Abstract
8-Acetoxycycloberberine (2) with a unique skeleton was first identified to display a potent activity profile against Gram-positive bacteria, especially methicillin-resistant S. aureus (MRSA) with minimum inhibitory concentration (MIC) values of 1–8 μg/mL, suggesting a possible novel mechanism of action against bacteria. Taking 2 as the lead, 23 new 8-substituted cycloberberine (CBBR) derivatives including ether, amine, and amide were synthesized and evaluated for their antibacterial effect. The structure–activity relationship revealed that the introduction of a suitable substituent at the 8-position could greatly enhance the potency against MRSA. Among them, compounds 5d and 9e demonstrated equally effective anti-MRSA potency as lead 2, with an advantage of having a more stable pharmacokinetics feature. A preliminary mechanism study indicated that compound 9e acted upon bacteria partly through catalyzing the cleavage of bacterial DNA. Therefore, we consider that 8-substituted CBBR derivatives constitute a promising class of antibacterial agents in the treatment of MRSA infections.
Keywords: Cycloberberine, berberine, structure−activity relationship, antibacterial, MRSA
Antimicrobial resistance is a major global health concern, and of the Gram-positive bacteria, drug-resistant Staphylococcus aureus (S. aureus) is a serious threat.1 Over a period of decades, S. aureus isolated from patients has developed increasing resistance to several classes of clinical antibacterials, such as β-lactam, fluoroquinolone, macrolide, glycopeptide, and oxazolidinone.2,3 Especially, the challenge of treating methicillin-resistant S. aureus (MRSA) was highlighted in a recent report published by the U.S. Centers for Disease Control and Prevention (CDC).4 Vancomycin, as one of the last resort therapies for MRSA infection, has issues that restrict its utility including slow bactericidal activity, low tissue penetration, and increasing reports of vancomycin-intermediate S. aureus (VISA).5−7 Although daptomycin remains as one of the main treatment options for MRSA, resistance cases in patients treated with daptomycin are a growing concern.8,9 This situation has resulted in a very pressing need for the discovery of novel antibacterial candidates for the treatment of infections arising from MRSA.
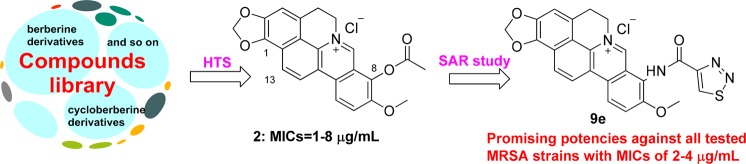
To explore and discover new chemical entity (NCE) against MRSA, a library of berberine (1, Figure 1) derivatives constructed in our lab was screened for their antibacterial activity using phenotype screening assay.10 Surprisingly, cycloberberine (CBBR, Figure 1) derivatives,11 such as 8-acetoxycyclo-berberine (2, Figure 1), were first identified to display satisfactory activity profile against both methicillin-susceptible S. aureus (MSSA) and MRSA strains with minimum inhibitory concentration (MIC) values ranging from of 1–8 μg/mL (Table 1), suggesting a possible novel mechanism of action against bacteria. The unique chemical scaffold and antibacterial activity profile of compound 2 against MRSA organisms, high-level resistant pathogens across the world, spurred us to further carry out structural modifications and optimization so as to develop them into a novel family of antimicrobial agents against MRSA.
Figure 1.
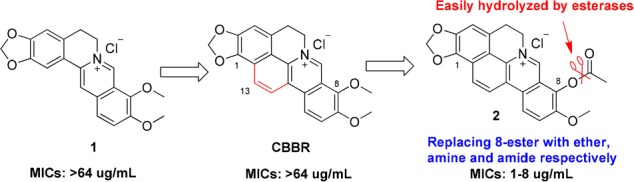
Structures and antibacterial activities of 1, CBBR, and 2, as well as the modification strategy.
Table 1. Antimicrobial Activities of the Target Compounds against Drug-Susceptible Gram-Positive Strains.
MIC (μg/mL), minimum inhibitory concentration.
The American Type Culture Collection (ATCC).
Strains isolated from patients in Chinese hospitals.
However, as shown in Figure 1, it is well-known that the ester bond at the 8-position of compound 2 could be easily hydrolyzed by esterases resulting in a poor in vivo pharmacokinetic (PK) profile. Therefore, a series of new 8-substituted CBBR ether, amine, and amide derivatives was then constructed and synthesized, aiming at overcoming PK instability of compound 2. In the present study, taking compound 2 as the lead, we describe 23 new 8-substituted CBBR analogues for their synthesis, in vitro effects against MSSA and MRSA, structure–activity relationship (SAR) analysis, and stability in blood as well as primary mechanism of action of the representative compound.
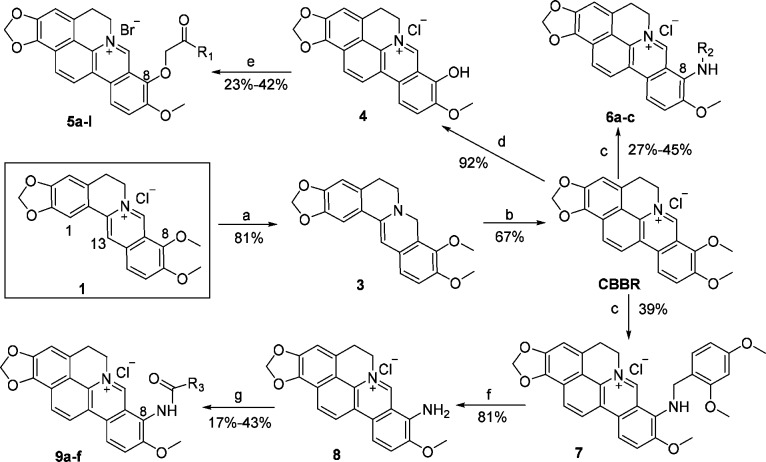
The synthetic route used for the preparation of the CBBR analogues is described in Scheme 1 with commercially available 1 as the starting material. Compound 1 was selectively reduced to the dihydroberberine (3) using NaBH4 as a reductive agent in the presence of 5% NaOH/K2CO3 in 81% yield. Glyoxal (40%) was added to the mixture of 3 and HOAc/CH3CN to give 13-acetaldehyde berberine, which was used for the next step without purification.11 A cyclization reaction took place on the addition of methanol/HCl (2:1 by vol.), and the desired CBBR was obtained with a combined yield of 67%, much higher than our previous report (38%).12 Then, CBBR was heated at 195–210 °C under vacuum (20–30 mmHg) and acidified in concentrated HCl/ethanol (5:95 by vol.) to get the key intermediate 4 in 92% yield using the previous procedures.11,12 Compound 4 was etherified to provide the final products 5a–l in 23–42% yield.
Scheme 1. Synthesis of All the Target Compounds.
Reagents and conditions: (a) NaBH4, 5% NaOH/K2CO3, CH3OH, rt, 3 h; (b) (1) 40% glyoxal, HOAc/CH3CN, reflux, 6 h; (2) methanol/HCl (2:1 by vol.), rt, 24 h; (c) 2,4-dimethoxybenzylamine/R2NH2, 100–116 °C, 4–32 h; (d) 20–30 mmHg, 195–210 °C, 40 min; (e) R1COCH2Br, KOH, DMF, 68–75 °C, 4–24 h; (f) 1:1 HCl/CH3OH, rt, 24 h; (g) R3COCl, pyridine, CH3CN, 40–91 °C, 3–72 h.
Compounds 6a–c were prepared using the corresponding amines in 27–45% yield. Similarly, intermediate 7 was prepared with 2,4-dimethoxybenzylamine as the nucleophilic reagent as well as the solvent. Free amine was then obtained by the removal of 2,4-dimethoxybenzyl using HCl/CH3OH in 81% yield. The desired products 9a–f were obtained by amidation with corresponding acyl chloride using pyridine as the base with 17–43% yield. All the final products were purified with flash column chromatography on silica gel using CH2Cl2 and MeOH as eluent.
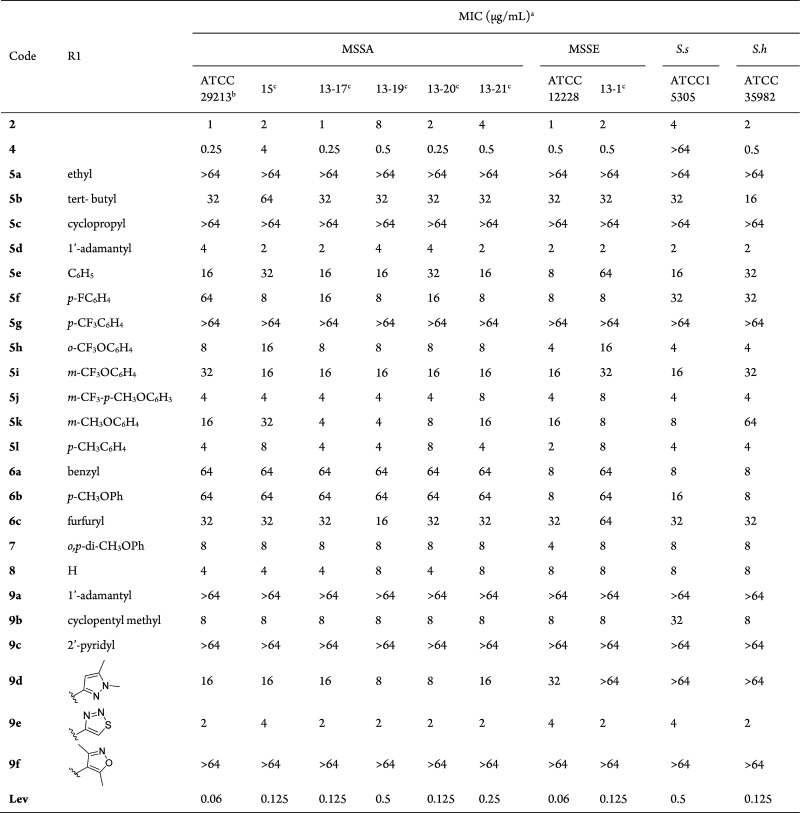
All of the newly synthesized compounds were initially examined for their activities using standard techniques13 against drug-susceptible Gram-positive strains including MSSA, methicillin-susceptible S. epidermidis (MSSE), S. saprophyticus (S. s), and S. hominis (S. h) taking levofloxacin (Lev) as a reference drug. Structures of the 23 CBBR analogues and their MIC values against Gram-positive bacteria are summarized in Table 1.
The SAR study was mainly focused on the influence of the substituent at the 8-position, and then a series of 8-substituted CBBR derivatives was obtained. First, 8-acetoxy in compound 2 was respectively replaced with several ether groups, by which CBBR ether analogues 5a–l were then generated and tested. Compounds 5a–c with small aliphatic chains or rings lost their antimicrobial activities completely; while compound 5d with large adamantyl14 exhibited activities with MICs ranging from 2 to 4 μg/mL, similar to that of lead 2. The results suggested that large volume at position 8 might be helpful for maintaining good activities. Based on the results, various benzenes with electron-withdrawing or electron-donating groups were respectively attached, and then new compounds 5e–l were created and tested. Compound 5g is much weaker than other analogs with similar phenyl substitutions, while compounds 5f, 5h, and 5i–l exhibited comparable antimicrobial activities with MICs values of 2–32 μg/mL, regardless of electron-withdrawing or electron-donating groups on the benzene ring.
Then, we moved our SAR investigation onto the amine or amide moiety at the 8-position. With an introduction of a free amine group, 2-furanmethanamine, and substituted benzylamines at position 8, respectively, five new CBBR amine analogues (6a–c, 7, and 8) were generated and measured. However, as described in Table 1, all of them abolished the activity completely or partially, suggesting that introduction of an amine substituent at the 8-position might not be helpful for potency. The potencies of compounds 7 and 8 reduced slightly, and the MIC values ranged from 4 to 8 μg/mL compared with the lead 2. Introduction of amide resulted in compounds 9a–f that were tested for activity. The activity of compound 9a with adamantyl was abolished, while the potencies of compounds 9b–d and 9f with cyclic or heterocyclic dropped obviously. Both compound 9a and 5d contain 1-adamantyl moiety, while the activity is quite different. Surprisingly, compound 9e with 1,2,3-thiadiazole15,16 attached exhibited comparable activity to the lead 2 (MICs = 1–8 μg/mL), suggesting that a thiadiazole group might be beneficial for antibacterial activity. The results illustrated that introducing a suitable substituent at the 8-position might be helpful for potency.
All of the target compounds were measured for their antibacterial activity using standard methods13 against ten drug-resistant S. aureus strains (MRSA/VISA) with Lev as a reference drug as summarized in Table 2. Their potencies against MRSA/VISA were basically consistent with that of drug-susceptible strains, suggesting a different mechanism of action from the current antibacterial drugs. Among them, most of compounds showed potential anti-MRSA effect on not only ATCC strains but also isolated ones from Chinese patients, with MIC values between 2 and 64 μg/mL. More importantly, compounds 5d, 7, 8, 9b, and 9e displayed promising effects against both MRSA and VISA strains with MICs values ranging from 2–64 μg/mL, similar to reference drug Lev. Especially, compounds 5d and 9e with different structures displayed excellent potencies with MIC of 2–4 μg/mL, and thus, both of them were selected as representative compounds for the next investigation. In addition, the key intermediate 4 was also tested for the activity, and results showed that it displayed a promising effect against both MRSA and VISA.
Table 2. Antibacterial Activities of the Target Compounds against Drug-Resistant Gram-Positive Strains.
| MIC
(μg/mL)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MRSA |
VISA |
|||||||||
| Code | ATCC 33591 | 13–18 | 13–23 | 12–3 | 12–8 | 12–13 | 12–16 | ATCC-BAA-1708b | ATCC 700698 | ATCC 700699 |
| 2 | 4 | 1 | 2 | 4 | 8 | 4 | 2 | 8 | 16 | 32 |
| 4 | 0.25 | 0.25 | 0.25 | 0.25 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 0.25 |
| 5a | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 5b | 32 | 32 | 32 | 32 | 64 | 32 | 32 | 32 | >64 | >64 |
| 5c | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 5d | 2 | 4 | 2 | 4 | 4 | 4 | 2 | 4 | 8 | 8 |
| 5e | 16 | 32 | 16 | 16 | 16 | 32 | 32 | 32 | >64 | >64 |
| 5f | 8 | 16 | 8 | 8 | 8 | 8 | 16 | 64 | >64 | >64 |
| 5g | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 5h | 8 | 8 | 8 | 16 | 8 | 4 | 4 | 32 | >64 | >64 |
| 5i | 16 | 16 | 16 | 16 | 8 | 32 | 16 | 64 | >64 | >64 |
| 5j | 4 | 8 | 4 | 4 | 8 | 4 | 8 | 16 | >64 | >64 |
| 5k | 4 | 4 | 16 | 16 | 16 | 8 | 8 | 64 | >64 | >64 |
| 5l | 4 | 4 | 4 | 4 | 8 | 4 | 4 | 8 | >64 | >64 |
| 6a | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 32 | >64 |
| 6b | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 64 | 16 | >64 |
| 6c | 64 | 64 | 64 | 32 | 64 | 32 | 64 | >64 | >64 | >64 |
| 7 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 16 | 4 | 8 |
| 8 | 4 | 8 | 8 | 8 | 4 | 4 | 4 | 4 | 64 | 4 |
| 9a | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 9b | 8 | 8 | 8 | 8 | 8 | 16 | 8 | 4 | 64 | 4 |
| 9c | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 9d | 64 | 32 | 8 | 32 | 32 | 16 | 16 | 16 | >64 | 4 |
| 9e | 2 | 2 | 2 | 2 | 2 | 4 | 2 | 2 | 16 | 8 |
| 9f | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Lev | 0.125 | 32 | 32 | 32 | 32 | 32 | 32 | 16 | 32 | 2 |
MIC (μg/mL), minimum inhibitory concentration.
mupA positive (isolates with mupirocin resistance).
Thus, compound 4 could be selected as a parent structure to make prodrugs for potential antibacterial agents against MRSA.
As displayed in Figure 2, CBBR ether 5d and amine 9e were chosen to further explore the in vitro stability in whole blood taking enalapril containing an ester bond (Figure 2) as the control.17,18 Compounds 2, 5d, and 9e and enalapril were incubated with blood isolated from the Sprague-Dawly (SD) rats, and samples were taken out at 0, 30, 60, 120, 240, and 420 min, respectively. As expected, after 420 min, the remaining ratios of the compounds 5d and 9e in blood were still 58.0 and 57.2%, respectively, much higher than that of lead 2 and enalapril, as described in Figure 2. The results suggested that compounds 5d and 9e possessed much more stable in vitro blood stability than lead 2.
Figure 2.
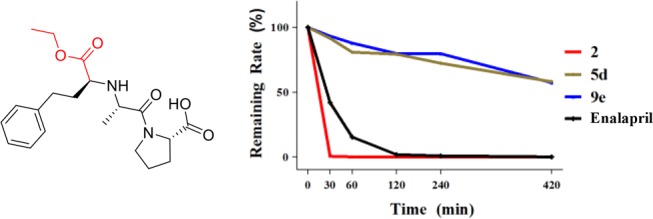
Structure of enalapril and metabolic stability of key compounds in whole blood.
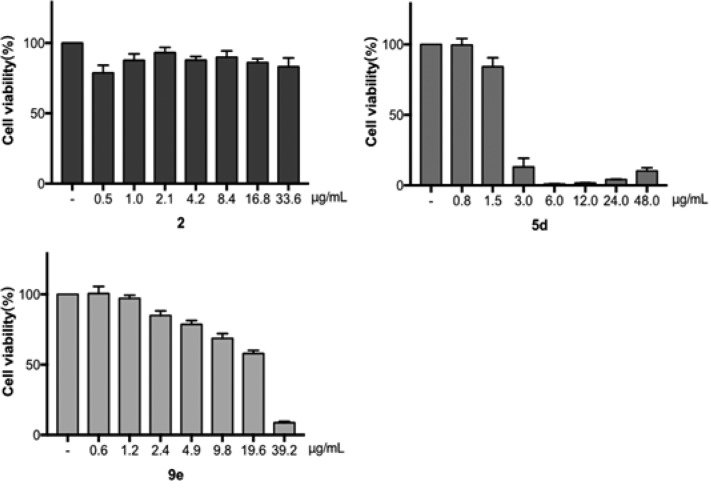
To evaluate the safety of this kind of compounds, the cytotoxic effects of the representative compounds 2, 5d, and 9e on A549 cells were carried out using MTT assay.19 As displayed in Figure 3, lead 2 had no cytotoxic activity in A549 cells with CC50 value over 33.6 μg/mL, while compound 9e demonstrated the moderate cytotoxicity with a CC50 of 16.1 μg/mL, much lower than that of compound 5d of 2.0 μg/mL. The quaternary ammonium structure at the 6-position in compound 9e made itself not easy to traverse the cellular membrane;20 therefore, compound 9e showed a moderate selectivity toward bacteria versus cells with the MIC values of 2–4 μg/mL.
Figure 3.
Cytotoxic effects of compounds 2, 5d, and 9e on A549 cells. Following pretreatment with compounds 2, 5d, and 9e at the indicated concentrations for 24 h, the cell viability of A549 cells were determined by MTT assay. Control cells were treated with 0.4% (v/v) DMSO.
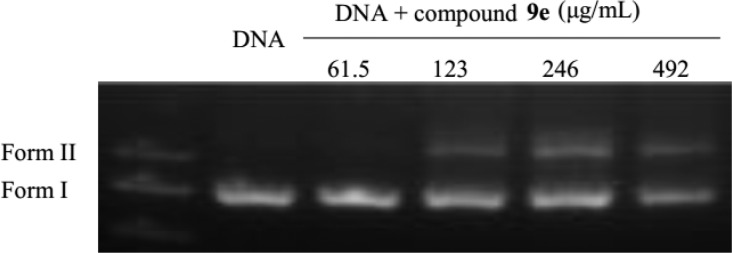
In order to further understand the mechanism of action of this kind of compounds, the preliminary mechanism of action of the representative compound 9e was carried out. Based on its plane rigid structure, we speculated that this kind of compounds might act upon microorganisms by intercalating into DNA of bacteria.21 Therefore, the activity of compound 9e toward supercoiled pET-32a (bacterial expression vector) DNA was conducted under physiological conditions (4 h, 37 °C) using agarose gel electrophoresis (GE). As shown in Figure 4, a strong dependence on the concentration (lanes 2–5), converting pET-32a DNA from CCC form (Form I) into open circular form (OC, Form II), could be detected on their cleaving activity. This result indicated that compound 9e acted upon bacteria partly through catalyzing the cleavage of bacterial DNA. Furthermore, compound 9e exerted the potent antibacterial activity with MIC values of 2–4 μg/mL, possibly due to other antibacterial mechanisms of this kind of compounds.22−24
Figure 4.
Agarose GE patterns for the cleavage of pET-32a DNA by compound 9e of increasing concentrations at 37 °C (4 h).
Twenty-three new 8-substituted CBBR derivatives with a unique chemical scaffold were synthesized and examined for their activity against Gram-positive bacteria including MRSA and VISA. SAR revealed that a suitable substituent at the 8-position could greatly enhance the antibacterial potency. Among them, compounds 5d and 9e exhibited potent activities against all tested drug-susceptible and drug-resistant strains with MICs ranging from 2 to 4 μg/mL, suggesting a possible novel mechanism of action against bacteria. Moreover, compound 9e showed much better stability in whole blood than that of lead 2. A preliminary mechanism study indicated that compound 9e acted upon bacteria partly through catalyzing the cleavage of bacterial DNA. Therefore, we consider 8-substituted CBBR derivatives to be a promising class of anti-MRSA agents, and compound 9e has been chosen for the further investigation.
Glossary
ABBREVIATIONS
- MRSA
methicillin-resistant S. aureus
- VISA
vancomycin-intermediate S. aureus
- MSSE
methicillin-susceptible S. epidermidis
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00094.
Synthetic procedures, analytical data, and antibacterial and cytotoxic assays (PDF)
Author Contributions
† T.F. and X.H. contributed equally.
This work was supported by the National Natural Science Foundation of China (81361138020 and 81621064), Beijing Natural Science Foundation (7172136), and the CAMS initiative for innovative medicine (2017-12M-1-012).
The authors declare no competing financial interest.
Supplementary Material
References
- Brown E. D.; Wright G. D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- Liu C.; Bayer A.; Cosgrove S. E.; Daum R. S.; Fridkin S. K.; Gorwitz R. J.; Kaplan S. L.; Karchmer A. W.; Levine D. P.; Murray B. E.; Rybak J. M.; Talan D. A.; Chambers H. F. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 2011, 52, 285–292. 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- Drebes J.; Künz M.; Pereira C. A.; Betzel C.; Wrenger C. MRSA infections: from classical treatment to suicide drugs. Curr. Med. Chem. 2014, 21, 1809–1819. 10.2174/0929867320666131119122520. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/threat-report-2013/.
- Mohammad H.; Mayhoub A. S.; Ghafoor A.; Soofi M.; Alajlouni R. A.; Cushman M.; Seleem M. N. Discovery and characterization of potent thiazoles versus methicillin-and vancomycin-resistant Staphylococcus aureus. J. Med. Chem. 2014, 57, 1609–1615. 10.1021/jm401905m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak M.; Lomaestro B.; Rotschafer J. C.; Moellering R.; Craig W. Jr; Billeter M.; Dalovisio J. R.; Levine D. P. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health-Syst. Pharm. 2009, 66, 82–98. 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- Han J. H.; Edelstein P. H.; Lautenbach E. Reduced vancomycin susceptibility and staphylococcal cassette chromosome mec (SCCmec) type distribution in methicillin-resistant Staphylococcus aureus bacteraemia. J. Antimicrob. Chemother. 2012, 67, 2346–2349. 10.1093/jac/dks255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullar R.; Casapao A. M.; Davis S. L.; Levine D. P.; Zhao J. J.; Crank C. W.; Segreti J.; Sakoulas G.; Cosgrove S. E.; Rybak M. J. A multicentre evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J. Antimicrob. Chemother. 2013, 68, 2921–2926. 10.1093/jac/dkt294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M.; Riederer K.; Chase P.; Khatib R. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 433–437. 10.1007/s10096-007-0455-5. [DOI] [PubMed] [Google Scholar]
- Wang Y. X.; Fu H. G.; Li Y. H.; Jiang J. D.; Song D. Q. Synthesis and biological evaluation of 8-substituted berberine derivatives as novel anti-mycobacterial agents. Acta Pharm. Sin. B 2012, 2, 581–587. 10.1016/j.apsb.2012.10.008. [DOI] [Google Scholar]
- Li Y. B.; Zhao W. L.; Wang Y. X.; Zhang C. X.; Jiang J. D.; Bi C. W.; Tang S.; Chen R. X.; Shao R. G.; Song D. Q. Discovery, synthesis and biological evaluation of cycloprotoberberine derivatives as potential antitumor agents. Eur. J. Med. Chem. 2013, 68, 463–472. 10.1016/j.ejmech.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Bi C. W.; Zhang C. X.; Li Y. B.; Zhao W. L.; Shao R. G.; Mei L.; Song D. Q. Synthesis and structure-activity relationship of cycloberberine as anti-cancer agent. Acta Pharmacol. Sin. 2013, 48, 1800–1806. [PubMed] [Google Scholar]
- MICs were determined as described by the NCCLS; see
- Liu J.; Obando D.; Liao V.; Lifa T.; Codd R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. 10.1016/j.ejmech.2011.01.047. [DOI] [PubMed] [Google Scholar]
- Sashidhara K. V.; Rao K. B.; Kushwaha P.; Modukuri R. K.; Singh P.; Soni I.; Shukla P. K.; Chopra S.; Pasupuleti M. Novel chalcone-thiazole hybrids as potent inhibitors of drug resistant Staphylococcus aureus. ACS Med. Chem. Lett. 2015, 6, 809–813. 10.1021/acsmedchemlett.5b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad H.; Reddy P. V. N.; Monteleone D.; Mayhoub A. S.; Cushman M.; Seleem M. N. Synthesis and antibacterial evaluation of a novel series of synthetic phenylthiazole compounds against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2015, 94, 306–316. 10.1016/j.ejmech.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiera S.; Kusa J. Evaluation of a rapid method for the therapeutic drug monitoring of aliskiren, enalapril and its active metabolite in plasma and urine by UHPLC-MS/MS. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2015, 980, 79–87. 10.1016/j.jchromb.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Ferron G. M.; Jusko W. J. Species differences in sirolimus stability in humans, rabbits, and rats. Drug Metab. Dispos. 1998, 26, 83–84. [PubMed] [Google Scholar]
- Wang Y. X.; Pang W. Q.; Zeng Q. X.; Deng Z. S.; Fan T. Y.; Jiang J. D.; Deng H. B.; Song D. Q. Synthesis and biological evaluation of new berberine derivatives as cancer immunotherapy agents through targeting IDO1. Eur. J. Med. Chem. 2018, 143, 1858–1868. 10.1016/j.ejmech.2017.10.078. [DOI] [PubMed] [Google Scholar]
- Richter M. F.; Drown B. S.; Riley A. P.; Garcia A.; Shirai T.; Svec R. L.; Hergenrother P. J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. 10.1038/nature22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakkumar P.; Zhang L.; Avula S. R.; Zhou C. H. Design, synthesis and biological evaluation of berberine-benzimidazole hybrids as new type of potentially DNA-targeting antimicrobial agents. Eur. J. Med. Chem. 2016, 122, 205–215. 10.1016/j.ejmech.2016.06.031. [DOI] [PubMed] [Google Scholar]
- Chu M.; Zhang M. B.; Liu Y. C.; Kang J. R.; Chu Z. Y.; Yin K. L.; Ding L. Y.; Ding R.; Xiao R. X.; Yin Y. N.; Liu X. Y.; Wang Y. D. Role of berberine in the treatment of methicillin-resistant Staphylococcus aureus infections. Sci. Rep. 2016, 6, 24748. 10.1038/srep24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N.; Du R. L.; Zheng Y. Y.; Huang B. H.; Guo Q.; Zhang R. F.; Wong K. Y.; Lu Y. J. Antibacterial activity of N-methyl benzofuro[3,2-b]quinoline and N-methylbenzoindolo[3,2-b]-quino line derivatives and study of their mode of action. Eur. J. Med. Chem. 2017, 135, 1–11. 10.1016/j.ejmech.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Kelley C.; Zhang Y.; Parhi A.; Kaul M.; Pilch D. S.; LaVoie E. J. 3-Phenyl substituted 6,7-dimethoxyisoquinoline derivatives as FtsZ-targeting antibacterial agents. Bioorg. Med. Chem. 2012, 20, 7012–7029. 10.1016/j.bmc.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.