Abstract
In this work, five new hybrids of phenylsulfonylfuroxan merging 3-benzyl coumarin and their seco-B-ring derivatives 2–6 were designed and synthesized. Among them, compound 3 showed the most potent antiproliferation activities with IC50 values range from 0.5 to 143 nM against nine drug-sensitive and four drug-resistant cancer cell lines. Preliminary pharmacologic studies showed that these compounds displayed lower toxicities than that of lead compound 1. Compound 3 obviously induced the early apoptosis and hardly affected the cell cycle of A2780, which was significantly different from compound 1. Especially, it gave 559- and 294-fold selectivity antiproliferation activity in P-gp overexpressed drug-resistant cancer cell lines MCF-7/ADR and KB-V compared to their drug-sensitive ones MCF-7 and KB, implying that compounds 2–6 might have an extra mechanism of anti-MDR-cancer with P-gp overexpression.
Keywords: Phenylsulfonylfuroxan, 3-benzyl coumarin, anticancer, multidrug resistance, P-gp overexpression
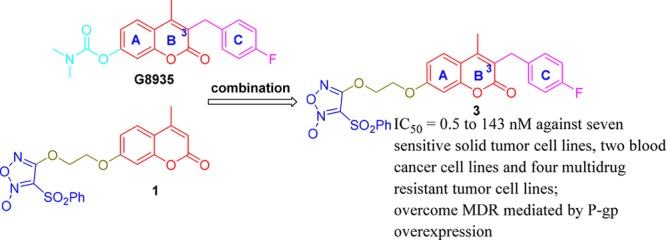
Nitric oxide (NO), which was reported by Furchgott and Zawadzki as an endothelium-derived relaxing factor (EDRF) in 1980,1,2 plays important roles in diverse physiological and pathophysiological processes.3−8 Additionally, NO can downregulate PI3K/Akt pathway and upregulate MEK/ERK pathway.9−11 Hideo Baba et al. reported that the combined administration of NO donor and MEK inhibitor can synergistically inhibit the viability of cancer cells through downregulating both PI3K/Akt and MEK/ERK pathways.12 (3,4-Bis(phenylsulfonyl)-1,2,5-oxadiazole 2-oxide (phenylsulfonylfuroxan) as an important NO donor was widely used in the design of anticancer agents.13−17 We previously reported that phenylsulfonylfuroxan and coumarin hybrid 1 showed remarkable antitumor activity through multitarget mechanism containing disruption of MEK pathway. However, its MEK inhibitory activity was relatively weak.18 Considering 4-fluorobenzyl at 3-position of coumarin skeleton in G8935, which was a MEK inhibitor,19,20 occupied a new binding pocket in the MEK docking model,21 we also introduced several benzyl groups covering 4-fluorobenzyl to the same position of lead compound 1 and obtained five new derivatives (2–6, Scheme 1). The structure optimization aimed at developing stronger synergistic antiproliferation activity with both NO donor and MEK inhibitory activity compared to lead compound 1. Besides, concerning coumarin core integrity for sustaining anticancer activity, two seco-B-ring derivatives were also synthesized.
Scheme 1. Design (a) and Synthesis (b) of 3-Benzyl Coumarin and Phenylsulfonylfuroxan Hybrids and Their Seco-B-ring Derivatives.
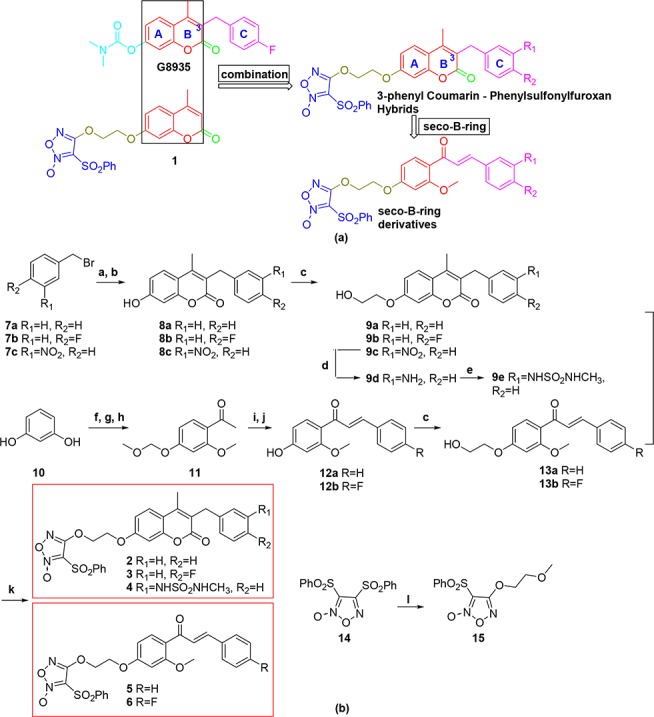
Reagents and conditions: (a) ethyl 3-oxobutanoate (1.0 equiv), NaH (1.2 equiv), dry THF, 60 °C, 2 h; (b) resorcinol (1.0 equiv), 70% H2SO4, rt, 2 h, 35–95% for two steps (a and b); (c) 2-chloro-1-ethanol (1.0 equiv), K2CO3 (3.0 equiv), KI (0.1 equiv), DMF, reflux, 2–10 h, 76–100%; (d) stannous chloride dehydrate (4.0 equiv), DMF, rt, 6 h, 99%; (e) N-methyl-2-oxooxazolidine-3-sulfonamide (2.0 equiv), NEt3 (3.0 equiv), MeCN, 80 °C, 8 h, 99%; (f) ZnCl2 (1.5 equiv), HOAc, reflux, 70 min, 51%; (g) chloromethyl methyl ether (2.0 equiv), K2CO3 (2.5 equiv), acetone, rt, overnight, 84%; (h) CH3I (1.2 equiv), K2CO3 (3.0 equiv), DMF, 80 °C, 30 min, 82%; (i) benzaldehyde or 4-fluorobenzaldehyde (1.05 equiv), 60% KOH aqueous solution (2 mL/mM compound 11), EtOH, 2–5 h, rt; (j) conc. HCl/EtOH = 1:25 (v/v), reflux, 30 min, 80–85% for two steps (i and j); (k) 14 (1.3 equiv), DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) (2.0 equiv), anhydrous DCM, rt, 3.5–12 h, 51–85%; (l) 2-methoxyethan-1-ol (1.1 equiv), DBU (2.0 equiv); anhydrous DCM, rt, overnight, 90%.
As shown in Scheme 1, compounds 8a–c, synthesized according to a previously described procedure,22,23 were treated with 2-chloro-1-ethanol to provide intermediates 9a–c. Compound 9c was reduced by stannous chloride dehydrate to form 9d and sulfonylated with N-methyl-2-oxooxazolidine-3-sulfonamide24 to obtain 9e. Compound 11 was prepared from resorcinol via Friedel–Crafts reaction, 4-hydroxyl protection, and 2-hydroxyl methylation. After aldol condensation of 11 with benzaldehyde or 4-fluorobenzaldehyde, deprotection of the 4-hydroxyl gave 12a,b. Seco-B-ring compounds 13a,b were synthesized by the same procedure used to obtain 9a–c. Finally, 9a–b,e and 13a,b were merged with phenylsulfonylfuroxan to provide compounds 2–6. Meanwhile, compound 15 containing phenylsulfonylfuroxan-linker fragment was also synthesized as a reference for bioevaluation analyses. Structures of 2–6 were confirmed by 1H NMR, 13C NMR, and MS spectra. In 1H NMR, the chemical shift of two alkenyl hydrogens of α,β-unsaturated ketone in compound 6 were 7.65 and 7.43 ppm, respectively, and the coupling constant is 15.8 Hz, indicating a trans-conformation of the ethylenic double bond structure.
Then 2–6 were screened for their bioactivities against nine drug-sensitive solid cancer cell lines, two hematological tumors, four drug-resistant cancer and four nontumorigenesis cell lines with lead compound 1 and NO donor 15 as references, and cisplatin, doxorubicin, gemcitabine, vincristine, SAHA, lenalidomide, and CC-88525 were chosen as positive controls. Except for MCF-7 and KB (Table 1), compounds 2–6 all showed higher antiproliferation activities (0.5 to 35.8 nM) than 1 and 15. Particularly, compound 3 with 4-fluorobenzyl at the 3-position of coumarin core was the most potent compound (0.8 to 8.3 nM). In A549, OVCA429, and MDA-MB-231, 2–6 all displayed significant activities in single digit nanomolar levels of IC50 values. While in HeLa, SKOV3, OVCA433, and A2780, 2 and 3 bearing 3-benzyl coumarin skeleton showed slightly better activities than that of their seco-B-ring derivatives (5 and 6). Introduction of the N-methylsulfomicamino group into the 3-position of phenyl group (4) resulted in slightly decreasing activities relative to compounds 3 and 6 bearing 4-fluorophenyl group. Additionally, they had strong antiproliferation bioactivities with a range from 5.1 to 156.8 nM of IC50 values against two hematological tumor cell lines MV-4-11 and MM-1S and four drug-resistant cancer cell lines A2780/CDDP, MDA-MB-231/Gem, MCF-7/ADR, and KB-V (Tables 2 and 3). Notably, the selective ratios of IC50 values about 3 were 559 and 294 (Table 3, 2.9 and 3.2 μM in MCF-7 and KB vs 5.1 and 11.0 nM in MCF-7/ADR and KB-V), whereas compounds 2–6 were almost at the same activity levels against A2780/CDDP vs A2780 and MDA-MB-231/Gem vs MDA-MB-231. The distinct selectivities presumed that new NO donors 2–6 probably had an extra pathway to inhibit certain MDR cancer. Moreover, 2–6 expressed far lower toxicities than compound 1 in HUVEC, T29, WI-38, and MCF-10A (Table 4, 0.19 to 20 μM), which indicated they have a noteworthy selectivity between tumor and nontumorigenesis cell lines.
Table 1. Antiproliferation Activities for 2–6 against Nine Solid Cancer Cell Linesa.
| IC50 (nM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| compd | HeLa | SKOV3 | A549 | OVCA429 | OVCA433 | A2780 | MDA-MB-231 | MCF-7 | KB |
| 15 | 8826 | 3909 | 4778 | 5173 | 4389 | 1635 | 1233 | 3503 | 3691 |
| 1 | 62.2 | 47.3 | 45.8 | 41.1 | 130.8 | 20.9 | 85.9 | 445 | 127 |
| 2 | 22.9 | 17.3 | 2.0 | 1.7 | 1.1 | 12.7 | 6.0 | 2351 | 4542 |
| 3 | 2.8 | 8.3 | 3.7 | 3.9 | 3.3 | 6.6 | 0.8 | 2853 | 3234 |
| 4 | 15.4 | 19.2 | 7.9 | 6.1 | 16.3 | 10.2 | 1.1 | 3131 | 1143 |
| 5 | 17.8 | 35.8 | 3.3 | 4.4 | 10.1 | 8.7 | 0.5 | 1865 | 2182 |
| 6 | 6.9 | 25.0 | 3.5 | 4.0 | 6.6 | 14.5 | 1.4 | 1413 | 1665 |
| cisplatin | 2741 | 1440 | 13220 | 7296 | 24630 | 2549 | NA | NA | NA |
| doxorubicin | NA | NA | NA | NA | NA | NA | NA | 879 | NA |
| gemcitabine | NA | NA | NA | NA | NA | NA | 36.4 | NA | NA |
| vincristine | NA | NA | NA | NA | NA | NA | NA | NA | 0.65 |
MTT assay. The data are the mean of triplicate determinations. NA: not available. HeLa: human cervical cancer cell lines. SKOV3, OVCA429, OVCA433 and A2780: human ovarian cancer cell lines. A549: human nonsmall cell lung cancer cell lines. MDA-MB-231 and MCF-7: human breast cancer cell lines. KB: human oral epidermoid cancer cell lines.
Table 2. Antiproliferation Activities for 2–6 in Hematological Tumor Cell Linesa.
| IC50 (nM) | 1 | 2 | 3 | 4 | 5 | 6 | SAHA | lenalidomide | CC-885 |
|---|---|---|---|---|---|---|---|---|---|
| MV-4–11 | 28.3 | 24.5 | 27.2 | 66.3 | 29.3 | 29.6 | NA | >20000 | 0.3 |
| MM-1S | 21.0 | 12.0 | 143.0 | 24.0 | 28.0 | 25.0 | 2844 | NA | NA |
MTS assay. The data are the mean of triplicate determinations. NA: not available. MV-4–11: human myeloid leukemia cell lines. MM-1S: human myeloma cell lines.
Table 3. Antiproliferation Activities for 2–6 against Drug-Resistant Tumor Cellsa.
| IC50 (nM) |
selectivity ratio |
|||||||
|---|---|---|---|---|---|---|---|---|
| compd | A2780/CDDP | MDA-MB-231/Gem | MCF-7/ADR | KB-V | IC50(A2780)/IC50(A2780/CDDP) | IC50(MDA-MB-231)/IC50(MDA-MB-231/Gem) | IC50(MCF-7)/IC50(MCF-7/ADR) | IC50(KB)/IC50(KB-V) |
| 15 | 4820 | 4747 | 7845 | 1959 | 0.3 | 0.3 | 0.5 | 1.9 |
| 1 | 85.7 | 85.9 | 156.8 | 24.9 | 0.2 | 1.0 | 2.8 | 5.1 |
| 2 | 94.0 | 25.2 | 16.3 | 16.0 | 0.1 | 0.2 | 144.2 | 283.9 |
| 3 | 22.8 | 6.9 | 5.1 | 11.0 | 0.3 | 0.1 | 559.4 | 294.0 |
| 4 | 47.2 | 50.4 | 50.1 | 65.4 | 0.2 | 0.02 | 62.5 | 17.5 |
| 5 | 51.7 | 10.5 | 18.0 | 13.9 | 0.2 | 0.05 | 103.6 | 157.0 |
| 6 | 34.1 | 93.0 | 47.9 | 10.1 | 0.4 | 0.02 | 29.5 | 164.9 |
| cisplatin | 201370 (r.r. = 79) | NA | NA | NA | NA | NA | NA | NA |
| doxorubicin | NA | NA | >100000 (r.r. > 113) | NA | NA | NA | NA | NA |
| gemcitabine | NA | >50000 (r.r. > 1373) | NA | NA | NA | NA | NA | NA |
| vincristine | NA | NA | NA | 417.0 (r.r. = 646) | NA | NA | NA | NA |
MTT assay. The data are the mean of triplicate determinations. r.r.: resistant ratio; r.r. = IC50 (drug-resistant cell lines)/IC50 (drug-sensitive cell lines). NA: not available. A2780/CDDP: cisplatin resistant human ovarian cancer cell lines. MDA-MB-231/Gem: gemcitabine resistant human breast cancer cell lines. MCF-7/ADR: doxorubicin resistant human breast cancer cell lines. KB-V: vincristine resistant human oral epidermoid cancer cell lines.
Table 4. Antiproliferation Activities for 2–6 in Nontumorigenesis Cells.
| IC50 (μM) | 1 | 2 | 3 | 4 | 5 | 6 | cisplatin | lenalimide | CC-885 |
|---|---|---|---|---|---|---|---|---|---|
| HUVECa | 0.13 | 0.44 | 0.27 | 0.19 | 0.41 | 0.36 | 1.10 | NA | NA |
| T29a | 1.8 | >5 | >5 | >5 | 2.5 | 3.5 | 1.8 | NA | NA |
| WI-38b | 0.09 | >20 | >20 | 1.36 | 0.44 | 0.56 | NA | >20 | >20 |
| MCF-10Ab | 0.66 | >20 | >20 | NA | NA | NA | NA | >20 | 0.005 |
MTT assay.
MTS assay. The data are the mean of triplicate determinations. NA: not available. HUVEC: human umbilical vein endothelial cell lines. T29: human immortalized but nontumorigenic ovarian epithelial cell lines. WI-38: human lung fibroblasts. MCF-10A: human nontumorigenic breast epithelial cell lines.
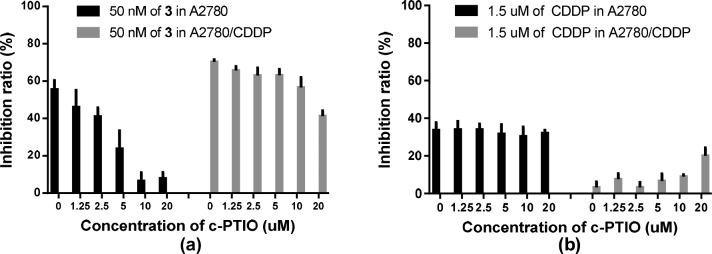
Assay of NO release showed that 2–6 produced much less NO compared to control 1 (see Figure S1), and the antiproliferation activities of compound 3 declined with the increasing concentration of scavenger c-PTIO in both A2780 and A2780/CDDP (Figure 1). However, compounds 2–6 showed much weaker MEK inhibiting potency than that of 1, which elucidated activities of 2–6 might be mainly related to the release of NO. Furthermore, compounds 3 and 6 could remarkably induce cell apoptosis but hardly affect cell cycle. Interestingly, 3 and 6 seemed to mainly induce early apoptosis, while reference 1 mainly induced late apoptosis (see Figure S3). Recently, some compounds including Dp44mT were reported to specifically inhibit the proliferation of drug-resistant cancer with P-gp overexpression.26−31 This promoted us to detect P-gp expression of the drug-resistant and their drug-sensitive cancer cell lines mentioned above. As expected (see Figure S4), P-gp expression was outstanding in KB-V and MCF-7/ADR, to which compounds 2–6 showed significant selective antiproliferation potency compared to KB and MCF-7; while P-gp overexpression was not observed in A2780/CDDP, MDA-MB-231/GEM, A2780, and MDA-MB-231, which have no relevant selective antiproliferation activities for 2–6. Further work is under way to find the possible pharmacologic mechanism of these compounds against P-gp overexpression MDR cancer.
Figure 1.
Antiproliferation activities of compound 3 at the concentration of 50 nM together with different concentrations of c-PTIO. Results were indicated as the mean ± SEM of two independent experiments.
In conclusion, novel NO donors 2–6 not only displayed significant antiproliferation activities in nine drug-sensitive tumor cell lines but also showed strong activities against four drug-resistant tumor cell lines with nanomolar, even subnanomolar, level IC50 values. The less NO-releasing levels and lower toxicity on nontumorigenesis cell lines compared to lead compound 1 suggested that they had a better selectivity against malignant cells in vitro. Notably, some of their preliminary pharmacologic study results were different from lead compound 1. Especially, compound 3 exhibited obvious selectivity ratios of anticancer potency in drug-resistant and their drug-sensitive cell lines MCF-7/ADR vs MCF-7 and KB-V vs KB with 559 and 294 folds, respectively. Western blot analysis discovered the overexpression of P-gp in MCF-7/ADR and KB-V, while it did not overexpress in MCF-7 and KB. The results suggested that there was a close relationship between P-gp overexpression and antiproliferation selectivity. Research of the detailed pharmacologic mechanism will further proceed for the development of desirable anticancer agents to overcome MDR mediated by P-gp overexpression.
Acknowledgments
Funding from “Major New Drugs Innovation and Development” of National Science and Technology Major Projects (2018ZX09711002-007-005). The authors wish to thank Zhimin Shao and Gong Yang for providing cancer cell lines.
Glossary
ABBREVIATIONS
- P-gp
P-glycoprotein
- MDR
multidrug resistant
- PI3K
phosphatidylinositol-3-kinase
- Akt
protein kinase B
- MEK
mitogen-activated protein kinase kinase
- ERK
extracellular regulated protein kinases
- NMR
nuclear magnetic resonance
- HSQC
heteronuclear single quantum correlation
- THF
tetrahydrofuran
- DMF
N,N-dimethylformamide
- DCM
dichloromethane
- SAHA
suberoylanilide hydroxamic acid
- DMSO
dimethyl sulfoxide
- PI
propidium iodide
- HRMS
high-resolution mass spectra
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00125.
Antiproliferative assay, inhibition activities of colony, nitrite measurement, cell apoptosis and Western blot analysis, HRMS, 1H and 13C NMR and HSQC spectra of compounds (PDF)
Author Contributions
§ Y.G. and Y.W. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Furchgott R. F.; Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Mocellin S.; Bronte V.; Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007, 27, 317–352. 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- Bonavida B.; Baritaki S.; Huerta-Yepez S.; Vega M. I.; Chatterjee D.; Yeung K. Novel therapeutic applications of nitric oxide donors in cancer: roles in chemo- and immunosensitization to apoptosis and inhibition of metastases. Nitric Oxide 2008, 19, 152–157. 10.1016/j.niox.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Riganti C.; Miraglia E.; Viarisio D.; Costamagna C.; Pescarmona G.; Ghigo D.; Bosia A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [PubMed] [Google Scholar]
- Kiechle F. L.; Zhang X. Apoptosis: biochemical aspects and clinical implications. Clin. Chim. Acta 2002, 326, 27–45. 10.1016/S0009-8981(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Sullivan R.; Graham C. H. Chemosensitization of cancer by nitric oxide. Curr. Pharm. Des. 2008, 14, 1113–1123. 10.2174/138161208784246225. [DOI] [PubMed] [Google Scholar]
- Dong R.; Wang X.; Wang H.; Liu Z.; Liu J.; Saavedra J. E. Effects of JS-K, a novel anti-cancer nitric oxide prodrug, on gene expression in human hepatoma Hep3B cells. Biomed. Pharmacother. 2017, 88, 367–373. 10.1016/j.biopha.2017.01.080. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Ning S.; Scicinski J.; Oronsky B.; Knox S. J.; Peehl D. M. Epigenetic effects of RRx-001: a possible unifying mechanism of anticancer activity. Oncotarget 2015, 6, 43172–43181. 10.18632/oncotarget.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita H.; Kaneki M.; Furuhashi S.; Hirota M.; Takamori H.; Baba H. Nitric oxide inhibits the proliferation and invasion of pancreatic cancer cells through degradation of insulin receptor substrate-1 protein. Mol. Cancer Res. 2010, 8, 1152–1163. 10.1158/1541-7786.MCR-09-0472. [DOI] [PubMed] [Google Scholar]
- Shields J. M.; Pruitt K.; McFall A.; Shaub A.; Der C. J. Understanding Ras: ‘it ain’t over ’til it’s over’. Trends Cell Biol. 2000, 10, 147–154. 10.1016/S0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- Lim K. H.; Ancrile B. B.; Kashatus D. F.; Counter C. M. Tumour maintenance is mediated by eNOS. Nature 2008, 452, 646–649. 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi S.; Sugita H.; Takamori H.; Horino K.; Nakahara O.; Okabe H.; Miyake K.; Tanaka H.; Beppu T.; Baba H. NO donor and MEK inhibitor synergistically inhibit proliferation and invasion of cancer cells. Int. J. Oncol. 2012, 40, 807–815. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Liu M.; Meng L.; Feng P.; Guo Y.; Ying M.; Zhu X.; Chen Y. Synthesis and antitumor evaluation of novel hybrids of phenylsulfonylfuroxan and epiandrosterone/dehydroepiandrosterone derivatives. Steroids 2015, 101, 7–14. 10.1016/j.steroids.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Ai Y.; Kang F.; Huang Z.; Xue X.; Lai Y.; Peng S.; Tian J.; Zhang Y. Synthesis of CDDO-amino acid-nitric oxide donor trihybrids as potential antitumor agents against both drug-sensitive and drug-resistant colon cancer. J. Med. Chem. 2015, 58, 2452–2464. 10.1021/jm5019302. [DOI] [PubMed] [Google Scholar]
- Ling Y.; Ye X.; Zhang Z.; Zhang Y.; Lai Y.; Ji H.; Peng S.; Tian J. Novel nitric oxide-releasing derivatives of farnesylthiosalicylic acid: synthesis and evaluation of antihepatocellular carcinoma activity. J. Med. Chem. 2011, 54, 3251–3259. 10.1021/jm1014814. [DOI] [PubMed] [Google Scholar]
- Gu X.; Huang Z.; Ren Z.; Tang X.; Xue R.; Luo X.; Peng S.; Peng H.; Lu B.; Tian J.; Zhang Y. Potent inhibition of nitric oxide-releasing bifendate derivatives against drug-resistant K562/A02 cells in vitro and in vivo. J. Med. Chem. 2017, 60, 928–940. 10.1021/acs.jmedchem.6b01075. [DOI] [PubMed] [Google Scholar]
- Chegaev K.; Riganti C.; Lazzarato L.; Rolando B.; Guglielmo S.; Campia I.; Fruttero R.; Bosia A.; Gasco A. Nitric oxide donor doxorubicins accumulate into Doxorubicin-resistant human colon cancer cells inducing cytotoxicity. ACS Med. Chem. Lett. 2011, 2, 494–497. 10.1021/ml100302t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.; Chen X.; Huang Y.; Feng P.; Guo Y.; Yang G.; Chen Y. Hybrids of phenylsulfonylfuroxan and coumarin as potent antitumor agents. J. Med. Chem. 2014, 57, 9343–9356. 10.1021/jm500613m. [DOI] [PubMed] [Google Scholar]
- Ohren J. F.; Chen H.; Pavlovsky A.; Whitehead C.; Zhang E.; Kuffa P.; Yan C.; McConnell P.; Spessard C.; Banotai C.; Mueller W. T.; Delaney A.; Omer C.; Sebolt-Leopold J.; Dudley D. T.; Leung I. K.; Flamme C.; Warmus J.; Kaufman M.; Barrett S.; Tecle H.; Hasemann C. A. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004, 11, 1192–1197. 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- Wallace E. M.; Lyssikatos J.; Blake J. F.; Seo J.; Yang H.; Yeh T. C.; Perrier M.; Jarski H.; Marsh V.; Poch G.; Livingston M. G.; Otten J.; Hingorani G.; Woessner R.; Lee P.; Winkler J.; Koch K. Potent and selective mitogen-activated protein kinase kinase (MEK) 1,2 inhibitors. 1. 4-(4-bromo-2-fluorophenylamino)-1-methylpyridin-2(1H)-ones. J. Med. Chem. 2006, 49, 441–444. 10.1021/jm050834y. [DOI] [PubMed] [Google Scholar]
- Han S.; Zhou V.; Pan S.; Liu Y.; Hornsby M.; McMullan D.; Klock H. E.; Haugen J.; Lesley S. A.; Gray N.; Caldwell J.; Gu X. J. Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5467–5473. 10.1016/j.bmcl.2005.08.097. [DOI] [PubMed] [Google Scholar]
- Aoki T.; Hyohdoh I.; Furuichi N.; Ozawa S.; Watanabe F.; Matsushita M.; Sakaitani M.; Morikami K.; Takanashi K.; Harada N.; Tomii Y.; Shiraki K.; Furumoto K.; Tabo M.; Yoshinari K.; Ori K.; Aoki Y.; Shimma N.; Iikura H. Optimizing the physicochemical properties of Raf/MEK inhibitors by nitrogen scanning. ACS Med. Chem. Lett. 2014, 5, 309–314. 10.1021/ml400379x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. F.; Chen M.; Wallace D.; Tith S.; Arrhenius T.; Kashiwagi H.; Ono Y.; Ishikawa A.; Sato H.; Kozono T.; Sato H.; Nadzan A. M. Discovery and structure-activity relationship of coumarin derivatives as TNF-alpha inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 2411–2415. 10.1016/j.bmcl.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Aoki T.; Hyohdoh I.; Furuichi N.; Ozawa S.; Watanabe F.; Matsushita M.; Sakaitani M.; Ori K.; Takanashi K.; Harada N.; Tomii Y.; Tabo M.; Yoshinari K.; Aoki Y.; Shimma N.; Iikura H. The sulfamide moiety affords higher inhibitory activity and oral bioavailability to a series of coumarin dual selective RAF/MEK inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6223–6227. 10.1016/j.bmcl.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Matyskiela M. E.; Lu G.; Ito T.; Pagarigan B.; Lu C.; Miller K.; Fang W.; Wang N.; Nguyen D.; Houston J.; Carmel G.; Tran T.; Riley M.; Nosaka L. A.; Lander G. C.; Gaidarova S.; Xu S.; Ruchelman A. L.; Handa H.; Carmichael J.; Daniel T. O.; Cathers B. E.; Lopez-Girona A.; Chamberlain P. P. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature 2016, 535, 252–257. 10.1038/nature18611. [DOI] [PubMed] [Google Scholar]
- Szakacs G.; Hall M. D.; Gottesman M. M.; Boumendjel A.; Kachadourian R.; Day B. J.; Baubichon-Cortay H.; Di Pietro A. Targeting the achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem. Rev. 2014, 114, 5753–5774. 10.1021/cr4006236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. J.; Yamagishi T.; Arvind A.; Seebacher N.; Gutierrez E.; Stacy A.; Maleki S.; Sharp D.; Sahni S.; Richardson D. R. Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein. J. Biol. Chem. 2015, 290, 9588–9603. 10.1074/jbc.M114.631283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez E. M.; Seebacher N. A.; Arzuman L.; Kovacevic Z.; Lane D. J.; Richardson V.; Merlot A. M.; Lok H.; Kalinowski D. S.; Sahni S.; Jansson P. J.; Richardson D. R. Lysosomal membrane stability plays a major role in the cytotoxic activity of the anti-proliferative agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT). Biochim. Biophys. Acta, Mol. Cell Res. 2016, 1863, 1665–1681. 10.1016/j.bbamcr.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Al-Eisawi Z.; Stefani C.; Jansson P. J.; Arvind A.; Sharpe P. C.; Basha M. T.; Iskander G. M.; Kumar N.; Kovacevic Z.; Lane D. J.; Sahni S.; Bernhardt P. V.; Richardson D. R.; Kalinowski D. S. Novel mechanism of cytotoxicity for the selective selenosemicarbazone, 2-acetylpyridine 4,4-dimethyl-3-selenosemicarbazone (Ap44mSe): lysosomal membrane permeabilization. J. Med. Chem. 2016, 59, 294–312. 10.1021/acs.jmedchem.5b01399. [DOI] [PubMed] [Google Scholar]
- Stacy A. E.; Palanimuthu D.; Bernhardt P. V.; Kalinowski D. S.; Jansson P. J.; Richardson D. R. Structure-activity relationships of di-2-pyridylketone, 2-benzoylpyridine, and 2-acetylpyridine thiosemicarbazones for overcoming pgp-mediated drug resistance. J. Med. Chem. 2016, 59, 8601–8620. 10.1021/acs.jmedchem.6b01050. [DOI] [PubMed] [Google Scholar]
- Seebacher N.; Lane D. J. R.; Richardson D. R.; Jansson P. J. Turning the gun on cancer: utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance. Free Radical Biol. Med. 2016, 96, 432–445. 10.1016/j.freeradbiomed.2016.04.201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.