Abstract
Background
Adenosquamous carcinoma (ASC) is a mixed glandular and squamous cell carcinoma (SCC) with more aggressive behavior than the other histologic subtypes of lung cancer. We aim to evaluate the prognosis of patients with ASC after surgical resection.
Methods
We reviewed records of patients who underwent surgical resection for lung cancer in two institutes between January 2010 and December 2015. Survival data were collected with a median follow-up of 59 (range from 10 to 85) months. Kaplan-Meier survival curve was determined for all patients.
Results
Patients with ASC accounted for 1.6% of all NSCLC patients (33 males, 25 females). The cumulative postoperative 3- and 5-year survival rates were 56% and 48%, respectively. Overall survival (OS) was significantly lower in ASC patients than in adenocarcinoma (AC) patients operated during the same period (P<0.01). Patients with ASC containing acinar predominant AC had better survival than those with non-acinar predominant ASC (P=0.03). No difference of OS was found in patients with or without visceral pleural invasion (VPI), vascular invasion (VI) or EGFR mutation status. Multivariate analysis showed gender, pathological subtype, and TNM staging to be independent prognostic factors.
Conclusions
We demonstrated that ASC were uncommon and aggressive lung tumors. Predominant histological subtype of AC might be an independent prognostic factor for ASC. Further prospective studies are warranted to clarify the characteristics of this rare tumor.
Keywords: Lung adenosquamous carcinoma, surgical resection, histological subtype, prognostic factor
Introduction
Lung adenosquamous carcinoma (ASC) is a rare biphasic malignant tumor with squamous cell carcinoma (SCC) and adenocarcinoma (AC) components that are detected in 0.4% to 4% of patients with lung cancer (1-3). Previous studies have reported on a series of ASCs with distinct clinical behavior and worse prognosis than AC and SCC (3,4). The potential prognostic factors include age, gender, histologic grade and tumor stage.
Recently, the new classification of lung AC was raised by the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS), and was applied by WHO classification. The growth pattern of AC is classified into lepidic-, acinar-, papillary-, micropapillary-, solid-predominant and invasive mucinous AC. Numerous studies have confirmed the predominant subtype to be associated with prognosis (5). However, the growth pattern of AC in ASC and the correlation with prognosis remains unclear.
In the present study, we analyzed the clinicopathologic features and histological subtype of ASC, to investigate the clinical characteristics and prognosis of this malignancy.
Methods
Patients and methods
We retrospectively analyzed the clinical records of patients who underwent surgical resection for lung cancer in two institutes between January 2010 and December 2015 (First affiliated hospital of Guangzhou Medical University and First People’s Hospital of Foshan City). This study was approved by the institutional ethics boards of the First Affiliated Hospital of Guangzhou Medical University and First People’s Hospital of Foshan City. Preoperative high resolution CT scans and pulmonary function tests (PFTs) were conducted in all patients. PET-CT has only been used in more recent years in the search for distant metastases. EBUS-TBNA was not performed in all patients but was routinely performed according to the results of preoperative evaluation, including tumor size, nodal extension, planned resection, and need for induction therapy. N3 disease and distant metastases precluded surgical treatment. Tumor stages were classified based on the 8th edition of the TNM classification for non-small cell lung cancer (NSCLC).
Histological evaluation
All resected specimens were fixed with formalin and stained with hematoxylin and eosin in the routine manner. Each of the slides was examined independently by two pathologists. Any controversy was solved with a third observer. Histologic features, such as the presence of keratinization or absence of the alveolar filling growth pattern, and immunohistochemical analyses, which could identify AC and SCC components, were used to distinguish between AC and SCC. The growth pattern of AC in ASC was classified according to the new IASLC/ATS/ERS classification. The predominant pattern is defined as the pattern with the largest percentage of carcinoma cells. Molecular analysis of EGFR mutation was performed using amplification mutation refractory system (AMRS) methods with formalin-fixed paraffin-embedded tissue blocks. The EGFR Mutation Detection Kit (Amoy Diagnostics, Xiamen, China) was used to detect the 29 most common types of EGFR mutations and the T790M mutation. All experiments were performed according to the user manual in our laboratory.
Statistical analysis
Survival analysis was estimated by the Kaplan-Meier method. Statistical comparisons between survival distributions were made by the log-rank test. A Cox proportional hazards regression model was used to simultaneously examine the effects of gender, smoking status, histological subtype, TNM classification, EGFR mutation, visceral pleural invasion (VPI) and vessel invasion on survival. All data analyses were conducted with the two-sided test: a P value less than 0.05 was considered statistically significant. All statistical analyses were performed by SPSS 20.0.
Results
Clinicopathological characteristics of ASC patients
A total of 58 (33 males, 25 females) patients that underwent surgical resection from two institutes were included for analysis. The mean age was 61±9 years. The tumor stage was I in 32 patients, II in 10 patients and III in 16 patients. We divided the patients into three groups according to predominant component of the tumors: 23 in AC predominant group (39.7%), 27 in squamous predominant group (46.6%), 8 in balance group (13.8%). The predominant growth pattern of AC was acinar in 39 patients, solid in 5 patients, papillary in 9 patients, lepidic in 2 patients, micropapillary in 1 patient and IMA in 2 patients. Vascular invasion (VI) was found in 11 patients and VPI was found in 21 patients. According to the EGFR mutation status, all the included tumor tissues were analyzed through ARMS PCR in our institutes. EGFR mutations were found in 17 patients (10 had 21L858 and 7 had 19-del). The patients’ demographic characteristics are shown in Table 1.
Table 1. Clinical characteristics of included patients (N=58).
| Variables | n (%) |
|---|---|
| Age (year) | |
| <50 | 6 (10.3) |
| >50, ≤60 | 15 (25.9) |
| >60 | 37 (63.8) |
| Gender | |
| Male | 33 (56.9) |
| Female | 25 (43.1) |
| Smoking status | |
| Former/current | 21 (36.2) |
| Never | 37 (63.8) |
| Predominant of the tumor | |
| Adenocarcinoma | 23 (39.7) |
| Squamous carcinoma | 27 (46.6) |
| Balance | 8 (13.8) |
| Predominant subtype of adenocarcinoma | |
| Lepidic | 2 (3.4) |
| Acinar | 39 (67.2) |
| Papillary | 9 (15.5) |
| Micropapillary | 1 (1.7) |
| Solid | 5 (8.6) |
| IMA | 2 (3.4) |
| TNM staging | |
| IA, IB | 32 (55.2) |
| IIA, IIB | 10 (17.2) |
| IIIA | 16 (27.6) |
| EGFR mutation | |
| Yes | 17 (29.3) |
| No | 41 (70.7) |
| Visceral pleural invasion | |
| Yes | 21 (36.2) |
| No | 37 (63.8) |
| Vessel invasion | |
| Yes | 11 (19.0) |
| No | 47 (81.0) |
IMA, invasive mucinous adenocarcinoma.
Survival results of ASC patients
The median follow-up time for all patients was 59 (range from 10 to 85) months. The cumulative postoperative 3- and 5-year survival rates were 56% and 48%, respectively. During the same period, a total of 2,702 patients with AC underwent surgical resection. Overall survival (OS) was significantly lower in ASC patients than in AC (P<0.01) (Figure 1). The OS according to TNM stage was shown in Figure 2. We divided patients into three groups: AC predominant (group 1), squamous carcinoma predominant (group 2), and balance group (group 3). The survival curves were shown in Figure 3. No differences were found between group 1 and 2 (P=0.81), group 1 and 3 (P=0.07). But group 3 had worse survival than group 2 (P=0.01). In regard to the histologic components, the OS for patients with acinar predominant ASC was significantly higher than that for non-acinar predominant ASC (P=0.03) (Figure 4). When ASC patients were considered according to EGFR mutation, we found the mutation status did not affect the OS in patients with ASC (P=0.23) (Figure 5). No differences were found between groups according to VPI (P=0.6) (Figure 6) and VI (P=81) (Figure 7).
Figure 1.
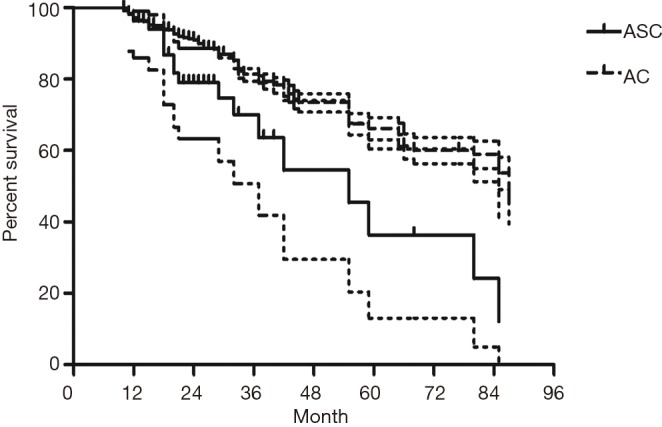
Survival of patients with adenosquamous carcinoma (ASC) and adenocarcinoma (AC) according to time periods.
Figure 2.
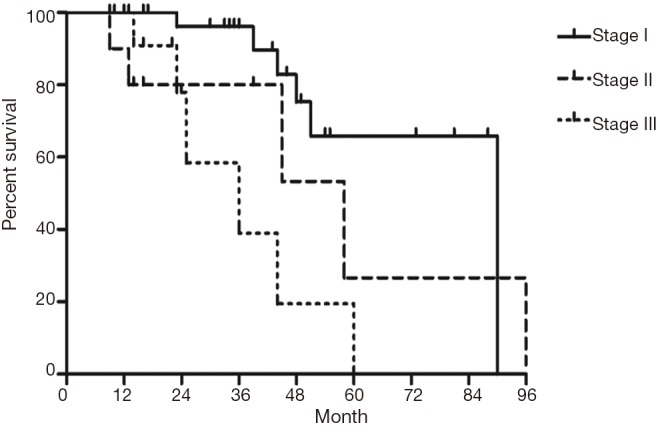
Survival of patients with adenosquamous carcinoma (ASC) according to TNM stage.
Figure 3.
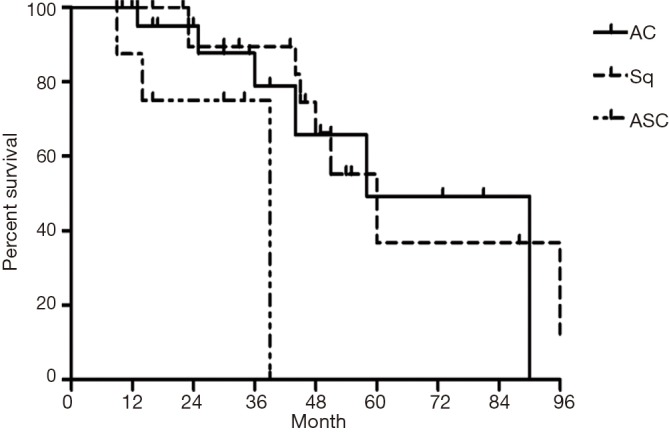
Survival of patients with adenosquamous carcinoma (ASC) according to predominant component of tumor. AC, adenocarcinoma; Sq, squamous carcinoma.
Figure 4.
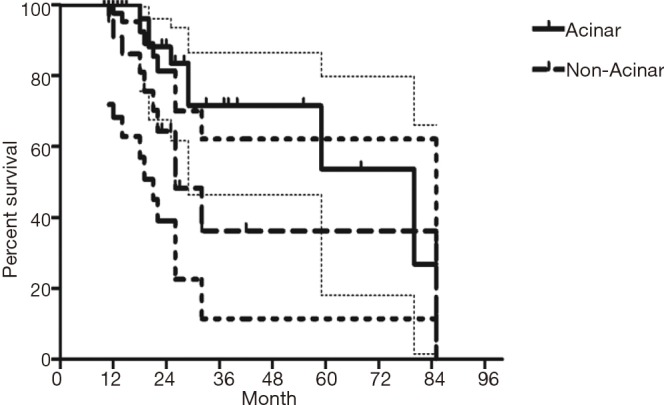
Survival of patients with adenosquamous carcinoma (ASC) according to growth pattern of adenocarcinoma in ASC.
Figure 5.
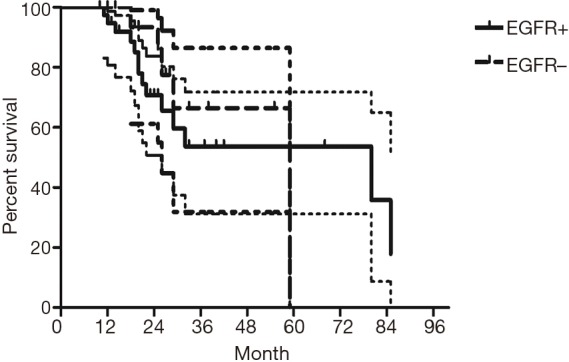
Survival of patients with adenosquamous carcinoma (ASC) according to EGFR mutation status.
Figure 6.
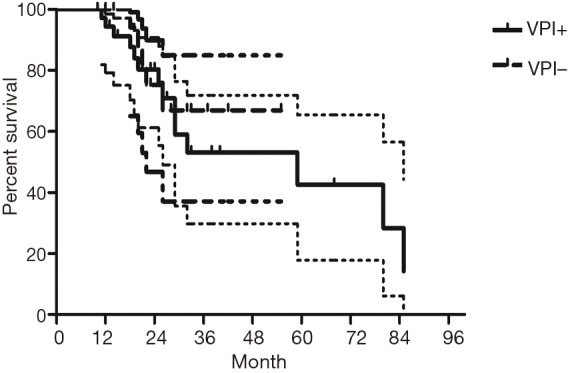
Survival of patients with adenosquamous carcinoma (ASC) according to visceral pleural invasion (VPI).
Figure 7.
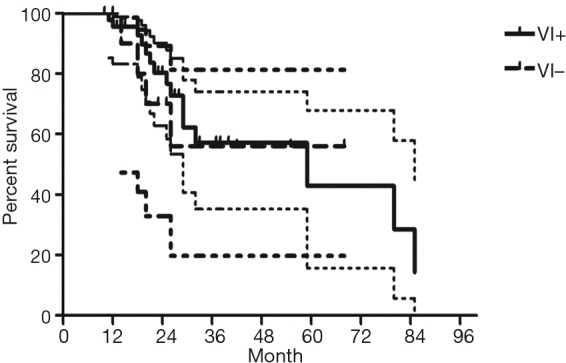
Survival of patients with adenosquamous carcinoma (ASC) according to vessel invasion (VI).
Multivariate analysis showed that gender, pathological subtype, and TNM staging were all independent factors that affected the prognosis of these patients. However, smoking status, EGFR mutation status, VPI and VI were not found to be significant prognostic factors (Table 2).
Table 2. Cox’s proportional hazards model analysis results.
| Prognosis factors | P value | Statistic difference |
|---|---|---|
| Gender | 0.003 | – |
| Smoking status | 0.662 | NS |
| Pathological subtype | 0.004 | – |
| TNM staging | 0.019 | – |
| EGFR mutation | 0.672 | NS |
| Visceral pleural invasion | 0.137 | NS |
| Vessel invasion | 0.458 | NS |
NS, non-significant.
Discussion
According to the World Health Organization’s classification, ASC is defined as a carcinoma showing components of both AC and SCC, with each component comprising at least 10% of the tumor (6). ASC is an unusual, aggressive form of non-small cell lung carcinoma and represents an independent prognostic variable suggesting poor outlook. The histogenesis of the neoplasm is unclear, but appears to be far more complex than a simple admixture of SCC and ADC components.
The frequency of ASC In previous studies of patients operated on for NSCLC varies from 1.4% to 4.5% in single-center series (7,8), and 2.4% to 4.1% in multicenter series (9,10). In the present study, the incidence of ASC was 1.6% of all NSCLC in our two institutes. Even though the incidence of such tumors is relatively low, due to its aggressive trait, ASC tends to present at higher stage than ADC or SCC. Nakagawa et al. (11) reported an overall cumulative 5-year survival rate of only 6.2% for patients with ASC, indicating a significantly poorer prognosis than AC or SCC (41.5%). Multivariate analyses have shown TNM staging, complete resection, age, gender and histology to be associated with poor prognosis. In our study, we found the cumulative postoperative 3- and 5-year survival rates were 56% and 48%, respectively. OS was significantly lower in ASC patients than in AC. Because the proportion of acinar predominant ASC is much higher than other sporadic subtypes, we divided into two subgroups. Besides gender and TNM staging, we demonstrated the histological subtype of AC in ASC had an influence on the patients’ survival. Patients with ASC containing acinar predominant AC had better survival than those with non-acinar predominant ASC. The better prognosis of ours might be due to the absence of stage IV patients and most were in early stage. Both Hsia et al. (12) and Shimizu et al. (3) have reported finding more advanced N factors in ASCs and suggest that this progression is linked with poorer prognosis for ASC. Here we found lymph node metastasis in 26 patients (44.8%), of which N2 was found in 16 patients (27.6%).
Some groups have identified identical EGFR abnormalities in ASCs. Kang et al. (13) reported that 15.4% EGFR mutations from ASCs of the lung and more frequent in female and never smokers in Korean patients. Toyooka et al. (14) found 27% for EGFR mutation and detected identical monoclonal patterns in the two tumor components of ASCs in Japanese patients. Moreover, Sasaki et al. (15) reported EGFR mutation status from ASCs was significantly correlated with gender and smoking history. Our study did not correspond to the previous studies, we did not find any difference between EGFR mutation status in smoking status, gender, stage, VPI or VI. The implication of these findings is that geographical differences may also determine different genetic susceptibility in the same race. The OS for patients with EGFR mutation seemed to be similar to wild-type. In addition, we found 36% of ASCs had VPI and 11% had VI. We further compared the survival between VPI+ and VPI−, VI+ and VI− groups, however no significant differences were found.
In our series, preoperative diagnosis of ASC proved to be difficult because the small size of the preoperative biopsy specimen often leads to removal of either an AC or a SCC component alone. Therefore, in most cases diagnosis is discovered at pathology. Some limitations need to be acknowledged. First, the study was retrospective and the sample was small, which may introduce bias in the results. Second, the results of OS might be influenced by adjuvant therapy. However, direct evidence of any significant prognostic impact of adjuvant therapy is still lacking. The survival improvement associated with a more recent time period may be explained by various factors, including improvements in patient selection (more than half the patients were in early-stage), surgical management, and perioperative care.
Conclusions
We demonstrated that ASC were uncommon and aggressive lung tumors. Predominant histological subtype of AC might be an independent prognostic factor for ASC. Further prospective studies are warranted to clarify the characteristics of this rare tumor.
Acknowledgements
None.
Ethical Statement: This study was approved by the institutional ethics boards of the First Affiliated Hospital of Guangzhou Medical University and First People’s Hospital of Foshan City.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Fitzgibbons PL, Kern WH. Adenosquamous carcinoma of the lung: a clinical and pathologic study of seven cases. Hum Pathol 1985;16:463-6. 10.1016/S0046-8177(85)80083-6 [DOI] [PubMed] [Google Scholar]
- 2.Ishida T, Kaneko S, Yokoyama H, et al. Adenosquamous carcinoma of the lung. Clinicopathologic and immunohistochemical features. Am J Clin Pathol 1992;97:678-85. 10.1093/ajcp/97.5.678 [DOI] [PubMed] [Google Scholar]
- 3.Shimizu J, Oda M, Hayashi Y, et al. A clinicopathologic study of resected cases of adenosquamous carcinoma of the lung. Chest 1996;109:989-94. 10.1378/chest.109.4.989 [DOI] [PubMed] [Google Scholar]
- 4.Mordant P, Grand B, Cazes A, et al. Adenosquamous carcinoma of the lung: surgical management, pathologic characteristics, and prognostic implications. Ann Thorac Surg 2013;95:1189-95. 10.1016/j.athoracsur.2012.12.037 [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis WD, Branbilla E, Muller-Hermelink HK. World Health Organization classification of tumours, pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC, 2004. [Google Scholar]
- 7.Uramoto H, Yamada S, Hanagiri T. Clinicopathological characteristics of resected adenosquamous cell carcinoma of the lung: risk of coexistent double cancer. J Cardiothorac Surg 2010;5:92. 10.1186/1749-8090-5-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gawrychowski J, Bruliński K, Malinowski E, et al. Prognosis and survival after radical resection of primary adenosquamous lung carcinoma. Eur J Cardiothorac Surg 2005;27:686-92. 10.1016/j.ejcts.2004.12.030 [DOI] [PubMed] [Google Scholar]
- 9.Maeda H, Matsumura A, Kawabata T, et al. Adenosquamous carcinoma of the lung: surgical results as compared with squamous cell and adenocarcinoma cases. Eur J Cardiothorac Surg 2012;41:357-61. 10.1016/j.ejcts.2011.05.050 [DOI] [PubMed] [Google Scholar]
- 10.Cooke DT, Nguyen DV, Yang Y, et al. Survival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomy. Ann Thorac Surg 2010;90:943-8. 10.1016/j.athoracsur.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa K, Yasumitu T, Fukuhara K, et al. Poor prognosis after lung resection for patients with adenosquamous carcinoma of the lung. Ann Thorac Surg 2003;75:1740-4. 10.1016/S0003-4975(03)00022-5 [DOI] [PubMed] [Google Scholar]
- 12.Hsia JY, Chen CY, Hsu CP, et al. Adenosquamous carcinoma of the lung. Surgical results compared with squamous cell and adenocarcinoma. Scand Cardiovasc J 1999;33:29-32. 10.1080/14017439950142000 [DOI] [PubMed] [Google Scholar]
- 13.Kang SM, Kang HJ, Shin JH, et al. Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer 2007;109:581-7. 10.1002/cncr.22413 [DOI] [PubMed] [Google Scholar]
- 14.Toyooka S, Yatabe Y, Tokumo M, et al. Mutations of epidermal growth factor receptor and K-ras genes in adenosquamous carcinoma of the lung. Int J Cancer 2006;118:1588-90. 10.1002/ijc.21500 [DOI] [PubMed] [Google Scholar]
- 15.Sasaki H, Endo K, Yukiue H, et al. Mutation of epidermal growth factor receptor gene in adenosquamous carcinoma of the lung. Lung Cancer 2007;55:129-30. 10.1016/j.lungcan.2006.09.003 [DOI] [PubMed] [Google Scholar]


