liver function and pre-renal azotemia significantly improved soon after cardiac radiotherapy (Figure 4). Five days later, despite the improving clinical condition and liver functions, echocardiography showed progression of pericardial effusion to massive amount (Figures S1,S2), therefore pericardiocentesis was performed and drained 1,080 mL bloody effusion with cytology confirming SCLC.
Figure 3.
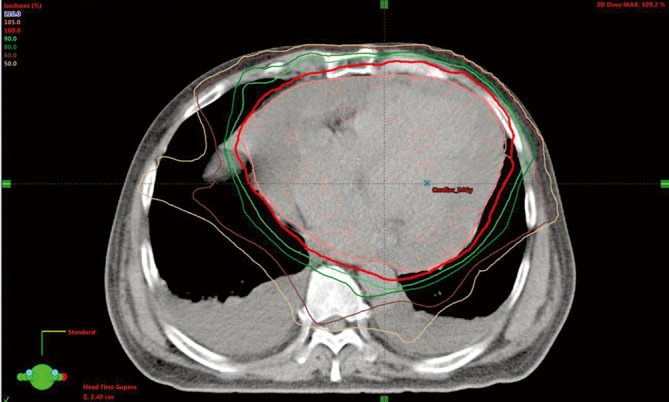
The radiation treatment plan for patient with cardiac metastasis from SCLC. The calculated iso-dose distribution was presented. The 100% prescribed dose was shown as red line (24 Gy). SCLC, small cell lung cancer.
Figure 4.
Trends of the liver and renal function data. Black arrow, cardiac radiotherapy on days 11–13 and days 16–20 (24 Gy in 8 fractions); dashed arrow, pericardiocentesis 1,080 mL on day 16. ALT, alanine aminotransferase; AST, aspartate aminotransferase; Cre, creatinine.
Figure S1.

Echocardiogram revealing bulky cardiac metastasis from small cell lung cancer (18). Available online: http://www.asvide.com/article/view/24502
Figure S2.
Explanation for the video. (A) First part (before cardiac radiotherapy): echocardiogram on long axis view, short axis view and 4 chamber view showed significant pericardial effusion and bulky epicardial mass encasement all over the heart, and with myocardial invasion around the right ventricle. The left ventricle ejection fraction was 63%, with constrictive effect on cardiac filling motion; (B) second part (five days after cardiac radiotherapy): echocardiogram 5 days after the beginning of cardiac radiotherapy (7 days after the first echocardiogram) showed progression of pericardial effusion to massive amount. Bulky cardiac metastasis especially around the right heart can be clearly delineated. Then pericardiocentesis was done at this day, and 1080ml bloody effusion was drained out; (C) third part (two weeks after cardiac radiotherapy): echocardiogram showed significant decreased size and thickness of the cardiac mass, and resolution of the pericardial effusion. The constrictive effect on heart chambers resolved and the diastolic function of heart also improved.
His exertional dyspnea and leg edema resolved, and follow-up echocardiography 2 weeks later revealed decreased size of the cardiac mass (Figures 5,S1,S2). He was discharged and lived at home for a short period. One month later, SCLC further progressed, and he expired in the hospice ward.
Figure 5.
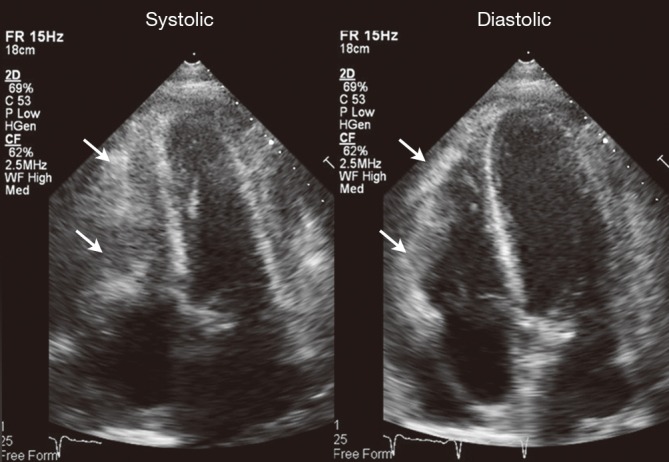
Echocardiogram 2 weeks after cardiac radiotherapy, showing the decreased size and thickness of the cardiac mass (arrows) and resolution of the pericardial effusion.
Discussion
The cardiac metastasis rate varies in different autopsy series and ranges from 2.7% to 25% in all patients with cancer (1,2). The tumors with the highest tendency of cardiac metastasis are melanoma, carcinomas of the breast and lung, and lymphoma (1). The parts of the heart involved in cardiac metastasis include the pericardium, myocardium, and endocardium, in the order of frequency (1,3).
Only about one-tenth of patients with autopsy-confirmed cardiac metastases presented with significant associated clinical symptoms before their deaths. Most cardiac metastases were small and clinically silent, some of which were masked by symptoms of diffuse tumor diseases (1).
Cardiac radiotherapy is overlooked and rarely used in clinical practice, probably because of the rarity of clinical evident cardiac tumors and for fear of cardiac toxicity from radiation. The first reported case of radiotherapy for cardiac metastasis could be traced back to the 1940s (2). Few case reports and small case series (Table 1) have described various cardiac tumors, and most of which showed symptomatic or image resolution after cardiac radiotherapy (4-13). Despite being unable to cure metastatic cancer, cardiac radiotherapy has beneficial effects on transient local tumor control, symptomatic relief, and may improve life quality.
Table 1. Summary of experiences in cardiac radiotherapy for cardiac tumors in published literature.
| Tumor origin | Radiotherapy dose/fractions | Survival since cardiac RT | Reference and case number |
|---|---|---|---|
| Primary cardiac tumor | |||
| Cardiac lymphoma | Median dose 34.8 [15–59] Gy | Median survival 22 mo (RT + C/T) | Petrich 2011 (4) (19 cases, survival no better than C/T alone) |
| Cardiac angiosarcoma | 42 Gy/21 Fr | 12–16 mo | Suderman 2011 (5), Nakamura-Horigome 2008 (6) (2 cases) |
| Metastatic cardiac tumor | |||
| Lung adenocarcinoma | 50 Gy/20 Fr, 20 Gy/5 Fr, and 6 Gy/1 Fr | 4 mo; 3.5 mo*; 0 mo* (died during RT course) | Lee 2012 (7) and Fotouhi Ghiam 2016 (8) (3 cases) |
| Melanoma | 45 Gy/25 Fr | 6 mo | Magnuson 2010 (9) (1 case) |
| Sarcoma | 25–60 Gy, 1.8–5 Gy per fraction | 5–24 mo | Fotouhi Ghiam 2016 (8), Takenaka 2011 (10) (9 cases) |
| Lymphoma | 25 Gy/10 Fr | 3 mo* | Fotouhi Ghiam 2016 (8) (1 case) |
| Rectal adenocarcinoma | 20 Gy/5 Fr; 16 Gy/4 Fr | 3 mo*; 0 mo* (died during RT course) | Fotouhi Ghiam 2016 (8) (2 cases) |
| Esophageal cancer | 20 Gy/5 Fr | 2.5 mo | Al-Mamgani 2008 (2) (1 case) |
| Cervical cancer | 28.8 Gy/16 Fr; 60 Gy/30 Fr | 1 mo; 7 mo | Lemus 1998 (11) (7 cases+) |
| Thyroid cancer | 37.5 Gy/15 Fr; 35 Gy/10 Fr | 2 mo; 4 mo | Dasgupta 2011 (12), Chen 2012 (13) (2 cases) |
| Thymoma/thymic carcinoma | 30 Gy/20 Fr; 36 Gy/18 Fr | 6 mo*; 11 mo* | Fotouhi Ghiam 2016 (8) (2 cases) |
| Hepatocellular carcinoma | 54 Gy/27 Fr | 6 mo* | Fotouhi Ghiam 2016 (8) (1 case) |
*, duration of response (from completion of cardiac radiotherapy to cardiac tumor progression or death without tumor regrowth); +, radiotherapy dose is available in only 2 of the 7 cases. C/T, chemotherapy; Fr, fraction; Gy, gray; mo, months; RT, radiotherapy.
Radiotherapy at the thoracic region has been reported to have several potential adverse effects on the heart, including pericarditis, pericardial effusion, coronary artery disease, heart failure, arrhythmia, and valvular disease (14). The risk is associated with the radiation dose, treatment duration, and radiation volume. Most cardiovascular complications are chronic, whereas acute complications of cardiac radiotherapy are uncommon (4-14). Recent literatures report acute cardiac toxicities as early as 1 month after radiation (15,16). Cuculich et al. also demonstrated cardiac radiation for ablation of ventricular tachycardia in five patients, with increased ventricular tachycardia episodes initially, and then became well controlled (17). However, in patients like our case, emergent radiation is still reasonable and necessary to stabilize the life-threatening condition. Therefore, the acute, subacute and chronic cardiac toxicities of radiation are of lesser importance.
Conclusions
In our case, cardiac metastasis from SCLC showed an immediately marked response to cardiac radiotherapy. This palliative treatment effectively relieved multi-organ failure, decreased the cardiac tumor burden, and also improved quality of life. We suggest that cardiac radiotherapy could be an effective and, at least in short-term, safe palliative treatment for radiosensitive malignancy with cardiac metastasis, especially in life-threatening conditions.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient’s relative for publication of this manuscript and any accompanying images
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Reynen K, Köckeritz U, Strasser RH. Metastases to the heart. Ann Oncol 2004;15:375-81. 10.1093/annonc/mdh086 [DOI] [PubMed] [Google Scholar]
- 2.Al-Mamgani A, Baartman L, Baaijens M, et al. Cardiac metastases. Int J Clin Oncol 2008;13:369-72. 10.1007/s10147-007-0749-8 [DOI] [PubMed] [Google Scholar]
- 3.Sosinska-Mielcarek K, Senkus-Konefka E, Jassem J, et al. Cardiac involvement at presentation of non-small-cell lung cancer. J Clin Oncol 2008;26:1010-1. 10.1200/JCO.2007.14.9328 [DOI] [PubMed] [Google Scholar]
- 4.Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer 2011;117:581-9. 10.1002/cncr.25444 [DOI] [PubMed] [Google Scholar]
- 5.Suderman D, Cooke A, Wong R, et al. Treatment of cardiac angiosarcoma with radiation and docetaxel: a case report with partial response and prolonged stable disease. J Thorac Oncol 2011;6:834-5. 10.1097/JTO.0b013e31820c2f18 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura-Horigome M, Koyama J, Eizawa T, et al. Successful treatment of primary cardiac angiosarcoma with docetaxel and radiotherapy. Angiology 2008;59:368-71. 10.1177/0003319707308212 [DOI] [PubMed] [Google Scholar]
- 7.Lee P, Kishan AU. Radiotherapy is effective for a primary lung cancer invading the left atrium. BMJ Case Rep 2012;2012. doi: . 10.1136/bcr-2012-006667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotouhi Ghiam A, Dawson LA, Abuzeid W, et al. Role of palliative radiotherapy in the management of mural cardiac metastases: who, when and how to treat? A case series of 10 patients. Cancer Med 2016;5:989-96. 10.1002/cam4.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnuson WJ, Halligan JB. Successful treatment of melanoma metastatic to the left atrium using external beam radiation therapy. Oncology (Williston Park) 2010;24:650-3. [PubMed] [Google Scholar]
- 10.Takenaka S, Hashimoto N, Araki N, et al. Eleven cases of cardiac metastases from soft-tissue sarcomas. Jpn J Clin Oncol 2011;41:514-8. 10.1093/jjco/hyq246 [DOI] [PubMed] [Google Scholar]
- 11.Lemus JF, Abdulhay G, Sobolewski C, et al. Cardiac metastasis from carcinoma of the cervix: report of two cases. Gynecol Oncol 1998;69:264-8. 10.1006/gyno.1998.5009 [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta T, Barani IJ, Roach M., 3rd Successful radiation treatment of anaplastic thyroid carcinoma metastatic to the right cardiac atrium and ventricle in a pacemaker-dependent patient. Radiat Oncol 2011;6:16. 10.1186/1748-717X-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen KH, Chou YH, Cheng AL. Primary squamous cell carcinoma of the thyroid with cardiac metastases and right ventricle outflow tract obstruction. J Clin Oncol 2012;30:e260-3. 10.1200/JCO.2011.39.9808 [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich PA, Kapoor JR. Radiation induced heart disease: systemic disorders in heart disease. Heart 2009;95:252-8. 10.1136/hrt.2008.149088 [DOI] [PubMed] [Google Scholar]
- 15.Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:1395-402. 10.1200/JCO.2016.71.6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol 2017;35:1387-94. 10.1200/JCO.2016.70.0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuculich PS, Schill MR, Kashani R, et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med 2017;377:2325-36. 10.1056/NEJMoa1613773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CF, Lin MH, Chu KA, et al. Echocardiogram revealing bulky cardiac metastasis from small cell lung cancer. Asvide 2018;5:440. Available online: http://www.asvide.com/article/view/24502




