Abstract
Rationale: Cognitive impairment is common among older adults, yet little is known about the association of pre–intensive care unit cognitive status with outcomes relevant to older adults maintaining independence after a critical illness.
Objectives: To evaluate whether pre–intensive care unit cognitive status is associated with post–intensive care unit disability, new nursing home admission, and mortality after a critical illness among older adults.
Methods: In this prospective cohort study, 754 persons aged 70 years or more were monitored from March 1998 to December 2013 with monthly assessments of disability. Cognitive status was assessed every 18 months, using the Mini-Mental State Examination (range, 0–30), with scores classified as 28 or higher (cognitively intact), 24–27 (minimal impairment), and less than 24 (moderate impairment). The primary outcome was disability count (range, 0–13), assessed monthly over 6 months after an intensive care unit stay. The secondary outcomes were incident nursing home admission and time to death after intensive care unit admission. The analytic sample included 391 intensive care unit admissions.
Results: The mean age was 83.5 years. The prevalence of moderate impairment, minimal impairment, and intact cognition (the comparison group) was 17.3, 46.2, and 36.5%, respectively. In the multivariable analysis, moderate impairment was associated with nearly a 20% increase in disability over the 6-month follow-up period (adjusted relative risk, 1.19; 95% confidence interval, 1.04–1.36), and minimal impairment was associated with a 16% increase in post–intensive care unit disability (adjusted relative risk, 1.16; 95% confidence interval, 1.02–1.32). Moderate impairment was associated with more than double the likelihood of a new nursing home admission (adjusted odds ratio, 2.37; 95% confidence interval, 1.01–5.55). Survival differed significantly across the three cognitive groups (log-rank P = 0.002), but neither moderate impairment (adjusted hazard ratio, 1.19; 95% confidence interval, 0.65–2.19) nor minimal impairment (adjusted hazard ratio, 1.00; 95% confidence interval, 0.61–1.62) was significantly associated with mortality in the multivariable analysis.
Conclusions: Among older adults, any impairment (even minimal) in pre–intensive care unit cognitive status was associated with an increase in post–intensive care unit disability over the 6 months after a critical illness; moderate cognitive impairment doubled the likelihood of a new nursing home admission. Pre–intensive care unit cognitive impairment was not associated with mortality from intensive care unit admission through 6 months of follow-up. Pre–intensive care unit cognitive status may provide prognostic information about the likelihood of older adults maintaining independence after a critical illness.
Keywords: critical illness, intensive care units, aged, cognitive dysfunction, activities of daily living
More than 1 million older adults survive an intensive care unit (ICU) stay in the United States each year (1), and this number is expected to increase as the population ages (2). Many ICU survivors suffer from physical (3, 4), cognitive (5, 6), and mental health impairments (7) after discharge, which can result in disability (8). Disability, defined as dependence in carrying out activities essential to independent living, can have devastating consequences for older adults, as it is associated with increased mortality (9), institutionalization, and greater use of home care services (10).
Pre-ICU vulnerability factors may strongly influence post-ICU outcomes, including disability (4, 11–13). In the United States, as many as 5 million older adults have dementia, and several million more are thought to have milder degrees of cognitive impairment that often go unrecognized by physicians (14, 15), including critical care physicians (16). Despite the high prevalence of cognitive impairment among older adults, little is known about the association of pre-ICU cognitive status with functional outcomes after a critical illness. Because it is difficult to accurately assess cognitive function in critically ill patients, many high-quality studies have approximated premorbid cognitive function using proxy questionnaires (17). To our knowledge, the association of pre-ICU cognitive status with important post-ICU functional outcomes, such as disability and new nursing home admission, has not been previously evaluated. Moreover, understanding whether pre-ICU cognitive status affects subsequent mortality can inform goals of care discussions in the ICU.
To address these knowledge gaps, we used data from a unique longitudinal study that includes objective assessments of cognitive status and monthly assessments of disability over 15+ years. Our objective was to evaluate the relationship between pre-ICU cognitive status and three distinct outcomes over the 6 months after a critical illness: disability, incident nursing home admission at hospital discharge, and all-cause mortality.
Methods
Study Population
Participants were drawn from the Precipitating Events Project (PEP), an ongoing longitudinal study of 754 community-dwelling adults aged 70+ years. The cohort was enrolled between March 1998 and October 1999; complete details have been provided elsewhere (18). The Yale Human Investigation Committee approved the study. All participants provided informed consent.
Data Collection
Comprehensive home-based assessments were completed at baseline and at 18-month intervals. Telephone interviews were completed monthly through June 2014. For participants who had significant cognitive impairment or were unavailable, a proxy informant was interviewed. Deaths were ascertained by review of obituaries and/or from a proxy. A total of 613 (81.3%) participants died after a median of 98 months, and 43 (5.7%) dropped out of the study after a median of 27 months. Data were otherwise available for 99.2% of 81,194 monthly interviews.
Assessment of cognitive status and covariates
Cognitive status was assessed during the comprehensive assessments, using the Mini-Mental State Examination (MMSE) (19), the most commonly used global measure of cognition. Scores were categorized as at least 28 (cognitively intact), 24–27 (minimal impairment), and not more than 24 (moderate impairment) (20, 21). In the absence of a formal clinical evaluation, we chose not to use the term “mild cognitive impairment,” which has specific diagnostic criteria (22). Data were also obtained on demographics, nine chronic conditions (18), and physical capabilities, using a modified Short Physical Performance Battery (23, 24), a validated and widely used measure of lower extremity physical performance among older adults.
Assessment of disability
During the monthly interviews, participants were asked, “At the present time, do you need help from another person [to complete the task]?” for four basic activities (bathing, dressing, walking, transferring), five instrumental activities (shopping, housework, meal preparation, taking medications, managing finances), and three mobility activities (walk a quarter-mile, climb a flight of stairs, lift/carry 10 pounds). Disability was defined as the need for personal assistance or inability to perform the task. Participants were also asked, “Have you driven a car during the past month?” Participants who responded “no” were classified as “disabled” in driving (25). To address the small amount (0.8%) of missing disability data, we used multiple imputation with 100 random draws per missing observation (26).
Assessment of hospitalizations and nursing home admissions
During the monthly interviews, participants were asked whether they had stayed overnight in the hospital since the prior interview. Based on an independent review of hospital records, the accuracy of these reports was high, with a sensitivity of 93.3% (95% confidence interval [CI], 90.5–96.1%) and a specificity of 99.3% (95% CI, 99.0–99.6%) (27). Participants were also asked whether they had been admitted to a nursing home during the prior month. The accuracy of these reports was also high, with a sensitivity of 96.3% (95% CI, 89.2–100%) and a specificity of 100% (95% CI, 88.1–100%) (28).
Ascertainment of intensive care unit admissions and acquisition of intensive care unit data
The majority of ICU admissions were identified through critical care revenue codes using linked Medicare claims data. We included codes for general, specialty, and coronary care units, while excluding psychiatric or intermediate critical care (29). For participants in managed Medicare, information was first obtained on hospitalizations from the monthly interviews. To identify ICU admissions, a medical record review of each hospitalization was then performed (4). For all ICU admissions included in this study, data were obtained about ICU length of stay, mechanical ventilation, and shock (see the online supplement).
Assembly of the analytic samples and outcomes
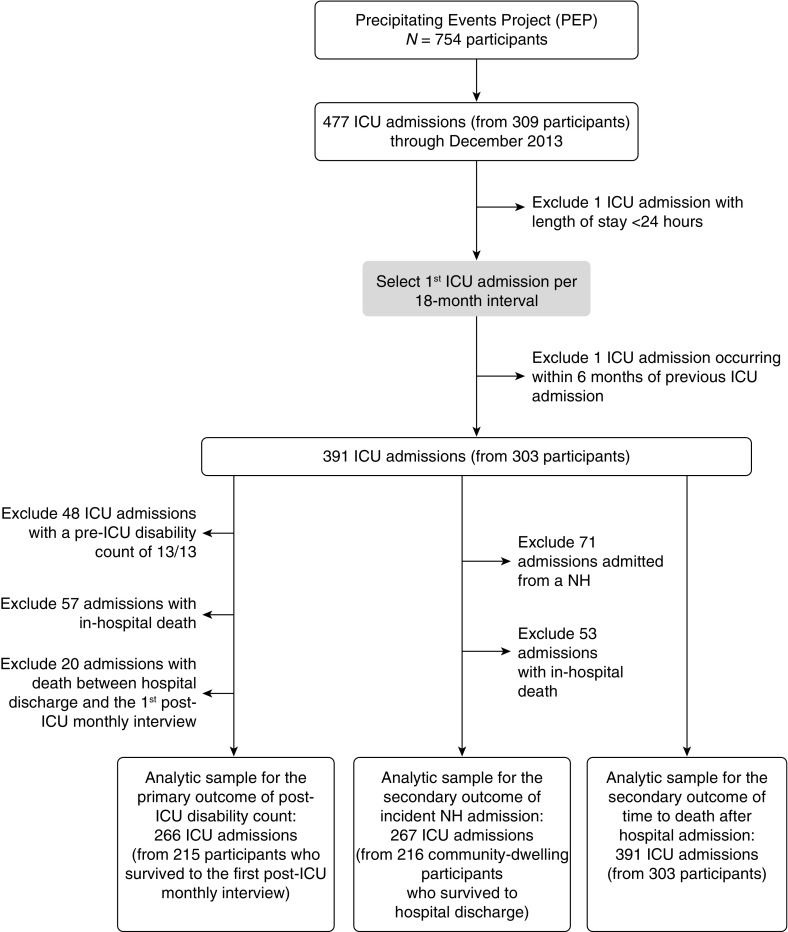
Figure 1 summarizes the assembly of the analytic samples. We considered all ICU admissions from enrollment through December 2013. One admission less than 24 hours was excluded. Because cognitive status was updated during the comprehensive assessments, only the first ICU admission per 18-month interval was included. One additional admission was excluded because we required 6 months or more between admissions occurring in consecutive intervals. Overall, 391 eligible ICU admissions were contributed by 303 participants.
Figure 1.
Assembly of the analytic samples from the parent cohort. All intensive care unit (ICU) admissions from study enrollment through December 2013 were identified. Because cognitive status was updated during the comprehensive assessments, only the first ICU admission per 18-month interval was included, yielding 391 eligible ICU admissions contributed by 303 participants. For the primary outcome of post-ICU disability count, the analytic sample included 266 ICU admissions contributed by 215 participants who survived to the first post-ICU monthly interview. For the secondary outcome of incident nursing home admission, the analytic sample included 267 ICU admissions contributed by 216 participants who were community-living before ICU admission. For the mortality outcome, the analytic sample included all 391 ICU admissions. NH = nursing home; PEP = Precipitating Events Project.
The primary outcome was the disability count (0–13) over the 6 months after an ICU admission. To reduce floor effects, admissions (n = 48) with the maximum disability count of 13/13 in the month before ICU admission were excluded. The resulting analytic sample included 266 ICU admissions contributed by 215 participants who survived to the first post-ICU monthly interview.
The secondary outcome was incident nursing home admission. The analytic sample included 267 ICU admissions contributed by 216 participants who were community-living before admission. Another secondary outcome was time to death in days from hospital admission through 6 months of follow-up. The analytic sample for this outcome included all 391 ICU admissions.
Statistical Analysis
We determined the mean number of disabilities by cognitive status over the 6 months after an ICU admission, with the pre-ICU disability count (from the month before admission) included as a reference point. For descriptive purposes, we categorized the disability scores as mild (<4), moderate (4–7), or severe (≥8).
For the multivariable model, we chose the following covariates a priori based on clinical relevance and prior research (4, 30): age, sex, race, education, number of chronic conditions, disability count in the month before ICU admission, physical capabilities, mechanical ventilation, shock, and ICU length of stay. We determined the adjusted associations between pre-ICU cognitive status and disability count over the six post-ICU months, using a negative-binomial distribution with generalized estimating equations. The model calculated the associations as relative risks (RRs), representing the proportional rise in the average post-ICU disability count corresponding to the level of impaired cognitive status, compared with intact cognition.
We calculated the rate of incident nursing home admission by pre-ICU cognitive status. We evaluated the association between cognitive status and nursing home admission using a multivariable logistic regression model with generalized estimating equations and the aforementioned set of covariates. We plotted survival by pre-ICU cognitive status using the Kaplan–Meier method from admission through 6 months of follow-up and compared the survival curves using the log-rank test. We tested for a mortality trend across the three cognitive groups with a Poisson distribution after graphically verifying linearity. Finally, we evaluated the association between pre-ICU cognitive status and time to death in days with a multivariable Cox proportional hazards regression model with the same covariates noted above.
For the two nonmortality outcomes, we completed a series of sensitivity analyses to assess for potential bias due to the competing risk of death. We imputed the missing outcomes from decedents to test the primary results against a range of hypothetical outcomes. This was the best statistical approach for our outcomes; the more common Fine and Gray approach is most appropriate for time-to-event outcomes (31). For the primary outcome, the disability counts of the 46 decedents who died between Months 1 and 6 of follow-up were imputed as both missing at random (MAR) and not missing at random (NMAR) (32). For the other nonmortality outcome, incident nursing home admissions were also imputed for hospital decedents under the same MAR and NMAR scenarios.
All analyses were performed with SAS (version 9.4; SAS Institute), and P < 0.05 (two-tailed) was used to indicate statistical significance.
Results
Descriptive statistics for the 266 ICU admissions in the primary analysis are presented in Table 1 according to pre-ICU cognitive status. The mean age was 83.5 years, and the prevalence of moderate impairment, minimal impairment, and intact cognition was 17.3, 46.2, and 36.5%, respectively. The markers of ICU severity of illness were comparable across the categories of cognitive status.
Table 1.
Characteristics of intensive care unit admissions contributed by participants who survived to the first post-ICU monthly assessment according to pre–intensive care unit cognitive status (N = 266)*†
| Characteristic | Operational Details | Mean ± SD or n (%) |
||
|---|---|---|---|---|
| Cognitively Intact (n = 97) | Minimal Impairment (n = 123) | Moderate Impairment (n = 46) | ||
| Age, yr | 82.4 ± 4.8 | 83.8 ± 5.7 | 85.1 ± 5.2 | |
| Female sex, n (%) | 54 (55.7) | 76 (61.8) | 26 (56.5) | |
| Non-Hispanic white, n (%) | 93 (95.9) | 102 (82.9) | 40 (87.0) | |
| Education, yr | 13.1 ± 2.5 | 11.6 ± 2.9 | 10.8 ± 2.9 | |
| Number of chronic conditions | Of a possible nine‡ | 2.5 ± 1.3 | 2.5 ± 1.3 | 2.5 ± 1.1 |
| Pre-ICU disability | From month before ICU admission (range, 0–13)§ | 3.2 ± 3.0 | 5.1 ± 3.5 | 6.2 ± 3.7 |
| Cognitive status | Folstein MMSE score (range, 0–30)† | 28.9 ± 0.8 | 25.8 ± 1.0 | 20.8 ± 3.4 |
| Physical capabilities|| | Short Performance Physical Battery (range, 0–12)|| | 6.2 ± 3.1 | 5.4 ± 2.8 | 4.5 ± 2.7 |
| Nursing home resident | In month before ICU admission | 4 (4.1) | 15 (12.2) | 3 (6.5) |
| ICU length of stay,¶ d | 3.0 ± 4.0 | 2.8 ± 4.8 | 2.8 ± 2.3 | |
| Use of mechanical ventilation** | 12 (12.4) | 15 (12.3) | 4 (8.7) | |
| Shock** | 5 (5.2) | 4 (3.3) | 3 (6.5) | |
Definition of abbreviation: ICU = intensive care unit; MMSE = Mini-Mental State Examination; SD = standard deviation.
The 266 ICU admissions were contributed by 215 participants.
Folstein MMSE (20): Scores range from 0 to 30, with higher scores representing better cognitive function. Moderate impairment, minimal impairment, and cognitively intact were defined as MMSE scores less than 24, 24–27, and 28–30, respectively.
Hypertension, myocardial infarction, heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer (other than minor skin cancers).
Disability was assessed monthly in each of 13 functional activities: four activities of daily living (bathing, dressing, walking, and transferring), five instrumental activities (shopping, housework, meal preparation, taking medications, and managing finances), and four mobility activities (walk a quarter-mile, climb a flight of stairs, lift or carry 10 pounds, and drive in the past month).
Short Physical Performance Battery (SPPB) (24): Scores range from 0 to 12, with higher scores indicating better performance on three objectively measured tasks (gait speed, chair stands, and balance), as described in the text.
When data were available from Medicare claims, ICU length of stay was based on the number of days billed in a critical care unit. For participants in managed Medicare, ICU length of stay was abstracted from the medical record.
Ascertained using International Classification of Diseases, Ninth Revision (ICD-9) codes and chart review, as described in the Methods in the online supplement.
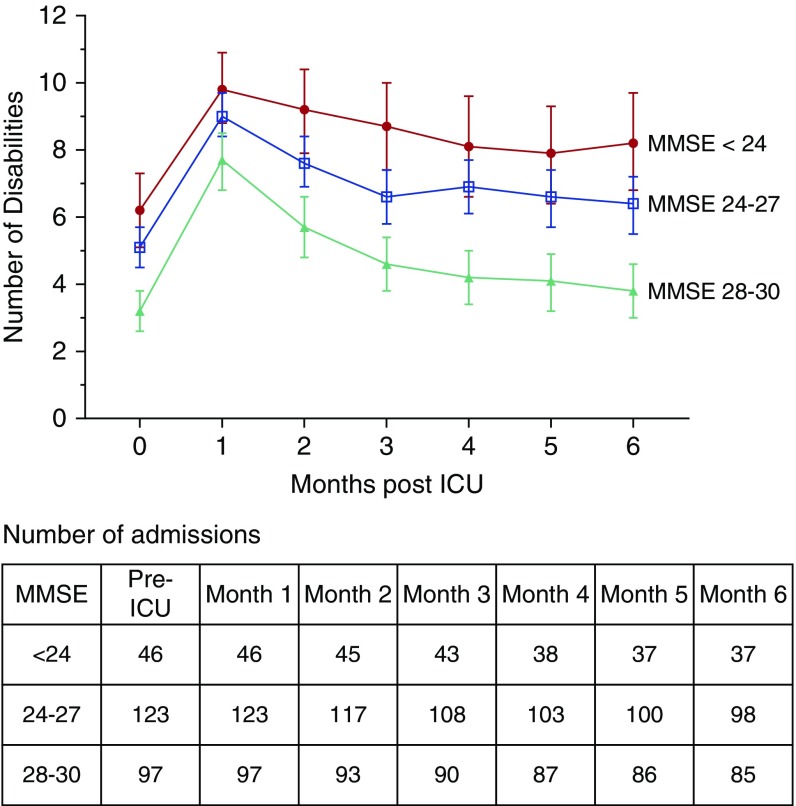
Figure 2 provides the mean number of disabilities over the six post-ICU months by pre-ICU cognitive status, with the pre-ICU disability count as a reference point. Although all three cognitive groups experienced increasing disability in the setting of a critical illness, the moderately impaired group experienced little functional recovery, with persistent severe disability (mean, 8.2) at the end of follow-up. The minimal impairment group experienced modest functional recovery, but remained moderately disabled (mean, 6.4). In contrast, the cognitively intact group experienced near-complete functional recovery, returning to a mean of 3.8 disabilities (median, 2.0) from their pre-ICU mean of 3.2 disabilities (median, 2.0). In the multivariable analysis, moderate impairment was associated with nearly a 20% increase in the average disability count over the 6 months after a critical illness (RR, 1.19; 95% CI, 1.04–1.36), referent to the cognitively intact group. The corresponding value for minimal impairment was a 16% increase (RR, 1.16; 95% CI, 1.02–1.32). In the sensitivity analysis, the associations between pre-ICU cognitive status and post-ICU disability remained robust to the competing risk of death (Table E1 in the online supplement).
Figure 2.
Disability count by pre–intensive care unit (ICU) cognitive status over 6 months of follow-up (N = 266). The 266 ICU admissions were contributed by 215 participants who survived to their first post-ICU monthly interview. The disability counts range from 0 to 13. As a reference point, the pre-ICU disability count (from the month before ICU admission) is included at Month 0. The table presents the number of observations contributed by participants alive at each month of follow-up by pre-ICU cognitive status. There was no attrition for reasons other than death. MMSE = Mini-Mental State Examination.
Table 2 provides descriptive statistics for the secondary analyses. The rate of incident nursing home admission increased with worsening pre-ICU cognitive status: intact, 35.1%; minimal impairment, 46.2%; moderate impairment, 62.7%. In the multivariable analysis, pre-ICU moderate impairment was associated with more than double the odds of new discharge to a nursing home after a critical illness (odds ratio, 2.37; 95% CI, 1.01–5.55). Minimal impairment was not significantly associated with incident nursing home admission (odds ratio, 1.61; 95% CI, 0.87–3.00). In the sensitivity analysis (Table E2), the associations remained robust to the competing risk of death.
Table 2.
Characteristics of intensive care unit admissions contributed by participants eligible for the secondary outcomes according to pre-ICU cognitive status
| Characteristic | Incident NH Admission (N = 267)* |
All-Cause Mortality (N = 391)† |
||||
|---|---|---|---|---|---|---|
| Cognitively Intact (n = 97) | Minimal Impairment (n = 119) | Moderate Impairment (n = 51) | Cognitively Intact (n = 127) | Minimal Impairment (n = 172) | Moderate Impairment (n = 92) | |
| Age, yr | 82.3 (±4.9) | 83.5 (±5.5) | 85.8 (±5.5) | 82.7 (±4.9) | 84.5 (±5.6) | 86.5 (±5.5) |
| Female sex | 53 (54.6) | 77 (64.7) | 28 (54.9) | 71 (55.9) | 107 (62.2) | 53 (57.6) |
| Non-Hispanic white | 91 (93.8) | 99 (83.2) | 45 (88.2) | 119 (93.7) | 148 (86.1) | 76 (82.6) |
| Education, yr | 13.1 (±2.5) | 11.7 (±3.0) | 10.7 (±2.9) | 13.1 (±2.6) | 11.8 (±3.0) | 10.5 (±3.1) |
| Cognitive function by MMSE score‡ | 28.9 (0.8) | 25.8 (±1.0) | 20.7 (±3.3) | 28.9 (0.7) | 25.8 (±1.1) | 18.5 (±5.0) |
| Chronic conditions§ | 2.4 (±1.3) | 2.5 (±1.3) | 2.5 (±1.1) | 2.4 (±1.3) | 2.6 (±1.3) | 2.5 (±1.3) |
| Pre-ICU disability|| | 2.9 (±2.9) | 4.7 (±3.4) | 6.5 (±3.9) | 4.1 (±3.7) | 6.0 (±4.1) | 8.7 (±4.1) |
| Physical capabilities¶ | 6.4 (±3.1) | 5.6 (±2.7) | 4.4 (±2.7) | 5.8 (±3.2)) | 5.1 (±2.9) | 3.1 (±2.8) |
| Nursing home resident in the month before ICU admission | NA | NA | NA | 11 (8.7) | 33 (19.2) | 27 (29.4) |
| ICU length of stay** | 3.5 (±5.8) | 3.1 (±4.9) | 2.7 (±2.1) | 4.1 (±6.4) | 3.9 (±5.7) | 3.2 (±3.0) |
| Use of mechanical ventilation†† | 14 (14.4) | 16 (13.6) | 4 (7.8) | 28 (22.1) | 42 (24.7) | 23 (25.0) |
| Shock†† | 6 (6.2) | 3 (2.5) | 4 (7.8) | 15 (11.8) | 15 (8.7) | 12 (13.0) |
Definition of abbreviations: ICU = intensive care unit; MMSE = Mini-Mental State Examination; NA = not applicable; NH = nursing home.
The 267 ICU admissions were contributed by 216 participants. Only ICU admissions where the participant was community-dwelling before hospital admission and survived to hospital discharge were eligible for the outcome of incident nursing home admission.
The 391 ICU admissions were contributed by 303 participants. Because the ICU admission date was not available in Medicare claims, the hospital admission date was used as a proxy.
Measured with the Folstein Mini-Mental State Exam (MMSE) (20): Scores range from 0 to 30, with higher scores representing better cognitive function. Moderate impairment, minimal impairment, and cognitively intact were defined as MMSE scores of less than 24, 24–27, and 28–30, respectively.
Of a possible nine: hypertension, myocardial infarction, heart failure, stroke, diabetes mellitus, arthritis, hip fracture, chronic lung disease, and cancer (other than minor skin cancers).
From the month before ICU admission, assessed in each of 13 functional activities: four activities of daily living (bathing, dressing, walking, and transferring), five instrumental activities (shopping, housework, meal preparation, taking medications, and managing finances), and four mobility activities (walk a quarter-mile, climb a flight of stairs, lift or carry 10 pounds, and drive in the past month).
Short Physical Performance Battery (SPPB) (24): Scores range from 0 to 12, with higher scores indicating better performance on three objectively measured tasks (gait speed, chair stands, and balance), as described in the text.
When data were available from Medicare claims, ICU length of stay was based on the number of days billed in a critical care unit. For participants in managed Medicare, ICU length of stay was abstracted from the medical record.
Ascertained using ICD-9 codes and chart review, as described in Methods in the online supplement.
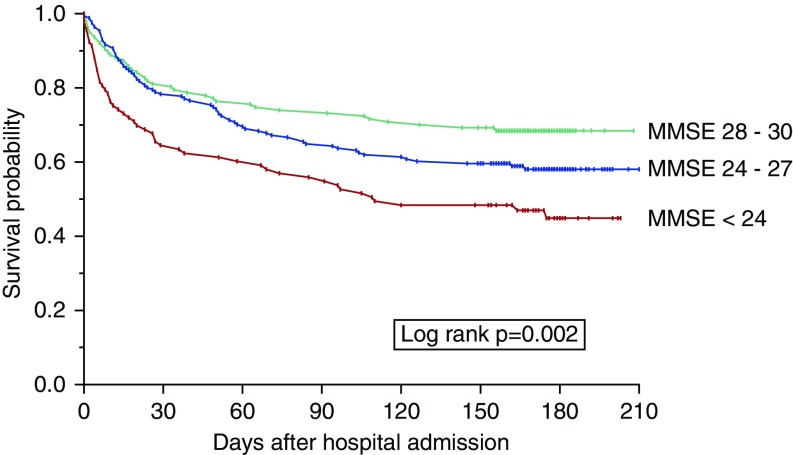
Survival by pre-ICU cognitive status is provided in Figure 3. The log-rank test demonstrated a statistically significant survival difference across the three cognitive groups (P = 0.002). Mortality rose with worsening cognitive status: cognitively intact, 31.5%; minimal impairment, 41.5%; moderate impairment, 53.8% (P < 0.001 for trend). However, in the multivariable analysis, compared with the cognitively intact group, neither moderate impairment (hazard ratio, 1.19; 95% CI, 0.65–2.19) nor minimal impairment (hazard ratio, 1.00; 95% CI, 0.61–1.62) was significantly associated with mortality.
Figure 3.
Kaplan–Meier graph demonstrating survival by MMSE group from admission through 6 months of posthospital follow-up (N = 391). Group sizes were as follows: cognitively intact, n = 127 (32.5%); minimal impairment, n = 172 (44.0%); and moderate impairment, n = 92 (23.5%). Because the intensive care unit admission date was not available in Medicare claims, the hospital admission date was used as a proxy. Follow-up extended to 210 days because each ICU admission was nested within a hospitalization, which sometimes extended beyond 30 days. MMSE = Mini-Mental State Examination.
Discussion
We found that any impairment in pre-ICU cognitive status was independently associated with greater disability over the 6 months after a critical illness, and that the relative increase in post-ICU disability was comparable between those with minimal and moderate cognitive impairment. Relative to intact cognition, moderate impairment was associated with more than double the likelihood of a new nursing home admission at hospital discharge. However, pre-ICU cognitive impairment was not associated with mortality from ICU admission through 6 months of follow-up. These findings provide new information about the association of pre-ICU cognitive status with post-ICU disability, incident nursing home admission, and mortality among critically ill older adults.
Outside of the ICU, cognitive impairment has been associated with diminished disability-free life expectancy (33) and incident ADL disability (34). In these longitudinal studies, increasingly severe cognitive impairment is generally associated with greater disability (35). Our study adds to this literature by evaluating, for the first time, the association of pre-ICU cognitive impairment and subsequent disability in a cohort of critically ill older adults. Some of our findings differed from those of the earlier non-ICU studies. For example, the association between cognitive impairment and post-ICU disability in our study was relatively modest, and the effect sizes of mild and moderate cognitive impairment were comparable. This may be partly due to selection effects, as the distribution of MMSE scores suggests that the most severely cognitively impaired older adults may not have been admitted to the ICU.
Older adults currently represent the majority of patients admitted to ICUs in the United States (2, 36). With the aging of the population, the number of persons with cognitive impairment has been increasing (15); by 2050, between 11 and 18.5 million adults will have some degree of cognitive impairment in the United States (14). Hence, the number of older adults presenting to the ICU with preexisting cognitive impairment will only increase. We did not find an association between cognitive status and mortality, indicating that these patients will join the growing ranks of ICU survivors. Our finding that pre-ICU cognitive impairment was associated with increased post-ICU disability and new nursing home admission—two outcomes strongly linked to a loss of independence among older adults—has implications for the burden of post-ICU care for patients, caregivers, and society.
To illustrate, let us consider an average patient in the moderate impairment group. An 84-year-old woman is admitted to the ICU. She had been living in her own home, with assistance from family for six activities (managing finances, shopping, driving, meal preparation, lifting 10 pounds, and housework). After her ICU admission, she becomes dependent in three additional activities: walking a quarter-mile, climbing a flight of stairs, and bathing. She is therefore discharged to a nursing home from the hospital for rehabilitation. Over the subsequent months, she has some functional recovery, but remains disabled in more than seven activities 6 months later. This magnitude of disability would have consequences for independent living, with (at best) greater dependence on formal home services and informal caregivers, and possibly, institutionalization.
In a study of health outcome prioritization, “maintaining independence” was ranked as the most important health outcome by a majority (76%) of older persons, with staying alive ranked a distant second (37). Because a patient’s values and preferences are the foundation of shared decision-making in the ICU (38), understanding that a loss of functional independence is more likely in those with cognitive impairment may help inform conversations about goals of care and treatment decisions. Moreover, understanding the expected increase in post-ICU disability and potential implications for independence may inform advance care planning discussions with cognitively impaired older patients and their families in the outpatient setting.
For older adults whose treatment preferences include ICU admission, providers can consider steps that may reduce the likelihood of adverse functional outcomes. First, providers can try to identify preexisting cognitive impairment, which is often underrecognized in older ICU patients (16). Prior research has demonstrated that it is feasible to detect cognitive impairment in the ICU, using validated tools administered to proxy informants (39). Although these tools may not provide a precise assessment of cognitive function, our results suggest that ascertaining any degree of cognitive impairment may be informative, given the comparable effect sizes of minimal and moderate cognitive impairment. Second, efforts to reduce the incidence of ICU delirium are particularly important in this population. Dementia is a strong predictor of ICU delirium (40), and delirium is associated with a host of adverse outcomes, including disability (5, 41, 42). Third, older ICU patients with cognitive impairment should be included in rehabilitative programs, including early ICU mobilization and postacute programs. Rehabilitation programs can be effectively implemented among cognitively impaired individuals in postacute care settings, as demonstrated in a systematic review of patients with hip fracture (43). Developing and testing rehabilitation approaches for ICU survivors across the spectrum of cognitive impairment should be a focus of future research.
The major strength of our study is its prospective design, allowing for objective assessments of cognitive status using the MMSE before a critical illness. In addition, the monthly assessments of functional status allowed us to rigorously evaluate the course of disability surrounding a critical illness. Additional strengths include minimal attrition for reasons other than death, the inclusion of managed Medicare patients, and an analytic strategy that assessed for the competing risk of death.
Our study has several limitations. First, the proportion of the sample with moderate impairment was lower than expected given the age of the population, suggesting that some critically ill patients with more severe cognitive impairment may not have been admitted to the ICU. This would have attenuated the magnitude of post-ICU disability seen in the moderate impairment group. Second, we did not have information about limitations on life-sustaining treatment, which may have varied by cognitive status. Third, it is possible that cognitive status may have changed between the prior assessment and ICU admission, although it would be unlikely to improve. Finally, because participants were drawn from one geographic region, our results may not be generalizable to other settings. However, the demographics of our cohort reflect those of older adults in greater New Haven County, which are similar to the U.S. population with the exception of race (44).
Conclusions
Preexisting cognitive impairment, whether mild or moderate, is independently associated with the course of disability after a critical illness, and moderate cognitive impairment is associated with new nursing home admission among previously community-living older adults; however, premorbid cognitive status is not associated with mortality from ICU admission through the subsequent 6 months. Pre-ICU cognitive status may provide important prognostic information about the likelihood of older adults maintaining their functional independence after a critical illness.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Andrea Benjamin, B.S.N., for assistance with data collection; Wanda Carr and Geraldine Hawthorne, B.S., for assistance with data entry and management; Peter Charpentier, M.P.H., for design and development of the study database and participant tracking system; and Joanne McGloin, M.Div., M.B.A., for leadership and advice as the project director.
Footnotes
Supported by a Paul B. Beeson Emerging Leaders Career Development Award in Aging from the National Institutes of Health/National Institute on Aging (NIH/NIA) (K76AG057023; L.E.F.), a GEMSSTAR award from the NIH/NIA (R03AG050874), a Pepper Scholar Award from the Yale Claude D. Pepper Older Americans Independence Center (NIH/NIA; P30AG021342), and a T. Franklin Williams Scholar Award, with funding provided by Atlantic Philanthropies, Inc., the John A. Hartford Foundation, the Alliance for Academic Internal Medicine-Association of Specialty Professors, and the American Thoracic Society Foundation. The PEP Study is supported by a grant from the NIA (R01AG017560). T.M.G. is the recipient of an Academic Leadership Award (K07AG043587) from the NIA. M.A.P. is funded by the Donaghue Foundation.
Author Contributions: Study concept and design: L.E.F., T.M.G.; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: L.E.F., M.A.P., T.E.M.; critical revision of the manuscript for important intellectual content: L.E.F., T.M.G., M.A.P.; statistical analysis: T.E.M., E.A.G.; obtained funding: L.E.F., T.M.G.; administrative, technical, or material support: E.A.G., L.S.L.-S.; study supervision: T.M.G., L.E.F., and T.M.G. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 3.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183:1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Int Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlenbach WJ, Hough CL, Crane PK, Haneuse S, Carson SS, Curtis JR, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303:763–770. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bienvenu OJ, Gellar J, Althouse BM, Colantuoni E, Sricharoenchai T, Mendez-Tellez PA, et al. Post-traumatic stress disorder symptoms after acute lung injury: a 2-year prospective longitudinal study. Psychol Med. 2013;43:2657–2671. doi: 10.1017/S0033291713000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummel NE, Balas MC, Morandi A, Ferrante LE, Gill TM, Ely EW. Understanding and reducing disability in older adults following critical illness. Crit Care Med. 2015;43:1265–1275. doi: 10.1097/CCM.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill TM, Robison JT, Tinetti ME. Difficulty and dependence: two components of the disability continuum among community-living older persons. Ann Intern Med. 1998;128:96–101. doi: 10.7326/0003-4819-128-2-199801150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kemper P. The use of formal and informal home care by the disabled elderly. Health Serv Res. 1992;27:421–451. [PMC free article] [PubMed] [Google Scholar]
- 11.Haas B, Wunsch H. How does prior health status (age, comorbidities and frailty) determine critical illness and outcome? Curr Opin Crit Care. 2016;22:500–505. doi: 10.1097/MCC.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 12.Cuthbertson BH, Wunsch H. Long-term outcomes after critical illness: the best predictor of the future is the past. Am J Respir Crit Care Med. 2016;194:132–134. doi: 10.1164/rccm.201602-0257ED. [DOI] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holsinger T, Deveau J, Boustani M, Williams JW. Does this patient have dementia? JAMA. 2007;297:2391–2404. doi: 10.1001/jama.297.21.2391. [DOI] [PubMed] [Google Scholar]
- 15.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisani MA, Redlich C, McNicoll L, Ely EW, Inouye SK. Underrecognition of preexisting cognitive impairment by physicians in older ICU patients. Chest. 2003;124:2267–2274. doi: 10.1378/chest.124.6.2267. [DOI] [PubMed] [Google Scholar]
- 17.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: practical method for grading cognitive state of patients for clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Saczynski JS, Inouye SK, Guess J, Jones RN, Fong TG, Nemeth E, et al. The Montreal Cognitive Assessment: creating a crosswalk with the Mini-Mental State Examination. J Am Geriatr Soc. 2015;63:2370–2374. doi: 10.1111/jgs.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spering CC, Hobson V, Lucas JA, Menon CV, Hall JR, O’Bryant SE. Diagnostic accuracy of the MMSE in detecting probable and possible Alzheimer’s disease in ethnically diverse highly educated individuals: an analysis of the NACC Database. J Gerontol Ser A Biol Sci Med Sci. 2012;67:890–896. doi: 10.1093/gerona/gls006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower-extremity function: association with self-reported disability and prediction of mortality and nursing-home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.Gill TM, Gahbauer EA, Han L, Allore HG. Factors associated with recovery of prehospital function among older persons admitted to a nursing home with disability after an acute hospitalization. J Gerontol Ser A Biol Sci Med Sci. 2009;64:1296–1303. doi: 10.1093/gerona/glp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156:131–140. doi: 10.1059/0003-4819-156-2-201201170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill TM, Guo Z, Allore HG. Subtypes of disability in older persons over the course of nearly 8 years. J Am Geriatr Soc. 2008;56:436–443. doi: 10.1111/j.1532-5415.2007.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill TM, Allore H, Holford TR, Guo ZC. The development of insidious disability in activities of daily living among community-living older persons. Am J Med. 2004;117:484–491. doi: 10.1016/j.amjmed.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Gill TM, Murphy TE, Gahbauer EA, Allore HG. Association of injurious falls with disability outcomes and nursing home admissions in community-living older persons. Am J Epidemiol. 2013;178:418–425. doi: 10.1093/aje/kws554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors associated with functional recovery among older intensive care unit survivors. Am J Respir Crit Care Med. 2016;194:299–307. doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 32.Little RJA, Rubin DB. Wiley series in probability and statistics: statistical analysis with missing data, 2nd ed. New York: Wiley-Interscience; 2002. [Google Scholar]
- 33.Jagger C, Matthews R, Matthews F, Robinson T, Robine JM, Brayne C, et al. The burden of diseases on disability-free life expectancy in later life. J Gerontol Ser A Biol Sci Med Sci. 2007;62:408–414. doi: 10.1093/gerona/62.4.408. [DOI] [PubMed] [Google Scholar]
- 34.Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, Cohen HJ. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc. 2007;55:885–891. doi: 10.1111/j.1532-5415.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 35.Gure TR, Langa KM, Fisher GG, Piette JD, Plassman BL. Functional limitations in older adults who have cognitive impairment without dementia. J Geriatr Psychiatry Neurol. 2013;26:78–85. doi: 10.1177/0891988713481264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullins PM, Goyal M, Pines JM. National growth in intensive care unit admissions from emergency departments in the United States from 2002 to 2009. Acad Emerg Med. 2013;20:479–486. doi: 10.1111/acem.12134. [DOI] [PubMed] [Google Scholar]
- 37.Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171:1854–1856. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kon AA, Davidson JE, Morrison W, Danis M, White DB. Shared decision-making in intensive care units: executive summary of the American College of Critical Care Medicine and American Thoracic Society: policy statement. Am J Respir Crit Care Med. 2016;193:1334–1336. doi: 10.1164/rccm.201602-0269ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisani MA, Inouye SK, McNicoll L, Redlich CA. Screening for preexisting cognitive impairment in older intensive care unit patients: use of proxy assessment. J Am Geriatr Soc. 2003;51:689–693. doi: 10.1034/j.1600-0579.2003.00215.x. [DOI] [PubMed] [Google Scholar]
- 40.Pisani MA, Murphy TE, Van Ness PH, Araujo KLB, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167:1629–1634. doi: 10.1001/archinte.167.15.1629. [DOI] [PubMed] [Google Scholar]
- 41.Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42:369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KLB, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Resnick B, Beaupre L, McGilton KS, Galik E, Liu W, Neuman MD, et al. Rehabilitation interventions for older individuals with cognitive impairment post-hip fracture: a systematic review. J Am Med Dir Assoc. 2016;17:200–205. doi: 10.1016/j.jamda.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gill TM, Allore HG, Holford TR, Guo ZC. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.