The chemotherapeutic agent cytarabine represents an effective treatment for acute myeloid leukemia and lymphomas. Cytarabine is a pyrimidine nucleoside that induces DNA damage in the S phase of the cell cycle. Moreover, cytarabine inhibits DNA and RNA polymerases as well as nucleotide reductase. Thereby, rapidly cycling cells are the most affected. Due to its strong biological effects on a variety of cells and tissues, important adverse reactions might occur during treatment with cytarabine, such as myelosuppression, gastrointestinal disorders, neurotoxicity, hepatitis and an immediate infusion reaction, known as “cytarabine syndrome”. This latter clinical entity is dose-dependent and includes fever, diaphoresis, myalgia and skin eruptions [1].
Although cases of hypersensitivity reactions to chemotherapeutic agents have been observed, allergy to cytarabine is uncommon and only sporadic reports exist [2]. Particularly, no delayed hypersensitivity reactions have been described in adults, so far.
Here, we report 2 cases of adult patients with delayed hypersensitivity to cytarabine that resolved successfully after desensitization.
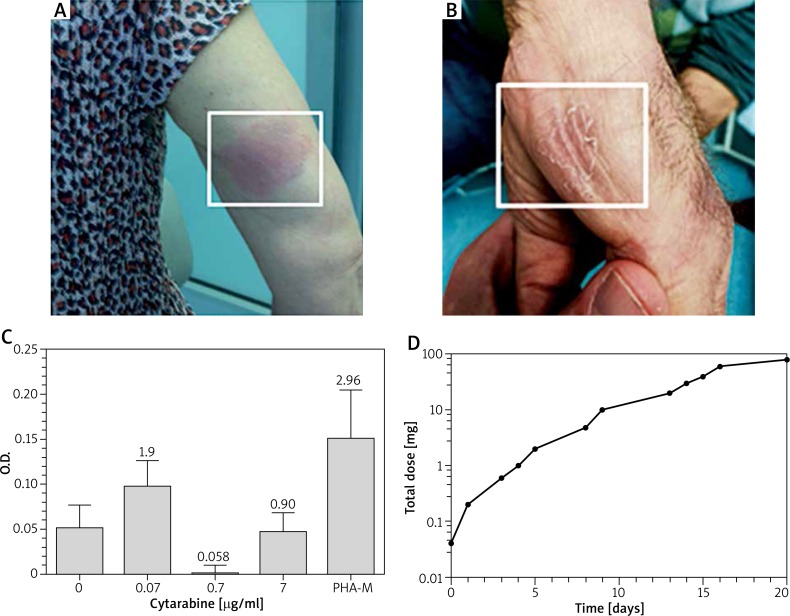
Case 1: A 66-year-old woman was diagnosed with acute myeloid leukemia in March 2012 and started a cytarabine treatment (160 mg/day, for 3 days), with no adverse effects. Likewise, in April and May 2012, two further cytarabine courses were well tolerated. However, in June 2012, she developed a severe cutaneous rash with intensively itchy and partially eroded erythematous maculae, widespread to the trunk and the extremities, 3 days after the treatment (Figure 1 A). Of note, the patient presented a marked eosinophilia (4730 cells/μl) and developed a sterile dental abscess after the cytarabine administration course, in the absence of any dental diseases prior to the therapy.
Figure 1.
A, B – Representative pictures of cutaneous lesions for case 1 and case 2. Similar lesions were scattered on the whole body. As for case 1, the lesion shown does not correspond to the site of injection of cytarabine. C – Case 2. Lymphocyte proliferation assessed after 5 days of culture with the indicated concentrations of cytarabine or phytohemagglutinin M (PHA-M). The ratio between the different cytarabine concentrations and negative control is shown above each Histogramm column. Error bars correspond to SEM. D – A dose-time desensitization curve of cytarabine
In order to exclude a possible cytarabine syndrome, a second cycle of treatment with reduced dosage was performed (80 mg/day, for 3 days). However, the patient developed the same symptoms, with similar time of onset as above (including the same sterile dental abscess), suggesting that the adverse reaction was immunologic in nature and delayed in presentation.
After 15 days, we performed skin tests, with two distinct techniques: skin prick testing and intradermal testing. The patient was first subjected to skin prick testing, using a 20 mg/ml cytarabine solution and, successively, to intradermal tests with 4 different 10-fold concentrations (0.02, 0.2, 2 and 20 mg/ml). Both skin testing procedures proved negative. Neither patch tests (the patient declined) nor any in vitro tests were performed. Lymphocyte proliferation test (LPT) was not yet established.
Thus, based on the clinical symptoms, the time of onset of the adverse reaction, the lack of dependency on the dose administered and the negative results of the skin tests, the patient was diagnosed with delayed hypersensitivity to cytarabine (clinically). Importantly, the eosinophilia at the time of diagnosis and the finding of the recurrent sterile dental abscess concomitant with cytarabine treatment rendered the adverse reaction compatible with a possible drug reaction with eosinophilia and systemic symptom (DRESS), according to RegiSCAR scoring system [3].
Case 2: A 69-year-old man, also diagnosed with acute myeloid leukemia, started a cytarabine treatment course in December 2015 with no adverse effects. Likewise, in January 2016, a second cytarabine course was well tolerated. In sharp contrast, in February 2016, during the third cycle of cytarabine treatment, the patient developed generalized severe cutaneous lesions (Figure 1 B), 3 days after the treatment, again suggesting a delayed hypersensitivity reaction.
After about 25 days, we performed both in vivo and in vitro diagnostic tests: patch tests and LPT. The patch tests, performed with different cytarabine concentrations (1, 10, 100 μg/ml) were negative. As for the LPT, the lymphocytes of the patient were incubated for 5 days with 3 different 10-fold cytarabine concentrations: 0.7 μg/ml, the “therapeutic concentration” calculated on a distribution volume of 2.6 l/kg, 0.07 μg/ml and 7 μg/ml, respectively. Upon incubation for 2 h with bromodeoxyuridine, lymphocyte proliferation was assessed. The test is deemed positive when the proliferation rate of any of the three concentrations tested (compared to the control) equals or exceeds 2. Although the test only provided a ratio of 1.91 for one of the three concentrations, it was considered suggestive of immunologic delayed nature of the adverse reaction, in consideration of the cytostatic effect of cytarabine and the concomitant corticosteroid therapy (prednisone, 12.5 mg/day) (Figure 1 C). Skin tests performed as described above, proved negative, thereby excluding the immediate nature of the allergic condition.
In both cases, cytarabine treatment was deemed indispensable. Therefore, we decided to desensitize the patients. To this aim a 12-step protocol was designed, using 4 cytarabine solutions: 0.2, 2, 20 and 100 mg/ml (Table 1). Cytarabine was administered subcutaneously at increasing doses and the desensitization was carried out over about 24 days, every other day (Figure 1 D). Each cytarabine dose was, in turn, fractionated into 2–4 injections, given every 30 min (Table 1). Remarkably, both patients completed the protocol and received the full planned dose of cytarabine thereafter. Importantly, no major adverse reactions were observed during the desensitization and during cytarabine treatment after the desensitization. Modest transient infiltrated nodules were occasionally observed at the site of injection, mostly during the first half of the desensitization course.
Table 1.
Allergen immunotherapy-like desensitization schedule for cytarabine-induced delayed hypersensitivity
| Step* | Solution [mg/ml] | Total volume injected [ml] | Number of injections** | Dose [mg] |
|---|---|---|---|---|
| 1 | 0.2 | 0.2 | 2 | 0.04 |
| 2 | 0.2 | 1 | 4 | 0.2 |
| 3 | 2 | 0.3 | 3 | 0.6 |
| 4 | 2 | 0.5 | 3 | 1 |
| 5 | 2 | 1 | 4 | 2 |
| 6 | 2 | 2.4 | 4 | 4.8 |
| 7 | 20 | 0.5 | 4 | 10 |
| 8 | 20 | 1 | 4 | 20 |
| 9 | 100 | 0.3 | 3 | 30 |
| 10 | 100 | 0.4 | 4 | 40 |
| 11 | 100 | 0.6 | 3 | 60 |
| 12 | 100 | 0.8 | 4 | 80 |
| Total | 42 | 248.64 |
Desensitization sessions were performed every other day (with few exceptions; e.g. patient availability, week-end days etc.).
Two to four subcutaneous injections were given 30 min apart during the same desensitization session.
Allergic reactions to chemotherapeutic agents are uncommon and only sporadic reports on cytarabine hypersensitivity exist [4, 5]. To our knowledge, the 2 cases presented here are the first cytarabine-induced delayed hypersensitivity cases described in adulthood.
Desensitization to cytarabine was previously proven to be effective in the treatment of immediate hypersensitivity reactions and cytarabine syndrome [6, 7], in both cases by intravenous rapid desensitization [8]. Exploiting our previous experience [9], we designed an “allergen-immunotherapy”- like desensitization protocol for delayed hypersensitivity to cytarabine. This subcutaneous desensitization procedure was both well tolerated and effective in preventing further hypersensitivity reactions. Importantly, the desensitization status was maintained over a certain period of time since, after the desensitization, patient 1 tolerated one further cytarabine course and patient 2 received 5 cytarabine courses, both with no adverse reactions.
In conclusion, the cases reported show that: i) in the case of delayed hypersensitivity to cytarabine, desensitization can be pursued effectively and safely; ii) this procedure can prevent cytarabine treatment withdrawal; and iii) LPT may be useful and informative.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Castleberry RP, Crist WM, Holbrook T, et al. The cytosine arabinoside (Ara-C) syndrome. Med Pediatr Oncol. 1981;9:257–64. doi: 10.1002/mpo.2950090309. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd GM. Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol. 2003;24:253–62. doi: 10.1385/CRIAI:24:3:253. [DOI] [PubMed] [Google Scholar]
- 3.Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–97.. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Rassiga AL, Schwartz HJ, Forman WB, Crum ED. Cytarabine-induced anaphylaxis. Demonstration of antibody and successful desensitization. Arch Intern Med. 1980;140:425–6. doi: 10.1001/archinte.140.3.425. [DOI] [PubMed] [Google Scholar]
- 5.Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38–40. doi: 10.1046/j.1525-1470.2001.018001038.x. [DOI] [PubMed] [Google Scholar]
- 6.Blanca M, Torres MJ, Giron M, et al. Successful administration of cytarabine after a previous anaphylactic reaction. Allergy. 1997;52:1009–11. doi: 10.1111/j.1398-9995.1997.tb02422.x. [DOI] [PubMed] [Google Scholar]
- 7.Lim KH, Kim JY, Kang MG, et al. Two cases of cytarabine syndrome successfully resolved by desensitization. J Investig Allergol Clin Immunol. 2015;25:80–2. [PubMed] [Google Scholar]
- 8.Castells M, Sancho-Serra Mdel C, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer Immunol Immunother. 2012;61:1575–84. doi: 10.1007/s00262-012-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Bona D, Albanesi M, Giliberti LA, et al. Desensitization for immediate hypersensitivity to oral dymethyl fumarate (Tecfidera®) J Allergy Clin Immunol Pract. 2017;5:821–2. doi: 10.1016/j.jaip.2016.09.044. [DOI] [PubMed] [Google Scholar]