Abstract
Background
Research studies rely on accurate assessment of entry criteria in order to maintain study integrity and participant safety, however, challenges can exist with HIV studies in international settings.
Objective
Examine the unexpectedly high proportion of study participants with an undetectable HIV viral load found in Ugandan and Russian research cohorts meeting antiretroviral therapy (ART) naïve entry criteria.
Methods
Russian participants with documented HIV and ART-naïve status were recruited between 2012–2015 from clinical and non-clinical sites in St. Petersburg. Participants in Uganda were recruited from Mbarara Regional Referral Hospital from 2011–2014 with documented HIV-infection via rapid diagnostic testing and recorded ART-naïve in the clinic database. HIV viral load testing of baseline samples was performed; the lower limit of detection was 500 copies/mL in Russia and 40 in Uganda. Due to an unexpectedly high proportion of participants with undetectable viremia, additional tests were performed: enzyme-linked immunosorbent assay HIV testing and testing for ART.
Results
In Russia, 16% (58/360) had undetectable viremia; 3% (9/360) re-tested HIV-seronegative and 4% (13/360) tested positive for ART. In Uganda 11% (55/482) had undetectable viremia; 5% (26/482) re-tested HIV-seronegative, while <1% (4/482) tested positive for ART.
Conclusions
In both Russia & Uganda, undetectable viremia was much higher than would be expected for an HIV-infected ART-naïve cohort. Misclassification of study participants was due to misdiagnosis of HIV with rapid diagnostic testing and inaccurate accounting of ART use. Confirmatory HIV testing could improve accuracy of participants meeting entry criteria for HIV-infection as might increased scrutiny of medication use in an ART-naïve cohort.
Keywords: HIV/AIDS, Rapid diagnostic testing, diagnostic screening, Uganda, Russia
Introduction
Diagnostic testing for HIV infection is implemented with various algorithms depending on the setting and resources available. Testing algorithms that maximize overall sensitivity and specificity are recommended to minimize misdiagnosis. Under ideal conditions, initial diagnostics detecting HIV antigen and/or HIV-specific antibodies are followed by supplemental confirmatory testing. The gold standard for HIV testing in the United States as recommended by Centers for Disease Control (CDC) guidelines (2014) includes a combination immunoassay of HIV antigen/antibody and HIV RNA testing.1 The trade-off to this type of rigorous testing is that most advanced diagnostic laboratory technologies are centralized, and require highly trained staff and specialized facilities. Also, this testing is not point-of-care and thus requires a waiting period for test results; consequently, individuals could be lost to follow-up.
Rapid diagnostic tests (RDTs) are validated as a screening test for initial HIV diagnosis where reactive results are further confirmed with supplemental testing. However, due to its low cost, rapid turnaround time for receipt of results, and point-of-care application, RDTs are often used alone for HIV diagnosis in settings where laboratory services may be limited.2 The benefit of this strategy is that it allows for a scale-up of HIV testing, widespread implementation of HIV programs including treatment and surveillance in resource limited settings.3–7 World Health Organization (WHO) guidelines call for a clinical sensitivity of 99% and a specificity of 98% for screening tests. These guidelines can be satisfied with validated testing algorithms involving a series of RDTs when carried out according to protocol.7 Despite these guidelines, a recent multi-center evaluation of the most widely used RDTs demonstrated that individual RDTs performed more poorly than WHO recommended thresholds.8 While RDT is optimized for initial diagnosis of HIV, it is at times relied upon for definitive determination of HIV status in which re-testing is not performed.
More recently, the WHO has issued guidance for the use of trained lay providers to perform HIV testing services using RDTs in order to increase access and coverage of HIV testing. 6 However, the risk of false-positive and false-negative diagnoses is a recognized problem and a growing concern,2,3,9–14 as recent data of proficiency testing suggest that lay counselors and nurses had more difficulty with interpretation of RDT results than laboratory personnel.15 Overall, there is a paucity of data on the magnitude of misdiagnosis within HIV testing programs. Data is primarily from retrospective audits and the false-positive rate has been found to range from 2.6% to 10.5%.2,9
In Uganda, the Ministry of Health (MOH) recommends serial testing algorithms for rapid HIV testing using test kits with different antigen reactivities (National HIV Testing Services Policy and Implementation Guidelines, 2016). Serial testing algorithms include stepwise testing, with a second independent test used only to confirm initial reactive results. Discordant results are subjected to a tie-breaker test. The Russian Ministry of Health requires confirmatory HIV antibody testing with two enzyme-linked immunosorbent assays (EIA) after any positive rapid test and Western blot after positive EIA testing. 16 HIV testing algorithms in both countries comply with WHO guidance.
Research studies that include HIV-infected participants rely on accurate inclusion and exclusion criteria to ensure participant safety and study integrity. This includes accurate HIV diagnosis which may be problematic in some settings, particularly resource limited countries. Oftentimes confirmatory HIV-testing is not feasible prior to study enrollment. In such cases, investigators rely on self-report, medical record reviews and other sources of HIV status documentation. However, information from medical histories, and alternate documentation may be unreliable in screening volunteers for research trials.17–19 Furthermore, self-report of infection status from potential study participants may be inaccurate due to an initial misdiagnosis, misunderstanding study entry criteria, or intentional misreporting in order to meet study inclusion criteria.18
The primary objective of this paper was to examine the unexpectedly high prevalence of low and undetectable vial load (VL) found in HIV-infected, antiretroviral therapy (ART) naïve participants from the Ugandan and Russian cohorts enrolled in the Uganda, Russia, Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS (URBAN ARCH) consortium. All participants in these cohorts, presumptively met HIV positive serostatus and ART-naïve criteria at study entry as reported by the clinical sites of recruitment. Given the unexpectedly high prevalence of undetectable (< 40 and <500 copies/mL in Ugandan and Russian cohorts, respectively) and low VL (between 40 and 500 copies/mL in Uganda) it was important to assess whether some research participants were HIV negative. Concurrently, we aimed to determine if participants were on ART, or had potentially misreported medical backgrounds. Our study is novel in that it reports on misdiagnosis of HIV in two very different research settings. We also verify current ART status of those with confirmed HIV infection and undetectable viremia by testing archived plasma for the presence of ART regimen.
Methods
Study Design and Participants
Participants from the Ugandan and Russian cohorts of the URBAN ARCH consortium were included. The Uganda and Russia ARCH cohorts were observational prospective studies aimed to assess the impact of alcohol consumption on aspects of HIV disease.20, 21
Uganda ARCH Cohort
From September 2011 to August 2014, 482 participants were enrolled from the Immune Suppression Syndrome (ISS) clinic of Mbarara regional referral hospital in southwestern Uganda, which is the primary municipal clinic for HIV-infected individuals in this area. Eligibility criteria for this cohort included: HIV-infection as tested by the ISS clinic (see below) or documented by a referring clinic, ART naïve status (and not scheduled to start ART within three months of entering the study); diagnosis of World Health Organization Stage I or II (asymptomatic or mild disease); and CD4 count of ≥ 350 cells/mm3 (later changed to 500 cells/mm3 in accordance with national ART guideline changes); fluency in Runyakole (local language) or English; residence within sixty kilometers or two hours of the ISS clinic; age 18 or older; and ability to give informed consent. Documented HIV-infection and ART naïve status was verified via electronic medical record review. No further HIV testing was performed as part of the screening for this study. After eligibility was verified and informed consent obtained, participants provided a blood sample for viral load testing and were given an interviewer-administered survey. The Institutional Review Boards of the Mbarara University of Science and Technology (MUST), University of California, San Francisco, Boston University Medical Campus, and the Uganda National Council for Science and Technology approved the protocol of this study.
Russia ARCH Cohort
A total of 360 participants were enrolled between November 2012 and June 2015 from clinical HIV and addiction care sites, non-clinical sites, and via snowball recruitment22 (i.e. in which existing study participants recruit from their social network) in St. Petersburg, Russia. Eligibility criteria included the following: 18–70 years old; documented HIV-infection ; documented ART-naïve status at enrollment; the ability to provide contact information for two contacts to assist with follow-up; stable address within St. Petersburg or districts within 100 kilometers of St. Petersburg; possession of a home or mobile phone. Documentation of HIV-infection and ART-naïve status took the form of letters from a medical provider, laboratory results (e.g. HIV test results, CD4 counts, and HIV viral load), and excerpts from medical histories. All records were paper documents supplied by the participant. No HIV testing was performed as part of the screening for this study. Participants were excluded if they were not fluent in Russian or had a cognitive impairment resulting in inability to provide informed consent. After eligibility was verified and informed consent obtained, participants provided a blood sample for viral load testing and were given an interviewer-administered survey. Participants had viral load testing at baseline, and subsequently at 12-month study visits. Institutional Review Boards of Boston University Medical Campus and First St. Petersburg Pavlov State Medical University approved this study.
Measures
In Uganda ARCH, the ISS clinic followed concurrent Uganda MOH recommendations of serial RDT for determining HIV status, using Determine™ HIV-1/2 (Alere, Chiba-ken, Japan) with sero-reactive testing followed by HIV-1/2 STAT PAK® rapid test (Chembio, Medford, NY, USA), and Uni-Gold™ (Trinity Biotech, Bray, Ireland) used as the tie-breaker following discordant test results. The sensitivity and specificity of Determine™ HIV-1/2 is 99.9% and 98.2% respectively in African populations. The confirmatory HIV-1/2 STAT PAK® rapid test has a sensitivity and specificity of 99.7% and 99.9% in populations that include Africa. The Uni-Gold™ tie-breaker test has very high sensitivity and specificity of 100% and 99.8% respectively. However, in some instances, the ISS clinic receives patients who recently underwent HIV testing at other public health facilities where the diagnostic testing algorithm used was presumed to follow Ugandan MOH guidelines and retesting prior to ISS clinic enrollment is not routinely performed.
In Russia ARCH, study personnel verified HIV and ART naïve status via medical documentation which was provided by participants during the screening process prior to study entry. In Russia, RDTs are performed as diagnostic screening tests, but in order for an individual to be officially registered as living with HIV/AIDS the Russian Ministry of Health requires confirmatory testing by two enzyme-linked immunosorbent assay (EIA) HIV antibody tests and, if positive on EIA, a confirmatory Western Blot (WB).
Russian ARCH samples were tested for HIV-1 VL using fresh venous blood EDTA plasma at the St. Petersburg Pasteur Institute using the AmpliSens® HIV-Monitor-FRT (Amplisens, Moscow, Russia), a polymerase chain reaction (PCR)-based diagnostic with a lower limit of quantitation (LLOQ) at 500 copies/mL. Baseline HIV viral load testing for Uganda ARCH samples was performed using venous blood EDTA plasma (cryopreserved at −80 C) at the University of California, San Francisco using the Abbott RealTime HIV-1 viral load test (Abbott Molecular, Inc, DesPlaines IL) with a LLOQ at 40 copies/mL.
Due to a high proportion of low (> 40 and < 500 copies/mL in Uganda) and undetectable viremia in both cohorts (< 40 copies/mL in Uganda, < 500 copies/mL in Russia), further testing was conducted to determine a potential explanation. Russian ARCH participants had VL testing at their 12 month study visit in addition to baseline testing, per protocol. Those with detectable viral loads at subsequent study visits (n=8) did not undergo any additional testing and were considered HIV-infected. All remaining Russian ARCH participants with undetectable viremia underwent enzyme-linked immunosorbent assay (EIA) HIV antibody testing at the St. Petersburg Pasteur Institute using the Genscreen™ ULTRA HIV Ag-Ab BIO-RAD, France and testing for the presence of ART in baseline plasma samples among those in whom HIV infection was confirmed. ART testing was performed at the University of North Carolina using a validated LC-MS/MS assay with a calibrated range of 1–4000ng/ml. All EIA and ART testing was performed on cryopreserved plasma at −80 C. Russian samples were tested for the presence of the following, most commonly available antiretroviral medications in Russia at the time of the study: tenofovir, emtricitabine, lamivudine, zidovudine, abacavir, and nevirapine.
The Uganda ARCH cohort had baseline viral load testing only, per protocol. All Ugandan ARCH participants with low or undetectable HIV viral loads had EIA testing (Abnova HIV-1/2 Ab ELISA) at Boston Medical Center, Boston, Massachusetts, and testing for the presence of ART in baseline plasma samples among those in whom HIV infection was confirmed. ART testing was performed at the University of California, San Francisco. Testing for nevirapine was done using a validated LC-MS/MS assay with a calibrated range of 100–9000ng/ml. Testing for efavirenze used a validated HPLC-UV system with a calibrated range of 100–10,000ng/mL. All EIA and ART testing was performed on cryopreserved plasma at −80 C. The baseline samples of Ugandan ARCH participants with low and undetectable viral loads who were HIV-seropositive with EIA were tested for the presence of the following antiretroviral medications: nevirapine and if negative were then tested for efavirenz as these constitute the most common 1st line regimen combinations in Uganda.
Data Analysis
Descriptive statistics were used to display sample characteristics stratified by cohort. We report on the proportion and exact binomial 95% confidence intervals (95% CI) of false-positive diagnoses in our sample and the proportion of participants that were subsequently found to be on ART in the both cohorts. We also compared those who were found to be HIV EIA negative to those who were positive within cohorts using descriptive statistics. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC).
Results
The Uganda ARCH cohort included 482 participants with a median age of 33 (interquartile range [IQR] 27, 41) years, 326 (68%) female, 148 (31%) with an education beyond primary school (9 years of school). Median years since HIV diagnosis was 1.7 (IQR 0.2, 6.0) and median CD4 cell count was 549 cells/mm3 (IQR 420, 686) at study entry. The Russian ARCH cohort included 360 participants with a median age of 33 (IQR 30, 37) years, 104 (29%) female, 283 (79%) with an education above primary school (9 years of school). On average, participants were aware of their HIV diagnosis for a median of 6.0 (IQR 3.0, 11.0) years and the median CD4 cell count was 485 cells/mm3 (IQR 328, 702) at study entry. The two cohorts differed with a greater percentage of females in the Uganda cohort, lower levels of primary education and more recent diagnosis of HIV. Median age and CD4 counts were similar between the cohorts.
Uganda ARCH Cohort
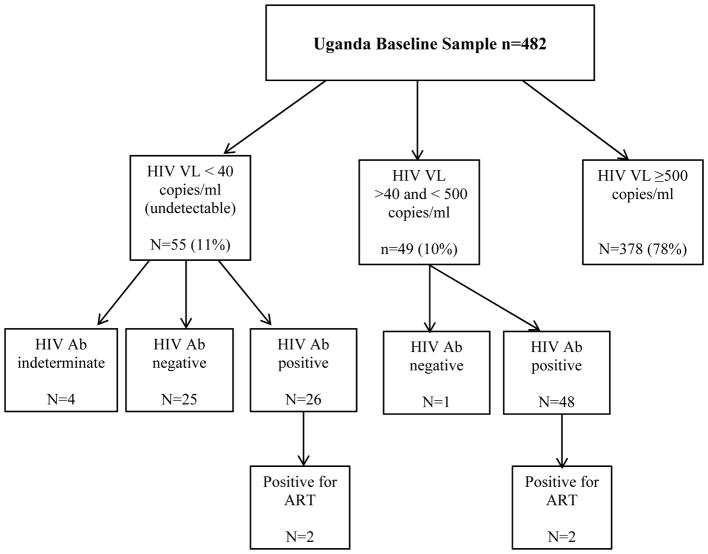
Among the Ugandan cohort participants (n=482), 55 (11%) had an undetectable HIV viral load (< 40 copies/mL), while 49 (10%) had a low but detectable HIV viral load (> 40 and < 500 copies/mL). Therefore, 104 (22%) of the cohort had either a low detectable or undetectable viral load. Among those with retrospective EIA HIV antibody testing on archived plasma, 25/55 with an undetectable viral load were found to be HIV-seronegative and 4 had indeterminate results. Twenty six of the 55 were HIV-seropositive and 2 of these tested positive for the presence of ART. Among the participants with a low but detectable viral load, 1 was found to be HIV-seronegative with EIA testing, and 48 were HIV seropositive by EIA, of which 2 tested positive for the presence of ART. Overall, 26 participants, 5.4% (95% CI: 3.6% – 7.8%) of the cohort were found to be HIV-seronegative and 4 seropositive participants (<1% of the cohort) were on ART. Five percent (24/482) of the Ugandan cohort had undetectable viral loads <40 copies/mL and were confirmed HIV-infected via EIA testing and were ART naïve at study entry; these were cautiously considered potential elite controllers, i.e. persons with HIV infection whose innate immunity controls HIV viral replication. When comparing the HIV-seronegative sample to the eligible enrolled sample, we found no notable differences in gender, level of education, employment status or CD4 cell count. The median CD4 cell count was 543 cells/mm3 (IQR 490, 776) in the EIA negative group vs. 550 cells/mm3 (IQR 416, 685) in the HIV-seropositive group. Those who were HIV-seronegative were older in age compared to the HIV-seropositive sample (median 38.5 [IQR 33.0, 46.0]) vs. (median 32.0 [IQR 27, 40.0]). Nearly 60% of the HIV-seronegative group was diagnosed from 2005 to 2009 while the remaining were diagnosed between 2010 and 2014.
Russia ARCH Cohort
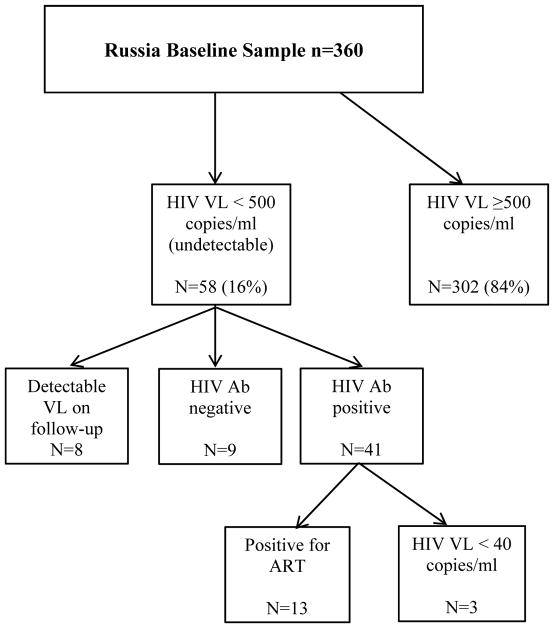
Among the Russia ARCH cohort (n=360), 58 (16%) had undetectable HIV viral load (< 500 copies/mL) at the initial study visit. Nine were found to be seronegative with EIA HIV antibody testing which represents 2.5% (95% CI 1.1% – 4.7%) of the Russia ARCH cohort. Five of the 9 HIV-negative participants had received their initial diagnosis in the prison system via RDT according to study staff. Detectable HIV viral load levels were subsequently found during the 12 month follow-up visit in 8 participants. Among the 41 with consecutive undetectable viremia and a positive EIA test, 13 (32%) were positive for ART. Samples for the 28 participants with undetectable viremia, positive EIA test, and negative ART tests were subsequently tested for HIV RNA at a lower limit of detection (40 copies/mL). Of those, three were found to be undetectable at the lower limit (< 40 copies/mL). Therefore, 3/360 (<1%) of the cohort were potential elite controllers which is similar to the expected population average for this rare phenotype.23 When comparing the HIV-seronegative sample to the eligible enrolled sample, we found no notable differences in gender, level of education, or employment status. Median CD4 cell count appeared higher, median 758 cells/mm3 (IQR 617, 921) in the EIA negative group vs. 483 cells/mm3 (IQR 323, 702) in the EIA positives. Those who were HIV-seronegative were older in age compared to the HIV-seropositive sample (median 36.0 [IQR 35.0, 40.0]) vs. (median 33.0 [IQR 30.0, 37.0]). There was no temporal trend noted in when misdiagnosis may have occurred.
Discussion
In Uganda and Russia, we examined the unexpectedly high proportion of undetectable viremia found in research cohorts meeting antiretroviral therapy (ART) naïve entry criteria. This study showed a potential for misdiagnosis of HIV in research cohorts as well as the potential for unreliable medical histories and documentation of HIV serostatus and/or ART naïve status. Between the 2 cohorts, 35 participants or 4.2% (95% CI: 2.9% – 5.7%) were found to be seronegative for HIV at study entry. Seventeen participants or 2.0% (95% CI: 2.7% – 5.3%) were taking ART at study entry.
The UNAIDS Fast-Track Strategy24 aims to greatly step up the HIV response in low- and middle-income countries to end the epidemic by 2030. The strategy sets 90-90-90 targets which means that 90% of all individuals with HIV will know their HIV status; 90% of all individuals living with HIV will receive ART, and 90% receiving ART will achieve viral suppression. In order to reach the first and second targets, a correct diagnosis is vital and countries will need to continue to improve the effectiveness and outreach of HIV testing, which includes updated WHO guidance.2 Given the growing number of reports indicating misdiagnosis of HIV status, serious concerns exist about the implications of the unnecessary initiation of life-long ART if one is given a false-positive HIV diagnosis.2 Initiating ART in these individuals would also waste scarce resources and be more taxing on health resources. Misdiagnosis can also have devastating individual consequences. In the Uganda and Russia cohorts the implications of the findings were shared with the participants and clinics as able. In Uganda, the investigators informed the clinic of the findings, and all the affected participants underwent counselling and were subsequently discharged from the clinic. This also prompted the clinic to re-test all HIV-infected patients in care who were not yet on ART and had persistently high CD4 counts and stage 1 disease. The research laboratory in Russia is not connected to a clinic and did not share findings regarding HIV seronegative individuals with any medical providers. All participants, however, were informed of the results, with a caveat that confirmatory testing was not performed and samples collected up to 2 years ago were tested. All participants were referred to the AIDS center in St. Petersburg for confirmatory testing.
The implications of HIV misdiagnosis can also undermine the integrity of research study results with individual and public health implications. Erroneous conclusions could be made if entry criteria are inaccurate. Falsification or mistaken reporting of clinical data is also of concern and methods to detect these issues have direct impact on protecting participant safety so participants do not receive unnecessary treatment. Ideally, HIV study participants would have their HIV status verified with confirmatory HIV testing, review of original medical record documentation or government registry review at study entry. ART documentation should also be carefully reviewed as individuals may not be aware that they are taking ART, or may be giving inaccurate reporting of ART use. The cost of conducting HIV testing to verify status must be weighed against the methodologic and ethical cost of including persons who are not infected with HIV.
Audits of HIV testing in several settings have determined that misdiagnosis can occur from limitations of the assay, operator-related factors, inappropriate storage of test kits, and suboptimal testing algorithms as well as non-HIV antibodies reacting with test antigens.2,3,25 Tie breaker algorithms similar to what was performed at the ISS clinic in Mbarara, Uganda have also been implicated in an increased rate of false positive HIV diagnoses due to cross-reactivity, especially in the presence of weak positive reactions on RDTs.26 Increasing availability of ART worldwide may also complicate interpretation of real HIV infection status, by increasing the numbers of those with seropositive, RNA negative status. ART testing may identify those with virologic suppression by ART, however drug testing may be impractical for many research studies.
In Russia, a history of incarceration has been found to be correlated with routine HIV testing in St. Petersburg, 26 but whether adequate post-test counselling and confirmatory testing are performed is unknown. There is some evidence, that WHO testing guidelines in prison settings may only be partially implemented in Europe and neighbouring countries and administrative barriers may exist in these institutional settings.27 In our study 5/9 HIV-negative participants had received their initial diagnosis in the prison system via RDT and never had confirmatory testing, required for registration in the national registry for HIV.
In some cases, the misrepresentation of ART-naïve status or HIV status could occur in research and clinical settings due to intentional or unintentional misrepresentation of medical history. There is evidence that both patients and potential study participants might choose to falsely report prior ART use for multiple reasons. Patients who start ART but are not adherent may feel a sense of shame and may choose not to disclose prior ART initiation.28 In the Russian cohort, the study team reported the potential for falsified paperwork especially among the participants that were screened as a result of snowball recruitment efforts. There are strategies that could address this problem such as the use of confirmatory testing to verify information disclosed by participants and using reinforcements to promote truthfulness and improve the accuracy of self-reported data. For example, researchers may inform participants that the accuracy of their information may be checked and that they will receive compensation (e.g., cash or gift vouchers) if no discrepancy is detected.29
Increased availability of relatively low cost supplemental/confirmatory assays with high specificity and sensitivity that measure reactivity to individual HIV 1/2 antigens that can be used outside a clinical laboratory setting will be useful in identifying those with false positive rapid test reactivity (independent of HIV RNA or drug level testing). Confirmatory HIV testing in resource limited settings has been proposed in study sites with Médecins Sans Frontières programs due to the unacceptably high rate of false-positive diagnoses. These confirmatory tests include the Orgenics Immunocomb Combfirm® HIV confirmation test (OIC-HIV) which is more feasible than Western blot in more remote settings. 14,30 Another test that identifies reactivity to HIV antigens is the Bio-Rad Geenius™ confirmatory test. The test is FDA approved for confirmation of HIV infection, is considered moderate complexity, is performed at ambient temperature, and is similar in procedure to RDT. The Geenius™ also has an automated reader that removes subjectivity in interpretation.31
This study has strengths and limitations. We did not use the Fourth Generation (HIV ½ Ab/p24 Ag) test for confirmatory testing using EIA on the Uganda samples. Therefore we could have the potential for a false-negative result in the Ugandan cohort where 1 individual had a viral load > 40 copies/mL and was found to be HIV-seronegative. We were unable to determine the reason for misclassification of HIV status for 4 of the 9 HIV-seronegative participants in the Russian cohort. We can only speculate that HIV test results provided to the study team were in error. Another limitation is that potential elite controllers among HIV-infected, ART naïve individuals cannot be verified without multiple undetectable viral loads recorded so we propose this possibility with caution. It could be that with further testing, there would be low but detectable VL in many of these subjects which is more compatible with a long term non-progressor definition. True elite controllers represent only 1% of the HIV population23, therefore the 5% in the Ugandan cohort may be the result of only testing HIV viral load at a single time point. The Ugandan cohort had no follow-up viral load testing so we were unable to determine if low or undetectable viral loads became detectable later in the study. Testing for the presence of ART was a strength in the both cohorts, however we only tested for first line ART combinations in Uganda. Some participants could have been on a second line regimen such as a protease inhibitor. The description of similar issues that occurred in two very different international cohorts in sub-Sahara Africa and Eastern Europe highlights the importance of the complexities of HIV diagnosis and verification of ART use in both clinical and research settings.
Conclusions
Similar proportions of false-positive HIV diagnoses in our Uganda and Russia ARCH cohorts from the URBAN ARCH Consortium that examines the impact of alcohol on HIV-infected persons were found. Unreported antiretroviral therapy use was also found in both cohorts. Between the two cohorts, 52 participants (6.2%) were subsequently found to not meet study eligibility criteria. Research studies need to be aware of the potential for misdiagnosis and unreliable medical documentation when screening potential study participants. HIV diagnosis by RDT may need to undergo further testing to confirm HIV status in both research and clinical settings. Strategies to improve the feasibility of confirmatory HIV testing and to ascertain accurate medical histories are strongly recommended. Research studies should invigorate efforts to avoid potential mischaracterization of participants in international HIV cohorts.
Figure 1.
Uganda HIV enzyme linked immunosorbent assay test results and antiretroviral therapy test results among those with low and undetectable viral load in the Uganda cohort of the Uganda, Russia, Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS (URBAN ARCH) consortium.
Figure 2.
Russia HIV enzyme linked immunosorbent assay test results and antiretroviral therapy test results among those with undetectable viral load in the Russia cohort of the Uganda, Russia, Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS (URBAN ARCH) consortium.
Table 1.
Characteristics of Uganda and Russia ARCH cohorts
| Uganda (n=482) Median (IQR) or N (%) |
Russia (n=360) Median (IQR) or N (%) |
|
|---|---|---|
| Demographic and Education | ||
| Age (years) | 33.0 (27, 41) | 33.0 (30, 37) |
| Female gender | 326 (68) | 104 (29) |
| Education above primary | 148 (31) | 283 (79) |
|
| ||
| Clinical | ||
| Years since HIV diagnosis | 1.7 (0.2, 6.0) | 6.7 (3.0, 11.0) |
| CD4 Cell Count (median/IQR)/mm3 | 549 (420, 686) | 485 (328, 702)† |
n=253
IQR= Interquartile range
Acknowledgments
The authors would like to thank the study participants, and our research staff who conducted the study; Kerrin M. Gallagher and Gregory Patts for providing editorial assistance in the preparation of this manuscript.
Footnotes
Declaration of Interest
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism(NIAAA) (K24AA022586, U01AA020780, U24AA020779, U24AA020778, U01AA020776 and U01AA021989) and the National Institute for Allergy and Infectious Diseases (NIAID) Center for AIDS Research (CFAR) (P30 AI027763, P30AI042853). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or other funding agencies. The authors report no declarations of interest.
References
- 1.Center for Disease Control and Prevention. [Accessed July 14, 2017];Laboratory Testing for the Diagnosis of HIV Infection: Updated Recommendations. 2014 https://stacks.cdc.gov/view/cdc/23447.
- 2.Johnson C, Fonner V, Sands A, et al. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. Geneva: World Health Organization; 2015. A report on the misdiagnosis of HIV status. Vol ANNEX 14. [PubMed] [Google Scholar]
- 3.Klarkowski D, O’Brien DP, Shanks L, Singh KP. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti Infect Ther. 2014;12(1):49–62. doi: 10.1586/14787210.2014.866516. [DOI] [PubMed] [Google Scholar]
- 4.Parisi MR, Soldini L, Di Perri G, Tiberi S, Lazzarin A, Lillo FB. Offer of rapid testing and alternative biological samples as practical tools to implement HIV screening programs. New Microbiol. 2009;32(4):391–396. [PubMed] [Google Scholar]
- 5.De Cock KM, Bunnell R, Mermin J. Unfinished business--expanding HIV testing in developing countries. N Engl J Med. 2006;354(5):440–442. doi: 10.1056/NEJMp058327. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Service delivery approaches to HIV testing and counselling (HTC): A strategic policy framework. Geneva: World Health Organization; 2012. [Accessed July 14, 2017]. http://www.who.int/hiv/pub/vct/htc_framework/en/ [Google Scholar]
- 7.World Health Organization. Consolidated guidelines on HIV testing services. Geneva: World Health Organization; 2015. [Accessed July 14, 2017]. http://www.who.int/hiv/pub/guidelines/hiv-testing-services/en/ [Google Scholar]
- 8.Kosack C, Page A, Beelaert G, et al. Towards more accurate HIV testing in sub-Saharan Africa: a multi-site evaluation of HIV RDTs and risk factors for false positives. Journal of the International Aids Society. 2017:20. doi: 10.7448/IAS.20.1.21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanks L, Klarkowski D, O’Brien DP. False positive HIV diagnoses in resource limited settings: operational lessons learned for HIV programmes. PLoS One. 2013;8(3):e59906. doi: 10.1371/journal.pone.0059906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanks L, Siddiqui MR, Abebe A, et al. Dilution testing using rapid diagnostic tests in a HIV diagnostic algorithm: a novel alternative for confirmation testing in resource limited settings. Virol J. 2015;12:75. doi: 10.1186/s12985-015-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn D, Johnson C, Sands A, et al. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection 2015. Geneva: World Health Organization; 2015. An analysis of 48 national HIV testing and counselling policies. Vol ANNEX 2. [PubMed] [Google Scholar]
- 12.Baveewo S, Kamya MR, Mayanja-Kizza H, et al. Potential for false positive HIV test results with the serial rapid HIV testing algorithm. BMC Res Notes. 2012;5:154. doi: 10.1186/1756-0500-5-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassett IV, Chetty S, Giddy J, et al. Screening for acute HIV infection in South Africa: finding acute and chronic disease. HIV Med. 2011;12(1):46–53. doi: 10.1111/j.1468-1293.2010.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klarkowski DB, Wazome JM, Lokuge KM, Shanks L, Mills CF, O’Brien DP. The evaluation of a rapid in situ HIV confirmation test in a programme with a high failure rate of the WHO HIV two-test diagnostic algorithm. PLoS One. 2009;4(2):e4351. doi: 10.1371/journal.pone.0004351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwangala S, Musonda K, Monze M, Musukwa K, Fylkesnes K. Accuracy in HIV Rapid Testing among Laboratory and Non-laboratory Personnel in Zambia: Observations from the National HIV Proficiency Testing System. Plos One. 2016;11(1) doi: 10.1371/journal.pone.0146700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Guidelines on dispensary observation and treatment of HIV-infected patients. (2015) In Russian http://nnoi.ru/news/50.
- 17.McCusker J, Stoddard AM, McCarthy E. The validity of self-reported HIV antibody test results. Am J Public Health. 1992;82(4):567–569. doi: 10.2105/ajph.82.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devine EG, Waters ME, Putnam M, et al. Concealment and fabrication by experienced research subjects. Clin Trials. 2013;10(6):935–948. doi: 10.1177/1740774513492917. [DOI] [PubMed] [Google Scholar]
- 19.Apseloff G, Swayne JK, Gerber N. Medical histories may be unreliable in screening volunteers for clinical trials. Clin Pharmacol Ther. 1996;60(3):353–356. doi: 10.1016/S0009-9236(96)90063-6. [DOI] [PubMed] [Google Scholar]
- 20.Muyindike WR, Lloyd-Travaglini C, Fatch R, et al. Phosphatidylethanol confirmed alcohol use among ART-naïve HIV-infected persons who denied consumption in rural Uganda. AIDS Care. 2017;29(11):1442–1447. doi: 10.1080/09540121.2017.1290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patts GJ, Cheng DM, Emenyonu N, et al. Alcohol Use and Food Insecurity Among People Living with HIV in Mbarara, Uganda and St. Petersburg, Russia. AIDS Behav. 2017;21(3):724–733. doi: 10.1007/s10461-016-1556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faugier J, Sargeant M. Sampling hard to reach populations. J Adv Nurs. 1997;26(4):790–797. doi: 10.1046/j.1365-2648.1997.00371.x. [DOI] [PubMed] [Google Scholar]
- 23.Olson AD, Meyer L, Prins M, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS One. 2014;9(1):e86719. doi: 10.1371/journal.pone.0086719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UNAIDS. [Accessed August 1, 2017];Fast Track Strategy. 2015 http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf.
- 25.Gray RH, Makumbi F, Serwadda D, et al. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. BMJ. 2007;335(7612):188. doi: 10.1136/bmj.39210.582801.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niccolai LM, Toussova OV, Verevochkin SV, Barbour R, Heimer R, Kozlov AP. High HIV prevalence, suboptimal HIV testing, and low knowledge of HIV-positive serostatus among injection drug users in St. Petersburg, Russia. AIDS Behav. 2010;14(4):932–941. doi: 10.1007/s10461-008-9469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deblonde J, De Koker P, Hamers FF, Fontaine J, Luchters S, Temmerman M. Barriers to HIV testing in Europe: a systematic review. Eur J Public Health. 2010;20(4):422–432. doi: 10.1093/eurpub/ckp231. [DOI] [PubMed] [Google Scholar]
- 28.Ware NC, Wyatt MA, Geng EH, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10(1):e1001369. doi: 10.1371/journal.pmed.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnik DB, McCann DJ. Deception by Research Participants. N Engl J Med. 2015;373(13):1192–1193. doi: 10.1056/NEJMp1506985. [DOI] [PubMed] [Google Scholar]
- 30.Shanks L, Siddiqui MR, Kliescikova J, et al. Evaluation of HIV testing algorithms in Ethiopia: the role of the tie-breaker algorithm and weakly reacting test lines in contributing to a high rate of false positive HIV diagnoses. BMC Infect Dis. 2015;15:39. doi: 10.1186/s12879-015-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montesinos I, Eykmans J, Delforge ML. Evaluation of the Bio-Rad Geenius HIV-1/2 test as a confirmatory assay. J Clin Virol. 2014;60(4):399–401. doi: 10.1016/j.jcv.2014.04.025. [DOI] [PubMed] [Google Scholar]