Abstract
Though cocaine abuse and addiction continue to have serious implications for health and society, no FDA-approved interventions have been developed. Anticocaine conjugate vaccines offer an attractive opportunity for addiction treatment; however, vaccines have thus far failed in clinical trials. As a result, anticocaine vaccines must be further optimized to achieve clinical translation. Herein, we report a study on the relationship between vaccine efficacy and hapten stability toward hydrolysis. Two haptens developed by our laboratory, GND and GNE, were conjugated to tetanus toxoid (TT) and formulated with alum and cytosine-guanine oligodeoxynucleotide 1826 (CpG ODN 1826) adjuvants, the optimal formulation in anticocaine vaccine design. GND, a diamide, is more hydrolytically stable than GNE, a monoamide, toward butyrylcholinesterases. Ultimately, both vaccines induced antibodies with high affinity for cocaine. In hyperlocomotion testing, GND-TT and GNE-TT vaccinated mice exhibited a robust blockade of cocaine’s stimulatory effects at all tested doses. Overall, antibodies raised against both haptens were highly effective in protecting mice from cocaine-induced psychostimulation.
Keywords: Addiction, antibody, cocaine, CpG ODN 1826, hapten, vaccine
Cocaine abuse and addiction remain grave health and societal problems in the United States, with 900,000 individuals meeting the criteria for substance use disorder and nearly 11,000 overdose deaths in 2015.1 The Drug Abuse Warning Network (DAWN) reported that approximately one in three drug-related visits to emergency departments were attributed to cocaine in 2011.2 Moreover, according to the National Survey on Drug Use and Health (NSDUH), cocaine use in the United States has remained comparatively high, with an estimated 1.5 million past-month users in 2014.3 Despite the high prevalence of cocaine use, no FDA-approved therapeutic for treating cocaine addiction has been achieved. Current treatment methods are limited to the alleviation of withdrawal symptoms.4,5
In view of the limited treatment options, interest has turned toward antibody-based pharmacokinetic (PK) strategies, which modulate receptor signaling by binding the drug in circulation, preventing its passage across the blood–brain barrier (BBB).6,7 In this strategy, administration of a protein–drug immunoconjugate prompts the immune system to generate highly specific antibodies that minimize free drug concentrations at their site of action.8 While this targeted PK approach has been effective in many preclinical studies, only a single anticocaine vaccine, termed TA-CD, has reached clinical trials. These trials failed in phase III due to inconsistent vaccine efficacy among trial participants.8−12 As a result, next generation anticocaine vaccines need to be optimized in hapten, adjuvant, and carrier protein formulations to induce broadly efficacious and highly specific anticocaine antibodies in wide-ranging patient populations.
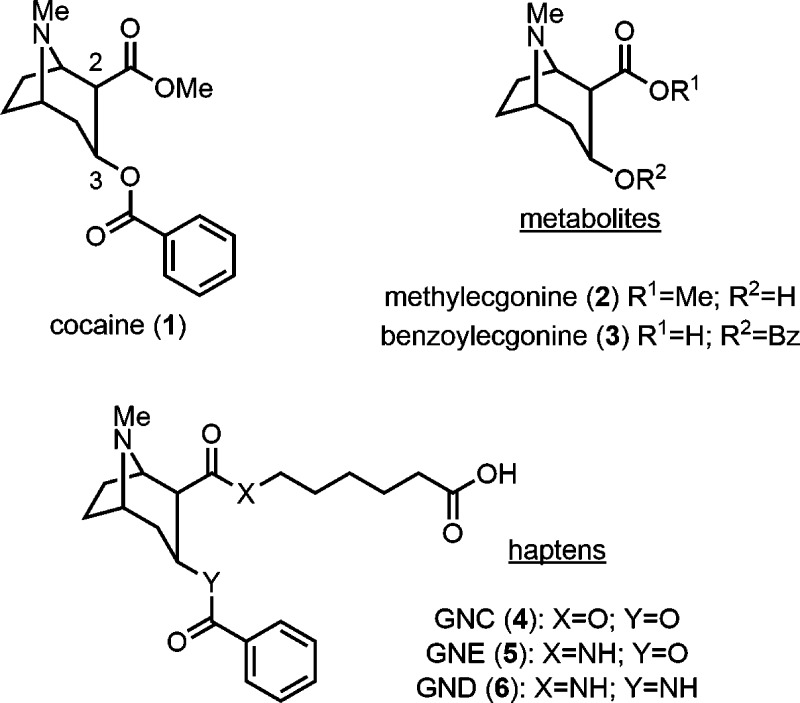
In the course of anticocaine vaccine development in the Janda Laboratory, we disclosed that hydrolytic stability of the cocaine hapten is a crucial determinant for vaccine efficacy.13,14 Cocaine (1) itself has a half-life (t1/2) of 1–2.5 h in humans with the most prevalent metabolites, methylecgonine (2) and benzoylecgonine (3), resulting from ester hydrolysis (Figure 1).15,16 Hydrolysis of the benzoyl ester is catalyzed by plasma butyrylcholinesterases (BChE), while hydrolysis of the methyl ester occurs spontaneously under basic biological conditions.15,16 Accordingly, cocaine haptens, which are designed with maximum congruence to the native drug, are likely susceptible to both spontaneous alkaline and enzymatic hydrolysis. To date, we have reported a number of cocaine haptens including GNC (4), GNE (5), and GND (6); a series characterized by increasing stability toward ester hydrolysis, respectively (Figure 1).13,14,17 Our design emphasized replacing the implicated esters with amides.
Figure 1.
Chemical structures of cocaine, cocaine metabolites, and haptens previously developed in the Janda Laboratory.
We rationalized the enhanced stability of GNE, and especially GND, toward hydrolysis would make for a more effective cocaine vaccine as a result of sustained antigen concentration and fewer unwanted, competing antigens, the latter resulting from hydrolytic degradation.13,14
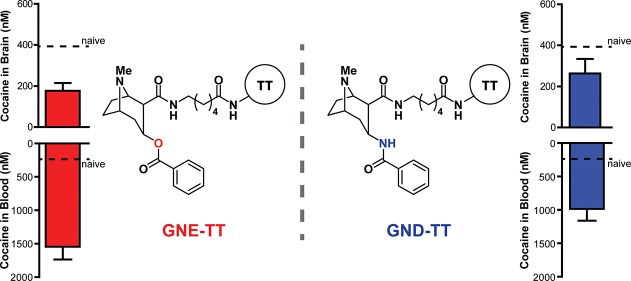
GNC, which incorporates ester linkages at C-2 and C-3, is the most structurally faithful to cocaine.17 While GNC does induce antibodies with the highest absolute titer value and affinity for cocaine, the hydrolytic instability of GNC translates to a lessened blockade of cocaine-induced psychomotor behavior.13 Early studies with GND, which incorporates amide linkages at C-2 and C-3, demonstrated longer-lasting titer values that translated to improved blockade of cocaine-induced stimulant effects when compared to GNC.13 Despite this promising finding, the low-yielding synthesis of GND limited further optimization of anticocaine vaccine formulations with this hapten. Accordingly, we disclosed a mixed amide/ester (C3:C2, respectively) hapten, termed GNE, with hydrolytic susceptibility only to BChEs to optimize additional vaccine components including the carrier protein and adjuvant(s).18−26 Employing GNE, we identified tetanus toxoid (TT) as a highly effective carrier protein and the adjuvants alum and cytosine-phosphodiester-guanine oligodeoxynucleotide (CpG ODN 1826) to be the optimal vaccine formulation.27 Our GNE-TT vaccine formulated with alum and CpG ODN 1826 reproducibly elicits high affinity anticocaine antibody production and robust blockade of cocaine-induced hyperlocomotor activity in mice and nonhuman primates.27
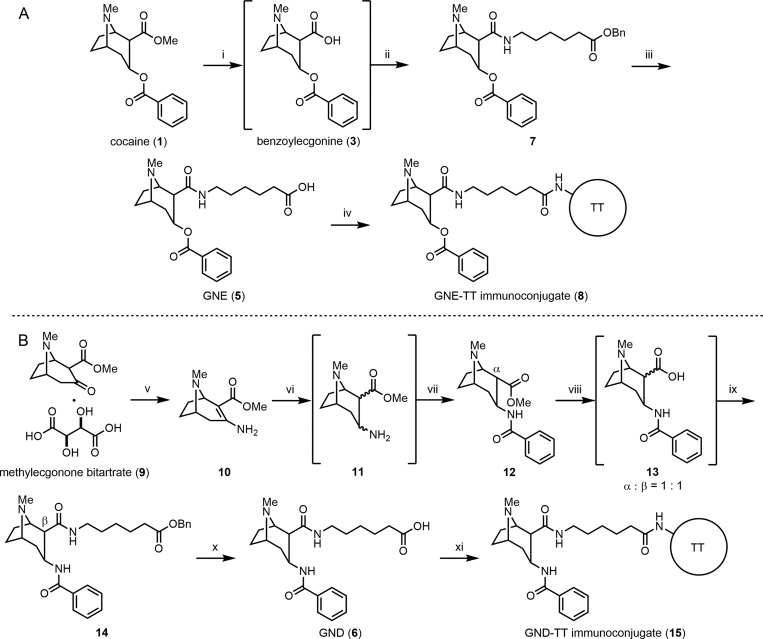
With an optimized vaccine formulation defined, we endeavored to evaluate a GND-TT vaccine formulated with alum and CpG ODN 1826, to compare this vaccine to the GNE-TT vaccine for the first time. Although we have previously reported syntheses for GNE and GND, these routes were particularly low yielding. Accordingly, we introduce herein refined syntheses to GNE and GND (Scheme 1).
Scheme 1.
Reagents and conditions: (i) H2O, 1,4-dioxane, MW, 40 W, 90 psi, 130 °C, 2 h, then evap.; (ii) benzyl 6-aminohexanoate, DMTMM, Et3N, THF, rt, 52% (one-pot); (iii) H2, Pd/C, EtOH, rt, 68%; (iv) sulfo-NHS, EDCI, DMF/H2O, rt, 12 h, then TT, PBS, 4 °C, 24 h; (v) sat. NH3 in MeOH, MW, 300 W, 150 psi, 100 °C, 4 h, 59%; (vi) NaBH3CN, 2 M HCl in 1,4-dioxane, MeOH, 33 h, then evap.; (vii) BzCl, NaHCO3, 1,4-dioxane/H2O, rt, 20% (one-pot); (viii) H2O, reflux, 12 h, then evap.; (ix) benzyl 6-aminohexanoate toluenesulfonic acid salt, EDCI, Et3N, DMAP, CH2Cl2, 16% (one-pot); (x) H2, Pd/C, EtOH, rt, 94%; (xi) sulfo-NHS, EDCI, DMF/H2O, rt, 12 h, then TT, PBS, 4 °C, 24 h.
The synthesis of GNE began with selective hydrolysis of the methyl ester in cocaine (1) using a microwave reactor to yield benzoylecgonine (3). Notably, this microwave-assisted hydrolysis was completed after 2 h, while conventional conditions required 7 days.28 After solvent removal, crude 3 was condensed with benzyl 6-aminohexanoate using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholium chloride (DMTMM) in the same pot to afford benzyl ester 7 in good yield. The benzyl ester was reduced upon treatment with H2 and Pd/C to give GNE (5) in 35% overall yield, improving on our first generation synthetic effort by 3-fold. Having GNE in hand, we pursued hapten–protein conjugation. The carboxylic acid of 5 was coupled with N-hydroxysulfosuccinimide (sulfo-NHS) using 1-ethyl-3-(3-(dimethylamino)propyl) carbodiimide (EDCI) and treated with TT to afford the desired GNE-TT immunoconjugate (8).
Our GND synthesis commenced with microwave-assisted one-pot desalinization/condensation of methylecgonone bitartrate (9) to afford enamine 10. Reduction of 10 with NaBH3CN under ca. pH 4 gave a highly polar amine (11). After solvent removal, crude 11 was converted to benzamide 12 using BzCl and NaHCO3 in the same pot. Although the stereochemistry of the methyl ester in 12 was undesired, epimerization occurred under aqueous refluxing conditions to afford a mixture of the desired α-carboxylic acid and the undesired β-carboxylic acid in a 1:1 ratio. This mixture of carboxylic acids was immediately subjected to amidation conditions using EDCI, Et3N, and 4-dimethylaminopyridine (DMAP) to give a mixture of stereoisomers. Benzyl ester 14 was isolated from this mixture by preparative TLC. Finally, the benzyl ester in 14 was cleaved under standard hydrogenation conditions to afford GND (6) in 1.8% overall yield. The significant improvement to this route includes the incorporation of multiple one-pot reactions, reducing the need for workup and purification steps. With the requisite 6 in hand, we undertook hapten–protein conjugation. After the activation of 6 with sulfo-NHS using the conditions described above, activated 6 was treated with TT to afford the desired GND-TT immunoconjugate (15).
Mice were intraperitoneally administered the immunoconjugates GNE-TT or GND-TT. Alum and CpG ODN 1826 were employed as adjuvants in all vaccines. Antibody titers were assessed by ELISA against a corresponding hapten-BSA conjugate, namely GNE- or GND-BSA. For all bleeds (21, 35, 70, and 84 days), mice vaccinated with GND-TT elicited higher antibody titers than the GNE-TT vaccinated mice, though no significant difference was observed (Figure 2). The highest GND-TT induced titers were observed after the second bleed, while the GNE-TT induced titers peaked at the first bleed and steadily decreased. In general, both vaccines successfully induced high anticocaine antibody titers throughout the course of this study.
Figure 2.
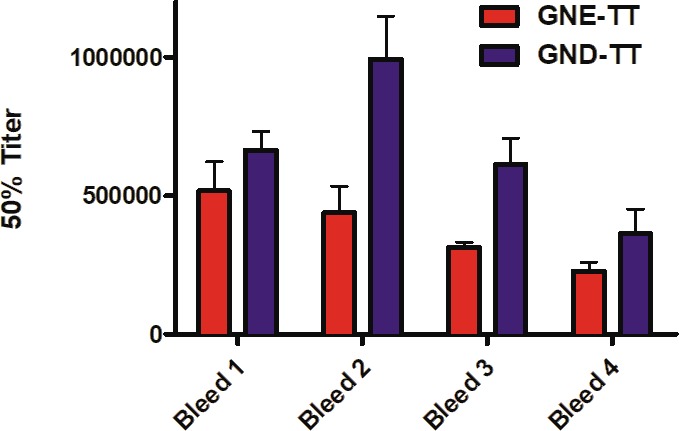
Anticocaine antibody midpoint titers measured by ELISA using serum from vaccinated mice (n = 6/group) on days 21 (bleed 1), 35 (bleed 2), 70 (bleed 3), and 84 (bleed 4) for each study group. Bars denote means ± SEM.
While ELISA titers are traditionally used to measure vaccine efficacy, this methodology can misrepresent the affinity and avidity of the generated antibodies for the native drug because ELISA evaluates binding to a coated antigen.29 To further quantify the antibody concentrations and anticocaine affinities raised by GNE-TT and GND-TT, a competitive radioimmunoassay (RIA) was conducted (Table 1). Mice in all groups were found to possess antibodies with high affinity for cocaine: Kd = 19 nM (GND) and 56 nM (GNE). The GND-TT vaccine induced antibodies with higher affinity for cocaine, although these antibodies were raised in lower concentrations. Demonstrating the opposite trend, serum from GNE-TT vaccinated mice exhibited higher antibody concentrations, though the affinities for cocaine were lower compared to the GND vaccine. This is a particularly interesting result because the GNE hapten is more structurally similar to cocaine; despite this, lower anticocaine antibody affinities were observed. The reduced affinities presumably result from the hydrolytic instability of GNE (versus GND). In other words, GNE can more readily undergo metabolic decomposition in vivo following immunization. Decomposition produces new antigenic motifs and thus the generation of nonspecific antibodies for cocaine. An alternative explanation is the increased hydrogen bonding capability of GND. Amides function as one of the best hydrogen bond donors/acceptors in molecular recognition.30 Because the GND hapten incorporates two amide functionalities in the correct stereochemical configuration (of cocaine), the antibody affinity for cocaine may be improved as a result of the increased hydrogen bonding interactions of GND in the antibody/antigen complex.13
Table 1. Anticocaine Antibody Kd Values and Concentrations Measured by Competitive RIA.
| vaccinea | Kd (nM) | Ab (μM) |
|---|---|---|
| GNE-TT | 55.8 ± 1.4 | 59.1 ± 5.0 |
| GND-TT | 18.8 ± 2.0 | 11.8 ± 3.8 |
Serum samples from vaccinated mice (n = 6/group) on day 21 were pooled for analysis. Data is reported as means ± SEM.
Being a stimulant, cocaine induces a quantifiable increase in rodent locomotor activity upon exposure. To assess vaccine performance, vaccinated mice were subjected to hyperlocomotion testing using ANY-Maze video tracking software (Stoelting Co.). Vaccine efficacy is measured in its ability to block cocaine-induced hyperlocomotion. As expected, naïve mice exhibited elevated hyperlocomotor activity with increasing doses of cocaine (Figure 3). Alternatively, GND-TT and GNE-TT exhibited robust blockade of cocaine’s psychostimulatory effects at 5, 10, and 20 mg/kg doses. Despite the higher induced titers and superior anticocaine antibody affinities of GND-TT, both vaccines were comparably effective in stunting cocaine-induced behavior.
Figure 3.
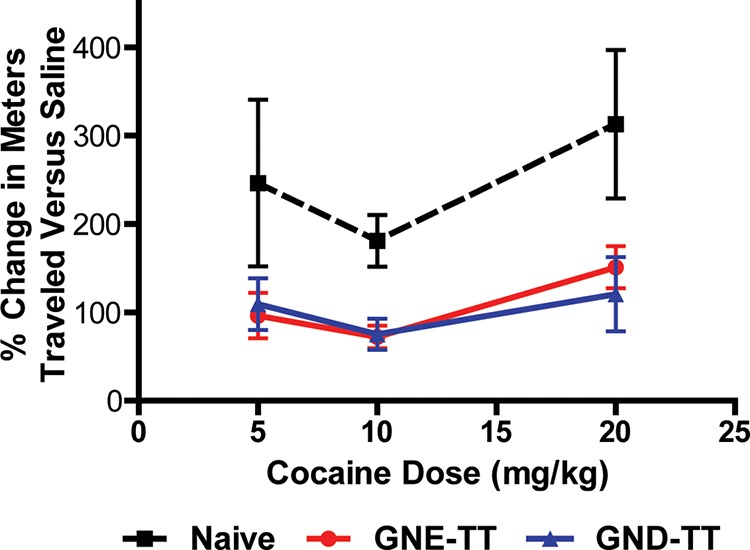
Cocaine-induced hyperlocomotor activity in vaccinated mice (n = 6/group) at 0, 5, 10, and 20 mg/kg on days 42 (0, 5 mg/kg), 44 (10 mg/kg), and 46 (20 mg/kg), respectively. Bars denote means ± SEM. Significance was observed.
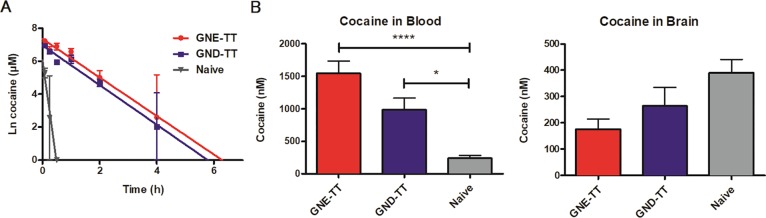
To evaluate the effect of GNE-TT and GND-TT vaccines on long-term drug metabolism, 20 mg/kg of cocaine was administered to all vaccinated and naïve mice. Blood from each mouse was serially collected over the course of 24 h.31 The amount of cocaine remaining in the blood at 0, 2, 4, and 6 h time points was quantified using LCMS (Figure 4A). In naïve mice, cocaine was found to have a serum half-life of 3.3 min. Vaccination was able to prolong cocaine’s half-life to 34.5 (GND) and 35.6 (GNE) minutes; a 10.4- to 10.7-fold increase over naïve animals, respectively (SI Table 1). As designed, the GNE-TT and GND-TT vaccines increased the peak concentration of cocaine in serum with comparable efficacy, demonstrating successful sequestration of free drug in circulation/outside the CNS.
Figure 4.
Serum pharmacokinetic results for vaccinated and naïve mice (n = 6 total, n = 2 per time point) on day 81 (A). Concentration of cocaine in blood and brain of vaccinated and naïve mice (n = 6) on day 101 (B). Bars denote means ± SEM; *P < 0.05 and ****P < 0.0001 were determined by one-way ANOVA.
To confirm these findings, biodistribution of the drug was investigated. After treating vaccinated and naïve mice with an additional 20 mg/kg cocaine, mice were sacrificed after 15 min by decapitation. Drug concentrations in the blood and brain were quantified by LCMS. As with the PK data discussed vide supra, the biodistribution data demonstrated that vaccine-generated antibodies effectively scavenged large amounts of free drug in the blood (Figure 4B). More specifically, mice vaccinated with GNE-TT appropriated nearly six times more cocaine in the blood than naïve mice, while GND-TT sequestered about four times more cocaine. Positively, both GNE-TT and GND-TT vaccines effectively lowered the cocaine concentration in the brain.
Comparing the results of ELISA, RIA, and behavioral testing in this study revealed that hydrolytically stable haptens, GNE and GND, are extremely effective anticocaine vaccines when conjugated to TT and adjuvanted with alum and CpG ODN 1826. Both GNE-TT and GND-TT elicited antibodies that effectively blocked the drug’s psychostimulatory activity at all tested concentrations. Accordingly, both the GND-TT and GNE-TT vaccines exhibited successful sequestration of cocaine outside the CNS. The results of this comparative analysis suggest that both GND-TT and GNE-TT present viable candidates for further consideration in more advanced rodent and nonhuman primate preclinical models, including self-administration studies to determine the surmountability and clinical-viability of these next-generation anticocaine vaccines.
Acknowledgments
The authors thank Beverly Ellis for her assistance with mouse blood collection. The authors thank Dr. Cody Wenthur and Dr. Paul Bremer for helpful discussions. This work was supported by generous funding from NIDA (R01 DA008590 to K.D.J. and F32 DA044692 to M.E.O.).
Biography
Dr. Kim D. Janda is the Ely R. Callaway, Jr. Chaired Professor in the Departments of Chemistry, Immunology, and Microbial Science at The Scripps Research Institute and is the Director of the Worm Institute of Research and Medicine. He is well known for his bridging of the fields of chemistry and immunology. Working at this interface, he has shown how antibodies could be transformed from simple binding entities to catalysts. He pioneered treating drug addiction though immunopharmacotherapy, the creation of synthetic diversity methods, including phage display, and small molecule DNA encoding methodologies. He has published over 550 peer-reviewed papers.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00051.
Experimental details, MALDI spectra, standard curve for LCMS quantification, half-life table, immobility times, and RIA-generated binding curves (PDF)
Author Present Address
† Specially Appointed Assistant Professor, Laboratory of Natural Products for Drug Discovery, Research Center for Drug Discovery, Graduate School of Pharmaceutical Sciences, Osaka University, 1-6 Yamadaoka, Suita, Osaka, 565-0871, Japan.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- U.S. Department of Health and Human Services (HHS), Office of the Surgeon General . Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health; HHS: Washington, DC, 2016. [PubMed]
- Drug Abuse Warning Network (DAWN) . HHS Publication No. (SMA) 13–4760, DAWN Series D-39 (ICPSR 34565); Substance Abuse and Mental Health Services Administration: Rockville, MD. 2011. [PubMed] [Google Scholar]
- Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. NSDUH Series H-44, HHS Publication No. (SMA) 15–4927; Substance Abuse and Mental Health Services Administration: Rockville, MD. 2015. [Google Scholar]
- Karila L.; Reynaud M.; Aubin H. J.; Rolland B.; Guardia D.; Cottencin O.; Benyamina A. Pharmacological treatments for cocaine dependence: is there something new?. Curr. Pharm. Des. 2011, 17, 1359–1368. 10.2174/138161211796150873. [DOI] [PubMed] [Google Scholar]
- Loftis J. M.; Huckans M. Substance use disorders: psychoneuroimmunological mechanisms and new targets for therapy. Pharmacol. Ther. 2013, 139, 289–300. 10.1016/j.pharmthera.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A. Y.; Janda K. D. Current challenges for the creation of effective vaccines against drugs of abuse. Expert Rev. Vaccines 2011, 10, 1637–1639. 10.1586/erv.11.145. [DOI] [PubMed] [Google Scholar]
- Janda K. D.; Treweek J. B. Vaccines targeting drugs of abuse: is the glass half-empty or half-full?. Nat. Rev. Immunol. 2012, 12, 67–72. 10.1038/nri3130. [DOI] [PubMed] [Google Scholar]
- Haney M.; Gunderson E. W.; Jiang H. P.; Collins E. D.; Foltin R. W. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol. Psychiatry 2010, 67, 59–65. 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell B. A.; Mitchell E.; Poling J.; Gonsai K.; Kosten T. R. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol. Psychiatry 2005, 58, 158–164. 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Martell B. A.; Orson F. M.; Poling J.; Mitchell E.; Rossen R. D.; Gardner T.; Kosten T. R. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch. Gen. Psychiatry 2009, 66, 1116–1123. 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T. R.; Domingo C. B.; Shorter D.; Orson F.; Green C.; Somoza E.; Sekerka R.; Levin F. R.; Mariani J. J.; Stitzer M.; Tompkins D. A.; Rotrosen J.; Thakkar V.; Smoak B.; Kampman K. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014, 140, 42–47. 10.1016/j.drugalcdep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T. R.; Rosen M.; Bond J.; Settles M.; St. Clair Roberts J.; Shields J.; Jack L.; Fox B. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine 2002, 20, 1196–1204. 10.1016/S0264-410X(01)00425-X. [DOI] [PubMed] [Google Scholar]
- Carrera M. R.; Ashley J. A.; Wirsching P.; Koob G. F.; Janda K. D. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 1988–1992. 10.1073/pnas.98.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M.; Wirsching P.; Janda K. D. Design and synthesis of a cocaine-diamide hapten for vaccine development. Tetrahedron Lett. 1996, 37, 5479–5482. 10.1016/0040-4039(96)01170-7. [DOI] [Google Scholar]
- Van Dyke C.; Barash P. G.; Jatlow P.; Byck R. Cocaine: plasma concentrations after intranasal application in man. Science 1976, 191, 859–861. 10.1126/science.56036. [DOI] [PubMed] [Google Scholar]
- Inaba T.; Stewart D. J.; Kalow W. Metabolism of cocaine in man. Clin. Pharmacol. Ther. 1978, 23, 547–552. 10.1002/cpt1978235547. [DOI] [PubMed] [Google Scholar]
- Carrera M. R.; Ashley J. A.; Parsons L. H.; Wirsching P.; Koob G. F.; Janda K. D. Suppression of psychoactive effects of cocaine by active immunization. Nature 1995, 378, 727–730. 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- Cai X. Q.; Whitfield T.; Moreno A. Y.; Grant Y.; Hixon M. S.; Koob G. F.; Janda K. D. Probing the effects of hapten stability on cocaine vaccine immunogenicity. Mol. Pharmaceutics 2013, 10, 4176–4184. 10.1021/mp400214w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoz A.; Hicks M. J.; Vallabhjosula S.; Synan M.; Kothari P. J.; Dyke J. P.; Ballon D. J.; Kaminsky S. M.; De B. P.; Rosenberg J. B.; Martinez D.; Koob G. F.; Janda K. D.; Crystal R. G. Adenovirus capsid-based anti-cocaine vaccine prevents cocaine from binding to the nonhuman primate CNS dopamine transporter. Neuropsychopharmacology 2013, 38, 2170–2178. 10.1038/npp.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks M. J.; Kaminsky S. M.; De B. P.; Rosenberg J. B.; Evans S. M.; Foltin R. W.; Andrenyak D. M.; Moody D. E.; Koob G. F.; Janda K. D.; Ricart Arbona R. J.; Lepherd M. L.; Crystal R. G. Fate of systemically administered cocaine in nonhuman primates treated with the dAd5GNE anticocaine vaccine. Hum. Gene Ther.: Clin. Dev. 2014, 25, 40–49. 10.1089/humc.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S. M.; Foltin R. W.; Hicks M. J.; Rosenberg J. B.; De B. P.; Janda K. D.; Kaminsky S. M.; Crystal R. G. Efficacy of an adenovirus-based anti-cocaine vaccine to reduce cocaine self-administration and reacqusition using a choice procedure in rhesus macaques. Pharmacol., Biochem. Behav. 2016, 150–151, 76–86. 10.1016/j.pbb.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P.; Pagovich O. E.; Hicks M. J.; Rosenberg J. B.; Moreno A. Y.; Janda K. D.; Koob G. F.; Worgall S.; Kaminsky S. M.; Sondhi D.; Crystal R. G. Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity. Hum. Gene Ther. 2013, 24, 58–66. 10.1089/hum.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks M. J.; De B. P.; Rosenberg J. B.; Davidson J. T.; Moreno A. Y.; Janda K. D.; Wee S.; Koob G. F.; Hackett N. R.; Kaminsky S. M.; Worgall S.; Toth M.; Mezey J. G.; Crystal R. G. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol. Ther. 2011, 19, 612–619. 10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.; Hicks M. J.; Wee S.; Rosenberg J. B.; De B. P.; Kaminsky S. M.; Moreno A.; Janda K. D.; Crystal R. G. Anti-cocaine vaccine based on coupling a cocaine analog to a disrupted adenovirus. CNS Neurol. Disord.: Drug Targets 2011, 10, 899–904. 10.2174/187152711799219334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. B.; Hicks M. J.; De B. P.; Pagovich O.; Frenk E.; Janda K. D.; Wee S.; Koob G. F.; Hackett N. R.; Kaminsky S. M.; Worgall S.; Tignor N.; Mezey J. G.; Crystal R. G. AAVrh.10-mediated expression of an anti-cocaine antibody mediates persistent passive immunization that suppresses cocaine-induced behavior. Hum. Gene Ther. 2012, 23, 451–459. 10.1089/hum.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S.; Hicks M. J.; De B. P.; Rosenberg J. B.; Moreno A. Y.; Kaminsky S. M.; Janda K. D.; Crystal R. G.; Koob G. F. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology 2012, 37, 1083–1091. 10.1038/npp.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimishima A.; Wenthur C. J.; Eubanks L. M.; Sato S.; Janda K. D. Cocaine vaccine development: Evaluation of carrier and adjuvant combinations that activate multiple Toll-like receptors. Mol. Pharmaceutics 2016, 13, 3884–3890. 10.1021/acs.molpharmaceut.6b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin A. H.; Hayes J. P.; Zhong D.. Methods for producing hydroxyalkyl tropane esters. WO2004018464 Apr. 3, 2004.
- Pravetoni M.; Keyler D. E.; Pidaparthi R. R.; Carroll F. I.; Runyon S. P.; Murtaugh M. P.; Earley C. A.; Pentel P. R. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem. Pharmacol. 2012, 83, 543–550. 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery G. A.; Saenger W.. Hydrogen Bonding in Biological Structures; Springer: New York, 1991. [Google Scholar]
- McCluskie M. J.; Evans D. M.; Zhang N.; Benoit M.; McElhiney S. P.; Unnithan M.; DeMarco S. C.; Clay B.; Huber C.; Deora A.; Thorn J. M.; Stead D. R.; Merson J. R.; Davis H. L. The effect of preexisting anti-carrier immunity on subsequent responses to CRM197 or Qb-VLP conjugate vaccines. Immunopharmacol. Immunotoxicol. 2016, 38, 184–196. 10.3109/08923973.2016.1165246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.