Abstract
In a recent study of N-acylphosphatidylethanolamine (NAPE) metabolism in elicitor-treated tobacco (Nicotiana tabacum L.) cells, we identified a rapid release and accumulation of medium-chain N-acylethanolamines (NAEs) (e.g. N-myristoylethanolamine or NAE 14:0) and a compensatory decrease in cellular NAPE (K.D. Chapman, S. Tripathy, B. Venables, A.D. Desouza [1998] Plant Physiol 116: 1163–1168). In the present study, we extend this observation and report a 10- to 50-fold increase in NAE 14:0 content in leaves of tobacco (cv Xanthi) plants treated with xylanase or cryptogein elicitors. Exogenously supplied synthetic NAE species affected characteristic elicitor-induced and short- and long-term defense responses in cell suspensions of tobacco and long-term defense responses in leaves of intact tobacco plants. In general, synthetic NAEs inhibited elicitor-induced medium alkalinization by tobacco cells in a time- and concentration-dependent manner. Exogenous NAE 14:0 induced expression of phenylalanine ammonia lyase in a manner similar to fungal elicitors in both cell suspensions and leaves of tobacco. NAE 14:0, but not myristic acid, activated phenylalanine ammonia lyase expression at submicromolar concentrations, well within the range of NAE 14:0 levels measured in elicitor-treated plants. Collectively, these results suggest that NAPE metabolism, specifically, the accumulation of NAE 14:0, are part of a signal transduction pathway that modulates cellular defense responses following the perception of fungal elicitors.
Several physiological studies of plant-pathogen interactions have established elicitor recognition as the initial source of signal(s) leading to incompatibility and activation of defense mechanisms (Lamb et al., 1989; Dixon and Lamb, 1990; Atkinson, 1993; Dixon et al., 1994; Boller, 1995). Elicitors are pathogen-derived compounds such as oligosaccharides, glycopeptides/glycoproteins, peptides/proteins, and/or lipids that trigger multiple defense responses (Ebel and Scheel, 1992). The earliest responses of cells in perception of elicitors include the activation of Ca2+ influx and K+/H+ exchange at the plasma membrane (Atkinson et al., 1985, 1990; Baker et al., 1991). These rapid changes in ion flux are followed by the oxidative burst (Adam et al., 1989; Keppler et al., 1989; Mehdy, 1994; Levine et al., 1994; Lamb and Dixon, 1997; Alvarez et al., 1998) and alteration of the phosphorylation status of proteins (Grab et al., 1989; Dietrich et al., 1990; Felix et al., 1991, 1993; Suzuki and Shinshi, 1995; Xing et al., 1996; Adam et al., 1997). These early events constitute a signaling pathway that leads to transcriptional activation of defense gene expression (Cramer et al., 1985; Lawton and Lamb, 1987; Suzuki et al., 1995; Jabs et al., 1997; Chamnongpol et al., 1998).
Among the best-characterized of plant defense genes are those encoding phenylalanine ammonia lyase (PAL), a key regulatory enzyme in phenylpropanoid metabolism (Lamb et al., 1989; Bowles, 1990; Dixon and Paiva, 1995). Several endogenous transmittable signals, such as salicylic acid, systemin, jasmonic acid, and ethylene, that are increased in plant cells following elicitor treatment or pathogen attack, can induce PAL expression (for review, see Enyedi et al., 1992) as part of a localized host defense-response. Recently, a pathogen-induced NO-signaling pathway was identified in plants (Delledonne et al., 1998), that involves production of cGMP and cADP-Rib as second messengers (Durner et al., 1998), and this pathway appears to selectively activate PAL expression.
Over the past several years a number of studies (Anderson et al., 1990; Lotan and Fluhr, 1990; Felix et al., 1993; Moreau et al., 1994) have characterized the multiple cellular responses of tobacco (Nicotiana tabacum L.) to xylanase (an elicitor protein from Trichoderma viride, Dean et al., 1989), including Ca2+ influx, K+/H+ exchange, induction of ethylene biosynthesis, production of phytoalexins, synthesis of pathogenesis-related proteins, and changes in membrane lipid composition. A plasma membrane receptor for xylanase was recently identified on tobacco cells (Hanania and Avni, 1997), and sensitivity to xylanase was linked to a single gene trait in tobacco (Bailey et al., 1993). In most cases these cellular responses of tobacco to xylanase are characteristic of resistant interactions in host plants.
In the present study we explore at the cellular and intact plant levels the physiological role of medium-chain N-acylethanolamine (NAE) accumulation with respect to defense-related signal transduction pathways in tobacco. In two earlier studies, N-acylphosphatidylethanolamine (NAPE) metabolism in tobacco cell suspensions was activated by the addition of xylanase. NAPE biosynthesis increased 1 to 2 h after xylanase treatment and this was preceded by a rapid hydrolysis of NAPE (Chapman et al., 1995, 1998). A phospholipase D (PLD)-type activity was identified in tobacco membranes that hydrolyzed NAPE to NAE in vitro, and this regulated PLD activity may be attributable to the recently discovered PLD β or γ isoforms (Pappan et al., 1997, 1998). Release and accumulation of NAE (Chapman et al., 1998) has prompted further interest in a possible role for NAE in elicitor-plant interactions.
In animal cells, NAEs and their precursors, NAPEs, have gained renewed interest as bioactive lipid molecules. Long-chain NAEs increased along with the corresponding NAPE under pathophysiological conditions (Epps et al., 1979, 1980; Cadas et al., 1997; Kondo et al., 1998; Sepe et al., 1998) and were involved in changes in membrane function (for review, see Schmid et al., 1990, 1996). The polyunsaturated anandamide (NAE 20:4) is an endogenous ligand for the cannabinoid receptor (CB1) in mammalian brain (Devane et al., 1992; Hanus et al., 1993), and is released from NAPE following signal-mediated activation of PLD (Cadas et al., 1997).
Recently, a number of other biological activities have been attributed to NAEs (mostly to anandamide) in vertebrates, including attenuation of pain (Jagger et al., 1998), embryo implantation (Das et al., 1995), and immuno-modulation (for review, see Di Marzo, 1998). In plants, medium-chain, saturated NAEs (e.g. NAE 14:0) accumulated in elicitor-treated tobacco cell suspensions (Chapman et al., 1998). Here we provide evidence that NAE 14:0 at nanomolar concentrations activates PAL expression in cell suspensions and leaves of tobacco, suggesting that NAE release is part of a signal transduction pathway(s) from elicitor perception to PAL expression. Our results also suggest that NAEs may modulate ion flux at the plasma membrane, as indicated by attenuation of elicitor-induced alkalinization of the culture medium. To our knowledge, this represents the first characterization of the biological activity of NAE in plant cells, and extends to plants the role of NAPE/NAE metabolism as a general mechanism for the production of lipid mediators in multicellular eukaryotes.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum cv KY 14) cell suspensions were grown and maintained as previously described (Chapman et al., 1995) and cell suspensions in log phase (72 h after subculture) were used for elicitor treatments. Cell suspensions were reinitiated from callus cultures periodically (every 3–4 months) to achieve consistent responses to elicitors and NAEs.
Tobacco (cv Xanthi) plants were grown in the greenhouse under a 14-h photoperiod (supplemented with high-intensity sodium lamps when necessary to extend daylength). Fully expanded leaves of 8- to 16-week-old plants were used for experiments.
Elicitor Treatment
Several elicitors of diverse origin were examined, including xylanase (Trichoderma viride, Sigma, St. Louis), cryptogein (Phytopthora cryptogea, kindly provided by Dr. R.A. Dixon, S.R. Noble Foundation, Ardmore, OK), harpinpss (He et al., 1993) (Pseudomonas syringae pv. syringae, kindly provided by Dr. J.C. Baker, Molecular Plant Pathology Laboratory, U.S. Department of Agriculture, Agricultural Research Services, Beltsville, MD), and ergosterol, a fungal membrane sterol. All of the elicitors were either infiltrated as aqueous solutions into tobacco leaves or added into aliquots of cell suspensions as aqueous solutions. Ergosterol was dissolved in culture medium with sonication and vortexing prior to addition to cultures.
NAE Treatment
Initially the NAEs 12:0, 14:0, 16:0, 18:0, 18:1, and 20:4 were kindly supplied by Dr. D. Piomelli (University of California, Irvine). Subsequently, we synthesized various NAE molecular species from acyl chlorides in ethanolamine (Devane et al., 1992). NAEs were synthesized in a reaction mixture of 25 mg of respective acylchlorides, 2.5 mL of dichloromethane, and 2.5 mL of ethanolamine (Sigma-Aldrich, Milwaukee, WI) at room temperature for 15 min with gentle swirling. The reaction was stopped with 10 mL of ultrapure water and washed twice with an equal volume of ultrapure water (MilliQUF Plus, Millipore, Bedford, MA). The NAEs were then collected in the organic layer, and the dichloromethane was evaporated under N2 gas. The NAE species were resuspended in anhydrous methanol, and purity was determined by gas chromatography-mass spectroscopy (GC-MS) (see below). For tobacco leaf infiltration, NAE species were dissolved in water (after removal of organic solvent under N2) with sonication and vortexing, whereas for treatment of cell suspensions, they were dissolved in culture supernatant prior to treatment.
Measurement of Medium Alkalinization
Aliquots of tobacco cell suspensions (20 mL/3–5 g wet weight) were equilibrated for 20 to 30 min with continuous stirring until a steady pH value was reached. The change in medium pH was monitored with a glass combination electrode (Ag/AgCl2, model 15 pH meter, Fisher Scientific, Houston, TX) for 40 min after the elicitor or NAE treatment.
RNA Isolation and Northern Analysis
Total RNA from tobacco leaves and cell suspensions was isolated according to the single-step guanidinium acid-phenol method of Chomczynski and Sacchi (1987). Prior to RNA isolation, tobacco leaves were treated for 12 h and tobacco cells for 4 h with elicitors and/or NAEs. Tobacco cells were collected by centrifugation at 300g. RNA was isolated from cells or leaves that were frozen in liquid N2 and precipitated by overnight incubation at −20°C with 1 volume of isopropanol. RNA samples (10 μg) were separated in agarose-formaldehyde gels (Sambrook et al., 1989) and incubated for 3 h in hydrolysis buffer (50 mm NaOH and 10 mm NaCl) and for 20 min (2×) in neutralization buffer (0.2 m Tris, pH 7.4, and 18× SSC) prior to blotting (Hybond N+, Amersham-Pharmacia Biotech, Uppsala) through capillary transfer in 20× SSC (3.0 m NaCl and 0.3 m sodium citrate, pH 7.4). Equal loading of samples was confirmed by including ethidium bromide in the gel-loading samples and also by methylene blue staining of blots (Herrin and Schmidt, 1988).
RNA was fixed to membranes by UV cross-linking (5-min exposure, G-30 T 8 UV lamp source, 0.5 m distance). RNA blots were prehybridized at 60°C for 2 h and hybridized (at 60°C for 18–20 h) with a 766-bp PCR fragment of PAL sequence amplified from tobacco (gift of R.A Dixon, S.R. Noble Foundation, Ardmore, OK) by using specific oligonucleotide primers complimentary to a portion of PAL cDNA (GenBank accession no. X78269) (at position +277, the forward primer, 5′-AAAAATGGCTGGTGTTGCACAA-3′ and at +1,052 bp, the reverse primer, 5′-CCATTCACAAGNGCAAGNCCTTCCTTAGG-3′). The PCR fragments were labeled directly (flourescein-11-dUTP) with a random prime labeling module (Gene Images, Amersham, Buckinghamshire, UK) and detected by the detection module (CDP-Star, Gene Images) following the manufacturer's instructions. Relative levels of PAL mRNA were estimated by normalizing to 28S RNA (in the same lanes) using scanning densitometry and the public domain NIH Image program (version 3.1, developed at the United States National Institutes of Health and available at http://rsb.info.nih.gov/nih-image).
NAE Quantification
Previous methods employed for NAE identification and quantification in cell suspensions were inadequate for analysis of more complex tissues of higher plants. Consequently, we adopted a new procedure for the routine identification and quantification of NAE species from tobacco leaves. Analysis of NAE relied on HPLC isolation of NAE-enriched fractions from crude lipid extracts, and the subsequent identification/quantification of trimethyl silyl-derivatized NAEs by GC-MS. This method is similar to that published by Piomelli and co-workers (Stella et al., 1997) for the analysis of anandamide in mammalian brain extracts, but with some modifications for quantification of saturated, medium-chain NAEs in chlorophyll-containing extracts. Following infiltration, tobacco leaves were harvested and immediately frozen (portions of approximately 1.0 g), powdered in liquid N2 in a mortar, and added to hot 2-propanol (to inactivate any endogenous phospholipases) (Chapman et al., 1998). Lipids were extracted into chloroform, filtered, and subjected to normal phase HPLC (4.6 × 250 mm Partisil 5 column, Whatman, Clifton, NJ). HPLC conditions involved a linear gradient of 2-propanol in hexane (up to 40% 2-propanol over 20 min), followed by 5 min at 50% 2-propanol, and then 5 min at 100% hexane. Under these conditions, NAEs eluted between 11 and 15 min, depending on the species, well away from most other lipids. A synthetic standard NAE 20:4, which has substantial UV absorbance (at 214 nm), was used to check column performance and NAE retention time on a daily basis.
The NAE-enriched HPLC fractions were collected and evaporated to dryness under N2 gas. NAEs were derivatized in bis(trimethylsilyl)trifluroacetamide at 50°C for 30 min. Trimethyl silyl-ether derivatives were suspended in hexane and analyzed by GC-MS. The gas chromatograph was a 5890 series II (Hewlett-Packard, Palo Alto, CA) equipped with a capillary column (30-m × 0.25-mm i.d. with a 0.25-μm film thickness, DB-5.625, J&W Scientific, Folsom, CA). The injector temperature was 260°C and the oven temperature was programmed from 40°C to 280°C at 10°C min−1. The GC was coupled to a mass spectrometer (model HP5970, Hewlett-Packard) equipped with an electron impact source (70 eV) and operated for ultimate sensitivity in the selective ion monitoring mode. The M+ and [M-15]+ fragmentation ions as well as two confirming masses were monitored for NAE 14:0. Standard curves and mass spectra were prepared using injected masses of 0.1 to 200 ng of synthetic NAE in the presence of 10 ng of internal standard (decachlorobiphenyl). Final quantification of NAE species was calculated from the ratio of analyte (NAE) response to that of the internal standard. Method efficiency was evaluated by recovery of synthetic NAE 17:0 “surrogate” added to the preparation at the time of tissue extraction, and replicate values were adjusted for NAE 17:0 recovery. Statistical comparisons of the data were made using an unpaired Student's t test.
RESULTS
Attenuation of Xylanase-Induced Alkalinization by NAEs in Cultured Tobacco Cells
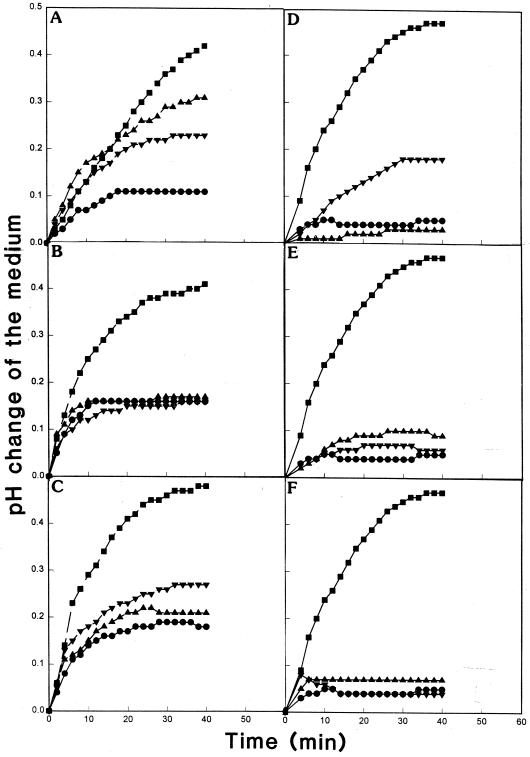
Xylanase induces extracellular medium alkalinization in cell suspension cultures of different plant species within minutes of treatment (Bailey et al., 1992; Felix et al., 1993). The addition of xylanase (1 μg mL−1) to tobacco cells triggered the extracellular pH to change rapidly (0.2–0.5 unit over 40 min, see Fig. 1). Since NAEs are released into tobacco cell culture medium within 10 min of xylanase treatment (Chapman et al., 1998), the effect of NAEs on this xylanase-induced alkalinization was analyzed. Several saturated and unsaturated species of NAE (12:0, 14:0, 16:0, 18:0, 18:1, and 20:4) at 100 μm were added either separately or in conjunction with xylanase to the cell suspensions. All of the NAEs inhibited the xylanase-induced alkalinization of the culture medium (Fig. 1). Of all of the NAEs tested, NAE 12:0 appeared to be the least effective in antagonizing the xylanase-induced alkalinization response. When added alone, the NAEs generally did not affect the medium pH, and results were comparable to control treatments with medium alone. In tobacco, NAE 14:0 was identified as a predominant endogenous NAE (Chapman et al., 1998), so the effect of this species was characterized in more detail in subsequent experiments.
Figure 1.
Effects of exogenously supplied synthetic NAEs (0.1 mm) on alkalinization of the tobacco cell culture medium induced by xylanase (1 μg mL−1). The specific NAE molecular species tested were: NAE 12:0 (A); NAE 14:0 (B); NAE 16:0 (C); NAE 18:0 (D); NAE 18:1 (E); and NAE 20:4 (F). NAEs were added to cultures as described in “Materials and Methods.” Cells in log phase (3–4 d after subculture) were treated with or without xylanase and incubated with or without NAE with continuous gentle stirring. The change in pH of the culture medium was recorded every 2 min for 40 min. Controls and experimental treatments were carried out on the same population of cells. Results presented are representative; similar trends were observed in experiments repeated three to six times. A, ▪, xylanase; ▴, xylanase plus NAE12:0; ▾, NAE12:0; ●, control. B, ▪, xylanase; ▴, xylanase plus NAE14:0; ▾, NAE14:0; ●, control. C, ▪, xylanase; ▾, xylanase plus NAE16:0; ▴, NAE16:0; ●, control. D, ▪, xylanase; ▾, xylanase plus NAE18:0; ●, control; ▴, NAE18:0. E, ▪, xylanase; ▴, xylanase plus NAE18:1; ▾, NAE18:1; ●, control. F, ▪, xylanase; ▴, xylanase plus NAE20:4; ●, control; ▾, NAE20:4.
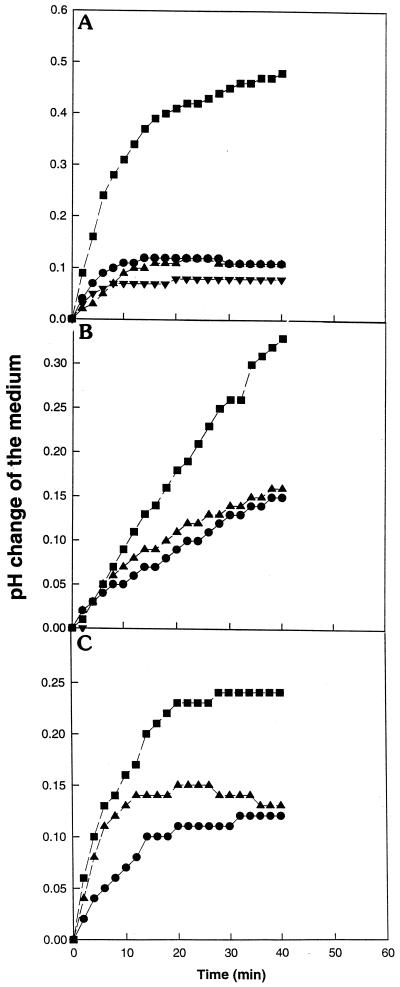
To analyze whether the inhibitory action of NAE 14:0 was elicitor specific or a more general phenomenon, other elicitors were tested (Fig. 2). The bacterial protein harpin (P. syringae) and the fungal protein cryptogein (Phytophthora cryptogea), both known to activate the alkalinization response in tobacco cell suspensions (Wei et al., 1992; Blein et al., 1991, respectively), and the fungal sterol, ergosterol, which elicits medium alkalinization in tomato cell suspensions (Granado et al., 1995), were tested with NAE 14:0. All of the elicitors induced medium alkalinization (between 0.15–0.4 pH unit), with harpin being the most pronounced, followed by cryptogein and ergosterol (Fig. 2). When NAE 14:0 at 100 μm was included in the treatment along with the elicitor, there was complete inhibition of medium alkalinization in harpin- and cryptogein-treated cell suspensions (Fig. 2, A and B), while inhibition was less obvious in ergosterol-treated cells (Fig. 2C). These results were consistent with the xylanase data (Fig. 1) and suggest that the inhibitory action of NAE can be extended to other elicitors of fungal and bacterial origin.
Figure 2.
Effect of exogenously supplied NAE 14:0 (0.1 mm) on the alkalinization of tobacco cell culture medium induced by different elicitors. A, Harpin 420 ng mL−1 (▪), control (●), hairpin plus NAE14:0 (▴), and NAE14:0 (▾). B, Cryptogein 150 nm (▪), cryptogein plus NAE14:0 (▴), and control (●). C, Ergosterol 10 nm (▪), ergosterol plus NAE14:0 (▴), and control (●). The elicitors and NAEs were added to the culture medium as described in “Materials and Methods” and the pH was recorded every 2 min for 40 min. Controls and experimental treatments were carried out on the same population of cells. Results presented are representative; similar trends were observed in experiments repeated three times.
Time and NAE 14:0 Concentration-Dependent Inhibition of Elicitor-Induced Medium Alkalinization
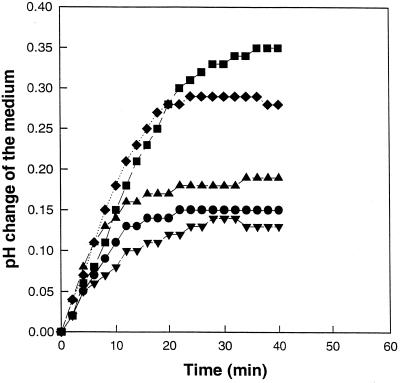
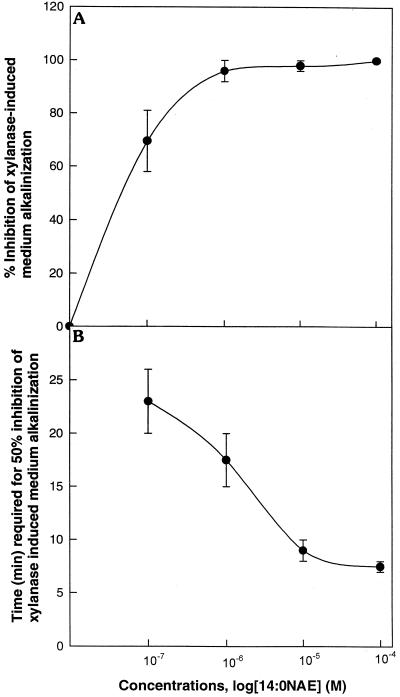
There was a defined time period in which NAE addition was effective in inhibiting elicitor-induced medium alkalinization (Fig. 3). NAE 14:0 inhibited xylanase-induced alkalinization when added 10 min prior to or at the same time as xylanase. Adding NAE 14:0 10 min after elicitor treatment was marginally effective, if at all, and did not reverse the alkalinization response. Inhibition by NAE 14:0 was concentration dependent, with some inhibition still at 10−7 m (Fig. 4A). At lower NAE 14:0 concentrations, a longer time was required to reach 50% inhibition (e.g. at 10−7 m, 22 min), emphasizing that the inhibitory effect of NAE was time and concentration dependent. Complete inhibition of the elicitor-induced alkalinization response by NAE is likely a manifestation of higher levels of exogenous NAE added at time of elicitor treatment. Endogenous levels of NAE are in the low- to mid-nanomolar range following elicitor treatment, and this may be responsible for the observed attenuation of elicitor-induced medium alkalinization that occurs between 20 and 40 min after elicitor treatment (elicitor only, Figs. 1 and 2). A clear answer will await the ability to block NAE release in vivo. Nonetheless, these results raise the possibility that endogenous release of NAE 14:0 may modulate the well-characterized elicitor-induced exchange response in vivo.
Figure 3.
Effect of NAE 14:0 (0.1 mm) on medium alkalinization prior to or during xylanase treatment. NAE 14:0 was added either 10 min before or after xylanase treatment (1 μg mL−1) and the pH of the medium was recorded every 2 min for 40 min. Controls and experimental treatments were carried out on the same population of cells. Results are representative of trends observed in two replicate experiments. ▪, Xylanase; ♦, NAE14:0 added 10 min after xylanase; ▴, xylanase plus NAE14:0 added at the same time; ●, control; ▾, NAE14:0 added 10 min before xylanase.
Figure 4.
Time- and concentration-dependent inhibition of the xylanase-induced alkalinization of tobacco cell culture medium by NAE 14:0. In A, the percent of overall inhibition (relative to medium controls) versus the log of the concentration of exogenously supplied NAE 14:0 is plotted. In B, the time required to achieve 50% inhibition versus the log of the concentration of exogenously supplied NAE 14:0 is plotted. The data points represent the averages ± sd of three independent experiments at each concentration.
Induction of Defense Gene Expression by Elicitors and NAE 14:0
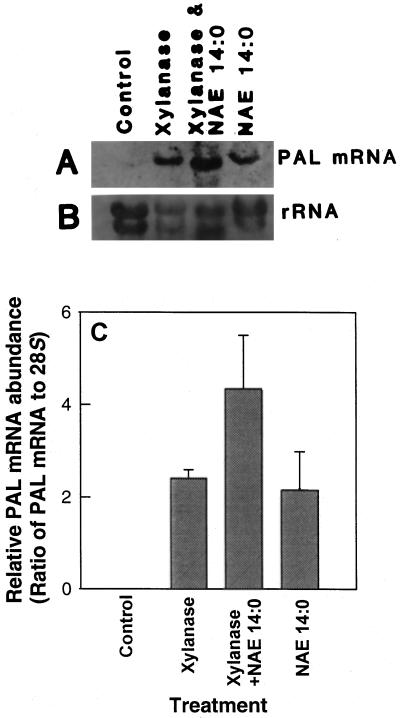
The effect of NAE 14:0 on PAL gene expression in these tobacco cell suspensions was striking. As expected, xylanase was found to induce PAL gene expression (Fig. 5). Relative PAL expression appeared to be greater when tobacco cells were treated with both xylanase and NAE 14:0 (100 μm). More importantly, PAL expression was induced by NAE 14:0 alone, and this induction was comparable to the relative expression levels induced by xylanase alone. Control cells treated with medium alone (lane 1) did not show any detectable PAL expression.
Figure 5.
Analysis of PAL mRNA expression in tobacco cell suspensions. A, Northern blot showing PAL expression in cell suspensions treated with medium only (control), xylanase (1 μg mL−1), xylanase in combination with NAE 14:0 (0.1 mm), and NAE 14:0 alone (0.1 mm). B, Methylene blue-stained blot showing relative amounts of RNA in each lane. C, Relative abundance of PAL mRNA (normalized to 28S rRNA by densitometric scanning and imaging analysis with NIH Image 3.1 software). Values represent the means ± sd of three independent experiments/extractions analyzed under identical conditions.
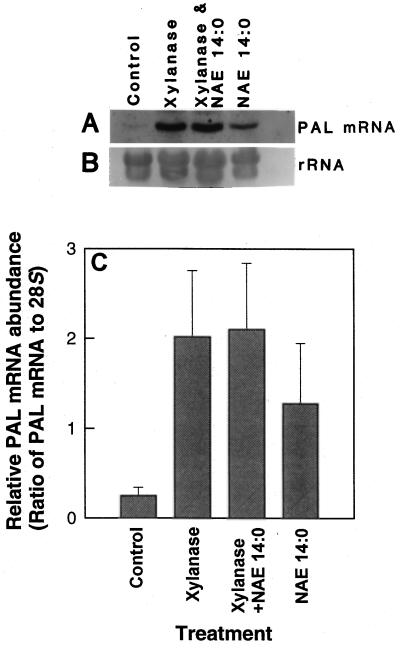
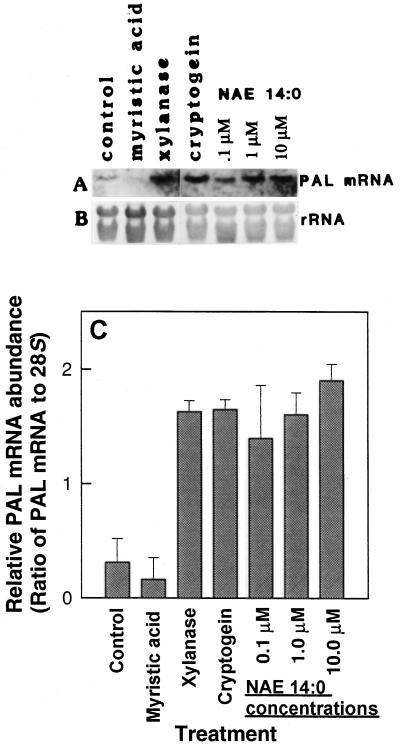
Since results in cell culture systems are sometimes inconsistent with responses in planta, the same experiments were performed with leaves of tobacco plants (Fig. 6). Tobacco leaves were infiltrated adaxially with xylanase and/or NAE 14:0, and total RNA was isolated after 12 h to analyze PAL expression. Unlike in cell cultures, there was no recognizable additive effect of xylanase and NAE 14:0. However, both xylanase and NAE 14:0 reproducibly activated PAL expression in leaves compared with controls (water only). NAE 14:0 activated PAL expression at concentrations down to 0.1 μm (Fig. 7), similar to treatments with xylanase or cryptogein. Moreover, the analogous fatty acid myristic acid showed no activation of PAL expression even at 100 μm (Fig. 7), ruling out nonspecific detergent effects of lipid treatments in these experiments.
Figure 6.
Analysis of PAL mRNA expression in tobacco leaves. A, Northern blot showing PAL expression in leaves treated with water only (control), xylanase (1 μg mL−1), xylanase in combination with NAE 14:0 (0.1 mm), and NAE 14:0 alone (0.1 mm). B, Methylene blue-stained blot showing relative amounts of RNA in each lane. C, Relative abundance of PAL mRNA (normalized to 28S rRNA by densitometric scanning and imaging analysis with NIH Image 3.1 software). Values represent the means ± sd of three independent experiments/extractions analyzed under identical conditions.
Figure 7.
Analysis of PAL mRNA expression in total RNA samples extracted from tobacco leaves treated with various amounts of NAE 14:0. Xylanase and cryptogein were included as positive controls. NAE 14:0 concentrations were varied from 0.1 to 10 μm and myristic acid, a 14:0 fatty acid, was tested at 100 μm. A, Northern blot showing PAL mRNA expression. B, Methylene blue-stained blot showing relative amounts of total RNA in each lane. C, Relative abundance of PAL mRNA (normalized to 28S rRNA by densitometric scanning and imaging analysis with NIH Image 3.1 software). Values represent the means ± sd of three independent experiments/extractions analyzed under identical conditions.
Accumulation of NAE 14:0 in Leaves
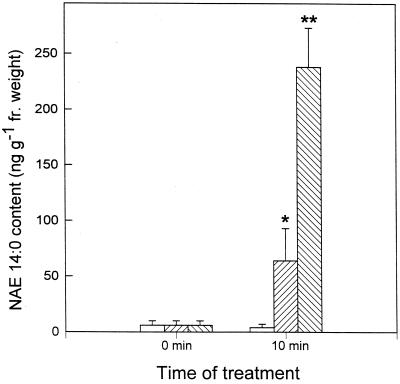
NAE 14:0 levels increased 10- to 50-fold in elicitor-treated tobacco leaves compared with leaves infiltrated with water only (Fig. 8). In leaves infiltrated for 10 min with xylanase, NAE 14:0 content increased from 6 ± 4 to 64 ± 29 ng g−1 fresh weight (n = 3; P < 0.03). In leaves infiltrated for 10 min with cryptogein, NAE 14:0 content increased from 6 ± 4 to 238 ± 35 ng g−1 fresh weight (n = 3; P < 0.0004). These results indicate that significant increases in NAE 14:0 content occur in elicitor-treated tobacco leaves, consistent with our prior observations in tobacco cell suspensions (Chapman et al., 1998). Increases in NAE 12:0 (previously seen with cell suspensions) were not particularly evident in leaves of intact plants (not shown). These results indicate that two well-characterized elicitors of tobacco defense responses trigger an accumulation of NAE 14:0 in vivo through a range sufficient to activate PAL expression (Fig. 7).
Figure 8.
Analysis of elicitor-induced NAE 14:0 content in cv Xanthi tobacco leaves as quantified by GC-MS. Tobacco leaves were infiltrated with xylanase (right-hatched bars; 1 μg mL−1), cryptogein (left-hatched bars; 150 nm), or water (white bars) as described in “Materials and Methods.” Each value represents the mean ± sd of three independent experiments and the asterisks (*) indicate statistically significant differences from controls (*, P < 0.03; **, P < 0.0004).
Cryptogein, as expected, also activated PAL expression in tobacco leaves (Fig. 7). Application of both xylanase (Bailey et al., 1990) and cryptogein (Ricci et al., 1989) to tobacco leaves induces development of lesions characteristic of the hypersensitive response that is commonly observed in resistant host-pathogen interactions. Our results indicate that NAPE metabolism is activated by these pathogen elicitors, and consequently imply that NAE release may be part of the initial signaling cascade that ultimately leads to plant disease resistance.
DISCUSSION
Membrane phospholipids are the precursors for second messengers produced through transmembrane signaling of activated ion channels, receptor kinases, or through receptor-activated effector enzymes (Munnik et al., 1998). Several phospholipases are known to be activated as a direct consequence of plant-pathogen interactions (for review, see Chapman, 1998). In tobacco cell suspensions, NAE 14:0 (and NAE 12:0) was formed from NAPE (likely by a PLD-type activity) following xylanase treatment and these NAEs accumulated extracellularly (Chapman et al., 1998). Here we demonstrate that NAE 14:0 levels also are increased in tobacco leaves in response to two different pathogen elicitors (Fig. 8). Our results are reminiscent of PLD-mediated NAE release in mammalian systems, and suggest that the N-acylation phosphodiesterase pathway (Schmid et al., 1996) operates in plant defense signaling.
Collectively, our results suggest that endogenous NAE (specifically NAE 14:0) acts as a lipid mediator in elicitor-induced cell signaling, and these results can be summarized as follows: (a) a 10- to 50-fold increase in NAE14:0 content was measured (by GC-MS) in planta in response to two different pathogen elicitors (Fig. 8), extending previous observations with cell suspensions (Chapman et al., 1998); (b) six different species of exogenous NAE (including NAE14:0) inhibited elicitor-induced medium alkalinization (Fig. 1); (c) this inhibitory effect was dependent on time and concentration of added NAE (Fig. 3), and was consistently observed for three different elicitors (Fig. 2); (d) NAE 14:0 alone was sufficient to activate PAL expression (in cell suspensions, Fig. 5, and in planta, Fig. 6) in a manner similar to that of the same elicitors that invoked NAE production in vivo; (e) NAE activated PAL expression at submicromolar levels well within the levels we actually quantified in vivo following elicitor treatment, and (f) myristic acid (14:0 fatty acid with no N-linked ethanolamine moiety) was inactive in terms of induction of PAL expression. In conclusion, NAEs, an endogenous family of lipid mediators in vertebrates, appear to have a related function in plant cell signal transduction. Future work is aimed at addressing the precise mechanisms of NAE action.
In this manuscript we examined the biological activity of NAE during elicitor perception and plant defense responses. In tobacco cells, attenuation of elicitor-induced medium alkalinization by NAE 14:0 as well as other NAE species suggests an involvement in the modulation of ion flux at the plasma membrane. Interestingly, in animals NAE inhibited the permeability-dependent Ca2+ release from mitochondria (Epps et al., 1982), N-type Ca2+ channel activity (Mackie et al., 1993), and gap-junction conductance (Venance et al., 1995). Although the complete mechanisms of NAE function in mammalian cells remain somewhat obscure, the cannabinoid receptors CB1 and CB2 for anandamide (Devane et al., 1992) and palimitoylethanolamide (Facci et al., 1995), respectively, are a likely site of action. A recent analysis of plant cell perception of systemin (the endogenous wound-induced signal; Pearce et al., 1991) indicated that the N-terminal domain was essential for receptor binding but it inhibited medium alkalinization induced by native systemin (Meindi et al., 1998). While we do not yet have any direct evidence, it seems reasonable to speculate that plants have a receptor for NAE 14:0 (similar to the CB receptor in vertebrates) that activates a pathway to regulate the elicitor-induced changes in cellular metabolism.
Recently, a pathogen-induced NO-signaling pathway that compliments events mediated by H2O2 has been identified in plants (for review, see Camp et al., 1998). Activation of this NO pathway leads to activation of PAL expression and potentiation of H2O2-mediated cell death in Arabidopsis leaves (Delledonne et al., 1998). In animal cells, NO is a well-known molecular component of signal transduction pathways controlling an array of physiological functions including host responses to infection (for review, see Hausladen and Stamler, 1998). In addition, the CB receptor (for NAE) is coupled to NO release in both vertebrate and invertebrate cells (Stefano et al., 1998a). Exogenously supplied anandamide also triggered NO release in leech and mussel ganglia in a concentration-dependent manner (Stefano et al., 1998b). Therefore, it is possible that NAE 14:0 release may act through a NO-mediated pathway to activate PAL expression. Consequently, NAPE/NAE metabolism may represent a conserved mechanism in multicellular eukaryotic organisms for signaling pathogen invasion at the cellular level.
It should be emphasized that perception of pathogen elicitors by plant cells involves the coordinate action of several phospholipases (Chapman, 1998) which together most certainly produce a multitude of lipid-derived signaling molecules. Clearly, NAE release represents only part of a complex scheme of signaling circuits that provides plant cells the flexibility to respond to multiple environmental stresses. A complete understanding of defense signal transduction pathways must take into account other PLD products (such as phosphatidic acid) as well as products of phosphatidylinositol-specific phospholipase C and phospholipase A activities. As efforts continue to focus on the identification and quantification of new lipid mediators, the interaction of phospholipase-mediated signaling pathways leading to plant defense responses will become better understood.
ACKNOWLEDGMENTS
Thanks to Drs. R. Dixon and C. Lamb for insightful discussions regarding plant defense signaling. Dr. Dixon provided the PAL PCR fragment and cryptogein; Dr. J.C. Baker provided the purified harpin; and Dr. Daniele Piomelli provided some of the NAE species initially used in these studies.
Footnotes
This research was supported initially by U.S. Department of Agriculture-National Research Initiative Competitive Grants Program (agreement no. 96–35304–3862) and also by the Texas Higher Education Coordinating Board (Advanced Research Program grant no. 003594–028).
LITERATURE CITED
- Adam A, Farkas T, Somiyal G, Hevesi M, Kiraly Z. Consequence of O2− generation during a bacterially induced hypersensitive reaction in tobacco: deterioration of membrane lipids. Physiol Mol Plant Pathol. 1989;34:13–26. [Google Scholar]
- Adam AL, Pike S, Hoyos ME, Stone JM, Walker JC, Novacky A. Rapid and transient activation of a myelin basic protein kinase in tobacco leaves treated with harpin from Erwinia amylovora. Plant Physiol. 1997;115:853–861. doi: 10.1104/pp.115.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Anderson JD, Bailey BA, Dean JFD, Taylor R. A fungal endoxylanase elicits ethylene biosynthesis in tobacco (Nicotiana tabacum L. cv. Xanthi) leaves. In: Flores HE, Arteca RN, Shannon JC, editors. Polyamines and Ethylene: Biochemistry, Physiology and Interactions. Rockville, MD: American Society of Plant Physiologists; 1990. pp. 146–156. [Google Scholar]
- Atkinson MM. Molecular mechanisms of pathogen recognition by plants. Adv Plant Pathol. 1993;10:35–64. [Google Scholar]
- Atkinson MM, Huang JS, Knopp JA. The hypersensitive reaction of tobacco to Pseudomonas syringae pv. Pisi: activation of a plasmalemma K+/H+ exchange mechanism. Plant Physiol. 1985;79:843–847. doi: 10.1104/pp.79.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MM, Keppler LD, Orlandi EW, Baker CJ, Mischke CF. Involvement of plasma membrane calcium influx in bacterial induction of the K+/H+ and hypersensitive responses in tobacco. Plant Physiol. 1990;92:215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA, Dean JF, Anderson JD. An ethylene biosynthesis-inducing endoxylanase elicits electrolyte leakage and necrosis in Nicotiana tabacum cv Xanthi leaves. Plant Physiol. 1990;94:1849–1854. doi: 10.1104/pp.94.4.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA, Korcak RF, Anderson JD. Alterations in Nicotiana tabacum L. cv Xanthi cell membrane function following treatment with an ethylene biosynthesis-inducing endoxylanase. Plant Physiol. 1992;100:749–755. doi: 10.1104/pp.100.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA, Korcak RF, Anderson JD. Sensitivity to an ethylene biosynthesis-inducing endoxylanase in Nicotiana tabacum L. cv. Xanthi is controlled by a single dominant gene. Plant Physiol. 1993;101:1081–1088. doi: 10.1104/pp.101.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, O'Neill NR, Keppler LD, Orlandi EW. Early responses during plant-bacterial interactions in tobacco cell suspensions. Phytopathology. 1991;81:1504–1507. [Google Scholar]
- Blein JP, Milat ML, Ricci P. Responses of cultured tobacco cells to cryptogein, a proteinaceous elicitor from Phytophthora cryptogea: possible plasmalemma involvement. Plant Physiol. 1991;95:486–491. doi: 10.1104/pp.95.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Bowles DJ. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Cadas H, Tomaso ED, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp WV, Montagu MV, Inze D. H2O2 and NO: redox signals in disease resistance. Trends Plant Sci. 1998;3:330–333. [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Montagu MV, Jr, Inze D, Camp WV. Defense activation and enhanced pathogen tolerance by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA. 1998;95:5818–5823. doi: 10.1073/pnas.95.10.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD. Phospholipase activity during plant growth and development and in response to environmental stress. Trends Plant Sci. 1998;3:419–426. [Google Scholar]
- Chapman KD, Conyers-Jackson A, Moreau A, Tripathy S. Increased N-acylphosphatidylethanolamine biosynthesis in elicitor-treated tobacco cells. Physiol Plant. 1995;95:120–126. [Google Scholar]
- Chapman KD, Tripathy S, Venables B, Desouza AD. N-Acylethanolamines: formation and molecular composition. Plant Physiol. 1998;116:1163–1168. doi: 10.1104/pp.116.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;102:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cramer CL, Ryder TB, Bell JN, Lamb CJ. Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985;227:1240–1242. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in mouse uterus. Proc Natl Acad Sci USA. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JFD, Gamble HR, Anderson JD. The ethylene biosynthesis inducing xylanase: its induction in Trichoderma viride and certain plant pathogens. Phytopathology. 1989;79:1071–1078. [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. ‘Endocanabinoids’ and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim Biophys Acta. 1998;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mayer JE, Hahlbrock K. Fungal elicitor triggers rapid, transient and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990;265:6360–6368. [PubMed] [Google Scholar]
- Dixon RA, Harrison MJ, Lamb CJ. Early events in the activation of plant defense responses. Annu Rev Plant Pathol. 1994;32:479–501. [Google Scholar]
- Dixon RA, Lamb CJ. Molecular communication in interactions between plants and microbial pathogens. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:339–367. [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Wendehenner D, Klessig D. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J, Cosio EG. Elicitors of plant defense responses. Int Rev Cytol. 1994;148:1–36. [Google Scholar]
- Ebel J, Scheel D. Elicitor recognition and signal transduction. In: Boller T, Meins Jr F, editors. Genes Involved in Plant Defense. New York: Springer-Verlag; 1992. pp. 183–205. [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin L. Signal molecules in systematic plant resistance to pathogens and pests. Cell. 1992;70:879–886. doi: 10.1016/0092-8674(92)90239-9. [DOI] [PubMed] [Google Scholar]
- Epps DE, Natarajan V, Schmid PC, Schmid HHO. Accumulation of N-acylethanolamine glycerophospholipids in infracted myocardium. Biochim Biophys Acta. 1980;618:420–430. doi: 10.1016/0005-2760(80)90260-x. [DOI] [PubMed] [Google Scholar]
- Epps DE, Pulmer JW, Schmid HHO, Pfeiffer DR. Inhibition of permeability-dependent Ca2+ release from mitochondria by N-acylethanolamines, a class of lipid synthesized in ischemic heart tissue. J Biol Chem. 1982;257:1383–1391. [PubMed] [Google Scholar]
- Epps DE, Schmid PC, Natarajan V, Schmid HHO. N-Acylethanolamine accumulation in infarcted myocardium. Biochem Biophys Res Commun. 1979;90:628–633. doi: 10.1016/0006-291x(79)91281-6. [DOI] [PubMed] [Google Scholar]
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Grosskopf DG, Regenass M, Boller T. Rapid changes in protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci USA. 1991;88:8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Regenass M, Boller T. Specific perception of sub-nanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Grab D, Feger M, Ebel J. An endogenous factor from soybean (Glycine max L.) cell cultures activates phosphorylation of protein which is dephosphorylated in vivo in elicitor-challenged cells. Planta. 1989;179:340–348. doi: 10.1007/BF00391079. [DOI] [PubMed] [Google Scholar]
- Granado J, Felix G, Boller T. Perception of fungal sterols in plants. Plant Physiol. 1995;107:485–490. doi: 10.1104/pp.107.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania U, Avni A. High-affinity binding site for ethylene-inducing xylanase elicitor on Nicotiana tabacum membranes. Plant J. 1997;12:113–120. [Google Scholar]
- Hanus L, Gopher A, Almog S, Mechoulam R. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J Med Chem. 1993;36:3032–3034. doi: 10.1021/jm00072a026. [DOI] [PubMed] [Google Scholar]
- Hausladen A, Stamler JS. Nitric oxide in plant immunity. Proc Natl Acad Sci USA. 1998;95:10345–10347. doi: 10.1073/pnas.95.18.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SY, Huang HC, Collmer A. Pseudomonas syringae pv syringae harpinpss a protein that is secreted via the hrp pathway and elicits the hypersensitive response in plants. Cell. 1993;73:1255–1266. doi: 10.1016/0092-8674(93)90354-s. [DOI] [PubMed] [Google Scholar]
- Herrin DL, Schmidt GW. Rapid, reversible staining of northern blots prior to hybridization. BioTechniques. 1988;6:196. [PubMed] [Google Scholar]
- Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger SI, Hasnie FS, Sellaturay S, Rice ASC. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ, Atkinson MM. Active oxygen production during a bacteria-induced hypersensitive reaction in tobacco suspension cells. Phytopathology. 1989;79:974–978. [Google Scholar]
- Kondo S, Sugiura T, Kodoka T, Kudo N, Waku K, Tokumura A. Accumulation of various N-acylethanolamines including N-arachidonoylethanolamine (anandamide) in cadmium chloride-administered rat testis. Arch Biochem Biophys. 1998;354:303–310. doi: 10.1006/abbi.1998.0688. [DOI] [PubMed] [Google Scholar]
- Lamb CJ, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lamb CJ, Lawton MA, Dron M, Dixon RA. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989;56:215–24. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Lawton MA, Lamb CJ. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987;7:335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon RA, Lamb CJ. H2O2 from the oxidative burst orchestrates the plant hypersensitive response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Lotan T, Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, use a non-ethylene pathway for induction. Plant Physiol. 1990;93:811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N 18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindi T, Boller T, Felix G. The plant wound hormone systemin binds with the N-terminal part to its receptor but needs the C-terminal part to activate it. Plant Cell. 1998;10:1561–1570. doi: 10.1105/tpc.10.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau RA, Powell MJ, Whitaker BD, Bailey BA, Anderson JD. Xylanase treatment of plant cells induces glycosylation and fatty acylation of phytosterols. Physiol Plant. 1994;91:575–580. [Google Scholar]
- Moreau RA, Preisig CL. Lipid changes in tobacco cell suspensions following treatment with cellulase elicitor. Physiol Plant. 1993;87:7–13. [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signaling in plants. Biochim Biophys Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Pappan K, Austin-Brown S, Chapman KD, Wang X. Substrate selectivities and lipid modulation of plant phospholipase Dα, -β, and -γ. Arch Biochem Biophys. 1998;353:131–140. doi: 10.1006/abbi.1998.0640. [DOI] [PubMed] [Google Scholar]
- Pappan K, Qin W, Dyer JH, Zheng L, Wang X. Molecular cloning and functional analysis of polyphosphoinositide-dependent phospholipase D, PLD β, from Arabidopsis. J Biol Chem. 1997;272:1–7. doi: 10.1074/jbc.272.11.7055. [DOI] [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA. A polypeptide from tomato leaves induces wound-inducible protease inhibitor proteins. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Ricci P, Bonnet P, Huet J-C, Sallantin M, Beauvais-Cante F, Bruneteau M, Billard V, Michel G, Pernollet JC. Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. Eur J Biochem. 1989;183:555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schmid HHO, Schmid PC, Natarajan V. N-Acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Schmid HHO, Schmid PC, Natarajan V. The N-acylation-phosphodiesterase pathway and cell signalling. Chem Phys Lipids. 1996;80:133–142. doi: 10.1016/0009-3084(96)02554-6. [DOI] [PubMed] [Google Scholar]
- Sepe N, Petrocelis LD, Montanaro F, Cimino G, Di Marzo V. Bioactive long chain N-acylethanolamines in five species of edible bivalve molluscs: possible implications for mollusc physiology and sea food industry. Biochim Biophys Acta. 1998;1389:101–111. doi: 10.1016/s0005-2760(97)00132-x. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Liu Y, Goligorsky Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes. J Biol Chem. 1998a;271:19238–19242. doi: 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Rialas CM, Deutsch DG, Salzet M. Anandamide amidase inhibition enhances anandamide-stimulated nitric oxide release in vertebrate neural tissues. Brain Res. 1998b;793:341–345. doi: 10.1016/s0006-8993(98)00273-x. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Fukuda Y, Shinshi H. Studies on elicitor signal transduction leading to differential expression of defense genes in cultured tobacco cells. Plant Cell Physiol. 1995;36:281–289. [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signaling in striatal astrocytes. Nature. 1995;376:590–593. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. Harpin: elicitor of hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E. Regulation of plant defense responses to fungal pathogens: two types protein kinases in the reversible phosphorylation of the host plasma membrane H+-ATPase. Plant Cell. 1996;8:555–564. doi: 10.1105/tpc.8.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]