Abstract
Introduction
Acne is a very common skin disease in adolescents and young adults, but it also affects adults. However, its aetiology is not yet fully understood. Demodex appears to be associated with multiple skin disorders, but controversy persists. Some reports indicate a connection between acne vulgaris and demodicosis.
Aim
To confirm the association between Demodex infestation and acne vulgaris.
Material and methods
A total of 108 patients were enrolled in the acne group. Acne severity was calculated as GASS and acne type (adolescent and post adolescent) was recorded. An age-sex matched healthy control group comprising 65 individuals were included in the study. Dermatological examinations were performed and an SSSB was used to determine the presence of Demodex.
Results
In our study, Demodex positivity was seen in 46 (42.6%) patients in the acne group and 8 (12.3%) in the control group; this difference was statistically significant (p < 0.001). A multivariate Backward Step-By-Step Logistic Regression analysis identified the most effective factors for acne development such as Demodex positivity (OR = 5.565, 95% CI: 2.384–12.99 and p < 0.001) and age under 25 years (OR = 2.3 and 95% CI: 1.183–4.473 and p = 0.014). Alcohol consumption was related to Demodex positivity (p = 0.019) in post adolescent acne.
Conclusions
Our study is the first one to evaluate acne severity, acne type and the relationship to Demodex prevalence. We suggest that Demodex infestation should be considered when the classical therapies are ineffective especially in cases of post adolescent acne.
Keywords: Demodex, acne, post adolescent acne, acne severity, alcohol
Introduction
Acne is one of the most common disorders treated by dermatologists. While it most often affects adolescents, it is not uncommon in adults. It is a multifactorial disease, originating in the pilosebaceous unit. Although much research has been conducted on acne, the aetiology of acne vulgaris remains poorly understood [1–4]. The current view is that acne is related to factors such as androgen, hyperseborrhea, hyperkeratosis of the pilosebaceous ducts, follicular orifice blockage, and proliferation of bacteria, such as Propionibacterium acnes, and Staphylococcus epidermidis [1, 2].
Post adolescent acne is seen in patients over the age of 25 years, regardless of the age of the onset. The clinical characteristics of adult and adolescent acne differ in several ways that need to be considered during the course of treatment. These factors may predispose certain individuals to suffer from post adolescent acne, but any differences in the skin microbiome of patients with adolescent, post adolescent acne have yet to be confirmed [5–8].
Demodicosis is an ectoparasitosis that applies to cutaneous diseases of the pilosebaceous unit caused by Demodex mites. The disease may be a primary skin disease, or it can also occur as secondary to inflammatory dermatoses. It can also act as a mimicker, as it can mimic many other dermatoses, as well as cutaneous lymphoma [9–14]. Human demodicosis may be primary or secondary [11, 15–17]. Demodicosis may be more prevalent than once thought in solid organ transplant recipients and haemodialysis patients [18–20], but some case reports also indicate that the disease could be seen in immunocompetent paediatric patients [21].
The infestation of Demodex has therefore been recognised by many researchers as important causes of skin diseases and has increasingly become a public health concern [14, 22].
Aim
In this paper, we discuss the relationship between acne vulgaris and Demodex mites.
Material and methods
Study population
The study included 108 patients ≥ 18 years old with acne vulgaris who attended our polyclinic. All acne patients had comedones; if no comedone was present, the patient was excluded. All acne patients had been using topical or systemic acne treatments previously; 12 (11.1%) patients had been using systemic retinoic acid therapy and 6 (5.6%) had used it before, and all of these (18) patients had Demodex infestations (16.6%). General and local retinoids can change the environment of the follicle. The wash out period was 1 month for the patients who used retinoids and systemic antibiotics.
A control group was formed from 65 healthy individuals (whose ages and genders were similar to the acne group) who attended our polyclinic. All participants were questioned about using cigarette smoking and alcohol consumption, family history of acne, whether pets (cats, dogs or birds) were kept at home. Dermatological examinations were performed.
Informed consent was obtained from all participants included in the study. The study was approved by the ethical committee of the Yildirim Beyazit University, Yenimahalle Training and Research Hospital and was conducted in accordance with the Declaration of Helsinki.
Exclusion criteria for the acne group were < 18 years old, pregnancy, breast feeding, menstrual irregularity, having acne caused by a drug, cosmetic acne, presence of any systemic disease, taking topical or systemic steroids, use of topical acaricide or immunosuppressive drugs and use of creams or gels containing tea tree oil. The healthy group were ≥ 18 years old, contained no pregnant or breast feeding persons, had no signs of dermatosis on either the face or the body, had no complaints of pruritus, and were not using topical or systemic steroids, topical acaricides, immunosuppressive drugs or creams or gels containing tea tree oil. The control group was chosen to have a skin type that was not considered as oily skin.
We accepted patients who were > 18 years old into the adolescent acne group (n = 65; 60.2%) and the patients who were > 25 into the post adolescent acne group (n = 43; 39.8%). The acne group was classified as having adolescent (18–25 years old) and post adolescent (> 25 years old) acne, according to literature [3, 5, 6]. In the acne group, the acne severity was assessed as grades 1–4 according to the Global Acne Severity Scale (GASS) proposed by Doshi et al. [23]. This system divides the face, chest and back into six locations (forehead, each cheek, nose, chin, chest and upper back). The six locations are graded separately on a 0–4 scale depending on the most severe lesion within that location (0 – no lesions, 1 – comedones, 2 – papules, 3 – pustules and 4 – nodules). The score for each area is the product of the most severe lesion, multiplied by the area factor. These individual scores are then added to obtain the total score. Patients with a total score between 1 and 18 were classified as mild while those with a total score between 19 and 30 were classified as moderate. Scores between 31 and 38 were classified as severe and those more than 39 were designated as very severe [24].
Demodex examination
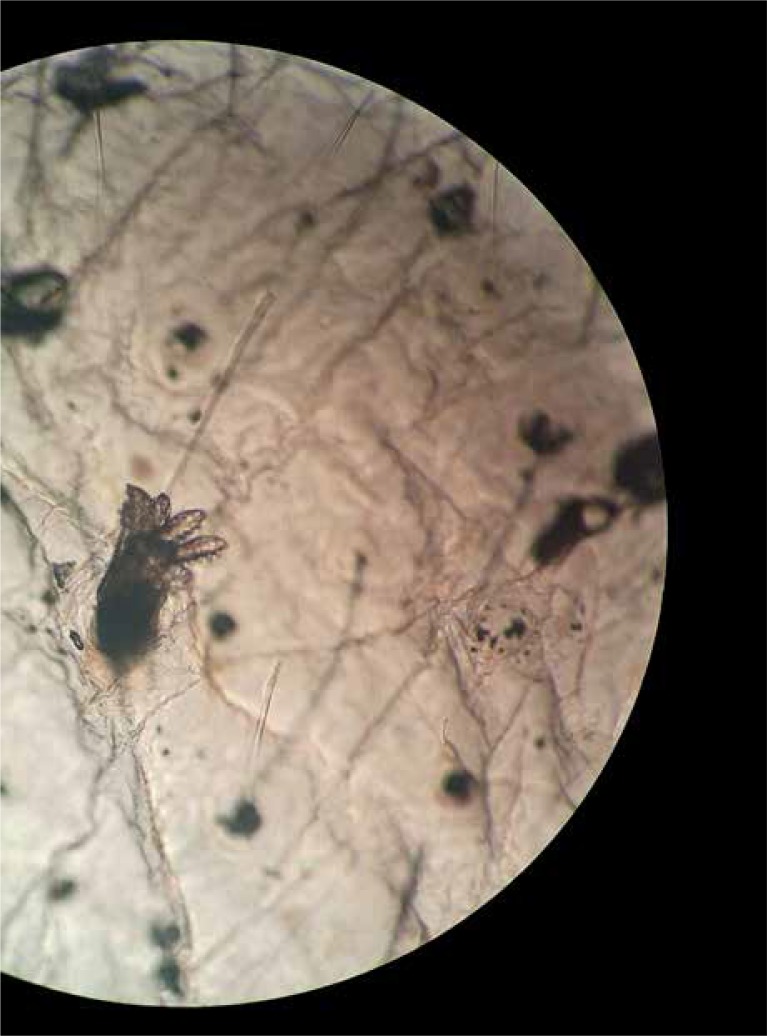
The ‘follicular biopsy’ is an extension of the non-invasive ‘surface biopsy’ technique originated by Dawber and Marks, which involves the use of a quick-setting cyanoacrylate polymer for extraction of the contents of sebaceous follicles [14, 25]. Depending on the surface area of the lesion, one sample was obtained using the Standardised Skin Surface Biopsy (SSSB) method. Parasites were removed using a non-invasive technique consisting of pressing a microscope slide with cyanoacrylate adhesive over the lesion to apply the adhesive to the skin. After 1 min, the specimen was removed from the skin. The SSSB removed the surface keratin layer, the top of the pilosebaceous follicle, and its contact. The sample was covered with a cover glass and examined for parasites by light microscopy at 10×, 40× and 100× magnification. The living parasites in a specimen were calculated for evaluating Demodex density related to Demodex severity. The total living parasite number was used for classification: 0–5 per cm2 was classified as 1+ density, 5–10 was 2+, 10–15 per cm2 was 3+, 15–20 per cm2 was 4+ and > 20 per cm2 was 5+ (Figure 1). All the patients were informed about the technique of SSSB.
Figure 1.
Demodex mites seen in SSSB (HE, 40×)
In the acne group, the SSSB was obtained from an inflammatory lesion. In the control group, the SSSB was obtained from the chin or forehead.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics version 17.0 software (IBM Corporation, Armonk, NY, USA). Normal distribution of continuous variables was determined by the Kolmogorov Smirnov test. Data were shown as medians (minimum–maximum) for continuous variables; otherwise, numbers of cases and percentages were used for categorical data.
The Mann Whitney U test was applied for the comparisons of variables not distributed normally, as well as for ordinal data (e.g. education status, severity of disease, etc.). Categorical data were analysed by χ2 or Fisher’s exact test, where appropriate. Degrees of association between ordinal variables were evaluated by Spearman’s Rank Correlation analyses.
The best predictor(s) that affected the existence of disease was determined by the Multiple Logistic Regression Backward procedure. Any variable whose univariate test had a p < 0.25 was accepted as a candidate for the multivariable model, along with all variables of known clinical importance. Adjusted odds ratios (ORs), 95% confidence intervals (CIs) and Wald statistics for each independent variable were also calculated.
A p-value less than 0.05 was considered statistically significant.
Results
Demodex positivity was significantly higher in the acne group (p < 0.001). Other clinical and demographical variables did not differ between the acne and control groups. The demographic and clinical features of all groups are presented in Table 1.
Table 1.
Demographic and clinical features in the groups
| Variables | Control group (n = 65) | Acne group (n = 108) | P-value |
|---|---|---|---|
| Age [year] | 28 (18–68) | 22 (18–54) | 0.072 |
| Age groups: | 0.017 | ||
| Adolescent (18–25) | 27 (41.5%) | 65 (60.2%) | |
| Post adolescent (> 25) | 38 (58.5%) | 43 (39.8%) | |
| Gender: | 0.294 | ||
| Female | 46 (70.8%) | 68 (63.0%) | |
| Male | 19 (29.2%) | 40 (37.0%) | |
| Educational status: | 0.143 | ||
| Primary school | 0 (0.0%) | 1 (0.9%) | |
| Secondary school | 1 (1.5%) | 9 (8.3%) | |
| High school | 31 (47.7%) | 52 (48.1%) | |
| University | 33 (50.8%) | 46 (42.6%) | |
| Cigarette smoking | 21 (32.3%) | 25 (23.1%) | 0.187 |
| Alcohol consumption | 7 (10.8%) | 7 (6.5%) | 0.317 |
| Demodex positivity | 8 (12.3%) | 46 (42.6%) | < 0.001 |
| History of familial acne | 16 (24.6%) | 41 (38.0%) | 0.070 |
| Blepharitis in family | 1 (1.5%) | 3 (2.8%) | 1.000 |
| Keeping animal at home | 21 (32.3%) | 24 (22.2%) | 0.143 |
Demodex positivity was significantly higher in the acne group (p < 0.001).
Demodex positivity was a risk factor for developing acne vulgaris (OR = 5.286; 95% CI: 2.299–12.153) (p < 0.001). Gender (p = 0.294), education level (p = 0.143), cigarette smoking (p = 0.187), alcohol consumption (p = 0.317), family history of acne (p = 0.070) and keeping a pet (cat, dog, or bird) at home (p = 0.143) had no predictive value on developing acne according to the monovariate statistical analyses (Table 2).
Table 2.
The variables that were likely predictive risk factors for developing acne
| Variables | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Male | 1.424 | 0.735–2.761 | 0.294 |
| Educational status | 0.637 | 0.379–1.069 | 0.143 |
| Cigarette smoking | 0.631 | 0.318–1.253 | 0.187 |
| Alcohol consumption | 0.574 | 0.192–1.719 | 0.317 |
| Demodex positivity | 5.286 | 2.299–12.153 | < 0.001 |
| Familial history of acne | 1.874 | 0.944–3.719 | 0.070 |
| Keeping an animal at home | 0.599 | 0.300–1.193 | 0.143 |
Demodex positivity was a risk factor for developing acne vulgaris (p < 0.001).
According to the multivariate Backward Step By Step Logistic Regression analyses, the most effective factors for acne development were Demodex positivity (OR = 5.565, 95% CI: 2.384–12.99 and p < 0.001) and being aged under 25 (OR = 2.3, 95% CI: 1.183–4.473 and p = 0.014).
The clinic features of adolescent and post adolescent acne are shown in Table 3.
Table 3.
Clinical and demographic features of acne groups
| Variables | Adolescent (n = 65) | Post adolescent (n = 43) | P-value |
|---|---|---|---|
| Cigarette | 13 (20.0%) | 12 (27.9%) | 0.340 |
| Alcohol consumption | 3 (4.6%) | 4 (9.3%) | 0.433 |
| Mean value of GASS | 26 (12–38) | 23 (13–41) | 0.165 |
| GASS: | 0.118 | ||
| Mild | 11 (16.9%) | 9 (20.9%) | |
| Moderate | 36 (55.4%) | 29 (67.4%) | |
| Severe | 18 (27.7%) | 4 (9.3%) | |
| Very severe | 0 (0.0%) | 1 (2.3%) | |
| Demodex: | 0.601 | ||
| Negative | 36 (55.4%) | 26 (60.5%) | |
| Positive | 29 (44.6%) | 17 (39.5%) |
There are no differences between adolescent and post adolescent acne according to variables.
Discussion
Demodex has been associated with the development of pityriasis folliculorum, rosacea [26–28], perioral dermatitis [29], seborrheic dermatitis [30], pustular eruption [31], blepharitis [32, 33], seborrheic alopecia [31], the dermatosis that persists and shows a resistance to classical therapies [34] and acne [34–36]. Polymerase chain reaction evaluation of tissue from rosacea patients has demonstrated upregulation of tumour necrosis factor α (TNF-α), ınterleukin 1b (IL-1b) and interleukin 8 (IL-8) [28] as P. acnes triggered secretion of TNF-α, IL-1α, IL-1β, IL-8, IL-10 and IL-12 in acne vulgaris [1].
Post adolescent acne is generally mild to moderate in severity and presents with more inflammatory lesions and fewer comedones when compared to adolescent acne. The aetiopathogenesis of post adolescent acne is yet to be fully elucidated. Hormonal parameters are normal in a majority of patients. Several environmental factors are emphasised, including stress, environmental pollution, ultraviolet exposure and smoking. Emotional stress is suggested to increase adrenal androgens, causing sebaceous hyperplasia, and may play a role in the etiopathogenesis of acne [3–6].
Some authors consider Demodex mites as simply passengers that can be typically found in normal adult skin or coincidentally in diseased skin. However, a growing number of case reports and epidemiological studies show that Demodex has an aetiopathogenic role in acne vulgaris. Lacey suggested that mites can easily become pathogenic [14, 34].
Baysal et al. [36] have investigated the relationship between acne and Demodex, and showed that 11.8% of 101 patients had Demodex positivity. Polat et al. [37] also studied the same subject and found positivity in 15.38% of 78 patients. However, other authors found no relationship between these two distinct diseases. The study conducted by Okyay et al. [38] concluded that the Demodex prevalence and parasite density were not significantly related with acne vulgaris [38].
Zhao et al. [34] reported a meta-analysis that determined an association between acne vulgaris and Demodex infestation. They evaluated 63 articles and 48 concluded a positive association. The total infestation rate of Demodex mites was 54.85% in acne patients; it was 31.54% higher than in the controls (OR = 2.80; 95% CI: 2.34–3.36). They suggested that the association between Demodex infestation and the development of acne vulgaris was statistically significant.
In our study, Demodex positivity was seen in 46 (42.6%) patients in the acne group and 8 (12.3%) in the control group and the differences were statistically significant (p < 0.001). Demodex positivity was found to be a risk factor for developing acne vulgaris (OR = 5.286; 95% CI: 2.299–12.153, p < 0.001). The multivariate Backward Step By Step Logistic Regression analyses revealed that the most effective factor for acne development was Demodex positivity (OR = 5.565, 95% CI: 2.384–12.99, Wald value 15.752 and p < 0.001). No significant difference was noted between Demodex positivity in the two acne groups in terms of age and sex (p > 0.05). Zhao et al. [22] and Dokuyucu et al. [39] found that gender was not statistically correlated with Demodex infestation, in agreement with other literature.
Demodex mites can be found in any age group except newborn infants, who are presumably infested soon after birth by direct contact. The extent of Demodex colonisation in the human population was reported as high (20–80%) and could reach 100% in elderly people [14, 22, 40]. No significant difference was found between age and Demodex positivity (p = 0.601) in the control and acne groups, but our study mean age was 22 (18–54) for the acne group and 28 (18–68) for the control group. Our findings may reflect the low mean age of the patients in our study.
Our study is the first to classify acne type according to age and evaluate its relationship to Demodex prevalence. The post adolescent group had 17 (39.5%) patients showing Demodex positivity and the age-sex matched control group had 8 (21.1%) positivity, but the difference was not statistically significant when compared with the age-matched control group (p = 0.072). All Demodex positive adult controls had hyperseborrhea. We think that increasing the number of post adolescent acne patients might reveal a significant positive difference. The adolescent group contained 29 (44.6%) positive patients and the age-sex matched controls had no Demodex positivity (p < 0.001) (Tables 4, 5).
Table 4.
The distribution of Demodex positivity
| Variable | Control group | Acne group | P-value |
|---|---|---|---|
| Adolescent: | < 0.001 | ||
| Demodex negative | 27 (100.0%) | 36 (55.4%) | |
| Demodex positive | 0 (0.0%) | 29 (44.6%) | |
| Post adolescent: | 0.072 | ||
| Demodex negative | 30 (78.9%) | 26 (60.5%) | |
| Demodex positive | 8 (21.1%) | 17 (39.5%) |
The difference in Demodex positivity was found statistically significant between the adolescent acne and control groups (p = 0.004).
Table 5.
The distribution of Demodex positivity (> 5 Demodex per cm2)
| Variable | Control group | Acne group | P-value |
|---|---|---|---|
| Adolescent: | 0.175 | ||
| Demodex negative | 27 (100.0%) | 59 (90.8%) | |
| Demodex positive | 0 (0.0%) | 6 (9.2%) | |
| Post adolescent: | 0.027 | ||
| Demodex negative | 38 (100.0%) | 37 (86.0%) | |
| Demodex positive | 0 (0.0%) | 6 (14.0%) |
The difference in Demodex positivity was found statistically significant between the post adolescent acne and control groups (p = 0.004).
Smoking has been suggested by some authors as an aetiopathogenic risk factor and aggravating factor for post adolescent acne [41, 42]. However, our study revealed no positive correlation between cigarette smoking in the adolescent and post adolescent acne groups when compared with their age- and sex-matched control groups (p = 0.187). Cigarette smoking (p = 0.901) and alcohol consumption (p = 0.247) did not influence the positivity and density of Demodex in the adolescent group. Cigarette smoking (p = 0.168) did not influence the positivity and density of Demodex in the post adolescent acne group, but alcohol consumption was related with Demodex positivity (p = 0.019). No previous study in the available literature has investigated the relationship between Demodex positivity and post adolescent acne.
Our study is the first to evaluate the acne severity with GASS and its relationship to Demodex prevalence. No statistically significant difference was found between acne severity and Demodex positivity (p = 0.347) and no positive correlation was noted between Demodex density and GASS (r = 0.094 and p = 0.333). However, the patients who had nodulocystic acne and severe acne scored +3, +4 and +5 for Demodex positivity. In the acne group, the number of patients who had severe acne (n = 23) was low. Most of the cases were mild (n = 20) and moderate (n = 65) acne. Our view is that if the number of patients with severe acne is numerically increased, statistical differences may be revealed according to acne severity.
In all groups, including the control group, the Demodex positivity was higher in patients with a family history of acne (p < 0.001). This finding is relevant with the literature because Demodex infection may be transferred by direct contact with an infected person’s skin or indirectly through contact with contaminated personal hygiene materials such as towels, combs, blankets, sponges or bedclothes [13, 43].
Some authors suggested that Demodex is a component of the microflora, but we and other authors disagree because of its potential as causative roles in the pathogenesis of some human skin disorders and because treatments given to patients that appear toxic to mites lead to clinical improvement in the relevant skin disorders. One suggestion is that when the mites multiply and reach a threshold number, they can become pathogenic due to their enhanced irritating action [14, 22]. The pathogenic potential is proposed to increase if the mite density is higher than 5 per cm2 [44]. If a density of Demodex is < 5 living parasites (+1 positivity) is accepted as negative, then no patients in the control group were infested with Demodex and 12 (11.1%) were infested in the acne group. According to this finding, the difference in Demodex positivity was still found statistically significant between the acne and control groups (p = 0.004). In this situation, in adolescent acne, the number of patients infected with Demodex was higher compared to the age-matched control group, but the difference was not statistically significant (p = 0.175). In the post adolescent group, the difference was significantly different (p = 0.027). The GASS and Demodex density did not show statistically significant differences with respect to acne (p = 0.655).
An association between Demodex infestation and acne vulgaris has been confirmed in some clinical research [22, 34, 45]. Acne vulgaris and Demodex folliculitis manifest several similar symptoms, including papules, pustules and nodules. For this reason, differential diagnosis may be difficult between two diseases. Demodex folliculitis, shows no comedones, but pityriasis folliculorum may be diagnosed with comedones. Zhao suggested that dermatologists in China may be misdiagnosing acne and Demodex folliculitis. In our study, we excluded the patients who did not have a comedone as the special sign of acne vulgaris.
The causal relationship of Demodex mites in skin lesions has been suspected to occur through several mechanisms. The mites may mechanically block the follicles, leading to distension and causing intra-follicular hyperkeratosis [34, 44]. The mites can migrate from one follicle to another at a speed of 8–16 mm over 7 h. The female mites are thought to deposit their ova in the deeper areas of the pilosebaceous unit, where the young will be able to continue their lifestyle to the adult form [14]. The mite body is covered with a hard exoskeleton and the presence of the mite’s chitinous external skeleton may act like a foreign body and contribute to the formation of granulomas. Most probably, when Demodex mites breach the epithelial barrier, their antigens influence the immune system of the host and induce a type IV hypersensitivity reaction. The waste products of Demodex mites and/or associated bacteria may activate the elements of the innate immune system or stimulate the immune system through the mechanism of delayed hypersensitivity reaction, and this hypersensitivity reaction may be the triggering factor for acne development [44].
Mumcuoglu and Akilov et al. [46] suggested that the parasite may act as a carrier of Bacillus oleronius bacteria, which most probably functions as a co-pathogen in the development of severe forms of blepharitis. A role for B. oleronius, originally isolated from within a Demodex folliculorum mite, has been suggested in the aetiology of the condition [47]. Akilov and Mumcuoglu et al. [48] showed the ability of B. oleronius proteins to induce neutrophil recruitment and activation. Neutrophils play a significant role in the inflammation associated with acne, and this activation of neutrophils could lead to the inflammation seen with acne and could act as an aggravating factor for acne.
A positive correlation between a high density of Demodex mites and the presence of antigens affecting tissue compatibility, HLA Cw2 and Cw4, has been established by Mumcuoglu and Akilov [46]. Increased numbers of mites have also been associated with a higher tendency of leukocytes to undergo apoptosis. This type of genetically conditioned decrease in immune performance may result in local immunosuppression, thereby facilitating the survival and replication of Demodex mites [48]. In our study, the acne severity and Demodex intensity was not correlated. We thought that a hypersensitivity to Demodex mites and an unknown immunological response to Demodex mites could possibly trigger the acne lesions.
Patients with mixed, oily or dry skin were more likely to be infested with Demodex when compared with patients with neutral skin [22]. Most of acne patients had oily or mixed skin type [2] and they could be readily infested with Demodex [22]. A high glycaemic index and insulin resistance causes hyperandrogenism and triggers the development of acne. A study that included adolescents and young adults (10–24 years old) found a lower risk of acne in patients with a low body mass index (BMI) than a high BMI. Dokuyucu et al. [39] reported that Demodex positivity was significantly higher in obese patients. Some authors suggested that poor blood glucose regulation, obesity and metabolic syndrome all increase the susceptibility to D. folliculorum mite infestation [39, 49–52]. A recent suggestion is that acne aetiology, and especially post adolescent acne, is triggered by insulin resistance [53]. Acne patients who show insulin resistance may have a higher tendency towards Demodex infestation. Yarim et al. [54] demonstrated that serum concentration and skin tissue expression of insulin-like growth factor 2 (IGF-2) increased in canine generalised demodicosis. The IGF-2 levels are high in acne patients as well.
A work group on acne has indicated that microbiologic testing of acne lesions is unnecessary because it does not affect management, and successful antibiotic treatment may not result from a complete reduction of bacterial colonisation [1, 2]. We suggest that Demodex infestation should be studied when the classical therapies are ineffective, as suggested by Zhao et al. [34].
Conclusions
Some clinicians deny any association between Demodex positivity and acne vulgaris; however, we suggest that when regular treatments for acne vulgaris are ineffective, examination for Demodex mites and therapy for Demodex should be considered. Our results need to be clarified in future studies, but this potential relationship may suggest a shared point where the human immunological system is activated to the microbiomes already associated with acne vulgaris [1, 2] and to Demodex mites.
Acknowledgments
The study was conducted at the Yildirim Beyazit University Yenimahalle Training and Research Hospital, Department of Dermatology, Ankara, Turkey.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Das S, Reynolds RV. Recent advances in acne pathogenesis: implications for therapy. Am J Clin Dermatol. 2014;15:479–88. doi: 10.1007/s40257-014-0099-z. [DOI] [PubMed] [Google Scholar]
- 2.Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945–73. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Kucharska A, Szmurło A, Siñska B. Significance of diet in treated and untreated acne vulgaris. Adv Dermatol Allergol. 2016;33:81–6. doi: 10.5114/ada.2016.59146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romañska-Gocka K, Woźniak M, Kaczmarek-Skamira E, Zegarska B. The possible role of diet in the pathogenesis of adult female acne. Adv Dermatol Allergol. 2016;33:416–42. doi: 10.5114/ada.2016.63880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian. J Dermatol Venereol Leprol. 2012;78:335–41. doi: 10.4103/0378-6323.95450. [DOI] [PubMed] [Google Scholar]
- 6.Goulden V, Clark SM, Cunliffe WJ. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136:66–70. [PubMed] [Google Scholar]
- 7.Capitanio B, Sinagra JL, Bordignon V, et al. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63:782–8. doi: 10.1016/j.jaad.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Balta I, Ekiz O, Ozuguz P, et al. Insulin resistance in patients with post-adolescent acne. Int J Dermatol. 2015;54:662–6. doi: 10.1111/ijd.12426. [DOI] [PubMed] [Google Scholar]
- 9.Aytekin S, Goktay F, Yasar S, et al. Tips and tricks on Demodex density examination by standardized skin surface biopsy. J Eur Acad Dermatol Venereol. 2016;30:126–8. doi: 10.1111/jdv.13402. [DOI] [PubMed] [Google Scholar]
- 10.Talghini S, Fouladi DF, Babaeinejad S, et al. Demodex mite, rosacea and skin melanoma; coincidence or association? Turkiye Parazitol Derg. 2015;39:41–6. doi: 10.5152/tpd.2015.3473. [DOI] [PubMed] [Google Scholar]
- 11.Forton FM, Germaux MA, Thibaut SC, et al. Demodicosis: descriptive classification and status of Rosacea, in response to prior classification proposed. J Eur Acad Dermatol Venereol. 2015;29:829–32. doi: 10.1111/jdv.12926. [DOI] [PubMed] [Google Scholar]
- 12.Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739–43. doi: 10.1016/j.clindermatol.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Rusiecka-Ziolkowska J, Nokiel M, Fleischer M. Demodex – an old pathogen or a new one? Adv Clin Exp Med. 2014;23:295–8. doi: 10.17219/acem/37081. [DOI] [PubMed] [Google Scholar]
- 14.Lacey N, Russell-Hallinan A, Powell FC. Study of Demodex mites. Challenges and solutions. J Eur Acad Dermatol Venereol. 2016;30:764–75. doi: 10.1111/jdv.13517. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219–25. doi: 10.1111/bjd.12850. [DOI] [PubMed] [Google Scholar]
- 16.Eismann R, Bramsiepe I, Danz B, et al. Abscessing nodular demodicosis: therapy with ivermectin and permethrin. J Eur Acad Dermatol Venereol. 2010;24:79–81. doi: 10.1111/j.1468-3083.2009.03273.x. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero-Gonzalez GA, Herz-Ruelas ME, Gomez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med 2014; 2014:458046. doi: 10.1155/2014/458046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chovatiya RJ, Colegio OR. Demodicosis in renal transplant recipients. Am J Transplant. 2016;16:712–16. doi: 10.1111/ajt.13462. [DOI] [PubMed] [Google Scholar]
- 19.Karincaoglu Y, Esrefoglu Seyhan M, Bayram N, et al. Incidence of Demodex folliculorum in patients with end stage chronic renal failure. Ren Fail. 2005;27:495–9. doi: 10.1080/08860220500198037. [DOI] [PubMed] [Google Scholar]
- 20.Yagdiran Duzgun O, Aytekin S. Comparison of Demodex folliculorum density in haemodialysis patients with a control group. J Eur Acad Dermatol Venereol. 2007;21:480–3. doi: 10.1111/j.1468-3083.2007.01926.x. [DOI] [PubMed] [Google Scholar]
- 21.Liang L, Safran S, Gao Y, et al. Ocular demodicosis as a potential cause of pediatric blepharoconjunctivitis. Cornea. 2010;29:1386–91. doi: 10.1097/ICO.0b013e3181e2eac5. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YE, Guo N, Xun M, et al. Sociodemographic characteristics and risk factor analysis of Demodex infestation (Acari: Demodicidae. J Zhejiang Univ Sci B. 2011;12:998–1007. doi: 10.1631/jzus.B1100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36:416–8. doi: 10.1046/j.1365-4362.1997.00099.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramli R, Malik AS, Hani AF, Jamil A. Acne analysis, grading and computational assessment methods: an overview. Skin Res Technol. 2012;18:1–14. doi: 10.1111/j.1600-0846.2011.00542.x. [DOI] [PubMed] [Google Scholar]
- 25.Askin U, Seckin D. Comparison of the two techniques for measurement of the density of Demodex folliculorum: standardized skin surface biopsy and direct microscopic examination. Br J Dermatol. 2010;162:1124–6. doi: 10.1111/j.1365-2133.2010.09645.x. [DOI] [PubMed] [Google Scholar]
- 26.Turgut Erdemir A, Gurel MS, Koku Aksu AE, et al. Demodex mites in acne rosacea: reflectance confocal microscopic study. Australas J Dermatol. 2017;58:e26–30. doi: 10.1111/ajd.12452. [DOI] [PubMed] [Google Scholar]
- 27.Firat PY, Gecit I, Depecik F, et al. [Demodex spp. positivity among laboratory staff, kitchen staff, cleaning workers and nurses working in a state hospital] Turkiye Parazitol Derg. 2010;34:164–7. doi: 10.5152/tpd.2010.05. [DOI] [PubMed] [Google Scholar]
- 28.Casas C, Paul C, Lahfa ML, et al. Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol. 2012;21:906–10. doi: 10.1111/exd.12030. [DOI] [PubMed] [Google Scholar]
- 29.Dolenc-Voljc M, Pohar M, Lunder T. Density of Demodex folliculorum in perioral dermatitis. Acta Derm Venereol. 2005;85:211–5. doi: 10.1080/00015550510030069. [DOI] [PubMed] [Google Scholar]
- 30.Karincaoglu Y, Tepe B, Kalayci B, et al. Is Demodex folliculorum an aetiological factor in seborrhoeic dermatitis? Clin Exp Dermatol. 2009;34:516–20. doi: 10.1111/j.1365-2230.2009.03343.x. [DOI] [PubMed] [Google Scholar]
- 31.Helou W, Avitan-Hersh E, Bergman R. Demodex folliculitis of the scalp: clinicopathological study of an uncommon entity. Am J Dermatopathol. 2016;38:658–63. doi: 10.1097/DAD.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 32.Kabatas N, Dogan AS, Kabatas EU, et al. The effect of Demodex infestation on blepharitis and the ocular symptoms. Eye Contact Lens. 2017;43:64–7. doi: 10.1097/ICL.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 33.Turk M, Ozturk I, Sener AG, et al. Comparison of incidence of Demodex folliculorum on the eyelash follicule in normal people and blepharitis patients. Turkiye Parazitol Derg. 2007;31:296–97. [PubMed] [Google Scholar]
- 34.Zhao YE, Hu L, Wu LP, Ma JX. A meta-analysis of association between acne vulgaris and Demodex infestation. J Zhejiang Univ Sci B. 2012;13:192–202. doi: 10.1631/jzus.B1100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khodaeiani E, Fouladi RF, Yousefi N, et al. Efficacy of 2% metronidazole gel in moderate acne vulgaris. Indian J Dermatol. 2012;57:279–81. doi: 10.4103/0019-5154.97666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baysal V, Aydemir M, Yorgancıgil B, Yıldırım M. Akne vulgaris etyopatogenezinde D. folliculorum’ların rolünün araştırılması. Türkiye Parazitol Derg. 1997;21:265–8. [Google Scholar]
- 37.Polat E, Aygün G, Ergin R, et al. The role of Demodex folliculorum and Propionibacterium acnes in pathogenesis of acne vulgaris. Türkiye Parazitol Derg. 2003;27:148–51. [Google Scholar]
- 38.Okyay P, Ertabaklar H, Savk E, Erfug S. Prevalence of Demodex folliculorum in young adults: relation with sociodemographic/hygienic factors and acne vulgaris. J Eur Acad Dermatol Venereol. 2006;20:474–6. doi: 10.1111/j.1468-3083.2006.01470.x. [DOI] [PubMed] [Google Scholar]
- 39.Dokuyucu R, Kaya OA, Yula E, et al. The presence of Demodex folliculorum in various obese groups according to BMI levels. Arch Iran Med. 2016;19:210–4. [PubMed] [Google Scholar]
- 40.Aytekin S, Yasar S, Goktay F, Gunes P. Spontaneous fluorescence of Demodex in the dark. J Eur Acad Dermatol Venereol. 2016;30:359–60. doi: 10.1111/jdv.12776. [DOI] [PubMed] [Google Scholar]
- 41.Yang YS, Lim HK, Hong KK, et al. Cigarette smoke-induced interleukin-1 alpha may be involved in the pathogenesis of adult acne. Ann Dermatol. 2014;26:11–6. doi: 10.5021/ad.2014.26.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafer T, Nienhaus A, Vieluf D, et al. Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol. 2001;145:100–4. doi: 10.1046/j.1365-2133.2001.04290.x. [DOI] [PubMed] [Google Scholar]
- 43.Aytekin S, Göktay F. Demodikosis. Türkiye Klinikleri. J Dermatol –Special Topics. 2015;8:35–41. [Google Scholar]
- 44.Lacey N, Ni Raghallaigh S, Powell FC. Demodex mites: commensals, parasites or mutualistic organisms? Dermatology. 2011;222:128–30. doi: 10.1159/000323009. [DOI] [PubMed] [Google Scholar]
- 45.Kapoor S. Rare facial dermatological lesions associated with Demodex infection, besides acne vulgaris. J Zhejiang Univ Sci B. 2012;13:421–2. doi: 10.1631/jzus.B1200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mumcuoglu KY, Akilov OE. The role of HLA A2 and Cw2 in the pathogenesis of human demodicosis. Dermatology. 2005;210:109–14. doi: 10.1159/000082565. [DOI] [PubMed] [Google Scholar]
- 47.Jarmuda S, O’Reilly N, Zaba R, et al. Potential role of Demodex mites and bacteria in the induction of rosacea. J Med Microbiol. 2012;61:1504–10. doi: 10.1099/jmm.0.048090-0. [DOI] [PubMed] [Google Scholar]
- 48.Akilov OE, Mumcuoglu KY. Immune response in demodicosis. J Eur Acad Dermatol Venereol. 2004;18:440–4. doi: 10.1111/j.1468-3083.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 49.Gokce C, Aycan-Kaya O, Yula E, et al. The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. J Int Med Res. 2013;41:1752–8. doi: 10.1177/0300060513494730. [DOI] [PubMed] [Google Scholar]
- 50.Keskin Kurt R, Aycan Kaya O, Karateke A, et al. Increased density of Demodex folliculorum mites in pregnancies with gestational diabetes. Med Princ Pract. 2014;23:369–72. doi: 10.1159/000363244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita LS, Cariello AJ, Geha NM, et al. Demodex folliculorum on the eyelash follicle of diabetic patients. Arq Bras Oftalmol. 2011;74:422–4. doi: 10.1590/s0004-27492011000600008. [DOI] [PubMed] [Google Scholar]
- 52.Enginyurt O, Karaman U, Cetin F, Ozer A. The prevalence of Demodex species and its relationship with the metabolic syndrome in women of Malatya Province, Turkey. Jundishapur J Microbiol. 2015;8:24322.. doi: 10.5812/jjm.24322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155–62. doi: 10.1016/j.jaad.2016.02.1220. [DOI] [PubMed] [Google Scholar]
- 54.Yarim GF, Yagci BB, Yarim M, et al. Serum concentration and skin tissue expression of insulin-like growth factor 2 in canine generalized demodicosis. Vet Dermatol. 2015;26:421–5. doi: 10.1111/vde.12270. [DOI] [PubMed] [Google Scholar]