Abstract
Ratiometric wide-field fluorescence microscopy with 1′,7′- bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF)-dextran demonstrated that gravistimulation leads to rapid changes in cytoplasmic pH (pHc) in columella cells of Arabidopsis roots. The pHc of unstimulated columella cells in tiers 2 and 3, known sites of graviperception (E.B. Blancaflor, J.B. Fasano, S. Gilroy [1998] Plant Physiol 116: 213–222), was 7.22 ± 0.02 pH units. Following gravistimulation, the magnitude and direction of pHc changes in these cells depended on their location in the columella. Cells in the lower side of tier 2 became more alkaline by 0.4 unit within 55 s of gravistimulation, whereas alkalinization of the cells on the upper side was slower (100 s). In contrast, all cells in tier 3 acidified by 0.4 pH unit within 480 s after gravistimulation. Disrupting these pHc changes in the columella cells using pHc modifiers at concentrations that do not affect root growth altered the gravitropic response. Acidifying agents, including bafilomycin A1, enhanced curvature, whereas alkalinizing agents disrupted gravitropic bending. These results imply that pHc changes in the gravisensing cells and the resultant pH gradients across the root cap are important at an early stage in the signal cascade leading to the gravitropic response.
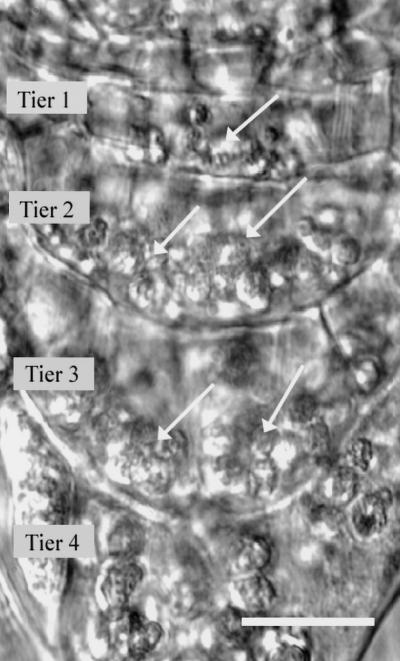
Gravity serves as an important guide for the growth of plant organs. The directed growth of roots or gravitropism involves the perception of gravity, followed by signal transduction that results in a differential growth response. The root cap perceives changes in root tip orientation and is essential for the gravitropic response (Darwin, 1896). Perception is confined to the columella cells within the root cap, where amyloplasts sediment in the direction of gravity (Sack, 1991). Using laser ablation of selected columella cells, Blancaflor et al. (1998) demonstrated that the tiers of columella cells in Arabidopsis roots that are proximal to the meristematic region (tiers 1–3; Fig. 1) are the sites of gravisensing. Once gravity is perceived, this signal is communicated to the elongation zone, where differential growth occurs (Selker and Sievers, 1987; Ishikawa et al., 1991; Ishikawa and Evans, 1993; for review, see Masson, 1995; Evans and Ishikawa, 1997; Chen et al., 1999).
Figure 1.
The root tip of Arabidopsis is the site of graviperception and early signaling events. Four tiers of cells comprise the columella of the Arabidopsis root cap, with tiers 1 through 3 being the most important for gravisensing. Each columella cell contains amyloplasts that sediment in the direction of gravity (arrows). Scale bar = 10 μm.
Very little is known about the signaling pathway linking the perception of gravity to differential growth. Logic would dictate that the earliest signaling events would also occur within the columella cells, since perception occurs there. While indirect evidence from a variety of experimental methods implicates calcium in signaling (Lee et al., 1983; Björkman and Cleland, 1991; Sievers and Busch, 1992; Stinemetz et al., 1992; for review, see Chen et al., 1999), ratiometric measurements of cytosolic free calcium ([Ca2+]c) in root columella cells has shown no apparent changes in [Ca2+]c following gravistimulation (Leagué et al., 1997).
Intracellular ionic currents in root cap cells during gravistimulation have been observed using microelectrodes and in vibrating probe studies (Behrens et al., 1985; Björkman and Leopold, 1987; Sievers et al., 1995). In plant cells, the plasma membrane H+-ATPase is primarily responsible for generating the membrane potential (Briskin, 1990; Assmann and Haubrick, 1996). Therefore, changes in membrane potential may cause or be a reflection of changes in cytosolic pH (pHc) that could be used in signaling. To date, no conclusive evidence has been provided pinpointing the earliest signals for root gravitropism after plants have perceived a change in orientation.
Many different physiological events in both animal and plant cells are regulated by changes in pHc. In animal cells, pHc is well characterized as a regulator of a number of processes, such as modulation of Ca2+ signaling (Malayev and Nelson, 1995), cytoskeletal polymerization (Yonezawa et al., 1985; Suprenant, 1991; Edmonds et al., 1995), longevity of cardiac action potentials (Steidl and Yool, 1999), protein synthesis (Dube et al., 1991), enzyme activity and secretion (Tapper and Sundler, 1995; Putnam, 1998), apoptosis (Thangaraju et al., 1999), and endocytosis and exocytosis (Cosson et al., 1989; Gluck et al., 1982). There is also increasing evidence that suggests changes in pHc can act as a second messenger in plants as well (for review, see Felle, 1989; Guern et al., 1992; Zimmermann et al., 1999). Intracellular pH changes are involved in signaling plant defense responses (Guern et al., 1992), tip growth (Gibbon and Kropf, 1994; Robson et al., 1996; Feijó et al., 1999), nodulation (Allen et al., 1994; Felle et al., 1996), elicitation of benzophenanthridine alkaloids (Roos et al., 1998), and response to hormone activity such as gibberellic acid (Swanson and Jones, 1996) and abscisic acid (Beffagna et al., 1997).
A role for intracellular pH changes occurring in response to a gravitropic stimulus has not been thoroughly investigated. We used two complimentary approaches to elucidate the role of pHc in the early signaling events of root gravitropism in Arabidopsis. In one approach, we demonstrated that perturbation of pHc in root cap cells modulates gravitropic bending. In the second approach, we monitored the pHc of root columella cells with a dextran-linked, pH-sensitive fluorescent dye, and observed gravity-induced pHc changes. These data strongly suggest that pHc changes occurring over time in specific columella cells are involved in the early signaling events of root gravitropism.
MATERIALS AND METHODS
Plant Material
Seeds of Arabidopsis were surface-sterilized (70% [w/v] ethanol, 2 min; 30% [w/v] bleach containing 0.01% [w/v] Triton X-100, 25 min) and plated on growth medium containing 3 mm KNO3, 2 mm Ca(NO3)2, 0.5 mm MgSO4, 1 mm (NH4)H2PO4, 1 mg/mL thiamine, 0.5 mg/mL pyridoxine-HCl, 2.8 mm myo-inositol, 2.6 mm MES, pH 5.7, 6% [w/v] Suc, 25 μm KCl, 17.5 μm H3BO3, 1 μm MnSO4, 0.25 μm CuSO4, 0.25 μm (NH4)6Mo7O24, 25 μm Fe-Na EDTA, and 0.5% (w/v) phytagel. Seeds were grown under sterile conditions for 3 to 4 d at 22°C and 24 h of light until roots were 10 mm in length. Seeds for growth and bending studies were grown in Petri dishes, while seedlings for microinjection purposes were grown on a coverslip coated with a 0.5-mm layer of growth medium containing phytagel and placed at a 45° angle to allow the roots to grow into the medium and along the surface of the coverslip.
pH Modifiers
Weak acids and bases and an inhibitor of the vacuolar H+-ATPase (V-ATPase), bafilomycin A1 (Sigma, St. Louis), were prepared in growth medium. To optimize uptake, the pH of the growth medium was adjusted to 5.5 for benzoic acid (100 μm), to 6.0 for bafilomycin A1 (0.3 and 1.3 μm) using 2-(N-morpholino)-ethanesulfonic acid (MES) buffer, and to 8.9 for procaine (50 and 100 μm) and methylamine (500 μm), replacing MES with Bis-Tris propane buffer.
Growth Measurements
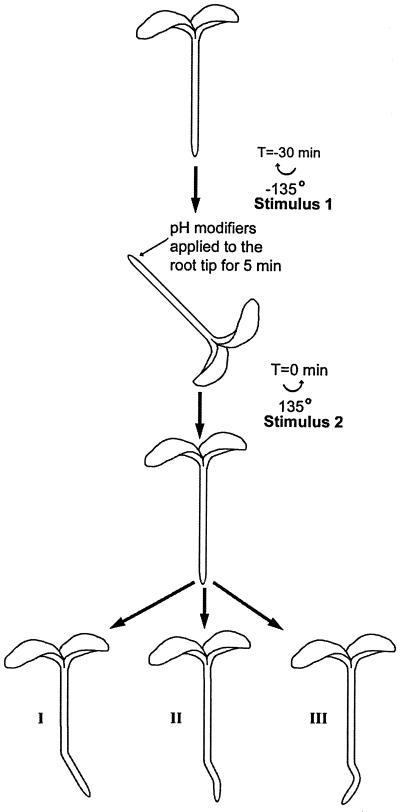
Roots were mounted vertically for 30 min and imaged using a horizontally mounted dissecting microscope (MZ12, Leica, Wetzlar, Germany). Roots were gravistimulated by rotating the plates 135° for 30 min (stimulus 1, Fig. 2). At the end of 30 min, 0.1 μL of control or modifier solution was applied specifically to the root cap while roots were maintained in this position for 5 min. Roots were then reoriented to the vertical orientation (stimulus 2) for 4 h more (Fig. 2). Images were captured at 10-min intervals (Newvicon camera, Hamamatsu, Bridgewater, NJ). Root growth and curvature were measured using image analysis software (Image-1, Universal Imaging, West Chester, PA), with curvature defined as the angle between the root tip and a reference point in the non-responding zone of differentiation. To assess movement of the modifier solution, 0.1 μL of carboxyfluorescein was applied to the tip in the same manner as the modifiers, and the location of the solution was viewed using fluorescence microscopy. These studies showed that the solution did not move into the elongation zone.
Figure 2.
Outline of the experimental protocol designed to test the effects of various pH modifiers on the signaling pathway for root gravitropism. At T = −30 min, vertical roots were rotated through 135° (Stimulus 1). At T = −5 min, 0.1 μL of control or pH-modiying solutions was applied solely to the root cap. Roots were returned to vertical at T = 0 (Stimulus 2). Three possible resultant morphologies are shown: I, Disrupted signaling; II, normal signaling; and III, enhanced signaling.
Ratiometric pH Measurements in Arabidopsis Root Columella Cells
Coverslips with 3-d-old seedlings were imaged on an Axiovert microscope with a ×25, numerical aperature 0.8, water immersion lens (Zeiss, Thornwood, NY), and columella cells were microinjected with quartz micropipettes (pulled with a P2000 puller, Sutter Instruments, Novato, CA) and positioned with an micromanipulator (Eppendorf, Madison, WI). 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) conjugated to a 10-kD dextran (5 mm in 100 mm KCl) was injected into the cytoplasm with two or three 1-s iontophoretic pulses of 10 nA or less (Current Generator 260, World Precision Instruments, Sarasota, FL). The micropipette was allowed to sit in the cell for 5 min following delivery of the dye into the cytoplasm and was then removed slowly. Roots were allowed to recover for at least 1 h before measurements were taken. Data acquisition was controlled using Metafluor (Universal Imaging) and 3 × 3-binned fluorescent images were collected at 510 to 560 nm with a cooled CCD camera (2.5 MHz PentaMAX camera ET/CCD-K1317, Princeton Instruments, Princeton, NJ) using dual excitation (490–500 nm for 3 s; 435–445 nm for 1 s; filter wheel model Lambda 10–2, Sutter Instruments).
For measurements of pHc during gravistimulation, seedlings that had recovered for 1 h after microinjection were placed vertically on a computer-controlled vertical traveling stage (H128 Series, Prior Scientific Instruments, Fulbourn, Cambridge, UK) for an additional 1 h prior to the start of the experiment. Images were collected every 10 s of plants positioned vertically for 5 min. The plants were then turned 90° and imaged every 10 s for an additional 15 min. The time taken to turn the plants and begin imaging was approximately 55 ± 10 s. As a control, other roots were imaged in the vertical orientation for 20 min. Ten replicates were taken of root columella cells within each tier for both the gravistimulated and control treatments.
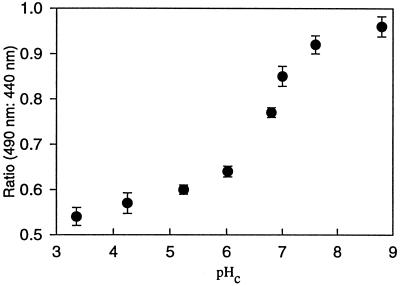
In vitro calibrations were unsatisfactory for determining pHc within Arabidopsis root columella cells because ratios obtained from cells did not lie on standard curves. Therefore, in situ standard curves were calculated using high K+/nigericin for a range of pH values with appropriate buffers (Vercesi et al., 1994). In situ calibrations had a fair degree of variability, therefore, calibrations were made following each experiment (Fig. 3). Although the observed pHc changes were reproducible, the presented absolute values for pHc may not be fully accurate, especially at pHc values away from the estimated pKa of BCECF (6.8).
Figure 3.
In situ calibrations for BCECF within Arabidopsis root columella cells showed a pH-dependent increase in ratio when the fluorescent emission using 490 nm of excitation (pH-dependent) was ratioed against emission with 440 nm excitation. Reliable pHc values may be obtained with BCECF between 5.5 and 8.0, since the pKa of BCECF was estimated at 6.8, which is close to the published value of 6.98.
RESULTS
pHc Modifiers Alter Gravitropic Bending but Not Growth
To determine if a change in pHc of columella cells could influence the gravitropic response, we measured the effects of weak acids and bases and bafilomycin A1 on the gravitropic bending response (Fig. 4), root growth, and pHc in tiers 2 and 3 of the root cap (Table I). When applied solely to the root cap and not the site of differential root growth, modifiers of pHc did not significantly affect root growth. However, all treatments apart from 0.3 μm bafilomycin A1 significantly altered the pHc of the inner columella cells (Table I). The addition of 100 μm benzoic acid had the greatest effect on pHc, decreasing the pH of the columella cells by 1.6, while the smallest effect was caused by 0.3 μm bafilomycin A1, which caused a nonsignificant decrease in pHc of 0.12 (Table I).
Figure 4.
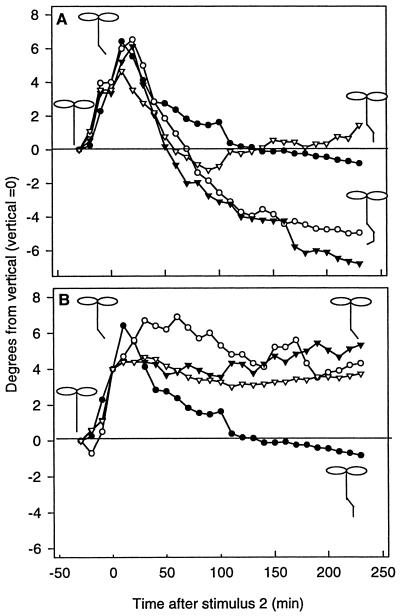
A, Results of modifier experiments showing that alkalinization of the root tip causes disruption of the signaling pathway. ●, Control; ○, 100 μm benzoic acid; ▾, 1.25 μm bafilomycin A1; ▿, 0.3 μm bafilomycin A1. B, Results of modifier experiments showing that acidification of the root tip causes enhancement of the signaling pathway. ●, Control; ○, 50 μm procaine; ▾, 500 μm methylamine; ▿, 100 μm procaine. Diagrams of Arabidopsis seedlings demonstrating the direction of roots bending at various times during the experiment. Stimulus 2 was applied at time = 0. Experiments are averages of 20 separate roots; for clarity, ses are not shown, but are approximately ±2 degrees of curvature for each point.
Table I.
pH modifiers alter the pHc of tier 2 and 3 root columella cells of Arabidopsis without affecting root growth
| Treatment | pHc | Statistical Significance | Root Growth | Statistical Significance |
|---|---|---|---|---|
| μm | ||||
| Control medium | 7.20 ± 0.02 | 582 ± 72 | ||
| 0.3 μm Bafilomycin A1 | 7.10 ± 0.04 | NS | 475 ± 97 | NS |
| 1.3 μm Bafilomycin A1 | 6.75 ± 0.02 | S | 431 ± 39 | NS |
| 100 μm Benzoic Acid | 5.52 ± 0.03 | S | 580 ± 57 | NS |
| 50 μm Procaine | 7.63 ± 0.08 | S | 640 ± 66 | NS |
| 100 μm Procaine | 7.65 ± 0.08 | S | 540 ± 131 | NS |
| 500 μm Methylamine | 8.12 ± 0.04 | S | 466 ± 63 | NS |
The average pHc from separate roots after a control treatment was 7.20 ± 0.02 (n = 5). Control or pH modifying solutions were applied solely to the root cap. Root growth was measured over 5 h and pHc of the same roots was measured in tier 2 and tier 3 columella cells. Data are means ± se (n = 5 for pHc data, n = 20 for growth data). A t test (95% confidence interval) was used to compare experimental and control values. NS, Not significant.
While pH modifiers did not affect net root growth, they did modify the gravitropic signaling pathway, as observed by changes in the differential growth response (Fig. 4). Modifier studies were designed to detect changes in the signaling pathway by applying modifiers to the region responsible for sensing after stimulus 1 and before stimulus 2 (Fig. 2). If the roots were able to signal a response to stimulus 1 and stimulus 2 (if the modifiers had no effect on signaling), a normal bending response would be observed. If the modifier blocked the signal for the second stimulus, the roots would continue to respond to stimulus 1. Alternatively, if the modifier enhanced signaling, the roots would grow in response to stimulus 1 and stimulus 2 and possibly over-respond to stimulus 2.
In the presence of both control solutions and pH modifiers, roots curved more than 4° within 30 min of gravistimulation. Agents that acidified the cytoplasm induced continuous curvature, indicating an enhancement of the signal (Fig. 4A). While low concentrations of bafilomycin A1 (0.3 μm) did not affect curvature and did not induce a significant change in pHc, the application of bafilomycin A1 at 1.25 μm, a concentration known to inhibit the V-ATPase (Calvert and Sanders, 1995), cytoplasmic acidification and enhanced curvature was observed.
Disruption of the signal by weak bases resulted in root growth in the orientation established prior to application of the bases (Fig. 4B). These treatments blocked curvature both in response to the primary and secondary gravity stimulation.
Gravity-Induced Changes in pHc Occur within Inner Columella Cells
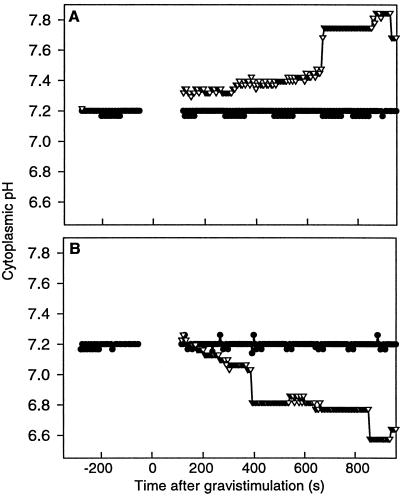
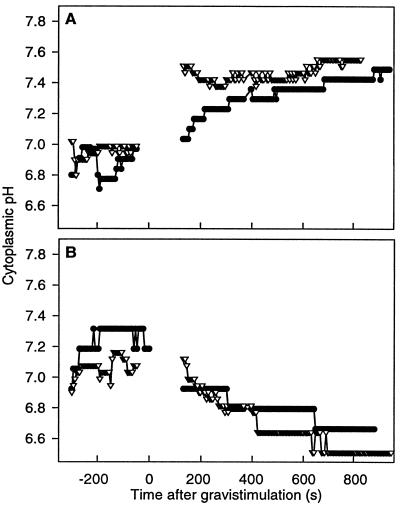
By measuring pHc in individual columella cells, we demonstrated a change in pHc after gravistimulation. The pHc of cells from tier 1 was not measured, since microinjection of these cells is difficult due to their small size and location in the root cap. Cells from tier 4 were not examined because of their limited role in gravitropic sensing (Blancaflor et al., 1998). The resting pHc of tier 2 and 3 cells in vertical roots was similar (7.22 ± 0.03 and 7.23 ± 0.02, respectively) and remained constant over 20 min (n = 20). In plants that were turned horizontally (n = 20), the pHc of columella cells from tier 2 and tier 3 was recorded for 15 min. Following gravistimulation (t = 0 s in Fig. 5), the cells in tier 2 showed an increase in pH to 7.35, with another increase to 7.75 at 690 s. In tier 2 cells, a difference in the onset of alkalinization was seen between the upper and lower cells with respect to gravity. The lower cells in tier 2 became more alkaline 100 s prior to an increase in pHc of the upper cells of tier 2 following plant rotation (Fig. 6A). The upper cells then reach the same pH as the lower cells 100 s following gravistimulation.
Figure 5.
Statistically significant changes in pHc occur in columella cells of Arabidopsis following gravistimulation (t = 0 s). A, Tier 2 cells (n = 10) alkalinize immediately following gravistimulation. ●, Control; ▿, after gravistimulation. B, Tier 3 cells (n = 10) acidify 180 s following gravistimulation. ●, Control; ▿, after gravistimulation.
Figure 6.
A pHc gradient is set up across tier 2 immediately following gravistimulation (t = 0 s), whereas there is no gradient across tier 3. A, Tier 2 shows a delay in the onset of alkalinization of the upper cells (●) compared with the lower cells (▿) by approximately 100 s, establishing a gradient across tier 2. B, The timing of the acidification of tier 3 is similar in upper (●) and lower (▿) cells following gravistimulation. Gravistimulation occurs at t = 0 s (n = 10). Error bars are not shown for clarity, but are approximately ±0.2 pH unit.
The pHc of cells in tier 3 showed a decrease to 6.80, 380 s following gravistimulation, with another drop to 6.60 at 850 s. In tier 2, these changes were apparent at the earliest time points that could be examined after gravistimulation, whereas in tier 3, a statistically significant change in pHc occurred 300 s after gravistimulation (Fig. 5). In tier 3 there appears to be no significant difference in the timing of acidification between the upper and lower cells (Fig. 6B).
DISCUSSION
The two lines of evidence presented here provide strong support for the idea that pHc changes are an early signaling event in Arabidopsis root columella cells. First, observable changes in pHc were seen in a cell-specific manner in tier 2 and 3 columella cells following gravistimulation. Second, experimentally induced changes in pHc of the columella cells modulate the gravitropic response.
pHc as a Second Messenger in Gravity Signaling
The gravity-induced changes that we have observed are comparable to pHc seen in other plant systems in which pHc is used as a second messenger. The onset of a pHc change used for signaling often occurs rapidly and may be transient, such as it is in C4 plant mesophyll cells responding to a dark/light transition (Yin et al., 1993), in leaves of Riccia fluitans in response to 1.0% (v/v) CO2 application (Ballesteros et al., 1998), and in response to light in the alga Eremosphaera viridis (Thaler et al., 1992). pHc changes may also occur as oscillations, such as in maize epidermal cells in response to auxin stimulation (Felle, 1988), or as persistent pHc gradients such as in tip growth of Fucus rhizoids (Gibbon and Kropf, 1994), fungal hyphae (Robson et al., 1996), and pollen tubes (Feijó et al., 1999), where the pH gradient is immediately set up and lasts as long as growth occurs. Both transient and persistent gradients were set up in columella cells following gravistimulation. The gradient across tier 2 occurs immediately following gravistimulation and is transient, with the gradient abolished by 300 s after gravistimulation. A large, persistent gradient between tier 2 and tier 3 occurs immediately following gravistimulation and lasts for at least 15 min.
The direction of pHc changes varies widely in plant systems from acidifications (Katsuhara et al., 1989; Yin et al., 1993; Ballesteros et al., 1998) or alkalinizations (Gehring et al., 1990) to directed pHc gradients that may be more alkaline (Robson et al., 1996) or more acidic (Gibbon et al., 1994; Feijó et al., 1999) toward the tip. The magnitude of pHc changes ranges from 0.05 (Gehring et al., 1990) to 1.4 pH units (Robson et al., 1996). The maximum pH gradient reported here is 1.2 pH units, which occurs between tier 2 and tier 3, 15 min after gravistimulation. The changes seen in columella cells are therefore typical of plant systems in which pHc is used as a second messenger and could be used as a second messenger for root gravitropism.
The Origin of pHc Changes in the Columella
Although the receptor for the gravity signal is still unknown, and we can only speculate as to events that occur before the observed changes in pHc, possible perception mechanisms include sedimentation of amyloplasts (Sack, 1991) or whole protoplast sedimentation (Staves, 1997). Mutants of Arabidopsis lacking statoliths demonstrated a delayed root gravitropic response (Caspar and Pickard, 1989; Sack and Kiss, 1989), which suggests that statolith sedimentation may be only one component of the perception mechanism in Arabidopsis roots. The force of gravity may also be sensed at cellular membranes and/or by cytoskeletal interaction.
A postulated second messenger in the signaling cascade linking perception of gravity to the differential growth response includes [Ca2+]c changes. However, gravity-induced changes, if present, have so far remained undetected (Leagué et al., 1997), possibly because of their small size or location to specific cellular microdomains. Changes in root cap cell membrane potentials following gravistimulation have been reported in the literature (Behrens et al., 1985; Sievers et al., 1995). Following gravistimulation of Lepidium sativum roots, Behrens et al. (1985) found a transient depolarization of statocytes on the lower side of the root, with repolarization occurring within 60 s to a membrane potential slightly more positive than the original resting potential. Statocytes on the upper side showed a slow hyperpolarization following gravistimulation. Sievers et al. (1995), also studying L. sativum roots, found that after gravistimulation there was a transient lowering of the membrane potential on both the upper and lower sides after 64 s. In some cases this was preceded by a transient increase. Although changes in membrane potential in response to gravistimulation are not entirely understood, they may play a role in the signaling as well. The pHc changes observed here may be a component of these electrical potential changes since pHc changes occur at the same time as potential changes and because the H+-ATPase is a key component to generation of membrane potential in plant cells (Briskin, 1990; Assmann and Haubrick, 1996).
However, our results demonstrate that it is not just the plasma membrane that regulates pHc and its involvement in root gravitropism. The enhancement of the gravity signal seen in the presence of bafilomycin A1, an inhibitor of the V-ATPase found in the tonoplast (Fig. 4A), suggests that the V-ATPase might be involved in the signaling pathway. While this result does not demonstrate a role for the V-ATPase in signaling per se, the V-ATPase plays a role in pHc maintenance (Putnam, 1998) and in this way contributes to gravisignaling. As far as we know, the changes in pHc reported here are the earliest potential second messenger seen in response to a gravity signal in Arabidopsis roots.
The Function(s) of Gravity-Induced pHc Changes
Our results show that pHc changes occur in tier 2 and 3 of Arabidopsis columella cells after gravistimulation. The alkalinization of tier 2 may be accomplished via a H+ efflux from the cytosol to the apoplast through the plasma membrane H+-ATPase, which would cause an acidification of the apoplast. This is consistent with preliminary results from E.B. Blancaflor (personal communication) that indicate gravistimulation induces acidification of the apoplast around all 4 tiers of columella. In addition, proton pumping into the vacuole, and/or transport of protons or buffering complexes through plasmodesmata from tier 2 to tier 3 may occur. The acidification of tier 3, which occurs 180 to 300 s after gravistimulation may be achieved through displacement of protons from tier 2 to tier 3. This is a possibility since the magnitude of the pH change in each tier is approximately the same, but in opposite directions, with tier 2 becoming more alkaline. However, the acidification of tier 3 cells could also be accomplished through the release of protons from the vacuole or other organelles or through inhibition of V-ATPases. The acidification of the tier 3 cytoplasm could enhance the activity of the H+-ATPase in the plasma membrane (Brummer et al., 1984), causing the apoplastic acidification seen by E.B. Blancaflor (personal communication).
Monshausen et al. (1996) suggested that there must be continuous signaling between the root cap (the site of perception) and the elongation zone (the area of response) through active maintenance of the resting membrane potential in statocytes due to continuous stimulation of roots, even in the vertical orientation (static stimulation; Sievers et al., 1991). Results of pHc modifier studies agree with this idea and suggest that maintenance of pHc is also involved in this continuous signaling. When the root cap was treated with modifiers that cause an increase in pHc, stimulus 2 did not result in bending. In addition, bending in response to stimulus 1 ceased, indicating that communication between the root cap and the elongation zone was abolished.
Exactly how these pH changes signal differential growth is still unclear. For example, immediately following gravistimulation, cells in the upper side of tier 2 are more acidic than those in the lower side. This may provide positional information to signal a differential growth response. This initial gradient across tier 2 is quickly dissipated, and the gradient between tier 2 and 3 remains for at least 15 min. The later gradient, however, appears to have no positional information and may serve to signal the distal elongation zone to continue the differential growth initiated by the gradient across tier 2. The possible effects of changes in pHc are numerous and may include changes in cytoskeletal tension (Grabski et al., 1994) and/or the activity of enzymes involved in signaling.
Our results imply that the H+ dynamics in the root cap during gravistimulation is highly complex. Other important stores for protons, such as the vacuole, must be further investigated using pH-sensitive dyes or organelle-targeted, pH-sensitive green fluorescent protein (Miesenböck et al., 1998). Since the pHc changes were observed within the columella cells that are necessary for a normal gravitropic response and because they occurred immediately following gravistimulation, we believe these changes occur early on in the signaling pathway and may be one of the first signaling events for root gravitropism. The search for the signaling mechanisms that link the perception of gravity to the observed curvature responses remains a goal of further research in our laboratory. Our present data suggest that changes in pHc are an early signaling event in root gravitropism. However, the generation of this signal and its incorporation into the rest of the signaling pathway needs further investigation.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Elison Blancaflor and Simon Gilroy for the training regarding microinjection of Arabidopsis root columella cells, and Drs. Wendy Boss, David Collings, Eva Johannes, and Gloria Muday for their scientific and editorial input in this paper.
Footnotes
This work was supported by a grant from the National Aeronautics and Space Administration (no. NAGW–4984) and Sigma Xi Grant-in-Aid-of-Research.
LITERATURE CITED
- Allen NS, Bennett MN, Cox DN, Shipley A, Ehrhardt DW, Long SR. Effects of nod factors on alfalfa root hair Ca++ and H+ currents and on cytoskeletal behavior. In: Daniels M, editor. Advances in Molecular Genetics. Vol. 3. Dordrecht, The Netherlands: Kluwer Academics; 1994. pp. 107–113. [Google Scholar]
- Assmann SM, Haubrick LL. Transport proteins of the plant plasma membrane. Curr Opin Cell Biol. 1996;8:458–467. doi: 10.1016/s0955-0674(96)80021-4. [DOI] [PubMed] [Google Scholar]
- Ballesteros D, Garcia-Sanchez MJ, Heredia MA, Felle H, Fernandez JA. Inorganic carbon acquisition of Riccia fluitans L. J Exp Bot. 1998;49:1741–1747. [Google Scholar]
- Beffagna N, Romai G, Meraviglia G, Pallini S. Effects of abscisic acid and cytoplasmic pH on potassium and chloride efflux in Arabidopsis thaliana seedlings. Plant Cell Physiol. 1997;38:503–510. doi: 10.1093/oxfordjournals.pcp.a029197. [DOI] [PubMed] [Google Scholar]
- Behrens HM, Gradmann D, Sievers A. Membrane-potential responses following gravistimulation in roots of Lepidium sativum L. Planta. 1985;163:463–472. doi: 10.1007/BF00392703. [DOI] [PubMed] [Google Scholar]
- Björkman T, Cleland RE. The role of extracellular free-calcium gradients in gravitropic signalling in maize roots. Planta. 1991;185:379–384. [PubMed] [Google Scholar]
- Björkman T, Leopold AC. An electric current associated with gravity sensing in maize roots. Plant Physiol. 1987;84:841–846. doi: 10.1104/pp.84.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JB, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin DP. Ca2+-translocating ATPase of the plant plasma membrane. Plant Physiol. 1990;94:397–400. doi: 10.1104/pp.94.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer B, Felle H, Parish RW. Evidence that acid solutions induce plant cell elongation by acidifying the cytosol and stimulating the proton pump. FEBS Lett. 1984;174:223–227. [Google Scholar]
- Calvert C, Sanders D. Inositol trisphosphate-dependent and -independent Ca2+ mobilization pathways at the vacuolar membrane of Candida albicans. J Biol Chem. 1995;270:7272–7280. doi: 10.1074/jbc.270.13.7272. [DOI] [PubMed] [Google Scholar]
- Caspar T, Pickard B. Gravitropism by a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta. 1989;177:185–197. [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH. Gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, de Curtis I, Pouyssegur J, Griffiths G, Davoust J. Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J Cell Biol. 1989;108:377–387. doi: 10.1083/jcb.108.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The Power of Movement in Plants. D. New York: Appleton; 1896. [Google Scholar]
- Dube F, Dufresne L, Coutu L, Clotteau G. Protein phosphorylation during activation of surf clam oocytes. Dev Biol. 1991;146:473–482. doi: 10.1016/0012-1606(91)90248-2. [DOI] [PubMed] [Google Scholar]
- Edmonds BT, Murray J, Condeelis J. pH regulation of the F-actin binding properties of Dictyostelium elongation factor 1. J Cell Biol. 1995;270:15222–15230. doi: 10.1074/jbc.270.25.15222. [DOI] [PubMed] [Google Scholar]
- Evans ML, Ishikawa H. Cellular specificity of the gravitropic motor response in plants. Planta Suppl. 1997;203:S115–S122. doi: 10.1007/pl00008099. [DOI] [PubMed] [Google Scholar]
- Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK. Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J Cell Biol. 1999;144:483–496. doi: 10.1083/jcb.144.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta. 1988;174:495–499. doi: 10.1007/BF00634478. [DOI] [PubMed] [Google Scholar]
- Felle H. Boss WF, Morré DJ, Second Messengers in Plant Growth and Development. Alan R. New York: Liss; 1989. pH as a second messenger in plants; pp. 145–166. [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Rapid alkalinization in alfalfa root hairs in response to rhizobial lipochitooligosaccharide signals. Plant J. 1996;10:295–301. [Google Scholar]
- Gehring CA, Irving HR, Parish RW. Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci USA. 1990;87:9645–9649. doi: 10.1073/pnas.87.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon BC, Kropf DL. Cytosolic pH gradients associated with tip growth. Science. 1994;263:1419–1421. doi: 10.1126/science.263.5152.1419. [DOI] [PubMed] [Google Scholar]
- Gluck S, Cannon C, Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci USA. 1982;79:4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski S, Xie XG, Holland JF, Schindler M. Lipids trigger changes in the elasticity of the cytoskeleton in plant cells: a cell optical displacement assay for live cell measurements. J Cell Biol. 1994;126:713–726. doi: 10.1083/jcb.126.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J, Mathieu Y, Thomine S, Jouanneau JP, Beloeil JC. Plant cells counteract cytoplasmic pH changes but likely use these pH changes as secondary messages in signal perception. Curr Top Plant Biochem Physiol. 1992;11:249–269. [Google Scholar]
- Ishikawa H, Evans ML. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 1993;102:1203–1210. doi: 10.1104/pp.102.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Hasenstein KH, Evans ML. Computer-based video digitizer analysis of surface extension in maize roots. Planta. 1991;183:381–390. doi: 10.1007/BF00197737. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Kuchitsu K, Takeshige K, Tazawa M. Salt stress-induced cytoplasmic acidification and vacuolar alkalization in Nitellopsis obtusa cells. Plant Physiol. 1989;90:1102–1107. doi: 10.1104/pp.90.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Mulkey TJ, Evans ML. Gravity-induced polar transport of calcium across root tips of maize. Plant Physiol. 1983;73:874–876. doi: 10.1104/pp.73.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–900. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malayev A, Nelson DJ. Extracellular pH modulates the Ca2+ current activated by depletion of intracellular Ca2+ stores in human macrophages. J Membr Biol. 1995;146:101–111. doi: 10.1007/BF00232684. [DOI] [PubMed] [Google Scholar]
- Masson PH. Root gravitropism. BioEssays. 1995;17:119–127. doi: 10.1002/bies.950170207. [DOI] [PubMed] [Google Scholar]
- Miesenböck G, de Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Zieschang HE, Sievers A. Differential proton secretion in the apical elongation zone caused by gravistimulation is induced by a signal from the root cap. Plant Cell Environ. 1996;19:1408–1414. doi: 10.1111/j.1365-3040.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Putnam R. Intracellular pH regulation. In: Sperelakis N, editor. Cell Physiology Source Book. San Diego: Academic Press; 1998. pp. 293–305. [Google Scholar]
- Robson GD, Prebble E, Rickers A, Hosking S, Denning DW, Trinci AP, Robertson W. Polarized growth of fungal hyphae is defined by an alkaline pH gradient. Fungal Gen Biol. 1996;20:289–298. doi: 10.1006/fgbi.1996.0043. [DOI] [PubMed] [Google Scholar]
- Roos W, Evers S, Hieke M, Tschöpe M, Schumann B. Shifts of intracellular pH distribution as a part of signal mechanism leading to the elicitation of benzophenanthridine alkaloids. Plant Physiol. 1998;118:349–364. doi: 10.1104/pp.118.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack F, Kiss J. Rootcap structure in wild type and in a starchless mutant of Arabidopsis. Am J Bot. 1989;76:454–464. [PubMed] [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Selker J, Sievers A. Analysis of extension and curvature during the graviresponse in Lepidium roots. Am J Bot. 1987;74:1863–1871. [Google Scholar]
- Sievers A, Buchen B, Volkmann D, Hejinowicz Z. Role of the cytoskeleton in gravity perception. In: Lloyd C, editor. The Cytoskeletal Basis of Plant Growth and Form. London: Academic Press; 1991. pp. 169–182. [Google Scholar]
- Sievers A, Busch MB. An inhibitor of the Ca2+-ATPase in the sarcoplasmic and endoplasmic reticula inhibits transduction of the gravity stimulus in cress roots. Planta. 1992;188:619–622. doi: 10.1007/BF00197057. [DOI] [PubMed] [Google Scholar]
- Sievers A, Sondag C, Trabacz K, Hejnowicz Z. Gravity induced changes in intracellular potentials in statocytes of cress roots. Planta. 1995;197:392–398. doi: 10.1007/BF00202662. [DOI] [PubMed] [Google Scholar]
- Staves M. Cytoplasmic streaming and gravity sensing in Chara internodal cells. Planta Suppl. 1997;203:S79–S84. doi: 10.1007/pl00008119. [DOI] [PubMed] [Google Scholar]
- Steidl JV, Yool AJ. Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identificaiton of pH sensor. Mol Pharmacol. 1999;55:812–820. [PubMed] [Google Scholar]
- Stinemetz CL, Hasenstein KH, Young LM, Evans ML. Effect of calmodulin antagonists on the growth and graviresponsiveness of primary roots of maize. Plant Growth Regul. 1992;11:419–427. doi: 10.1007/BF00130651. [DOI] [PubMed] [Google Scholar]
- Suprenant K. Unidirectional microtubule assembly in cell-free extracts of Spisula solidissima oocytes is regulated by subtle changes in pH. Cell Motil Cytoskeleton. 1991;19:207–220. doi: 10.1002/cm.970190308. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Jones RL. Gibberellic acid induces vacuolar acidification in barley aleurone. Plant Cell. 1996;8:2211–2221. doi: 10.1105/tpc.8.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper H, Sundler R. Protein-kinase-C and intracellular pH regulate zymosan-induced lysosomal-enzyme secretion in macrophages. J Leukocyte Biol. 1995;58:485–494. doi: 10.1002/jlb.58.4.485. [DOI] [PubMed] [Google Scholar]
- Thaler M, Simonis W, Schonknecht G. Light-dependent changes of the cytoplasmic H+ and Cl− activity in the green alga Eremosphaera viridis. Plant Physiol. 1992;99:103–110. doi: 10.1104/pp.99.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraju M, Sharma K, Liu DN, Shen SH, Srikant CB. Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res. 1999;59:1649–1654. [PubMed] [Google Scholar]
- Vercesi AB, Moreno SNJ, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304:227–233. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin ZH, Heber U, Raghavendra AS. Light-induced pH changes in leaves of C4 plants: comparison of cytosolic alkalinization and vacuolar acidification with that of C3 plants. Planta. 1993;189:267–277. [Google Scholar]
- Yonezawa N, Nishida E, Sakai H. pH control of actin polymerization by cofilin. J Biol Chem. 1985;260:14410–14412. [PubMed] [Google Scholar]
- Zimmermann S, Ehrhardt T, Plesch G, Muller-Rober B. Ion channels in plant signaling. Cell Mol Life Sci. 1999;55:183–203. doi: 10.1007/s000180050284. [DOI] [PMC free article] [PubMed] [Google Scholar]