Abstract
Rationale/aim
Despite the increasing efficacy of recanalization therapies for acute ischemic stroke, a large number of patients are left with long-term functional impairment, devoid of efficacious treatments. CD34+ cells comprise a subset of bone marrow-derived mononuclear cells with the capacity to promote angiogenesis in ischemic lesions and have shown promising results in observational and in vitro studies. In this study, we aim to assess the efficacy of an autotransplant of CD34+ cells in acute ischemic stroke.
Sample size estimates
30 patients will be randomized for a power of 90% and alpha of 0.05 to detect a difference in 3 months infarct volume.
Methods and design
We will screen 18–80 years old patients with acute ischemic stroke due to occlusion of a middle cerebral artery (MCA) for randomization. Persistent arterial occlusions, contra-indications to magnetic resonance imaging (MRI), premorbid dependency, or other severe diseases will be excluded. Treatment will involve bone marrow aspiration, selection of CD34+ cells, and their administration intra-arterially in the symptomatic MCA by angiography. Patients will be randomized for treatment at 7 (±2) days, 20 (±5 days) or sham procedure, 10 in each group.
Study outcomes
The primary outcome will be infarct volume in MRI performed at 3 months. Secondary outcomes will include adverse events and multidimensional functional and neurological measures.
Discussion/conclusion
STROKE34 is a PROBE design phase IIa clinical trial to assess the efficacy of intra-arterial administration of CD34+ cells 7 and 20 days after acute ischemic stroke.
Trial registration (EU Clinical Trials Register)
2017-002456-88.
Keywords: clinical trial, intervention, ischemic stroke, magnetic resonance imaging, stem cells, stroke
Introduction and Rationale
Current treatment algorithms for ischemic stroke have their primary focus on promoting vascular reperfusion, leaving tissue recovery devoid of directed and efficient therapies. In fact, despite the increasing efficacy of recanalization methods, up to half of patients who survive ischemic stroke develop long-term functional impairment. To overcome this therapeutic need, cell therapies have emerged as a potential method of promoting neurological recovery (1–5). However, many quandaries still limit their translation into clinical practice, especially in therapies aiming at neuro and synaptogenesis (6). Recently, CD34+ cells have shown promising results in observational and in vitro studies, urging research translation into randomized trials (7–9). They comprise a subset of bone marrow-derived mononuclear cells representing the main source of endogenous endothelial progenitor cells (EPC), with the capacity to promote angiogenesis in ischemic injuries. A phase I clinical trial demonstrated the safety and feasibility of administering intra-arterially autologous, bone marrow-derived CD34+ cells (10). We aim to design a phase IIa clinical trial to evaluate the efficacy of the intra-arterial administration of CD34+ at two timepoints: 7 ± 2 and 20 ± 5 days.
Methods and Analysis
Design
STROKE34 is a phase IIa superiority, unicenter, randomized, blinded outcome assessment, sham-controlled clinical trial evaluating the efficacy of intra-arterially administered CD34+ cells 7 ± 2 and 20 ± 5 days after ischemic stroke (PROBE design).
Patient Population
We will screen for inclusion consecutive patients with non-lacunar acute ischemic stroke infarctions within the territory supplied by a middle cerebral artery (MCA). In Table 1, we present the study’s inclusion and exclusion criteria. Demonstration of arterial recanalization at the time of inclusion will be determined by angiography (modified TICI grades 2b or 3) or transcranial color coded Doppler (TIBI grades 4 or 5) (11, 12).
Table 1.
Inclusion and exclusion criteria for the study.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Randomization
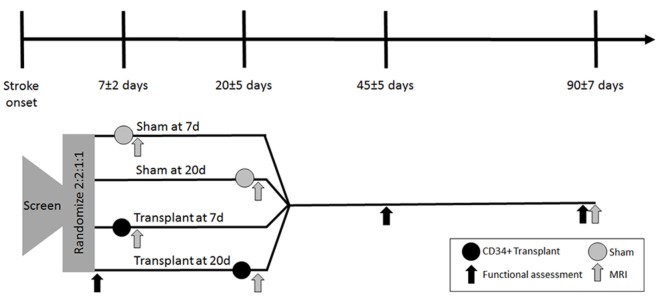
Subjects will be randomized, stratified for age, gender, baseline NIHSS (at randomization), laterality and performance of recanalization therapies (intravenous or intra-arterial) into intervention at day 7 ± 2; intervention at day 20 ± 5; sham procedure at day 7 ± 2; sham at day 20 ± 5, in a 2:2:1:1 ratio, using a computerized stratified randomization program (Figure 1).
Figure 1.
Trial design flow chart. Abbreviations: MRI, magnetic resonance imaging; d, days.
Intervention
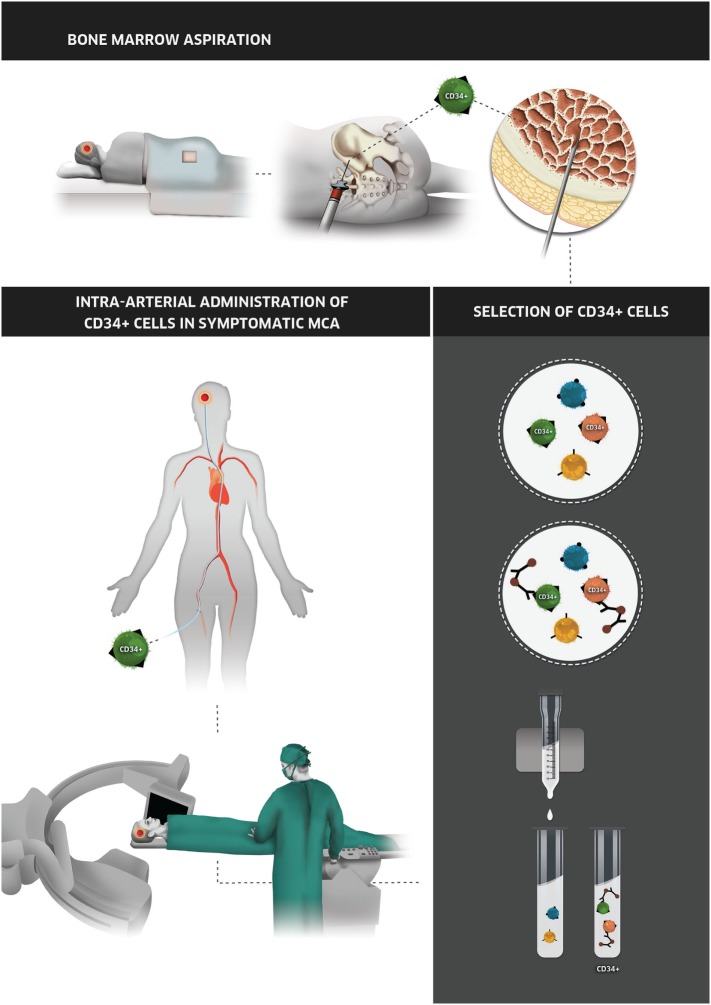
First, a single-pull 10-mL aspirate of bone marrow from the iliac crest will be collected by experienced Hematologist under aseptic conditions, using only local anesthesia at puncture site. CD34+ cells will then be isolated by magnetic cell sorting using CliniMACS Plus® (Miltenyi Biotec) without cellular manipulation and suspended in 10–100 mL of saline solution. 24–48 h after collection, 1 × 106 up to 1 × 108 cells suspended in human albumin solution will be administered intra-arterially in the symptomatic MCA (Figure 2). Before infusion, all solutions will be screened for infections according to national human tissue transplantation requirements (national legislation 12/2009): Syphilis, HIV 1 and 2, Hepatitis B and C in all patients, as well as human T lymphotropic virus 1 and 2 whenever indicated.
Figure 2.
Illustrative representation of autotransplant of CD34+ cells. In the top of the image, bone marrow aspiration by hematologist is represented. The second step (bottom right) is the isolation of CD34+ cells, followed by the intra-arterial administration of the CD34+ in the symptomatic MCA (bottom left). Abbreviation: MCA, middle cerebral artery.
In the angio-suite, catheterization of the symptomatic MCA by neuroradiologist will follow routine practice. In short, all patients will initially be submitted to local anesthesia of the groin for femoral artery puncture and intravenous administration of 5,000 units of unfractionated heparin. Then, a guide catheter selected according anatomical features of the patient will be navigated up to the initial segment of the symptomatic internal carotid artery. At that point, a microcatheter will navigate up to the beginning of the M1-MCA, where the solution containing the CD34+ cells will be slowly infused during approximately 10 min. The expected total duration of the procedure will be 30 min. All procedures will be accompanied by anesthesiologist. Sedation will not be used routinely, but will be possible according to the anesthesiologist’s choice. No other drug will be used routinely during the procedure, but the treating physicians will be free to use any other treatment considered necessary according to clinical condition. In case the interventionalist encounters a previously undiagnosed high-grade stenosis (>70%), vascular occlusion or any vascular malformation in the symptomatic arteries considered to be potentially hazardous in case of catheterization the treatment will be aborted.
Patients in the sham group will initially be subject to local anesthesia of the iliac crest by Hematologist (without bone marrow aspiration) and brought to the angio-suite 24–48 h later. All study participants will be blindfolded during the angiographic procedure and submitted to local anesthesia of the groin. Patients in the sham group will not be subject to arterial puncture and will remain in the angio-suite for approximately 30 min simulating cellular infusion. No information about treatment allocation will be transferred to the recovery staff at stroke unit. Subsequent medical caregivers will also be blinded to treatment allocation. The study physicians present during the angiographic procedure will have no further contact with the patient during the study.
Until clinical stabilization, the patients will remain in the hospital and perform a control magnetic resonance imaging (MRI) 48 h after treatment, followed by orientation by a dedicated rehabilitation center.
Allocation Concealment
Stroke unit staff, patients, and outcome assessors are blinded to treatment allocation for the duration of the study. Unblinding will only take place whenever the treating physician decides that clinical management will critically depend on knowledge of treating allocation. Unblinding will be monitored and audited.
Outcomes Assessed
The primary outcome will be ischemic stroke volume, measured by automated planimetric method on FLAIR imaging sequences of MRI performed 3 months after index event. The secondary outcomes are presented in Table 2. Neuroimaging assessment will include analysis of the three MRI performed during the study: before treatment, 48 h after treatment, and at 3 months. The neuroradiologist that will evaluate lesion volumes and physicians that will evaluate the secondary outcomes will not be involved in the initial management of the patient nor intervention and will be blinded for treatment allocation.
Table 2.
Study outcomes assessed.
| Primary outcome |
|---|
|
| Secondary outcomes |
|
Data Monitoring Board
An independent Data and Safety and Monitoring Board (DSMB) will monitor any serious adverse events. It will be constituted by Neurologists and Internal Medicine physicians not involved in any part of the study design or execution. In the event of any safety concern, namely, rate of hemorrhagic transformation, cancer, procedural complications, or any other unexpected serious adverse event regarding the intervention group, the DSMB will make a recommendation to continue, stop, or modify the trial.
Ethics
A signed written informed consent form will be sought by co-investigator stroke physicians and mandatory for participation in the trial. In the form all study procedures, potential risks and benefits are explained in simple terms. The study physicians will be responsible to explain and make sure the protocol is correctly understood by participants. All study subjects will be free to withdraw consent at any time. In case the patient is handicapped and not able to write down the required information, a legally acceptable representative will be allowed to fill out the informed consent form. Participants will be given a study number, which will be kept throughout all the analysis, maintaining confidentiality. All study participants will have a prespecified insurance to cover any adverse event of study participation.
Possible safety concerns rely on MRI acquisition, bone marrow aspiration, and angiographic catheterization (that will all follow routine protocols), as well as the autotransplant of CD34+ (that has been suggested to be safe in a phase I trial). Clinical follow-up after study completion will be the discretion of the treating physician.
An additional ethical concern will be to maintain blinding of the participants throughout the trial. Apart from the above mentioned design, all study and hospital staff personnel will receive training specific to this trial.
The study will be conducted in accordance with the principles of Good Clinical Practice, the Medical Research Involving Human Subjects act and the Declaration of Helsinki. The study has been approved by the funding body (COMPETE 2020, Ref: 3386). All protocol changes such as modifications in eligibility criteria, outcome measures, analyses, or study procedures will be resubmitted.
Sample Size Estimates
No human randomized trials are available for effect estimate on intra-arterial administration of CD34+ for acute ischemic stroke. Moreover, animal studies are not ideal considering the specificity and inherent heterogeneity of human subjects. As such, for sample size calculation, we have used data from human observational studies on EPCs (7, 13). These studies indicated a large effect of EPCs in final ischemic lesion volume, with reported treatment effect sizes ranging from 1.2 to 2.0 (7, 13). For our sample size calculation of a unicenter pivotal phase IIa trial, we used an effect size of 1.2 for 90% power and a significance level of 5% to identify a difference between independent means of final infarct volume. A total of 30 patients was estimated for the trial: 10 patients treated at day 7 ± 2, 10 at day 20 ± 5, and 10 in the control group (5 sham procedures at each timepoint to ensure blinding).
Expected Results
The authors expect that the autotransplant of CD34+ will be associated with lower infarct volumes at 3 months. Observational and in vitro data do not allow a rational expectation regarding the optimal time window for CD34+ administration (7 ± 2 vs. 20 ± 5 days). Considering the validated cellular protocol using autotransplantation and angiographic delivery using routine catheterization techniques, we expect to confirm the overall safety of this procedure, with no serious adverse events.
Dissemination and Data Availability
The results will be disseminated through peer-reviewed publications, presentation at relevant scientific conferences and the general public.
The datasets generated during the study will be available on reasonable request.
Study Organization and Funding
STROKE34 is part of StrokeTherapy (POCI-01-0247-FEDER-003386), executed by Stemlab, S.A. in Co-Promotion with Centro de Reabilitação do Centro - Rovisco Pais and Universidade de Coimbra, in partnership with Centro Hospitalar e Universitário de Coimbra. The trial is funded by the Operational Program Portugal 2020 and the European Regional Development Fund through COMPETE 2020.
Discussion
The potential integration of stem cells in the treatment algorithm of acute ischemic stroke is still limited by several uncertainties, with most data coming from observational studies. STROKE34 is a randomized trial comparing different timepoints of CD34+ autotransplant: subacute (7 days) and late subacute stages (20 days). CD34+ have been confirmed to have a contributing role in the development of angiogenesis in vivo and in vitro. Angiogenesis plays a pivotal role in poststroke recovery; however, animal models have raised the possibility of hemorrhagic transformation as a potential complication of promoting new, immature vessels (14). Nonetheless, this link shows an apparent time-dependent response with acute disruption of the blood–brain barrier and hemorrhagic transformation in the first hours after stroke, whereas enhancing effective neoangiogenesis with improved outcomes in the subacute stage (8, 15). These facts reinforce the need to define an optimized therapeutic window for the design of future phase III trials.
Three methods are potential candidates for delivery of CD34+ cells: surgical cranial implantation, intravenous, and intra-arterial. Considering the invasiveness and potential hazards of surgical implantation as well as the significant absorption by non-target organs with intravenous delivery, intra-arterial administration seems to be the most promising candidate. This method is further supported by the increasing use of intra-arterial access for the treatment of acute stroke, and the safety and feasibility demonstrated in phase I study (10).
Another critical point in the design of this trial is the choice of therapeutic time window. Angiogenesis after stroke has been demonstrated from 3 to 4 days up to 5 weeks after injury (16–18). However, it is still unknown when CD34+ transplantation would be clinically effective. Early administration would imply exposing the highly active and metabolically demanding CD34+ cells to an inhospitable ischemic environment and would potentially promote ineffective angiogenesis and hemorrhagic transformation. Moreover, within the first 24 h, bone marrow aspiration would be contra-indicated in patients submitted to intravenous thrombolysis. Nonetheless, it is uncertain if later time windows would still have any clinical meaning, and the need for a permeable blood–brain barrier will ultimately limit cell delivery beyond the first 5 weeks (19). Altogether, a wide time frame is still potentially usable (from the first days up to 5 weeks) with presumably different biological effects and clinical implications. In this trial, we hope to answer if an earlier time window (5–9 days) is preferable compared to a later window (15–25 days).
A potential limitation of this study design is the small sample size planned. However, we have defined the primary endpoint as a neuroimaging variable (3 months infarct volume), to allow the inclusion of a smaller number of patients in this exploratory phase IIa trial, and have included comprehensive functional assessments as secondary outcomes.
Ethics Statement
A signed written informed consent form will be sought by co-investigator stroke physicians and mandatory for participation in the trial. In the form all study procedures, potential risks and benefits are explained in simple terms. The study physicians will be responsible to explain and make sure the protocol is correctly understood by participants. In case the patient is handicapped and not able to write down the required information, a legally acceptable representative will be allowed to fill out the informed consent form. The study will be conducted in accordance with the principles of Good Clinical Practice, the Medical Research Involving Human Subjects act and the Declaration of Helsinki.
Author Contributions
JS-F wrote the article. JS-F, LF, FS, GS, and LC defined the inclusion criteria and clinical design. LF, AG, TM, and CC defined the therapeutic product. CN and OG defined the neuroimaging protocol. JC defined the hematological protocol. AP, PA, JB, and VL defined the rehabilitation program and functional assessments.
Conflict of Interest Statement
The authors declare that this study protocol was designed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Nélia Gouveia (from NOVA Clinical Research Unit, Lisbon, Portugal) for the help in design of the clinical trial.
Footnotes
Funding. This work is part of the project StrokeTherapy (POCI-01-0247-FEDER-003386), executed by Stemlab in co-promotion with Centro de Reabilitação do Centro—Rovisco Pais and Universidade de Coimbra in partnership with Centro Hospitalar e Universitário de Coimbra. The project is financed by the Portugal 2020 program, through COMPETE 2020.
References
- 1.Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol (2017) 16:360–8. 10.1016/S1474-4422(17)30046-7 [DOI] [PubMed] [Google Scholar]
- 2.Mizuma A, Yamashita T, Kono S, Nakayama T, Baba Y, Itoh S, et al. Phase II trial of intravenous low-dose granulocyte colony-stimulating factor in acute ischemic stroke. J Stroke Cerebrovasc Dis (2016) 25:1451–7. 10.1016/j.jstrokecerebrovasdis.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 3.Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke (2014) 45:3618–24. 10.1161/STROKEAHA.114.007028 [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med (2014) 372(1):11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 5.Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet (2016) 388:787–96. 10.1016/S0140-6736(16)30513-X [DOI] [PubMed] [Google Scholar]
- 6.Kenmuir CL, Wechsler LR. Update on cell therapy for stroke. Stroke Vasc Neurol (2017) 2:59–64. 10.1136/svn-2017-000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogoslovsky T, Chaudhry A, Latour L, Maric D, Luby M, Spatz M, et al. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology (2010) 75:2059–62. 10.1212/WNL.0b013e318200d741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sargento-freitas J, Aday S, Nunes C, Cordeiro M, Gouveia A, Silva F, et al. Endothelial progenitor cells enhance blood – brain barrier permeability in subacute stroke. Neurology (2018) 90:e127–34. 10.1212/WNL.0000000000004801 [DOI] [PubMed] [Google Scholar]
- 9.Sargento-Freitas J, Aday S, Nunes C, Cordeiro M, Gouveia A, Silva F, et al. Endothelial progenitor cells influence acute and subacute stroke hemodynamics. J Neurol Sci (2018) 15:119–25. 10.1016/j.jns.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S, Bentley P, Hamady M, Marley S, Davis J, Shlebak A, et al. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med (2014) 3:1322–30. 10.5966/sctm.2013-0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demchuk AM, Burgin WS, Christou I, Felberg RA, Barber PA, Hill MD, et al. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke (2001) 32:89–93. 10.1161/01.STR.32.1.89 [DOI] [PubMed] [Google Scholar]
- 12.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, Von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke (2013) 44:2650–63. 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke (2007) 38:2759–64. 10.1161/STROKEAHA.107.484386 [DOI] [PubMed] [Google Scholar]
- 14.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab (2014) 34:185–99. 10.1038/jcbfm.2013.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest (2000) 106:829–38. 10.1172/JCI9369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke (2008) 39:1563–8. 10.1161/STROKEAHA.107.502146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin K-J, Hamblin M, Chen YE. Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol (2015) 13:352–65. 10.2174/15701611113119990016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CY, Chang C, Cheung WM, Lin MH, Chen JJ, Hsu CY, et al. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: evaluation with contrast-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab (2008) 28:1491–501. 10.1038/jcbfm.2008.42 [DOI] [PubMed] [Google Scholar]
- 19.Merali Z, Huang K, Mikulis D, Silver F, Kassner A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One (2017) 12(2):e0171558. 10.1371/journal.pone.0171558 [DOI] [PMC free article] [PubMed] [Google Scholar]