Abstract
Traumatic orthopedic injuries, particularly extremity wounds, are a significant cause of morbidity. Despite prophylactic antibiotic treatment and surgical intervention, persistent infectious complications can and do occur. Persistent bacterial infections are often caused by biofilms, communities of antibiotic tolerant bacteria encased within a matrix. The structural and metabolic differences in this mode of growth make treatment difficult. Herein, we describe both established and novel, experimental treatments targeted at various stages of wound healing that are specifically aimed at reducing and eliminating biofilm bacteria. Importantly, the highly tolerant nature of these bacterial communities suggests that most singular approaches could be circumvented and a multifaceted, combinatorial approach will be the most effective strategy for treating these complicated infections.
Keywords: Biofilm, wound care, chronic infection, persistent infection, antimicrobial treatment
Introduction
Trauma injuries are a significant cause of morbidity and disability, effecting quality of life as well as causing a financial and physical burden, with extremity injuries making up a significant majority 1-4. Importantly, complications associated with extremity trauma continue to occur, resulting in failed or delayed healing. These continued issues further increase the physical and financial burden associated with extremity injuries. While a number of causes exist that pose a detriment to healing, persistent bacterial infections play a large role in the formation and severity of these complications 5. It is now widely accepted that many instances of persistent wound infections are mediated by structures known as biofilms 6, 7. Of note, microscopic analyses of chronic wounds have shown that over 60% exhibit presence of a biofilm 8. These microbial communities exhibit a distinct phenotype that contributes to their ability to remain in wounds despite primary treatment. Thus, this review will discuss current and developing treatments aimed at preventing, dispersing, and eradicating these communities. The complex nature of biofilm bacteria makes treatment difficult, as most individual treatments fail to completely remove the offending pathogens. Proper treatment of biofilm based infections will require a multifaceted approach that employs a variety of physical treatments over the full span of the infectious process. Importantly, many of the treatments described herein are still in their infancy; the goal of this review is to highlight up and coming therapies, and how they may be utilized in conjunction with established methodologies to drastically improve treatment options.
Clinical burden of extremity trauma
Extremity trauma injuries represent a significant portion of traumatic injuries 1-3, and contribute to a large proportion of associated costs and resources. These costs, estimated by one study to near $2 billion 4, are due to an association between these injuries and repeated hospitalizations, ongoing therapies and treatments, and extended hospital stays, indicating that ongoing complications are still a significant issue even after initial treatment 3, 4. Hospital stays due to these injuries average 10.7 days in length in one study, and were associated with 60% of injury related costs 4. Extremity injuries are also associated with delayed amputations and subsequent rehospitalizations, particularly among patients with lower extremity injuries 9. When infectious complications are present, failure rates of revision surgeries can be high, adding to costs. Furthermore, extremity injuries often lead to a loss of function or mobility, resulting in costs associated with disability 1, 4.
Biofilm bacteria present unique challenges to effective treatment
Despite timely and appropriate treatment following extremity trauma, infections can and do still occur. While a number of pathogens have been recovered from wounds, a few specific species have been found to be most frequently isolated. These include Staphylococcus species (including S. aureus), Klebsiella spp., Enterobacter spp., P. aeruginosa, and A. baumannii 10, 11. Both Gram positive and Gram negative organisms have been cultured from infected wounds, with Gram negative species appearing to be common during the beginning of the wound healing process, while Gram positive species are often isolated from chronically infected wounds 10, 11. Late stage infections exhibit an increased chance of being caused by uncommon species or commensal, opportunistic pathogens. Importantly, many of the species isolated are capable of forming biofilms. Furthermore, many clinical isolates have been shown to be particularly high biofilm forming strains 6, 7, 12. While the degree of biofilm formation in vitro does not necessarily match in vivo abilities, there is a correlation between the two, suggesting that these isolates may be more prone to form these structures during infections. This is demonstrated by the fact that isolates that fail to form biofilms in vitro are often poor biofilm formers in vivo 13.
Biofilms often occur on implanted material; a hydrated surface where a solid-liquid interface exists is the ideal location for attachment and community establishment. These structures are defined in the simplest term as microbial communities attached to a surface and encased within a polymeric matrix. This matrix is composed of a variety of factors, which are largely bacterial in origin, but may also be scavenged from the host 14. Additionally, bacteria within a biofilm exhibit an altered phenotype and are metabolically distinct from their planktonic counterparts. They differ in their levels of gene expression, protein production, and growth rates 15, 16. Furthermore, bacteria within the biofilm exhibit gradients of gene expression and altered phenotypes, depending on their location within the community; bacteria closer to the outside surface may have a dramatically different phenotype than those deep within the biofilm 17. Persister cells, a subpopulation of resistant bacteria, are more commonly isolated from biofilms than planktonic cultures. These cells exhibit an inactive or drastically decreased metabolic profile, rendering them essentially dormant 18-20. The altered metabolic rate also regulates the efficacy of certain antibiotics, as many treatments are bacteriostatic in nature and dependent on cell division 21, 22. The presence of the biofilm matrix also acts as a barrier to effective treatment. The thick, polysaccharide rich structure acts as a physical shield against antimicrobials, slowing down their penetration and diffusion within the community. While some cells on the outer edges of the biofilm may be exposed to administered antibiotics, cells deep within the biofilm are likely well protected. Furthermore, bacteria within biofilms are known to exhibit an increased rate of genetic exchange, resulting in transfer of antibiotic tolerance genes and microevolution of bacteria within the biofilm 23, 24. Mobile genetic elements containing tolerance genes have been detected in a number of clinical isolates; these elements may likely be transferred to other organisms during co-colonization within a biofilm 25. Thus, the presence of some bacteria that display antibiotic tolerance can quickly give rise to a larger population of treatment resistant bacteria.
Lastly, biofilm infections are not likely to spontaneously resolve, therefore resulting in chronic infection. This is due to a known inability of the immune system to combat this particular mode of bacterial growth. Once a biofilm is well established, a persistent infection can occur, resulting in continuous complications and levels of inflammation that are inadequate at clearing the community. While planktonic bacteria can be readily recognized and phagocytized, aggregate cells present a challenge as they are two large for the capacity of the phagocytic cell 26. These cells then undergo apoptosis, resulting in a release of proinflammatory factors. Similar to the inability of antimicrobials to enter the biofilm matrix, host immune effectors may also be unable to penetrate the substance, thus protecting the bacteria from clearance. However, recent research has shown that some leukocytes are able to bypass the matrix 27. However, these cells lost their phagocytic capabilities once inside the matrix, indicating that there is a mechanism protecting biofilm bacteria from this process.
Therefore, biofilm bacteria present a significant problem in regard to treatment. They have shown to be incredibly tolerant to a number of conventional and first line treatments, and treatment failure often leads to persistent infections that can have far reaching consequences. Thus, determining multi-level treatment options that best reduce the risk of biofilm infection, as well as treatments that disperse and resolve existing infections, is essential to promoting healing and reducing complications of extremity wounds. The tenants of the various aspects of these treatments are best described by the 5D concept introduced by Winkler; debridement, detection, dead space management, disruption, and decontamination are all essential aspects to combating biofilm mediated disease 28. This review will focus on the types of treatments available within these categories, and will discuss why combination therapies are the likely solution to effectively treating these persistent infections. Emerging concepts and therapies will also be explored, as there are a number of complications for which current treatment options are largely insufficient, and innovative therapies are likely the only solution. The recalcitrant nature of biofilms suggests that the optimal approach to treatment will require some combination of chemical, pharmacological, and physical/mechanical methods, all of which will be discussed herein.
Initial Treatment of Wounds Is Critical to Decrease Likelihood of Biofilm Infections
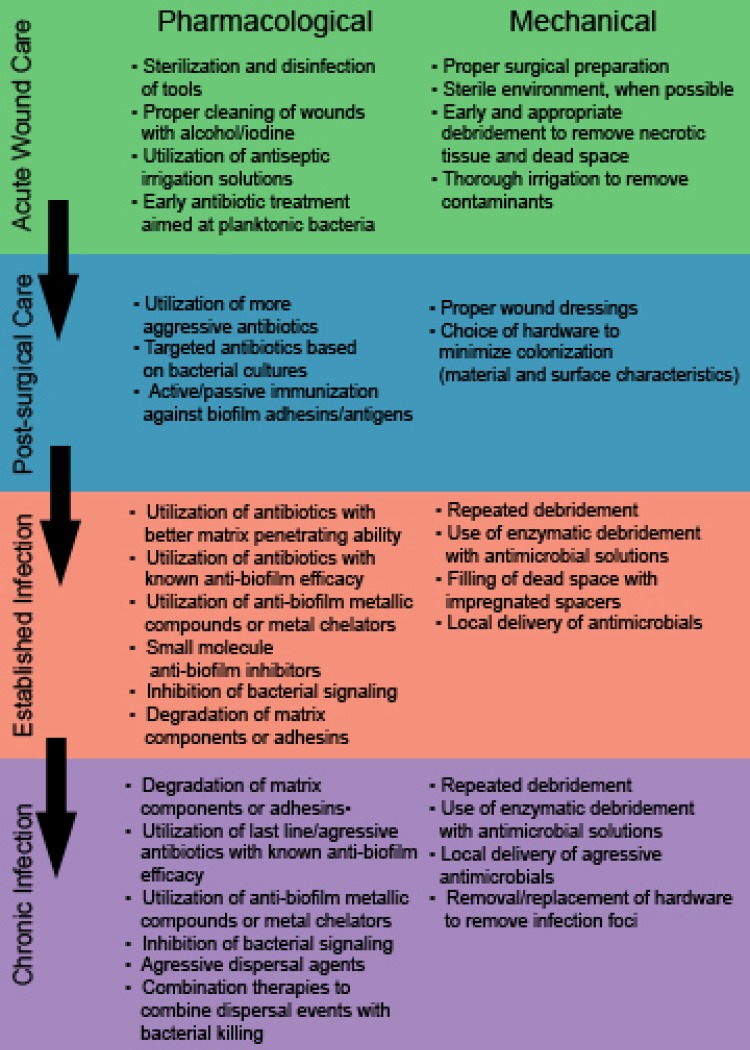
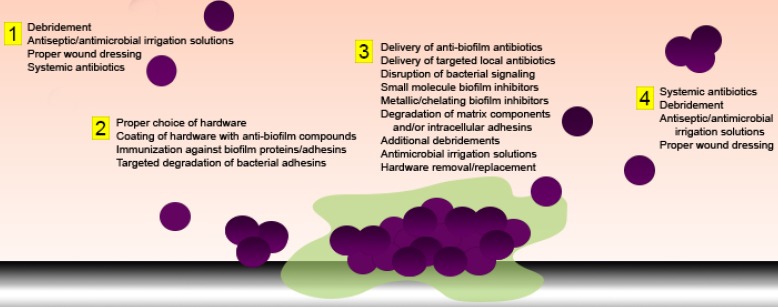
Treatment during the time period immediately following an extremity trauma event is critical to both healing and reducing the risk of complications (Figure 1). First and foremost, proper emergency treatment and surgical preparation during initial wound care is essential to reducing the exposure of the wound to contaminants 29, 30. Maintaining an environment as sterile as possible can help prevent the entrance of bacteria into the wound and the seeding of biofilm infections. Sterilization and disinfection of tools and instruments prior to use is critical to prevent nosocomial infection. Additionally, proper cleaning of the wound area using alcohol, iodine, or chlorohexidine can help reduce the bacterial burden surrounding the wound, thus decreasing the chances of introduction of commensal bacteria into deeper tissues. This is particularly pertinent in the case of Staphylococcal species residing near hair follicles 30. These basic steps can help reduce the amount of bacteria introduced into the wound immediately following injury. However, these precautions do not completely eliminate microorganisms, and the traumatic event itself can introduce contaminants into the wound upon its creation. Thus, further steps must be taken to remove debris and bacteria from the wound before they are able to attach and form biofilm structures (Figure 2).
Figure 1.
Pharmacological and mechanical approaches to preventing or treating biofilm based infections at various stages of wound development and care, ranging from immediately after creation of the wound (acute care) to prolonged, persistent infections (established and chronic infections).
Figure 2.
Anti-biofilm strategies aimed at various stages of bacterial growth and attachment. Both pharmacological and mechanical strategies can be employed in attempts to: 1) remove or eradicate planktonic bacteria, 2) prevent primary attachment, 3) degrade matrix components and/or facilitate active dispersal of biofilm bacteria, or 4) remove or eradicate dispersed or sloughed bacteria or biofilm aggregates.
An important further step during acute wound care is physical removal of both necrotic tissue and contaminants. This can be accomplished through a combination of debridement and irrigation techniques. Debridement aids in the reduction of pathogenic bacteria in a number of ways. This can include physical removal of bacteria during the debridement process. Additionally, the necrotic tissue and the associated cellular products found in acute wounds provide an ideal environment for bacterial growth and proliferation. Thus, removal of such tissue is crucial to reducing burden. Debridement can also uncover and remove “dead spaces” in which bacteria can reside. Lastly, drainage of purulence can help remove bacterial cells and debris. In a thorough review of strategies for wound management, Schultz et al list a number of debridement techniques and provide rationale behind the appropriateness of various methods as it pertains to control of infection 31. In this paper, surgical debridement is listed as the most effective for reducing infection, followed by mechanical, enzymatic and autolytic. Biological therapy utilizing sterile larvae is also suggested as a promising method for reducing bacterial counts. However, each of these methods has both advantages and drawbacks which must be considered based on the specific wound and status of the patient. A number of different solutions for wound cleansing and irrigation have also been developed, many of which play a large role in eliminating problematic bacteria. There is significant debate over which substances are most effective at reducing the risk of infection. Sterile saline alone is commonly utilized, but additive soaps and/or antiseptics show promise in further reducing bacterial burden 31, 32. The use of antiseptics remains controversial as, while harsh chemicals are effective against bacteria, they may also cause detrimental damage to host cells 31, 32. Additionally, some irrigation techniques, namely pulsatile lavage, may cause additional soft damage and drive bacteria deeper within the wound 31, 33, 34. Thus, an optimal combination of proper irrigation solution and technique must be determined to maximize the role of this step in reducing infection.
Wound dressing is also an important determinant of both healing and microbial colonization. A variety of wound dressing materials are available and individual wounds must be assessed to determine the dressing best suited for optimal healing. However, there are a number of specific dressings recommended for wounds that show evidence of contamination or are at high risk for contamination. Proactively choosing an appropriate dressing is important, as the goal of the dressing is to maintain a moist wound environment which can accelerate healing, but supports as little detrimental microbial growth as possible 35. Crystalline sodium chloride gauze is particularly well suited for infected wounds, as this dressing is highly absorbent and exhibits antimicrobial properties 31. Calcium alginate dressings are also highly recommended for infected wounds, as they absorb a large amount of fluid and are able to sequester contaminating pathogens within the wound exudate 36. Additionally, new dressings are being developed that directly inhibit bacteria, such as those that provide a sustained release of antimicrobial silver 37, 38.
Lastly, initial treatment with antibiotics can be effective at preventing infection with certain bacteria. These antibiotics can be administered both locally and systemically. Early antibiotic treatment is usually executed via administration of systemic cephalosporins as quickly as possible following injury. These antibiotics are effective against most Gram positive bacteria, as well as Gram negative rods 11. As their coverage is not complete, an aminoglycoside will often be co-administered to combat other Gram negative organisms. However, if antibiotic tolerant bacteria are present, or deep seeded bacteria have begun to attach to biological surfaces and form resistant communities, these treatments may be insufficient.
In an ideal situation, proper and timely debridement and wound cleaning will result in a properly healing wound with no signs of clinical infection. However, this is often not the case. Generally, a wound is considered “chronic” when 4-6 weeks have elapsed and healing has not occurred. As mentioned, biofilm infections can lead to this type of delay in healing. When infection develops despite these initial steps, further care must be administered to combat the established infection and allow healing of chronic wounds (Figure 1). Schultz, et al have described an approach to healing chronic wounds using the acronym TIME (Tissue, Inflammation/ Infection, Moisture balance, and Epithelial edge advancement) 31, 39. While the guidelines summarized by these categories are meant to facilitate overall healing, each can be adapted to reflect methods of antimicrobial treatment. For instance, non-viable tissue must be removed for a chronic wound to heal. As stated above, this is accomplished via debridement. In chronic wounds, repeated debridement is often required to both remove necrotic tissue and clear readily detachable bacteria. Methods aimed at reduction of inflammation/infection are obviously a major component of treating biofilm mediated infections; approaches to this subset will be discussed in later sections. Moisture balance refers to the necessity to create a moist wound environment that promotes healing, but also controls excess exudate. Wound dressings play a large role in accomplishing this task, but also double as a means to control infection via sequestration and seeding with antimicrobial compounds. The last step, epithelial edge advancement, likely requires prior treatment of infection, as many therapies aimed at priming wound edges for closure are not recommended in the face of an active infection.
Pharmacological Methods of Controlling Established Infections
Targeted use of antibiotics to treat biofilm infections
If initial treatment fails, bacterial proliferation and attachment can occur, eventually resulting in formation of a biofilm. The first attempt at combating an established infection is to treat with more aggressive antibiotics (Figure 1). One major issue presented by biofilm bacteria is their recalcitrance to antibiotics. While the optimal approach is to provide antibiotic treatment as soon as possible after initial debridement, at the earliest possible point following injury, when any remaining bacteria are most likely in a planktonic form (Figure 2), this is not always possible or successful. Once biofilms have become established, treatment options change drastically. Much of this is due to the metabolically dormant state that deep seeded biofilm bacteria exhibit. In addition co-colonization with multiple species can provide a protective effect within the group and result in biofilm facilitated transfer of genetic material and resistance genes. Thus, new classes of antibiotics must be developed to circumvent these tolerance mechanisms. One very intriguing new class of inhibitors are acyldepsipeptide (ADEP) antibiotics. ADEPs are naturally produced antimicrobials that have a number of semi synthetic derivatives of varying activity 40. One particular derivative, ADEP4, has shown a large amount of promise as a future therapeutic. ADEP4 induces bacterial death by inducing the dysregulation of the ClpP protease. Activated ClpP cleaves largely nonspecifically, resulting in a widespread destruction of bacterial proteins 40, 41. Mutants deficient in ClpP are more tolerant to ADEP4, however, they become more susceptible to other antibiotics upon treatment, indicating ADEP4 may have additional targets, and that combination therapy may circumvent tolerance mechanisms 41. While this compound is still in the early stages of development, it provides an attractive option for future treatments and is a current focus in the development of anti-biofilm treatments. Rifampin, a RNA polymerase inhibitor, has also been shown to have some effectiveness against biofilms, although it likely does not provide complete clearance and is often utilized in combination therapies 42-46. Daptomycin and moxifloxacin have also shown efficacy against biofilms formed by Gram positive organisms 45, 47, 48. For Gram negative populations, tobramycin has shown some evidence of efficacy against formed biofilms, although this is likely bolstered by combination therapies with targeted anti-biofilm compounds 49, 50.
In addition to active tolerance to antimicrobials, the matrix of the biofilm can also increase resistance by preventing the diffusion of antimicrobials or containing a number of drug inactivating compounds 51, 52. The matrix can also be directly inhibitory, as it possesses a negative charge and can interact with cationic antimicrobials 53. Some antibiotics, such as delafloxacin, are naturally more permeable than other antibiotics, rendering them intriguing candidates for biofilm therapeutics 47. Other quinolones, as well as tetracyclines, macrolides, and lincosamides tend to penetrate the matrix better than other antibiotic classes 54-56. However, their diffusion is often not complete and, in some cases, these antibiotics are not ideal due to the nature of the causative organism. In order to circumvent this issue, a number of agents that either degrade the matrix or increase its permeability are in development. However, degradation of the matrix has implications on biofilm eradication that extend further than simply antibiotic penetration, and will thus be discussed in the following sections.
Administration of novel antimicrobials can be systemic or local. While systemic antibiotics are useful during early stages of infection, chronic biofilm wound infection will likely be treated most efficiently with local antibiotics. Local administration of antibiotics allows for delivery of therapeutics at significantly higher doses, allowing the minimal biofilm inhibitory concentration to be obtained without the risk of toxicity encountered during systemic delivery of high dose antibiotics 57. A variety of mechanisms exist to optimize delivery. Still, proper delivery does not solve the issue of intrinsic tolerance, and will likely need to be accompanied by further pharmacological or mechanical strategies to completely eradicate the biofilm. This, combined with careful selection of antibiotics and agents that increase matrix permeability, may allow for targeted treatment of attached bacteria. The choice of delivery method is an important one, with each type of compound having risks and benefits. These will be discussed with other mechanically based approaches.
Notably, the specific recommendations for antibiotic use are organism specific. Therefore, administration of antibiotics once a chronic infection has been detected is best delayed until proper microbial determination has been made. The time at which the infection is diagnosed is a general indicator of contaminant, with S. aureus being common in delayed infections and in those who have spent considerable time in healthcare facilities. Immunocompromised, elderly, and diabetic patients are at an increased risk of Gram negative infections, and those presenting with late stage emergence of infection have a high possibility of infection due to coagulase negative staphylococci or other less common, opportunistic pathogens 58, 59. Upon proper diagnosis of the offending pathogen, high dose systemic antibiotics are often the first approach, followed by surgical intervention and local antibiotic application once the infection proves to be persistent (Figure 1).
A caveat to the assertion that antibiotics must be administered in an organism specific manner is that diagnosis of deep seeded, persistent infections can often prove difficult. The metabolic dormancy and irregularity of biofilm bacteria versus their planktonic counterparts may contribute to false negative results during culture analysis. Multiple studies have observed culture negative rates of 40-50% among patients with active osteomyelitis, indicating that microbial culture alone may not be effective at determining causative agents 60-62. A number of other methods of microbiological identification have been proposed and utilized, including using enriched culture media, polymerase chain reaction (PCR) and imaging 63-65. However, some studies have shown the efficacy of these methods to be less than ideal, and results are limited by primer and probe choices, respectively 66, 67. In addition, PCR based diagnosis has a higher rate of false positives66. Mass spectrometry based diagnostic methods have also gained steam in recent years, providing a highly accurate and specific method for identification of offending organisms 68-70. Utilizing these techniques in cases where culture methods alone cannot detect a pathogen will likely prove useful in detection of biofilm associated bacteria, allowing for more targeted antibiotic delivery.
Degradation of biofilm structures using biologically derived compounds
One of the defining features of the biofilm is the matrix that encases the associated bacteria. This matrix plays many roles, participating in protection, nutrient delivery, genetic transfer, and structural stability. Therefore, disruption of this matrix is a prime candidate for lessening the defenses of contained bacteria (Figure 2). A number of compounds have been shown to actively degrade matrix components. As the three main components of the matrix are proteins, carbohydrates, and extracellular DNA (eDNA), likely candidates for optimal dissolution of the matrix will specifically target these molecules. Recombinant DNase has been used with great success to disrupt the EPS of in vitro biofilms 71-74. Clinically, DNases have been utilized for disruption of mucous in cystic fibrosis patients, indicating it is a viable potential therapy for human use 75-77. In vivo studies utilizing DNase have shown that degradation of eDNA can disrupt biofilms present in a mammalian host 78, 79. However, there are some limitations. Firstly, DNase has been shown to only be effective against nascent biofilms, and its efficacy decreases with maturity of the biofilm. Practically, DNase is expensive to produce, which would likely limit its distribution and use.
A number of proteases and amylases have been tested for their ability to disrupt biofilms, with varying degrees of success 80, 81. Serine proteases in particular have been shown to be effective at disrupting the matrix. This is not entirely surprising, as a number of biofilm forming microbes produce their own serine proteases which likely aid in active dispersal and biofilm structural arrangement 80, 82, 83.The efficacy of these enzymes in breaking down matrix material will likely be dependent on the offending species, as each organisms exhibits a slightly different matrix profile 14. Additionally, a few emerging therapies have begun to gain attention and may eventually be available for clinical use. Antimicrobial peptides (AMPs), known to be produced by almost all organisms from bacteria to mammals, are also highly effective at breaking down matrix components. Many AMPs produced by microorganisms are glycoside or acid hydrolases that disrupt structural integrity through degradation of polysaccharide 84. Perhaps the most well-known of these peptides is Dispersin B, an AMP produced by A. actinomycetemcomitans that exhibits efficacy against biofilms formed by a wide range of pathogens 85, 86. Urea has also been investigated as a matrix disrupting agent, as it causes disruption of hydrogen bonds that provide matrix stability 84, 87. Bismuth thiols are a chemical approach to degradation of matrices. These compounds likely have dual functionality, as they interact with production of exopolysaccharide as well as chelate metal ions, which are known to be critical in matrix stabilization 88, 89.
Removal of the matrix ultimately leads to dispersal of biofilm communities, as the structural integrity of the biofilm is no longer intact. At this point, combination therapies will likely play a role, and may utilize treatments including continued debridement (to remove necrotic tissue and sloughed biofilm) and local antibiotic delivery (as the matrix is no longer providing a protective encasement) (Figure 1).
Targeting bacterial signaling as a method of biofilm inhibition
As biofilms function as living communities of cells, cell to cell communication plays an important role in their formation and structure. Many bacteria accomplish this communication via the process of quorum sensing (QS), where bacteria produce a small signaling molecule that can be received by surrounding bacteria. There are three main QS systems known to exist among bacteria, with each being defined by the specific signaling molecule produced: the autoinducing peptide (AIP) system (common in Gram positive bacteria), the acylhomoserine lactone (AHL) system (Gram negative bacteria), and the autoinducer 2 (AI-2) system (both Gram positive and Gram negative bacteria). When these molecules are being produced in significant amounts, they are indicative of the size of the community and have the ability to stimulate a cell density dependent expression of biofilm specific genes 90, 91. Therefore, inhibition of quorum sensing would essentially trick bacteria into thinking they are no longer within the community, thus reducing the production of biofilm mediators (Figure 2). A number of compounds have been shown to interact and disrupt quorum sensing systems. As QS systems in different species of bacteria utilize different signaling molecules, each class of inhibitor exhibits differential efficacy based on system specificity. For instance, glucosamines have been shown to disrupt QS signaling in the Gram negative bacteria P. aeruginosa and E. coli, likely through competitive inhibition of the signal receptor binding site 92, 93. Bergamottin compounds, isolated from grapefruit juice, also utilize this mechanism, blocking the binding side for AHL QS systems 91, 94. Fusaric acid analogues also show efficacy against Gram negative QS systems 95. The polyphenolic compounds epigallocatechin and baicalin hydrate were found to be effective against mature biofilms formed by a number of Gram negative species 96-99. Norspermadine, a polyamine, induces dispersal and inhibits biofilm formation in A. baumannii, likely through inhibiting the expression of QS genes 100. A small molecule inhibitor, RNAIII-inhibiting peptide (RIP), and its analogues are effective at inhibiting agr mediated QS in S. aureus as well as other AIP based systems 101, 102. RIP is thought to block the signaling receptor. Interestingly, some inhibitors appear to be able to block the signal transduction cascade downstream of the signal receptor. Both natural furanone and cinnamaldehyde are two well studies examples of such inhibitors 103-108. This method of inhibition has important implications, as it can be more broadly applicable across bacterial species.
There is also evidence to suggest that combination therapy with a QS inhibitor and antibiotics can increase the efficacy of the antibiotic 99. For instance, components of garlic extract, including ajoene, inhibited AHL receptors and increased the susceptibility of P. aeruginosa biofilms to tobramycin 109, 110. RIP and its analogs also exhibit this effect; various studies examining treatment of Staphylococcal biofilms with these compounds have shown increased susceptibility to daptomycin, clindamycin, and vancomycin 99, 111. Combining QS inhibition and antibiotic treatment may therefore be a viable method for eliminating a number of bacterial biofilms.
Another aspect of bacterial communication that can be exploited for therapeutic use is the existence of molecules that act as dispersal signals for biofilm communities. A number of compounds have been identified that appear to trigger active dispersal. D-amino acids, produced by a number of bacterial species, are thought to lead to dispersal of preformed biofilms via disruption of the bacterial cell wall, where they are incorporated into the peptidoglycan in place of D-alanine 12, 112, 113. D-amino acids mediated dispersal is also correlated with an increased susceptibility to antibiotics 12. Similarly, dispersin B, the previously discussed glycoside hydrolase that can degrade poly-N-acetylglucosamine (PNAG),a major matrix component, also appears to induce biofilm dispersal signaling 85. Nitric oxide (NO),a ubiquitous molecule, has a demonstrated ability to induce bacterial signaling and has been shown to contribute to biofilm dispersal at low concentrations 114, 115. A number of NO based therapeutics are being investigated, rendering it a promising potential antibiofilm agent 115. Cis-2-deconic-acid, a fatty acid signaling molecule produced by P. aeruginosa has been shown to not only disrupt preformed biofilms, but also to induce reversion of persister cells 116, 117. The dispersal effect of this compound was shown to occur in a number of species, including S. aureus, S. pyogenes, E. coli, and K. pneumoniae 116.
The majority of dispersal agents do not exert any type of antimicrobial effect leading to cell death. Therefore, use of these compounds would be primarily as an adjuvant with antibiotics that exhibit activity against liberated planktonic bacteria (Figure 2). However, their lack of bactericidal properties makes them less likely to induce bacterial tolerance via selective pressure. Notably, the majority of studies regarding dispersal agents have been performed utilizing in vitro systems; the in vivo efficacy of these compounds has yet to be properly evaluated. However, the gap in treatment options for inducing dispersal of biofilms cannot be ignored, thus these agents represent a class of treatments that must continue to be investigated and developed.
Degradation of biofilm adhesins
The first step of biofilm formation is bacterial attachment to a surface. Attachment occurs in two steps: an initial non-specific, reversible attachment and a second, irreversible, specific attachment mediated by bacterial adhesins. This step-wise formation has been studied extensively in vitro, and studies on nasopharyngeal and oral biofilms suggest it occurs in vivo, as well 13, 118. Targeting these temporal events may assist in combating biofilms infections. Therapeutics that eliminate these attachments during early infection can potentially prevent the formation of a biofilm (Figure 2). Additionally, degrading specific adhesins present in a formed biofilm may lead to biofilm dispersal and increased susceptibility to other treatments.
In Staphylococci, biofilm formation can be polysaccharide matrix based or proteinaceous in nature 119. One of the main specific adhesins involved in the former type of Staphylococcal biofilm formation is the polysaccharide intercellular adhesion (PIA). PIA promotes bacterial aggregation and substantially contributes to the matrix during polysaccharide dependent biofilm formation. Adhesion through PIA is thought to be based on electrostatic interactions with bacterial teichoic acids, as PIA is positively charged 120. Recently, methods have been developed to disrupt the adhesive properties of PIA using cationic peptides, providing a promising avenue for disruption of proteinaceous adhesion 121. Conversely, protein based biofilm formation may be mediated by a number of other adhesins, many containing LPxTG motifs that anchor the proteins to the bacterial cell wall. A large subset of these cell wall anchored adhesins (CWAs) is comprised of microbial surface component recognizing adhesive matrix molecules (MSCRAMMs). These proteins are named as such due to their ability to bind to components of the mammalian cellular matrix, including fibronectin, fibrinogen, and collagen. While these proteins aide in adhesion to biotic surfaces, they also facilitate binding to implanted/abiotic surfaces that have been coated in host matrix components 122, 123. Thus, MSCRAMMs present a viable target for dispersing or disrupting biofilms on a multitude of surfaces. Additionally, many of these proteins have multiple functions, a number of which are involved in inter-bacterial binding and attachment. Fibronectin-binding protein A (FnBPA) is thought to contribute to intercellular adhesion via homophilic binding between specific domains on adjacent proteins 124. Accumulation associated protein (Aap) also appears to contribute to aggregation this way, as it is a known biofilm contributor and purified Aap is able to form dimers in solution via interaction of adjacent B domains 125, 126. Aap can also promote surface attachment via its N-terminal A domain 125, 126. The large adhesion extracellular matrix binding protein (EmbP) of S. epidermidis is also a fibronectin binding protein, and promotes biofilm formation via adhesion to the matrix 127. Biofilm associated protein (Bap), is a multi-domain S. aureus surface protein that has been shown to be essential for biofilm formation in vitro. Homologues have been identified in a number of other organisms, including other staphylococcal species, A. baumanii, E. faecalis, P. fluorescens, and S. typhimurium 128, 129. While the bap gene itself has not been identified in human S. aureus isolates, homologues from other species have been identified in clinical samples, making its contribution to in vivo biofilms uncertain 128, 129. Interestingly, a number of anti-biofilm proteases have been discovered, many of which show activity against Bap, including Aureolysin and the V8 serine protease 84. SraP is another multifunction CWA. This protein can dimerize via its N-terminal region, which contains cadherin like domains, allowing for bacterial aggregation. Additionally, this same domain is known to be involved in binding to host cell glycoconjugates, thereby initiating primary adhesion to the host 130. S. epidermidis surface protein C (SesC), also appears to be involved in specific bacterial interactions, as introduction of its gene into deficient bacteria resulted in a proteinaceous biofilm matrix, and blocking of the protein resulted in a disruption of biofilm formation 131.
While these proteins represent an attractive target for therapeutics, as their disruption could potentially dislodge tightly adhered communities, development of such treatments remains challenging. Specific cleavage of proteins is not common in therapeutics due to the possibility of off-target effects and difficulties in obtaining efficient dosages. However, the number of protease treatments currently under investigation suggests that this may be changing 81. The challenges of developing targeted treatments for biofilm mediated infections will like revolve around protease specificity; protein engineering will be of utmost importance. A small handful of broad activity proteases have been studied and are currently utilized for enzymatic debridement. In the United States, only collagenase is currently approved for use. Collagenase is a bacterial derived metalloprotease that specifically degrades triple helical collagen via hydrolysis of peptide bonds 132. While collagenase appears to act slowly, it is also relatively gentle and generally well tolerated. Importantly, collagen is a major point of attachment for bacteria through MSCRAMMs 133, thus its degradation may dislodge attached organisms. Papain is a cysteine protease derived from the papaya 134. Addition of urea enhances the activity of papain. The tolerance and safety of papain is debated, as usage of this enzymatic agent can lead to patient discomfort and increased amounts of exudate 135. As of 2008, the FDA no longer recommends papain/urea for use. However, papain itself has been shown to have anti-biofilm properties in vitro, suggesting it possesses activity against bacterial proteins 136. In fact, papain was shown to directly degrade the Actinomyces fimbrial proteins FimA and FimP in a dental plaque model 137. Bromelain is a pineapple derived protease that is currently approved in Europe 138. Like papain, bromelain has been shown to have activity against bacterial biofilms in vitro 136. In addition, bromelain possesses anti-inflammatory properties, suggesting it may be useful in controlling both the biofilm and the detrimental inflammatory response within the wound bed 138. Fibrinolysin/ desoxyribonuclease is another enzymatic compound that can be utilized for debridement. This compound specifically degrades necrotic tissue via disruption of fibrin molecules. As with collagenase, degradation of matrix components may disrupt bacterial attachment via MSCRAMMs, leading to biofilm detachment. Trypsin, a serine protease commonly used in laboratory applications, has also been investigated for use against biofilms and during wound healing 137, 139.
Lastly, recent studies have adopted the approach of preventing cell surface expression of adhesins as a method of preventing biofilm formation. In Gram positive bacteria such as S. aureus, translocation of proteins to the cell surface requires the function of Sortase A. The diarylacrylonitriles, curcumin, aryl ethyl ketones, and their derivatives have all been shown to inhibit sortase function and expression 140-142.
Use of metals and metal chelators in preventing biofilms
Another group of antimicrobials with a steadily increasing amount of supportive literature are compounds which disrupt fundamental bacterial processes. One of the largest classes of this group is chelators, which effect biofilm bacteria via the sequestration of critical metal ions. Bacteria within biofilms are less sensitive to high metal concentrations, due to the ability of the matrix to bind a large percentage of cations, and the decreased metabolic state of the bacteria themselves 143. There is some evidence to suggest that the presence of these cations actively contributes to biofilm formation. Magnesium, calcium, and iron have all been shown to be correlated with increased production of biofilm related proteins, including adhesins and extracellular proteases 144-147. Specifically, iron appears to be required for the production of PIA by S. aureus 146. Lactoferrin is an iron binding protein that can be found in both blood and secreted fluids, with the highest concentration being found in milk. A number of studies have shown lactoferrin to have marked antimicrobial activity. In addition, biofilm specific studies suggest that this protein is active against metabolically distinct sessile bacteria 148-150. While the majority of lactoferrin activity is likely due to its iron sequestration abilities, it may also function as a protease and is a known anti-inflammatory molecule 151. Its current usage in both clinical and nutritional settings renders it an attractive candidate for future treatment options. Another iron chelator, 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG), was shown to cause reduced adhesion and decreased production of the PIA adhesin in S. aureus 146. Various calcium chelators have also been investigated for their antibiofilm properties. Ethylene glycol tetraacetic acid (EGTA) has been shown by multiple investigators to prevent bacterial attachment and biofilm formation in vitro 144, 152. However, these effects were largely strain dependent 144, and another calcium chelator, trisodium citrate, exhibited the same pattern of inhibition. Furthermore, EGTA did not disrupt preformed biofilms 152, indicating that successful use of calcium chelators would require early administration as a part of a combination therapy. Ethylenediaminetetraacetic acid (EDTA) is a commonly used and broadly active chelator that can bind a number of metal cations, including calcium, magnesium, and iron 153. A number of studies have shown that EDTA has antibacterial and antibiofilm properties 153, 154. However, other studies have failed to observe an effect or noticed a strain specificity to EDTAs activity 155. Importantly, while chelators may ultimately result in cell death due to sequestration of key nutrients and signaling molecules, they are likely not directly bacteriocidal.
In addition to sequestration of metallic components, there is a wealth of evidence suggesting that use of heavy metal nanoparticles is a very effective method for disrupting bacterial growth. Silver, in particular, has long been known to have antibacterial properties 156. Silver nanoparticles (AgNP) are thought to be the most effective and least cytotoxic form of silver delivery. Clinically, these particles can be engineered into a paste that is able to be applied locally, making it an attractive compound for wound treatment. AgNP activity is thought to result from release of silver ions upon hydration. The exact antibacterial mechanism of these ions is currently unknown, but is thought to involve disruption of the membrane and therefore the proton motive force of the bacteria, as well as induction of oxidative damage 157. Collodial silver has also been shown to have activity against biofilms, both in vitro and in vivo 158, 159, suggesting a number of silver formulations should be further investigated for use in wound care. Importantly, application of silver appears to disrupt preformed biofilms, making it an attractive candidate for treatment of preexisting infections 157. Gold nanoparticles (AuNPs) have also been investigated as a potential antimicrobial and have been found to be effective against both planktonic and biofilm bacteria 160, 161. These effects were seen against both Gram positive and Gram negative species of clinical relevance, suggesting that addition of AuNPs could provide a broad spectrum treatment. The action of gold mediated bacterial death is thought to be similar to the mechanisms described for silver 162. Importantly, use of AuNPs would likely require careful engineering and therapeutic dosing, as gold has been shown to have cytotoxic effects 163.Gallium has also been shown to have antibacterial properties, reducing the burden of a number of strains of drug resistant bacteria grown both planktonically and in a biofilm. Additionally, gallium has been shown to potentiate the activity of the antimicrobials gentamycin and ciprofloxacin 164, 165. Lastly, copper nanoparticles also have documented antimicrobial properties. Its antibacterial mechanisms are thought to be multifactorial, and include binding to DNA and preventing replication and interacting with amine and carboxyl groups on the bacterial cell surface 162. Additionally, copper appears to have specific inhibitory effects against biofilm bacteria, and was shown to significantly decrease biofilm biomass and polysaccharide matrix production in P. aeruginosa 166. A 2016 study by Ahire, et al showed that addition of copper nanofibers to wound dressings resulted in a significant decrease in biofilm formation 167. Thus, treatment of contaminated wounds with different metallic compounds is a promising avenue of future research. Additionally, metallic nanoparticles may be used as a delivery system for antibiotics, allowing for engineering of highly effective anti biofilm combination therapies 168-170.
Mechanical Approaches to Biofilm Prevention and Disruption
Surgical removal of biofilm bacteria and infected hardware
Once a determination of the offending pathogen has been made, every effort should be made to treat infections with systemic antibiotics. However, the longer the infection persists, the more likely it is that biofilm bacteria are involved. Implanted hardware provides a significant level of stabilization and are, overall, a great asset in wound management. Unfortunately, their abiotic nature and surface properties made them ideal points of attachment for biofilm communities. Therefore, assurance that a formed biofilm has been eradicated can essentially only be made upon removal of the hardware. However, removal of hardware must be done as a last possible step, particularly at time points prior to complete union or in cases of non-union (Figure 1). Destabilized fractures by and large do not heal, and are therefore also more prone to worsening infection. The existence of dead space within the fracture gives bacteria a focus in which they can persist and thrive. Thus, determination of surgical intervention and hardware retention or removal must be made on an individual basis using standardized guidelines. The therapies discussed above are likely to work in conjunction with these determinations.
When surgical intervention is deemed necessary, repeated debridements are often indicated for removal of bacteria and infected or necrotic tissue, with many patients needing two or more 59. As biofilm formation is expected at this point, enzymatic debridement or debridement involving solutions mentioned above may be an attractive option to clear out as much bacteria as possible. At this point it is also recommended to fill dead space via administration of stabilizing bone fillers. These fillers have the added benefit of acting as local antibiotic carriers, delivering high doses directly into the wound site, allowing therapeutics to enter deep and non-vascularized sites that systemic antibiotics cannot 57. A combination of aggressive debridement and high dose antibiotic treatment may be able to quell an infection sufficiently, allowing retention of hardware until the bone has fully healed 171. At this point, hardware can be removed to ensure excision of any remaining attached bacteria 28, 59. Treatment with retention of hardware has been shown to be successful in about 70% of cases 172. In cases where these treatments do not sufficiently ablate the infection, or in cases where the infection has caused destabilization of the hardware, removal and replacement is generally recommended 171. By removing the original hardware, the focus of infection is removed and debridement and antibiotic treatment may be better able to clear pathogens. Additionally, temporary removal of hardware exposes hard to reach sites around the implant which may have been inaccessible during initial debridement efforts 59. Implants can then be exchanged, allowing for bone healing to continue in an environment with a drastically reduced bacterial burden. If these methods fail and marked infection persists alongside a lack of bone healing, complete removal of hardware is likely necessary. In these cases, fixation conversion is recommended and external fixation devices may be used to allow for healing while the infection is being treated 59. In these cases, external fixation allows for an optimal success rate, but may lead to soft tissue infections and patient discomfort. Additionally, the type of fixation device used has an impact on microbial detection and hardware retention. For instance, while IM nail fixation is associated with a later determination of infection and increased difficulty of surgical intervention, success rates of hardware retention are often high 59.
Utilization of anti-biofilm materials
Currently, stainless steel and titanium or titanium alloy implants are the most commonly used implant materials 173, 174. While titanium has become the optimal standard for use, there is still some debate over which material results in the lowest rate of infectious complications. Stainless steel has been shown to become colonized more readily in murine models, however medical grade stainless steel is highly polished and is not thought to provide an optimal surface for bacterial attachment. Conversely, titanium often exhibits a grittier surface, potentially making it more prone to colonization 175. However, a study comparing biofilm formation on stainless steel and titanium wire fixation of toe injuries found that titanium implants were far less likely to allow for biofilm formation 176. Interestingly, one retrospective study showed a higher rate of failure in fixations using titanium plates versus those using stainless steel 177. Conversely, titanium plates appear to elicit less of a soft tissue response and may be less inflammatory than their steel counterparts 178. However, Rotini et al showed that titanium alloy hardware appears to be more readily colonized than solid titanium formulations, suggesting specific formulation of titanium implants may make a difference in infection risk. A number of new materials have recently been suggested and are under development. These include hardware constructed of tantalum, carbon fiber and PEEK plastic formulations, and cobalt based alloys 179-181. The ability of bacteria to colonize both commonly used materials and new hardware configurations has been studied by multiple investigators. Multiple studies have shown that cobalt alloy hardware has lower rates of colonization than titanium formulations. However, the biocompatibility and efficacy of these plates in successful healing and fixation must be considered 179. Tantalum was also shown to have a reduced capacity for bacterial colonization 181. Thus, use of newer hardware biomaterials may be an attractive method for biofilm reduction. In addition to the material itself, the surface treatments of hardware have a pronounced effect on the ability of bacteria to colonize. Both surface roughness and wettability have been investigated as determinants of colonization ability 182. Techniques such as acid etching and sandblasting change the surface characteristics of implanted hardware. Studies of biofilm formation on varying titanium surfaces used for dental implants suggest that sandblasted titanium exhibits a slightly increased propensity for colonization 182, 183. Likewise, a systemic review of clinical reports of dental implant colonization found that increased surface roughness correlated with an increased likelihood of infection 184. However, implant material must allow for the attachment of host derived cells and components. Multiple studies have shown that the presence of host cells and tissues on implants decreases the likelihood of bacterial colonization 185, 186 This phenomenon was termed the 'race to the surface' by Gristina, where the first cell type to attach (host or bacterial) gains the advantage during colonization or integration 186. Unfortunately, a number of techniques aimed at reducing bacterial colonization may also inhibit tissue integration of implants. Subbiahdoss, et al found that a hydrophilic polymer-brush coating reduced bacterial burden, but also decreased the degree of host cell adhesion 187. A study on dental implants by Zhao, et al, showed that fibroblasts were outcompeted in the race to the surface on a number of surfaces with varying roughness and wettability, with the exception of smooth titanium 188. Therefore, when choosing implant hardware, the material and the surface treatments must be carefully selected to allow for a balance of decreased infection risk and increased tissue integration and proper fracture fixation (Figure 1). While the ideal biomaterial for the achievement of this balance is currently unknown, the advances in material development and coating techniques make this strategy an important future consideration.
An additional consideration regarding fracture hardware is the use of implant coatings with various antimicrobial properties. Some coating options have been discussed above as separate treatment options. For instance, AMPs have been considered for implant coating due to the large number available, their wide range of mechanisms, and low occurrence of bacterial tolerance. However, immobilization of AMPs may decrease their function, as many require direct contact with membranes to exert their optimal antimicrobial activities. One approach to circumvent this is utilization of AMP containing hydrogels which can be applied to implants 189. Silver has also shown a great deal of promise as an antimicrobial coating; a systematic review of implant coatings showed silver treated implants to have significantly decreased rates of infection while maintaining a high level of biocompatibility and low rates of tolerance190. Implant coatings that allow for the release of NO have also been described 191. Additionally, a number of surface treatments that change the surface properties of the implant, including charge and hydrophobicity, have been described. Hydrophobic polycationic coatings were examined by Schaer, et al, who found that coated steel and titanium were more resistant to bacteria than untreated materials 192. Trimethylsilane coating was also shown to reduce biofilm formation on both stainless steel and titanium plates in an in vitro model 193. Coating of surfaces with polytetrafluoroethylene (ePTFE) has also been shown to reduce biofilm formation 194. Chitosan, a polysaccharide derived from the exoskeletons of crustaceans, has been investigated as a potential titanium coating 195. Chitosan exhibits antibacterial and anti-biofilm properties, and has been shown to increase the efficacy of co-administered antibiotics 196, 197.
While biofilm formation primarily occurs on the hardware implants themselves, another facet of material choice is the selection of spacers to provide stability and fill dead space within fracture wounds. A number of these materials also function as antibiotic delivery systems, with varying pharmacokinetics and efficacy. PMMA spacers are commonly used to deliver antibiotics. However, PMMA may not be a particularly ideal material, as it exhibits a lower biocompatibility than other options and elicits a low grade inflammatory response 28. Additionally, antibiotic release from PMMA may not be complete, resulting in initially high levels of antibiotic which then transition to sub-inhibitory concentrations 28, 57. This may contribute to development of antibiotic tolerance. Furthermore, up to 50% of spacers removed from patients show evidence of biofilm formation on their surface, indicating that if the delivery method is not effective at eradicating infection, it may itself become a focal point for persistent infection 198. Antibiotic loaded cement spacers are another potential filler material. Calcium sulfate has been used in treatment of orthopedic injuries since the 1980's. It dissolves relatively quickly within the wound, making it less than ideal for structural support. However, this same property allows for high levels of local antibiotic delivery 57. Conversely, calcium phosphate formulations, including hydroxyapatite, dissolve slowly. This allow for greater stabilization and increased potential for healing 57. However, this delayed resorption may be detrimental during infection, as antibiotic release may not be as sharp and quick, and the cement filling itself may retain offending bacteria 57. Allografted bone is considered to be the optimal material for healing, as it is highly biocompatible, particularly when processed and sterilized. Unfortunately, the unvascularized nature of these grafts renders them highly susceptible to colonization 28. New techniques involving removal of the donor bone marrow, and replacement with loaded antibiotics, show promise in offering a highly biocompatible delivery system with a high antibiotic surface area and, therefore, high concentrations of local antibiotic delivery 28.
Targeting the Host Response Against Biofilms
While the presence of persistent infection may indicate that the immune response would play little role in clearance of biofilms, that may not be the case. The issue in biofilm formation is not a complete lack of immune response, but instead, a largely inefficient one. Innate immune mechanisms are ineffective due to the barriers described above, and antibodies against largely planktonic antigens are ineffective in clearing the bacteria, as biofilm and planktonic bacteria have largely distinct antigens profiles 199. In fact, a robust immune response is thought to occur during biofilm mediated infections, but ultimately causes collateral damage while failing to eradicate the infection 200. Therefore, a significant amount of research has focused on shifting the immune response to one more efficient in targeting biofilm bacteria.
Vaccination against bacterial proteins known to be important during adhesion or biofilm formation is a common approach that has had varying degrees of success (Figure 2). An effective vaccine could reduce morbidity and mortality, decrease surgeries and hospital stays, and be incredibly cost effective 201. Many of the vaccination candidates for S. aureus are MSCRAMMs or other molecules important during the initial stages of biofilm formation. These include: fibronectin binding proteins, clumping factors A and B (ClfA and ClfB), iron regulated surface determinants A and B (IsdA and IsdB) and PIA 202, 203. Use of many of these candidates as antigens has shown to provide at least partial protection against biofilm mediated disease. Antibodies against the biofilm upregulated protein PhnD (a phosphate ABC transporter), have been shown to inhibit biofilm formation at the initial attachment stage 204. Vaccination against extracted biofilm matrix components has been shown to provide significant protection from S. aureus biofilm formation, as well as limiting seeding dispersal to other organs 205. Another study examined the effect of immunization against a quadrivalent vaccine against glucosaminidase, a conserved hypothetical protein, and two lipoproteins. While the quadrivalent vaccine alone did not completely eliminate bacterial burden, it was effective when used in conjunction with an antibiotic, suggesting that the vaccine was able to specifically prevent biofilm formation, allowing the planktonically focused antibiotic to remain effective 206. Glucosaminidase, a subunit of the major S. aureus autolysin, is a very attractive target for vaccine development as it is non-redundant and largely conserved amongst S. aureus strains 207, 208. Biofilm antigens from S. epidermidis have also been investigated; SesC, a surface protein, was shown to provide protection against biofilm formation in a catheter model 209. Vaccines targeting the capsular polysaccharide have also been tested and remain a promising approach, as similar vaccines have been successful for other Gram positive species. The obvious caveat with prophylactic vaccination is that it requires a period of immune priming and activation prior to introduction of the pathogen, particularly when considering vaccine candidates that prevent initial attachment. In the case of traumatic injuries, this is likely not possible. Interestingly, therapeutic immunization has shown promise in other biofilm mediated conditions, such as otitis media. In this approach, vaccines against major matrix components are administered after the identification of a persistent infection. Recent studies have shown that these vaccines may stimulate the immune system to disperse the biofilm through sequestration of matrix materials 210, 211. For S. aureus, therapeutic use of a conjugate capsular polysaccharide and protein vaccine conferred protection in a rat model of osteomyelitis 212. Use of these vaccines in conjunction with antibiotics may be effective at treating established biofilm infections. A few limitations do exist regarding standard vaccination. Vaccination may not be recommended for certain patients, particularly those with compromised immune systems. Additionally, a problem specific to S. aureus antigen vaccines is the problematic adverse effects of high antibody titers against certain proteins. A number of clinical trials have failed due to the collateral organ damage induced by high levels of anti-staphylococcal antibodies and robust immune response 189. To circumvent this, passive immunization has been investigated as a therapeutic. Varrone, et al used a murine model of osteomyelitis to shown that passive immunization against glucosaminidase resulted in increased opsonophagocytosis of aggregated bacterial clusters 208. Similarly, passive immunization with a polyclonal antibody against S. aureus was shown to be effective in a murine model of sepsis through increased opsonophagocytosis and neutralization of antigens 213. Passive immunization has also been investigated for treatment of infections caused by A. baumannii, another biofilm forming strain known to cause orthopedic infections. Antibodies against the capsular polysaccharide resulted in serotype specific protection in a rat soft tissue infection model 214. Another study using a highly conserved A. baumannii protein antigen as a vaccine target showed passive immunization provided opsonophagocytic mediated protection in a diabetic mouse model.
Importantly, clinical studies involving both active and passive immunization against S. aureus have been unsuccessful 215. Active immunization against capsular polysaccharide conferred only partial and short lived protection 216, 217. A clinical trial examining immunization against IsdB was halted due to safety concerns and low efficacy 218. Likewise, passive immunization against ClfA also proved to be unsuccessful 219. Importantly, many of these trials looked at bacterial burden and infection rates as a study endpoint, as the vaccines were developed to prevent general staphylococcal infections. However, anti-biofilm vaccines will likely have a different primary goal. Preventing the formation of biofilms on orthopedic implants may increase the efficacy of commonly used antibiotics. Thus, more studies on these combination therapies are warranted.
Conclusions: Multi-Level Combination Therapy Is Necessary to Combat Orthopedic Biofilm Infections
Orthopedic infections mediated by biofilm forming bacteria are often complex in nature and difficult to treat. The approaches highlighted above demonstrate that a variety of well-studied and newly emerging methods are available, and that they can be applied at a multitude of different stages in the infectious process. First and foremost, appropriate initial treatment can dramatically reduce the incidence of microbial contamination. These methods, including sterility, timely and patient appropriate debridement, early antibiotic therapy, and optimal wound dressings are currently utilized to stave off infectious complications, with much success. However, there is always room for improvement. In cases where patients have an increased risk of contamination, specific methods may be favored due to their antimicrobial properties. Furthermore, while the nature of the orthopedic injury largely dictates the hardware that will be implanted, a number of materials and coatings have shown great efficacy in preventing biofilm infections. Anti-bacterial hardware, whether plates or pins, may be used during initial fixation, or in the event that postoperative infection does occur, as an extra elimination method during revision surgery. Importantly, many of these coatings simply prevent bacterial attachment, but are not bacteriocidal in nature, necessitating the concurrent use of antibiotics. If revision surgery is necessary, all steps should be taken to try to preserve the presence of hardware. Anti-biofilm hardware lessens the chance that retained fixation devices become a focal point for bacteria. In these cases, thorough debridement upon revision is an important step, and may be aided by the use of enzymatic debridement agents that decrease the adhesive properties of the bacteria while physically removing them from the wound. Revision surgery also offers the opportunity to reduce necrotic tissue and dead spaces, thereby removing areas that are prone to infection. Dead spaces can then be filled with material that is both conducive to bone healing and acts a carrier for local antimicrobials. These substances can be released in the high concentrations necessary to combat biofilm infections. Antimicrobials administered during this stage may be solely last line or biofilm active antibiotics, or may consist of a cocktail of matrix and adhesion degrading molecules, dispersal agents, and antimicrobials that can clear the newly liberated bacteria. Lastly, therapeutic vaccinations targeting the immune response may also be a candidate for combination therapy in cases where other treatments have failed. As opsonophagocytic based therapies have shown little success in clinical trials, these will likely need to be focused on targeting biofilm bacterial phenotypes, thus requiring delivery with antibiotics or other antimicrobials.
The recent insights into the genetic, proteomic, and signaling based properties of biofilms have increased the pool of potential therapeutics exponentially. While a number of the therapies described are still in their infancy, they provide promise for filling the current gaps in treatment options. The ability of bacteria to switch between phenotypes, and to circumvent both immune responses and antimicrobial therapy means that effective therapies will need to consider a truly multifaceted approach to combat complicated infections. These combination therapies will likely incorporate both established protocols and emerging, cutting edge technologies and pharmaceutical advances, allowing for optimal and more effective care of chronically infected wounds.
Disclaimer
The opinions or assertions contained herein are the private views of the authors and are not to be constructed as official or as reflecting the views of the Department of the Army or the Department of Defense.
References
- 1.Cross JD, Ficke JR, Hsu JR, Masini BD, Wenke JC. Battlefield orthopaedic injuries cause the majority of long-term disabilities. J Am Acad Orthop Surg. 2011;19(Suppl 1):S1–7. doi: 10.5435/00124635-201102001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Owens BD, Kragh JF Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma. 2008;64:295–9. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 3.Masini BD, Owens BD, Hsu JR, Wenke JC. Rehospitalization after combat injury. J Trauma. 2011;71:S98–102. doi: 10.1097/TA.0b013e3182218fbc. [DOI] [PubMed] [Google Scholar]
- 4.Masini BD, Waterman SM, Wenke JC, Owens BD, Hsu JR, Ficke JR. Resource utilization and disability outcome assessment of combat casualties from Operation Iraqi Freedom and Operation Enduring Freedom. J Orthop Trauma. 2009;23:261–6. doi: 10.1097/BOT.0b013e31819dfa04. [DOI] [PubMed] [Google Scholar]
- 5.Santolini E, West R, Giannoudis PV. Risk factors for long bone fracture non-union: a stratification approach based on the level of the existing scientific evidence. Injury. 2015;46(Suppl 8):S8–S19. doi: 10.1016/S0020-1383(15)30049-8. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez CJ Jr, Mende K, Beckius ML, Akers KS, Romano DR, Wenke JC. et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwiecinski J, Kahlmeter G, Jin T. Biofilm formation by Staphylococcus aureus isolates from skin and soft tissue infections. Curr Microbiol. 2015;70:698–703. doi: 10.1007/s00284-014-0770-x. [DOI] [PubMed] [Google Scholar]
- 8.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J. et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen AT, Vallier HA. Noncontiguous and open fractures of the lower extremity: Epidemiology, complications, and unplanned procedures. Injury. 2016;47:742–7. doi: 10.1016/j.injury.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis. 2007;45:409–15. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 11.Murray CK, Obremskey WT, Hsu JR, Andersen RC, Calhoun JH, Clasper JC. et al. Prevention of infections associated with combat-related extremity injuries. J Trauma. 2011;71:S235–57. doi: 10.1097/TA.0b013e318227ac5f. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez CJ Jr, Akers KS, Romano DR, Woodbury RL, Hardy SK, Murray CK. et al. D-amino acids enhance the activity of antimicrobials against biofilms of clinical wound isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58:4353–61. doi: 10.1128/AAC.02468-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchette-Cain K, Hinojosa CA, Akula Suresh Babu R, Lizcano A, Gonzalez-Juarbe N, Munoz-Almagro C. et al. Streptococcus pneumoniae biofilm formation is strain dependent, multifactorial, and associated with reduced invasiveness and immunoreactivity during colonization. MBio. 2013;4:e00745–13. doi: 10.1128/mBio.00745-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–33. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 15.Becker P, Hufnagle W, Peters G, Herrmann M. Detection of differential gene expression in biofilm-forming versus planktonic populations of Staphylococcus aureus using micro-representational-difference analysis. Appl Environ Microbiol. 2001;67:2958–65. doi: 10.1128/AEM.67.7.2958-2965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beenken KE, Dunman PM, McAleese F, Macapagal D, Murphy E, Projan SJ. et al. Global gene expression in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:4665–84. doi: 10.1128/JB.186.14.4665-4684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pabst B, Pitts B, Lauchnor E, Stewart PS. Gel-Entrapped Staphylococcus aureus Bacteria as Models of Biofilm Infection Exhibit Growth in Dense Aggregates, Oxygen Limitation, Antibiotic Tolerance, and Heterogeneous Gene Expression. Antimicrob Agents Chemother. 2016;60:6294–301. doi: 10.1128/AAC.01336-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis K. Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol; 2012. pp. 121–33. [DOI] [PubMed] [Google Scholar]
- 19.Percival SL, Hill KE, Malic S, Thomas DW, Williams DW. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 2011;19:1–9. doi: 10.1111/j.1524-475X.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry (Mosc) 2005;70:267–74. doi: 10.1007/s10541-005-0111-6. [DOI] [PubMed] [Google Scholar]
- 21.Walters MC 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47:317–23. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown MR, Allison DG, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988;22:777–80. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- 23.Madsen JS, Burmolle M, Hansen LH, Sorensen SJ. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol. 2012;65:183–95. doi: 10.1111/j.1574-695X.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 24.Marks LR, Reddinger RM, Hakansson AP. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. MBio; 2012. p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguila-Arcos S, Alvarez-Rodriguez I, Garaiyurrebaso O, Garbisu C, Grohmann E, Alkorta I. Biofilm-Forming Clinical Staphylococcus Isolates Harbor Horizontal Transfer and Antibiotic Resistance Genes. Front Microbiol. 2017;8:2018. doi: 10.3389/fmicb.2017.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wettero J, Tengvall P, Bengtsson T. Platelets stimulated by IgG-coated surfaces bind and activate neutrophils through a selectin-dependent pathway. Biomaterials. 2003;24:1559–73. doi: 10.1016/s0142-9612(02)00543-4. [DOI] [PubMed] [Google Scholar]
- 27.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339–45. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkler H. Treatment of chronic orthopaedic infection. EFORT Open Rev. 2017;2:110–6. doi: 10.1302/2058-5241.2.160063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McConoughey SJ, Howlin R, Granger JF, Manring MM, Calhoun JH, Shirtliff M. et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9:987–1007. doi: 10.2217/fmb.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Percival SL. Importance of biofilm formation in surgical infection. Br J Surg. 2017;104:e85–e94. doi: 10.1002/bjs.10433. [DOI] [PubMed] [Google Scholar]
- 31.Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K. et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1–28. doi: 10.1046/j.1524-475x.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 32.Harries RL, Bosanquet DC, Harding KG. Wound bed preparation: TIME for an update. Int Wound J. 2016;13(Suppl 3):8–14. doi: 10.1111/iwj.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassinger SM, Harding G, Wongworawat MD. High-pressure pulsatile lavage propagates bacteria into soft tissue. Clin Orthop Relat Res. 2005;439:27–31. doi: 10.1097/01.blo.0000182246.37454.b2. [DOI] [PubMed] [Google Scholar]
- 34.Boyd JI 3rd, Wongworawat MD. High-pressure pulsatile lavage causes soft tissue damage. Clin Orthop Relat Res; 2004. pp. 13–7. [DOI] [PubMed] [Google Scholar]
- 35.Sibbald RG, Williamson D, Orsted HL, Campbell K, Keast D, Krasner D. et al. Preparing the wound bed-debridement, bacterial balance, and moisture balance. Ostomy Wound Manage. 2000;46:14–22. 4-8, 30-5; quiz 6-7. [PubMed] [Google Scholar]
- 36.Bowler PG, Jones SA, Davies BJ, Coyle E. Infection control properties of some wound dressings. J Wound Care. 1999;8:499–502. doi: 10.12968/jowc.1999.8.10.26356. [DOI] [PubMed] [Google Scholar]
- 37.Percival SL, Slone W, Linton S, Okel T, Corum L, Thomas JG. The antimicrobial efficacy of a silver alginate dressing against a broad spectrum of clinically relevant wound isolates. Int Wound J. 2011;8:237–43. doi: 10.1111/j.1742-481X.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meaume S, Vallet D, Morere MN, Teot L. Evaluation of a silver-releasing hydroalginate dressing in chronic wounds with signs of local infection. J Wound Care. 2005;14:411–9. doi: 10.12968/jowc.2005.14.9.26835. [DOI] [PubMed] [Google Scholar]
- 39.Schultz GS, Barillo DJ, Mozingo DW, Chin GA, Wound Bed Advisory Board M. Wound bed preparation and a brief history of TIME. Int Wound J. 2004;1:19–32. doi: 10.1111/j.1742-481x.2004.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brotz-Oesterhelt H, Beyer D, Kroll HP, Endermann R, Ladel C, Schroeder W. et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat Med. 2005;11:1082–7. doi: 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- 41.Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K. et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503:365–70. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina-Manso D, del Prado G, Ortiz-Perez A, Manrubia-Cobo M, Gomez-Barrena E, Cordero-Ampuero J. et al. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int J Antimicrob Agents. 2013;41:521–3. doi: 10.1016/j.ijantimicag.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez CJ Jr, Shiels SM, Tennent DJ, Hardy SK, Murray CK, Wenke JC. Rifamycin Derivatives Are Effective Against Staphylococcal Biofilms In Vitro and Elutable From PMMA. Clin Orthop Relat Res. 2015;473:2874–84. doi: 10.1007/s11999-015-4300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rose WE, Otto DP, Aucamp ME, Miller Z, de Villiers MM. Prevention of biofilm formation by methacrylate-based copolymer films loaded with rifampin, clarithromycin, doxycycline alone or in combination. Pharm Res. 2015;32:61–73. doi: 10.1007/s11095-014-1444-x. [DOI] [PubMed] [Google Scholar]
- 45.Ozturk I, Yurtman AN, Erac B, Gul-Yurtsever S, Ermertcan S, Hosgor-Limoncu M. In vitro effect of moxifloxacin and rifampicin on biofilm formation by clinical MRSA isolates. Bratisl Lek Listy. 2014;115:483–6. doi: 10.4149/bll_2014_093. [DOI] [PubMed] [Google Scholar]
- 46.Fazly Bazzaz BS, Khameneh B, Zarei H, Golmohammadzadeh S. Antibacterial efficacy of rifampin loaded solid lipid nanoparticles against Staphylococcus epidermidis biofilm. Microb Pathog. 2016;93:137–44. doi: 10.1016/j.micpath.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 47.Siala W, Mingeot-Leclercq MP, Tulkens PM, Hallin M, Denis O, Van Bambeke F. Comparison of the antibiotic activities of Daptomycin, Vancomycin, and the investigational Fluoroquinolone Delafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob Agents Chemother. 2014;58:6385–97. doi: 10.1128/AAC.03482-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferreira IS, Bettencourt AF, Goncalves LM, Kasper S, Betrisey B, Kikhney J. et al. Activity of daptomycin- and vancomycin-loaded poly-epsilon-caprolactone microparticles against mature staphylococcal biofilms. Int J Nanomedicine. 2015;10:4351–66. doi: 10.2147/IJN.S84108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du J, Bandara HM, Du P, Huang H, Hoang K, Nguyen D. et al. Improved Biofilm Antimicrobial Activity of Polyethylene Glycol Conjugated Tobramycin Compared to Tobramycin in Pseudomonas aeruginosa Biofilms. Mol Pharm. 2015;12:1544–53. doi: 10.1021/mp500846u. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy S, Beaudoin T, Yau YC, Caraher E, Zlosnik JE, Speert DP. et al. Activity of Tobramycin against Cystic Fibrosis Isolates of Burkholderia cepacia Complex Grown as Biofilms. Antimicrob Agents Chemother. 2015;60:348–55. doi: 10.1128/AAC.02068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunne WM Jr. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–66. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 53.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 54.Yasuda H, Ajiki Y, Koga T, Kawada H, Yokota T. Interaction between biofilms formed by Pseudomonas aeruginosa and clarithromycin. Antimicrob Agents Chemother. 1993;37:1749–55. doi: 10.1128/aac.37.9.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2010;65:1955–8. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 56.Stone G, Wood P, Dixon L, Keyhan M, Matin A. Tetracycline rapidly reaches all the constituent cells of uropathogenic Escherichia coli biofilms. Antimicrob Agents Chemother. 2002;46:2458–61. doi: 10.1128/AAC.46.8.2458-2461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson J, Diefenbeck M, McNally M. Ceramic Biocomposites as Biodegradable Antibiotic Carriers in the Treatment of Bone Infections. J Bone Jt Infect. 2017;2:38–51. doi: 10.7150/jbji.17234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang C, Wong TM, Lau TW, To KK, Wong SS, Leung F. Infection after fracture osteosynthesis - Part I. J Orthop Surg (Hong Kong) 2017;25:2309499017692712. doi: 10.1177/2309499017692712. [DOI] [PubMed] [Google Scholar]
- 59.Fang C, Wong TM, To KK, Wong SS, Lau TW, Leung F. Infection after fracture osteosynthesis - Part II. J Orthop Surg (Hong Kong) 2017;25:2309499017692714. doi: 10.1177/2309499017692714. [DOI] [PubMed] [Google Scholar]
- 60.Yoon HK, Cho SH, Lee DY, Kang BH, Lee SH, Moon DG. et al. A Review of the Literature on Culture-Negative Periprosthetic Joint Infection: Epidemiology, Diagnosis and Treatment. Knee Surg Relat Res. 2017;29:155–64. doi: 10.5792/ksrr.16.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White LM, Schweitzer ME, Deely DM, Gannon F. Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiology. 1995;197:840–2. doi: 10.1148/radiology.197.3.7480765. [DOI] [PubMed] [Google Scholar]
- 62.Floyed RL, Steele RW. Culture-negative osteomyelitis. Pediatr Infect Dis J. 2003;22:731–6. doi: 10.1097/01.inf.0000078901.26909.cf. [DOI] [PubMed] [Google Scholar]
- 63.Termaat MF, Raijmakers PG, Scholten HJ, Bakker FC, Patka P, Haarman HJ. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:2464–71. doi: 10.2106/JBJS.D.02691. [DOI] [PubMed] [Google Scholar]
- 64.Minassian AM, Newnham R, Kalimeris E, Bejon P, Atkins BL, Bowler IC. Use of an automated blood culture system (BD BACTEC) for diagnosis of prosthetic joint infections: easy and fast. BMC Infect Dis. 2014;14:233. doi: 10.1186/1471-2334-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48:1208–14. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzeng A, Tzeng TH, Vasdev S, Korth K, Healey T, Parvizi J. et al. Treating periprosthetic joint infections as biofilms: key diagnosis and management strategies. Diagn Microbiol Infect Dis. 2015;81:192–200. doi: 10.1016/j.diagmicrobio.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 67.Mariaux S, Tafin UF, Borens O. Diagnosis Of Persistent Infection In Prosthetic Two-Stage Exchange: PCR analysis of Sonication fluid From Bone Cement Spacers. J Bone Jt Infect. 2017;2:218–23. doi: 10.7150/jbji.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, Parvizi J. Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2012;94:2247–54. doi: 10.2106/JBJS.L.00210. [DOI] [PubMed] [Google Scholar]
- 69.Bizzini A, Greub G. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry, a revolution in clinical microbial identification. Clin Microbiol Infect. 2010;16:1614–9. doi: 10.1111/j.1469-0691.2010.03311.x. [DOI] [PubMed] [Google Scholar]
- 70.Bizzini A, Durussel C, Bille J, Greub G, Prod'hom G. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol. 2010;48:1549–54. doi: 10.1128/JCM.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tetz VV, Tetz GV. Effect of extracellular DNA destruction by DNase I on characteristics of forming biofilms. DNA Cell Biol. 2010;29:399–405. doi: 10.1089/dna.2009.1011. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen UT, Burrows LL. DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int J Food Microbiol. 2014;187:26–32. doi: 10.1016/j.ijfoodmicro.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 73.Kaplan JB, LoVetri K, Cardona ST, Madhyastha S, Sadovskaya I, Jabbouri S. et al. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J Antibiot (Tokyo) 2012;65:73–7. doi: 10.1038/ja.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baelo A, Levato R, Julian E, Crespo A, Astola J, Gavalda J. et al. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J Control Release. 2015;209:150–8. doi: 10.1016/j.jconrel.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 75.Shak S. Aerosolized recombinant human DNase I for the treatment of cystic fibrosis. Chest. 1995;107:65S–70S. doi: 10.1378/chest.107.2_supplement.65s. [DOI] [PubMed] [Google Scholar]
- 76.Sawicki GS, Chou W, Raimundo K, Trzaskoma B, Konstan MW. Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. J Cyst Fibros. 2015;14:777–83. doi: 10.1016/j.jcf.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A. 1990;87:9188–92. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis. 2013;207:1491–7. doi: 10.1093/infdis/jit047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conover MS, Mishra M, Deora R. Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice. PLoS One. 2011;6:e16861. doi: 10.1371/journal.pone.0016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loughran AJ, Atwood DN, Anthony AC, Harik NS, Spencer HJ, Beenken KE. et al. Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. Microbiologyopen. 2014;3:897–909. doi: 10.1002/mbo3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Craik CS, Page MJ, Madison EL. Proteases as therapeutics. Biochem J. 2011;435:1–16. doi: 10.1042/BJ20100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marti M, Trotonda MP, Tormo-Mas MA, Vergara-Irigaray M, Cheung AL, Lasa I. et al. Extracellular proteases inhibit protein-dependent biofilm formation in Staphylococcus aureus. Microbes Infect. 2010;12:55–64. doi: 10.1016/j.micinf.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Chen C, Krishnan V, Macon K, Manne K, Narayana SV, Schneewind O. Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J Biol Chem. 2013;288:29440–52. doi: 10.1074/jbc.M113.502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fleming D, Rumbaugh KP. Approaches to Dispersing Medical Biofilms. Microorganisms; 2017. p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kerrigan JE, Ragunath C, Kandra L, Gyemant G, Liptak A, Janossy L. et al. Modeling and biochemical analysis of the activity of antibiofilm agent Dispersin B. Acta Biol Hung. 2008;59:439–51. doi: 10.1556/ABiol.59.2008.4.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramasubbu N, Thomas LM, Ragunath C, Kaplan JB. Structural analysis of dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J Mol Biol. 2005;349:475–86. doi: 10.1016/j.jmb.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 87.Brindle ER, Miller DA, Stewart PS. Hydrodynamic deformation and removal of Staphylococcus epidermidis biofilms treated with urea, chlorhexidine, iron chloride, or DispersinB. Biotechnol Bioeng. 2011;108:2968–77. doi: 10.1002/bit.23245. [DOI] [PubMed] [Google Scholar]
- 88.Varposhti M, Abdi Ali A, Mohammadi P. Synergistic Effects of Bismuth Thiols and Various Antibiotics Against Pseudomonas aeruginosa Biofilm. Jundishapur J Microbiol. 2014;7:e9142. doi: 10.5812/jjm.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Folsom JP, Baker B, Stewart PS. In vitro efficacy of bismuth thiols against biofilms formed by bacteria isolated from human chronic wounds. J Appl Microbiol. 2011;111:989–96. doi: 10.1111/j.1365-2672.2011.05110.x. [DOI] [PubMed] [Google Scholar]
- 90.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 91.Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des. 2015;21:5–11. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- 92.Kimyon O, Uluturk ZI, Nizalapur S, Lee M, Kutty SK, Beckmann S. et al. N-Acetylglucosamine Inhibits LuxR, LasR and CviR Based Quorum Sensing Regulated Gene Expression Levels. Front Microbiol. 2016;7:1313. doi: 10.3389/fmicb.2016.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Biswas NN, Yu TT, Kimyon O, Nizalapur S, Gardner CR, Manefield M. et al. Synthesis of antimicrobial glucosamides as bacterial quorum sensing mechanism inhibitors. Bioorg Med Chem. 2017;25:1183–94. doi: 10.1016/j.bmc.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 94.Girennavar B, Cepeda ML, Soni KA, Vikram A, Jesudhasan P, Jayaprakasha GK. et al. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and biofilm formation in bacteria. Int J Food Microbiol. 2008;125:204–8. doi: 10.1016/j.ijfoodmicro.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 95.Tung TT, Jakobsen TH, Dao TT, Fuglsang AT, Givskov M, Christensen SB. et al. Fusaric acid and analogues as Gram-negative bacterial quorum sensing inhibitors. Eur J Med Chem. 2017;126:1011–20. doi: 10.1016/j.ejmech.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 96.Xu X, Zhou XD, Wu CD. Tea catechin epigallocatechin gallate inhibits Streptococcus mutans biofilm formation by suppressing gtf genes. Arch Oral Biol. 2012;57:678–83. doi: 10.1016/j.archoralbio.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 97.Lee P, Tan KS. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch Oral Biol. 2015;60:393–9. doi: 10.1016/j.archoralbio.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 98.Asahi Y, Noiri Y, Miura J, Maezono H, Yamaguchi M, Yamamoto R. et al. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J Appl Microbiol. 2014;116:1164–71. doi: 10.1111/jam.12458. [DOI] [PubMed] [Google Scholar]
- 99.Brackman G, Cos P, Maes L, Nelis HJ, Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011;55:2655–61. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Green B, Elhamshary A, Gomez R, Rahimi S, Brennan PA. An update on the current management of head and neck mucosal melanoma. J Oral Pathol Med. 2017;46:475–9. doi: 10.1111/jop.12526. [DOI] [PubMed] [Google Scholar]
- 101.Giacometti A, Cirioni O, Gov Y, Ghiselli R, Del Prete MS, Mocchegiani F. et al. RNA III inhibiting peptide inhibits in vivo biofilm formation by drug-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:1979–83. doi: 10.1128/AAC.47.6.1979-1983.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kiran MD, Adikesavan NV, Cirioni O, Giacometti A, Silvestri C, Scalise G. et al. Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol Pharmacol. 2008;73:1578–86. doi: 10.1124/mol.107.044164. [DOI] [PubMed] [Google Scholar]
- 103.Yang S, Abdel-Razek OA, Cheng F, Bandyopadhyay D, Shetye GS, Wang G. et al. Bicyclic brominated furanones: a new class of quorum sensing modulators that inhibit bacterial biofilm formation. Bioorg Med Chem. 2014;22:1313–7. doi: 10.1016/j.bmc.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niu C, Afre S, Gilbert ES. Subinhibitory concentrations of cinnamaldehyde interfere with quorum sensing. Lett Appl Microbiol. 2006;43:489–94. doi: 10.1111/j.1472-765X.2006.02001.x. [DOI] [PubMed] [Google Scholar]
- 105.Kim SG, Yoon YH, Choi JW, Rha KS, Park YH. Effect of furanone on experimentally induced Pseudomonas aeruginosa biofilm formation: in vitro study. Int J Pediatr Otorhinolaryngol. 2012;76:1575–8. doi: 10.1016/j.ijporl.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 106.He Z, Wang Q, Hu Y, Liang J, Jiang Y, Ma R. et al. Use of the quorum sensing inhibitor furanone C-30 to interfere with biofilm formation by Streptococcus mutans and its luxS mutant strain. Int J Antimicrob Agents. 2012;40:30–5. doi: 10.1016/j.ijantimicag.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 107.Brackman G, Defoirdt T, Miyamoto C, Bossier P, Van Calenbergh S, Nelis H. et al. Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp. by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol. 2008;8:149. doi: 10.1186/1471-2180-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beema Shafreen RM, Selvaraj C, Singh SK, Karutha Pandian S. In silico and in vitro studies of cinnamaldehyde and their derivatives against LuxS in Streptococcus pyogenes: effects on biofilm and virulence genes. J Mol Recognit. 2014;27:106–16. doi: 10.1002/jmr.2339. [DOI] [PubMed] [Google Scholar]
- 109.Jakobsen TH, van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M. et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother. 2012;56:2314–25. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christensen LD, van Gennip M, Jakobsen TH, Alhede M, Hougen HP, Hoiby N. et al. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J Antimicrob Chemother. 2012;67:1198–206. doi: 10.1093/jac/dks002. [DOI] [PubMed] [Google Scholar]
- 111.Cirioni O, Giacometti A, Ghiselli R, Dell'Acqua G, Orlando F, Mocchegiani F. et al. RNAIII-inhibiting peptide significantly reduces bacterial load and enhances the effect of antibiotics in the treatment of central venous catheter-associated Staphylococcus aureus infections. J Infect Dis. 2006;193:180–6. doi: 10.1086/498914. [DOI] [PubMed] [Google Scholar]
- 112.Harmata AJ, Ma Y, Sanchez CJ, Zienkiewicz KJ, Elefteriou F, Wenke JC. et al. D-amino acid inhibits biofilm but not new bone formation in an ovine model. Clin Orthop Relat Res. 2015;473:3951–61. doi: 10.1007/s11999-015-4465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–9. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duong HT, Jung K, Kutty SK, Agustina S, Adnan NN, Basuki JS. et al. Nanoparticle (star polymer) delivery of nitric oxide effectively negates Pseudomonas aeruginosa biofilm formation. Biomacromolecules. 2014;15:2583–9. doi: 10.1021/bm500422v. [DOI] [PubMed] [Google Scholar]
- 115.Barraud N, Kelso MJ, Rice SA, Kjelleberg S. Nitric oxide: a key mediator of biofilm dispersal with applications in infectious diseases. Curr Pharm Des. 2015;21:31–42. doi: 10.2174/1381612820666140905112822. [DOI] [PubMed] [Google Scholar]
- 116.Davies DG, Marques CN. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol. 2009;191:1393–403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marques CN, Davies DG, Sauer K. Control of Biofilms with the Fatty Acid Signaling Molecule cis-2-Decenoic Acid. Pharmaceuticals (Basel) 2015;8:816–35. doi: 10.3390/ph8040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wake N, Asahi Y, Noiri Y, Hayashi M, Motooka D, Nakamura S. et al. Temporal dynamics of bacterial microbiota in the human oral cavity determined using an in situ model of dental biofilms. NPJ Biofilms Microbiomes. 2016;2:16018. doi: 10.1038/npjbiofilms.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Speziale P, Pietrocola G, Foster TJ, Geoghegan JA. Protein-based biofilm matrices in Staphylococci. Front Cell Infect Microbiol. 2014;4:171. doi: 10.3389/fcimb.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Formosa-Dague C, Feuillie C, Beaussart A, Derclaye S, Kucharikova S, Lasa I. et al. Sticky Matrix: Adhesion Mechanism of the Staphylococcal Polysaccharide Intercellular Adhesin. ACS Nano. 2016;10:3443–52. doi: 10.1021/acsnano.5b07515. [DOI] [PubMed] [Google Scholar]
- 121.Hofmann CM, Bednar KJ, Anderson JM, Marchant RE. Disruption of Staphylococcus epidermidis biofilm formation using a targeted cationic peptide. J Biomed Mater Res A. 2012;100:1061–7. doi: 10.1002/jbm.a.33273. [DOI] [PubMed] [Google Scholar]
- 122.Fong JN, Yildiz FH. Biofilm Matrix Proteins. Microbiol Spectr; 2015. p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paharik AE, Horswill AR. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol Spectr; 2016. p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Herman-Bausier P, El-Kirat-Chatel S, Foster TJ, Geoghegan JA, Dufrene YF. Staphylococcus aureus Fibronectin-Binding Protein A Mediates Cell-Cell Adhesion through Low-Affinity Homophilic Bonds. MBio. 2015;6:e00413–15. doi: 10.1128/mBio.00413-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schaeffer CR, Woods KM, Longo GM, Kiedrowski MR, Paharik AE, Buttner H. et al. Accumulation-associated protein enhances Staphylococcus epidermidis biofilm formation under dynamic conditions and is required for infection in a rat catheter model. Infect Immun. 2015;83:214–26. doi: 10.1128/IAI.02177-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Conlon BP, Geoghegan JA, Waters EM, McCarthy H, Rowe SE, Davies JR. et al. Role for the A domain of unprocessed accumulation-associated protein (Aap) in the attachment phase of the Staphylococcus epidermidis biofilm phenotype. J Bacteriol. 2014;196:4268–75. doi: 10.1128/JB.01946-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Christner M, Franke GC, Schommer NN, Wendt U, Wegert K, Pehle P. et al. The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol Microbiol. 2010;75:187–207. doi: 10.1111/j.1365-2958.2009.06981.x. [DOI] [PubMed] [Google Scholar]
- 128.Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190:1036–44. doi: 10.1128/JB.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lasa I, Penades JR. Bap: a family of surface proteins involved in biofilm formation. Res Microbiol. 2006;157:99–107. doi: 10.1016/j.resmic.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 130.Yang YH, Jiang YL, Zhang J, Wang L, Bai XH, Zhang SJ. et al. Structural insights into SraP-mediated Staphylococcus aureus adhesion to host cells. PLoS Pathog. 2014;10:e1004169. doi: 10.1371/journal.ppat.1004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khodaparast L, Khodaparast L, Shahrooei M, Stijlemans B, Merckx R, Baatsen P. et al. The Possible Role of Staphylococcus epidermidis LPxTG Surface Protein SesC in Biofilm Formation. PLoS One. 2016;11:e0146704. doi: 10.1371/journal.pone.0146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R. et al. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23:3020–30. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Birkenhauer E, Neethirajan S, Weese JS. Collagen and hyaluronan at wound sites influence early polymicrobial biofilm adhesive events. BMC Microbiol. 2014;14:191. doi: 10.1186/1471-2180-14-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith RG. Enzymatic debriding agents: an evaluation of the medical literature. Ostomy Wound Manage. 2008;54:16–34. [PubMed] [Google Scholar]
- 135.Langer V, Bhandari PS, Rajagopalan S, Mukherjee MK. Enzymatic debridement of large burn wounds with papain-urea: Is it safe? Med J Armed Forces India. 2013;69:144–50. doi: 10.1016/j.mjafi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Watters CM, Burton T, Kirui DK, Millenbaugh NJ. Enzymatic degradation of in vitro Staphylococcus aureus biofilms supplemented with human plasma. Infect Drug Resist. 2016;9:71–8. doi: 10.2147/IDR.S103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mugita N, Nambu T, Takahashi K, Wang PL, Komasa Y. Proteases, actinidin, papain and trypsin reduce oral biofilm on the tongue in elderly subjects and in vitro. Arch Oral Biol. 2017;82:233–40. doi: 10.1016/j.archoralbio.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 138.Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001;58:1234–45. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cetrulo GI. Use of trypsin intravenously in a gunshot wound. J Am Med Assoc. 1953;152:605–6. doi: 10.1001/jama.1953.63690070001010. [DOI] [PubMed] [Google Scholar]
- 140.Maresso AW, Wu R, Kern JW, Zhang R, Janik D, Missiakas DM. et al. Activation of inhibitors by sortase triggers irreversible modification of the active site. J Biol Chem. 2007;282:23129–39. doi: 10.1074/jbc.M701857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oh KB, Kim SH, Lee J, Cho WJ, Lee T, Kim S. Discovery of diarylacrylonitriles as a novel series of small molecule sortase A inhibitors. J Med Chem. 2004;47:2418–21. doi: 10.1021/jm0498708. [DOI] [PubMed] [Google Scholar]
- 142.Hu P, Huang P, Chen WM. Curcumin inhibits the Sortase A activity of the Streptococcus mutans UA159. Appl Biochem Biotechnol. 2013;171:396–402. doi: 10.1007/s12010-013-0378-9. [DOI] [PubMed] [Google Scholar]
- 143.Harrison JJ, Turner RJ, Ceri H. Persister cells, the biofilm matrix and tolerance to metal cations in biofilm and planktonic Pseudomonas aeruginosa. Environ Microbiol. 2005;7:981–94. doi: 10.1111/j.1462-2920.2005.00777.x. [DOI] [PubMed] [Google Scholar]
- 144.Abraham NM, Lamlertthon S, Fowler VG, Jefferson KK. Chelating agents exert distinct effects on biofilm formation in Staphylococcus aureus depending on strain background: role for clumping factor B. J Med Microbiol. 2012;61:1062–70. doi: 10.1099/jmm.0.040758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dunne WM Jr, Burd EM. The effects of magnesium, calcium, EDTA, and pH on the in vitro adhesion of Staphylococcus epidermidis to plastic. Microbiol Immunol. 1992;36:1019–27. doi: 10.1111/j.1348-0421.1992.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 146.Lin MH, Shu JC, Huang HY, Cheng YC. Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS One. 2012;7:e34388. doi: 10.1371/journal.pone.0034388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sarkisova S, Patrauchan MA, Berglund D, Nivens DE, Franklin MJ. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J Bacteriol. 2005;187:4327–37. doi: 10.1128/JB.187.13.4327-4337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kamiya H, Ehara T, Matsumoto T. Inhibitory effects of lactoferrin on biofilm formation in clinical isolates of Pseudomonas aeruginosa. J Infect Chemother. 2012;18:47–52. doi: 10.1007/s10156-011-0287-1. [DOI] [PubMed] [Google Scholar]
- 149.Dashper SG, Pan Y, Veith PD, Chen YY, Toh EC, Liu SW. et al. Lactoferrin inhibits Porphyromonas gingivalis proteinases and has sustained biofilm inhibitory activity. Antimicrob Agents Chemother. 2012;56:1548–56. doi: 10.1128/AAC.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Allison LM, Walker LA, Sanders BJ, Yang Z, Eckert G, Gregory RL. Effect of Human Milk and its Components on Streptococcus Mutans Biofilm Formation. J Clin Pediatr Dent. 2015;39:255–61. doi: 10.17796/1053-4628-39.3.255. [DOI] [PubMed] [Google Scholar]
- 151.Ammons MC, Copie V. Mini-review: Lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling. 2013;29:443–55. doi: 10.1080/08927014.2013.773317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liesse Iyamba JM, Seil M, Nagant C, Dulanto S, Deplano A, El Khattabi C. et al. Inhibition by EGTA of the formation of a biofilm by clinical strains of Staphylococcus aureus. J Basic Microbiol. 2014;54:700–10. doi: 10.1002/jobm.201200511. [DOI] [PubMed] [Google Scholar]
- 153.Finnegan S, Percival SL. EDTA: An Antimicrobial and Antibiofilm Agent for Use in Wound Care. Adv Wound Care (New Rochelle) 2015;4:415–21. doi: 10.1089/wound.2014.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Z, Lin Y, Lu Q, Li F, Yu J, Wang Z. et al. In vitro and in vivo activity of EDTA and antibacterial agents against the biofilm of mucoid Pseudomonas aeruginosa. Infection. 2017;45:23–31. doi: 10.1007/s15010-016-0905-z. [DOI] [PubMed] [Google Scholar]
- 155.Zenga J, Gagnon PM, Vogel J, Chole RA. Biofilm formation by otopathogenic strains of Pseudomonas aeruginosa is not consistently inhibited by ethylenediaminetetraacetic acid. Otol Neurotol. 2012;33:1007–12. doi: 10.1097/MAO.0b013e31825f249e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alexander JW. History of the medical use of silver. Surg Infect (Larchmt) 2009;10:289–92. doi: 10.1089/sur.2008.9941. [DOI] [PubMed] [Google Scholar]
- 157.Markowska K, Grudniak AM, Wolska KI. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol. 2013;60:523–30. [PubMed] [Google Scholar]
- 158.Goggin R, Jardeleza C, Wormald PJ, Vreugde S. Colloidal silver: a novel treatment for Staphylococcus aureus biofilms? Int Forum Allergy Rhinol. 2014;4:171–5. doi: 10.1002/alr.21259. [DOI] [PubMed] [Google Scholar]
- 159.Rajiv S, Drilling A, Bassiouni A, James C, Vreugde S, Wormald PJ. Topical colloidal silver as an anti-biofilm agent in a Staphylococcus aureus chronic rhinosinusitis sheep model. Int Forum Allergy Rhinol. 2015;5:283–8. doi: 10.1002/alr.21459. [DOI] [PubMed] [Google Scholar]
- 160.Li X, Robinson SM, Gupta A, Saha K, Jiang Z, Moyano DF. et al. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano. 2014;8:10682–6. doi: 10.1021/nn5042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Boda SK, Broda J, Schiefer F, Weber-Heynemann J, Hoss M, Simon U. et al. Cytotoxicity of Ultrasmall Gold Nanoparticles on Planktonic and Biofilm Encapsulated Gram-Positive Staphylococci. Small. 2015;11:3183–93. doi: 10.1002/smll.201403014. [DOI] [PubMed] [Google Scholar]
- 162.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:278–84. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 163.Giri K, Yepes LR, Duncan B, Parameswaran PK, Yan B, Jiang Y. et al. Targeting bacterial biofilms via surface engineering of gold nanoparticles. RSC Adv. 2015;5:105551–9. doi: 10.1039/C5RA16305F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Richter K, Thomas N, Claeys J, McGuane J, Prestidge CA, Coenye T, A Topical Hydrogel with Deferiprone and Gallium-Protoporphyrin Targets Bacterial Iron Metabolism and Has Antibiofilm Activity. Antimicrob Agents Chemother; 2017. p. 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Richter K, Thomas N, Zhang G, Prestidge CA, Coenye T, Wormald PJ. et al. Deferiprone and Gallium-Protoporphyrin Have the Capacity to Potentiate the Activity of Antibiotics in Staphylococcus aureus Small Colony Variants. Front Cell Infect Microbiol. 2017;7:280. doi: 10.3389/fcimb.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.LewisOscar F, MubarakAli D, Nithya C, Priyanka R, Gopinath V, Alharbi NS. et al. One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling. 2015;31:379–91. doi: 10.1080/08927014.2015.1048686. [DOI] [PubMed] [Google Scholar]
- 167.Ahire JJ, Hattingh M, Neveling DP, Dicks LM. Copper-Containing Anti-Biofilm Nanofiber Scaffolds as a Wound Dressing Material. PLoS One. 2016;11:e0152755. doi: 10.1371/journal.pone.0152755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yadollahi M, Farhoudian S, Namazi H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol. 2015;79:37–43. doi: 10.1016/j.ijbiomac.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 169.Ahangari A, Salouti M, Heidari Z, Kazemizadeh AR, Safari AA. Development of gentamicin-gold nanospheres for antimicrobial drug delivery to Staphylococcal infected foci. Drug Deliv. 2013;20:34–9. doi: 10.3109/10717544.2012.746402. [DOI] [PubMed] [Google Scholar]
- 170.Rastogi L, Kora AJ, J A. Highly stable, protein capped gold nanoparticles as effective drug delivery vehicles for amino-glycosidic antibiotics. Mater Sci Eng C Mater Biol Appl. 2012;32:1571–7. doi: 10.1016/j.msec.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 171.Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS. 2017;125:353–64. doi: 10.1111/apm.12687. [DOI] [PubMed] [Google Scholar]
- 172.Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop Relat Res. 2008;466:466–72. doi: 10.1007/s11999-007-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mantripragada VP, Lecka-Czernik B, Ebraheim NA, Jayasuriya AC. An overview of recent advances in designing orthopedic and craniofacial implants. J Biomed Mater Res A. 2013;101:3349–64. doi: 10.1002/jbm.a.34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ferraris S, Spriano S. Antibacterial titanium surfaces for medical implants. Mater Sci Eng C Mater Biol Appl. 2016;61:965–78. doi: 10.1016/j.msec.2015.12.062. [DOI] [PubMed] [Google Scholar]
- 175.Yeo IS, Kim HY, Lim KS, Han JS. Implant surface factors and bacterial adhesion: a review of the literature. Int J Artif Organs. 2012;35:762–72. doi: 10.5301/ijao.5000154. [DOI] [PubMed] [Google Scholar]
- 176.Clauss M, Graf S, Gersbach S, Hintermann B, Ilchmann T, Knupp M. Material and biofilm load of K wires in toe surgery: titanium versus stainless steel. Clin Orthop Relat Res. 2013;471:2312–7. doi: 10.1007/s11999-013-2919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Banovetz JM, Sharp R, Probe RA, Anglen JO. Titanium plate fixation: a review of implant failures. J Orthop Trauma. 1996;10:389–94. doi: 10.1097/00005131-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 178.Uhthoff HK, Bardos DI, Liskova-Kiar M. The advantages of titanium alloy over stainless steel plates for the internal fixation of fractures. An experimental study in dogs. J Bone Joint Surg Br. 1981;63B:427–84. doi: 10.1302/0301-620X.63B3.7263759. [DOI] [PubMed] [Google Scholar]
- 179.Koseki H, Yonekura A, Shida T, Yoda I, Horiuchi H, Morinaga Y. et al. Early staphylococcal biofilm formation on solid orthopaedic implant materials: in vitro study. PLoS One. 2014;9:e107588. doi: 10.1371/journal.pone.0107588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rotini R, Cavaciocchi M, Fabbri D, Bettelli G, Catani F, Campochiaro G. et al. Proximal humeral fracture fixation: multicenter study with carbon fiber peek plate. Musculoskelet Surg. 2015;99(Suppl 1):S1–8. doi: 10.1007/s12306-015-0371-2. [DOI] [PubMed] [Google Scholar]
- 181.Schildhauer TA, Robie B, Muhr G, Koller M. Bacterial adherence to tantalum versus commonly used orthopedic metallic implant materials. J Orthop Trauma. 2006;20:476–84. doi: 10.1097/00005131-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 182.Schmidlin PR, Muller P, Attin T, Wieland M, Hofer D, Guggenheim B. Polyspecies biofilm formation on implant surfaces with different surface characteristics. J Appl Oral Sci. 2013;21:48–55. doi: 10.1590/1678-7757201302312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Burgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, Gosau M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res. 2010;21:156–64. doi: 10.1111/j.1600-0501.2009.01815.x. [DOI] [PubMed] [Google Scholar]
- 184.Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17(Suppl 2):68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 185.Perez-Tanoira R, Aarnisalo AA, Eklund KK, Han X, Soininen A, Tiainen VM. et al. Prevention of Biomaterial Infection by Pre-Operative Incubation with Human Cells. Surg Infect (Larchmt) 2017;18:336–44. doi: 10.1089/sur.2016.263. [DOI] [PubMed] [Google Scholar]
- 186.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 187.Subbiahdoss G, Grijpma DW, van der Mei HC, Busscher HJ, Kuijer R. Microbial biofilm growth versus tissue integration on biomaterials with different wettabilities and a polymer-brush coating. J Biomed Mater Res A. 2010;94:533–8. doi: 10.1002/jbm.a.32731. [DOI] [PubMed] [Google Scholar]
- 188.Zhao B, van der Mei HC, Subbiahdoss G, de Vries J, Rustema-Abbing M, Kuijer R. et al. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent Mater. 2014;30:716–27. doi: 10.1016/j.dental.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 189.Moriarty TF, Kuehl R, Coenye T, Metsemakers WJ, Morgenstern M, Schwarz EM. et al. Orthopaedic device-related infection: current and future interventions for improved prevention and treatment. EFORT Open Rev. 2016;1:89–99. doi: 10.1302/2058-5241.1.000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Alt V. Antimicrobial coated implants in trauma and orthopaedics-A clinical review and risk-benefit analysis. Injury. 2017;48:599–607. doi: 10.1016/j.injury.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 191.Zhou X, Zhang J, Feng G, Shen J, Kong D, Zhao Q. Nitric Oxide-Releasing Biomaterials for Biomedical Applications. Curr Med Chem. 2016;23:2579–601. doi: 10.2174/0929867323666160729104647. [DOI] [PubMed] [Google Scholar]
- 192.Schaer TP, Stewart S, Hsu BB, Klibanov AM. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials. 2012;33:1245–54. doi: 10.1016/j.biomaterials.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 193.Ma Y, Chen M, Jones JE, Ritts AC, Yu Q, Sun H. Inhibition of Staphylococcus epidermidis biofilm by trimethylsilane plasma coating. Antimicrob Agents Chemother. 2012;56:5923–37. doi: 10.1128/AAC.01739-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen AF, Winkler H. Local Antimicrobial Treatment in Orthopaedic Surgery. J Bone Jt Infect. 2017;2:1–2. doi: 10.7150/jbji.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peng ZX, Tu B, Shen Y, Du L, Wang L, Guo SR. et al. Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface. Antimicrob Agents Chemother. 2011;55:860–6. doi: 10.1128/AAC.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carlson RP, Taffs R, Davison WM, Stewart PS. Anti-biofilm properties of chitosan-coated surfaces. J Biomater Sci Polym Ed. 2008;19:1035–46. doi: 10.1163/156856208784909372. [DOI] [PubMed] [Google Scholar]
- 197.Paramasivan S, Jones D, Baker L, Hanton L, Robinson S, Wormald PJ. et al. The use of chitosan-dextran gel shows anti-inflammatory, antibiofilm, and antiproliferative properties in fibroblast cell culture. Am J Rhinol Allergy. 2014;28:361–5. doi: 10.2500/ajra.2014.28.4069. [DOI] [PubMed] [Google Scholar]
- 198.Nelson CL, Jones RB, Wingert NC, Foltzer M, Bowen TR. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin Orthop Relat Res. 2014;472:2208–14. doi: 10.1007/s11999-014-3571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sanchez CJ, Hurtgen BJ, Lizcano A, Shivshankar P, Cole GT, Orihuela CJ. Biofilm and planktonic pneumococci demonstrate disparate immunoreactivity to human convalescent sera. BMC Microbiol. 2011;11:245. doi: 10.1186/1471-2180-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Blanchette KA, Prabhakara R, Shirtliff ME, Wenke JC. Inhibition of fracture healing in the presence of contamination by Staphylococcus aureus: Effects of growth state and immune response. J Orthop Res; 2017. [DOI] [PubMed] [Google Scholar]
- 201.Lee BY, Wiringa AE, Bailey RR, Lewis GJ, Feura J, Muder RR. Staphylococcus aureus vaccine for orthopedic patients: an economic model and analysis. Vaccine. 2010;28:2465–71. doi: 10.1016/j.vaccine.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Josefsson E, Hartford O, O'Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184:1572–80. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 203.McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Doring G. et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–7. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 204.Lam H, Kesselly A, Stegalkina S, Kleanthous H, Yethon JA. Antibodies to PhnD inhibit staphylococcal biofilms. Infect Immun. 2014;82:3764–74. doi: 10.1128/IAI.02168-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gil C, Solano C, Burgui S, Latasa C, Garcia B, Toledo-Arana A. et al. Biofilm matrix exoproteins induce a protective immune response against Staphylococcus aureus biofilm infection. Infect Immun. 2014;82:1017–29. doi: 10.1128/IAI.01419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brady RA, O'May GA, Leid JG, Prior ML, Costerton JW, Shirtliff ME. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment. Infect Immun. 2011;79:1797–803. doi: 10.1128/IAI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gedbjerg N, LaRosa R, Hunter JG, Varrone JJ, Kates SL, Schwarz EM. et al. Anti-glucosaminidase IgG in sera as a biomarker of host immunity against Staphylococcus aureus in orthopaedic surgery patients. J Bone Joint Surg Am. 2013;95:e171. doi: 10.2106/JBJS.L.01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Varrone JJ, de Mesy Bentley KL, Bello-Irizarry SN, Nishitani K, Mack S, Hunter JG. et al. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. J Orthop Res. 2014;32:1389–96. doi: 10.1002/jor.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shahrooei M, Hira V, Khodaparast L, Khodaparast L, Stijlemans B, Kucharikova S. et al. Vaccination with SesC decreases Staphylococcus epidermidis biofilm formation. Infect Immun. 2012;80:3660–8. doi: 10.1128/IAI.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Novotny LA, Clements JD, Goodman SD, Bakaletz LO. Transcutaneous Immunization with a Band-Aid Prevents Experimental Otitis Media in a Polymicrobial Model. Clin Vaccine Immunol; 2017. p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Novotny LA, Jurcisek JA, Ward MO Jr, Jordan ZB, Goodman SD, Bakaletz LO. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol Microbiol. 2015;96:276–92. doi: 10.1111/mmi.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lattar SM, Noto Llana M, Denoel P, Germain S, Buzzola FR, Lee JC. et al. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun. 2014;82:83–91. doi: 10.1128/IAI.01050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang J, Yang F, Zhang X, Jing H, Ren C, Cai C. et al. Protective Efficacy and Mechanism of Passive Immunization with Polyclonal Antibodies in a Sepsis Model of Staphylococcus aureus Infection. Sci Rep. 2015;5:15553. doi: 10.1038/srep15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Russo TA, Beanan JM, Olson R, MacDonald U, Cox AD, St Michael F. et al. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun. 2013;81:915–22. doi: 10.1128/IAI.01184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fowler VG Jr, Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect. 2014;20(Suppl 5):66–75. doi: 10.1111/1469-0691.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J. et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 217.Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother. 2015;11:632–41. doi: 10.4161/hv.34414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW. et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–78. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 219.Capparelli EV, Bloom BT, Kueser TJ, Oelberg DG, Bifano EM, White RD. et al. Multicenter study to determine antibody concentrations and assess the safety of administration of INH-A21, a donor-selected human Staphylococcal immune globulin, in low-birth-weight infants. Antimicrob Agents Chemother. 2005;49:4121–7. doi: 10.1128/AAC.49.10.4121-4127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]