Abstract
GNE myopathy is a rare, autosomal recessive, inborn error of sialic acid metabolism, caused by mutations in GNE, the gene encoding UDP-N-acetyl-glucosamine-2-epimerase/N-acetylmannosamine kinase. The disease manifests as an adult-onset myopathy characterized by progressive skeletal muscle weakness and atrophy. There is no medical therapy available for this debilitating disease. Hyposialylation of muscle glycoproteins likely contributes to the pathophysiology of this disease. N-acetyl-D-mannosamine (ManNAc), an uncharged monosaccharide and the first committed precursor in the sialic acid biosynthetic pathway, is a therapeutic candidate that prevents muscle weakness in the mouse model of GNE myopathy. We conducted a first-in-human, randomized, placebo-controlled, double-blind, single-ascending dose study to evaluate safety and pharmacokinetics of ManNAc in GNE myopathy subjects. Single doses of 3 and 6 g of oral ManNAc were safe and well tolerated; 10 g was associated with diarrhea likely due to unabsorbed ManNAc. Oral ManNAc was absorbed rapidly and exhibited a short half-life (~2.4 h). Following administration of a single dose of ManNAc, there was a significant and sustained increase in plasma free sialic acid (Neu5Ac) (Tmax of 8-11 h). Neu5Ac levels remained above baseline 48 h post-dose in subjects who received a dose of 6 or 10 g. Given that Neu5Ac is known to have a short half-life, the prolonged elevation of Neu5Ac after a single dose of ManNAc suggests that intracellular biosynthesis of sialic acid was restored in subjects with GNE myopathy, including those homozygous for mutations in the kinase domain. Simulated plasma concentration-time profiles support a dosing regimen of 6 g twice daily for future clinical trials.
Keywords: First-in-human study, GlcNAc kinase, GNE, N-acetylneuraminic acid, pharmacokinetics, sialylation
1. Introduction
GNE myopathy is an autosomal recessive inborn error of sialic acid metabolism with an estimated prevalence of ~6/1,000,000 and no approved therapy [1]. The typical presentation is distal extremity weakness in young adults, with progression to other skeletal muscle groups and significant disability, including wheelchair use and dependent care [2, 3].
GNE myopathy is caused by biallelic mutations in the GNE gene, which encodes the bifunctional enzyme UDP-N-acetylglucosamine (GlcNAc) 2-epimerase/N-acetylmannosamine (ManNAc) kinase (EC:3.2.1.183, EC:2.7.1.60) [1, 4]. This enzyme catalyzes the rate-limiting step in the biosynthesis of sialic acid (N-acetylneuraminic acid; Neu5Ac) [5], which enters the nucleus to become activated into cytidine-5′-monophospho-N-acetylneuraminic acid (CMP-sialic acid); CMP-sialic acid returns to the cytoplasm, where it is used by the Golgi as a substrate for sialyltransferases to enzymatically sialylate glycans (glycoproteins and glycolipids) (Fig. 1) [6, 7]. Alternatively, cytoplasmic CMP-sialic acid can feedback inhibit the UDP-GlcNAc 2-epimerase activity of GNE by binding to the enzyme’s allosteric site, creating a mechanism for controlling intracellular sialic acid production (Fig. 1) [8, 9]. Sialic acids are the most abundant terminal monosaccharides on human glycans, and Neu5Ac (commonly referred to as ‘sialic acid’) is the most abundant mammalian sialic acid and the precursor of most other sialic acids [10].
Figure 1. Sialic Acid Biosynthetic Pathway.
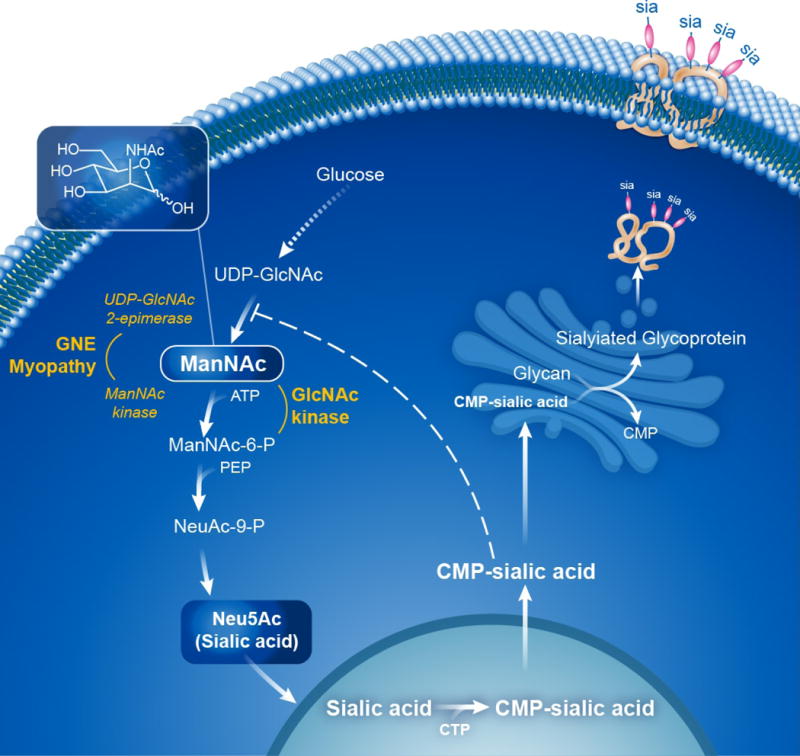
The biosynthesis of Neu5Ac (sialic acid) occurs in the cytosol, where glucose undergoes several modifications to become UDP-GlcNAc. The UDP-GlcNAc 2-epimerase activity of the bifunctional GNE enzyme then epimerises UDP-GlcNAc into ManNAc, after which the GNE ManNAc kinase activity further converts this to ManNAc-6-P, which then undergoes two more steps to form cytoplasmic free sialic acid. A nuclear step activates free sialic acid to CMP-sialic acid, which translocates back to the cytosol, where is utilized by the Golgi complex to sialylate glycans (glycoproteins and glycolipids). Cytoplasmic CMP-sialic acid strongly feedback-inhibits the UDP-GlcNAc 2-epimerase enzymatic activity in its allosteric site (dashed line). PEP=phosphoenolpyruvate. Patients with GNE myopathy have deficiency of GNE UDP-GlcNAc 2-epimerase and ManNAc kinase activity, resulting in decreased sialic acid production. Supplementation of ManNAc (uncharged small molecule that crossed membranes readily) circumvents the feedback inhibition step in this pathway and increases sialic acid production. ManNAc can be converted to ManNAc-6-P by the ancillary enzyme GlcNAc kinase, which likely occurs in GNE myopathy patients with severe ManNAc kinase deficiency. See text for further details and references. Figure courtesy of Daryl Leja and Julia Fekecs, NHGRI, NIH.
Mutations in the GNE gene lead to decreased sialic acid synthesis and decreased sialylation of (muscle) glycans. GNE myopathy-related mutations in GNE are predominantly missense, distributed throughout the entire protein-coding region [1], and result in decreased, but never absent, epimerase and kinase enzymatic activities [1, 11–13]. It was recognized that, compared with enzyme activities in a cell-free system, fibroblasts from GNE myopathy patients in culture exhibited higher residual activities of ManNAc kinase, suggesting the presence of additional sugar kinases with overlapping substrate specificity [12]. In fact, GlcNAc kinase has been identified as the only other enzyme that can phosphorylate ManNAc to ManNAc-6P in cells lacking GNE activity [14], as depicted in Fig. 1.
In GNE myopathy, overall sialylation of tissues is normal, but muscle proteins appear hyposialylated [3, 11, 15, 16]. In addition, mouse and cellular models of GNE myopathy reveal that muscle glycan hyposialylation and/or muscle atrophy and weakness can be prevented or treated by treatment with sialic acid metabolites [17–20]. Specifically, there is in vitro and in vivo evidence that the neutral sialic acid precursor N-acetyl-D-mannosamine (ManNAc) increases sialic acid production and sialylation of hyposialylated glycans in models of GNE myopathy [17, 18, 20]. Of note, sialic acid production through ManNAc supplementation in GNE myopathy patients is not influenced by the feedback-inhibition step of the sialic acid synthesis pathway, and ManNAc can efficiently be converted to ManNAc-6P by GlcNAc kinase, rather than the deficient ManNAc kinase [14] (Fig. 1).
In studies of GNE-deficient lymphoid or hematopoietic cells [21], as well as Gne-deficient murine embryonic stem cells [22], ManNAc supplementation restored sialylation. In addition, a gene-targeted knock-in Gne mouse homozygous for the Persian-Jewish GNE myopathy founder mutation, p.Met743Thr [20], showed therapeutic normalization of hyposialylated muscle glycans after 12 weeks of oral ManNAc treatment (1 g/kg/day and 2 g/kg/day; the human equivalent dose (HED) was ~5.6 g/day and ~11.2 g/day) [18]. A transgenic mouse model (on a Gne null background) for a founder mutation in Japanese patients, p.Asp207Val, received prophylactic oral treatment with ManNAc at 0.02 g/kg/day (HED ~ 0.112 g/day), 0.2 g/kg/day (HED ~ 1.12 g/day), and 2 g/kg/day (HED ~ 11.2 g/day), which resulted in prevention of muscle weakness and atrophy, improved survival, and improved sialylation of muscle membrane-bound glycans in muscle and other tissues [17].
We now describe a first-in-human clinical trial of ManNAc in subjects with GNE myopathy, to determine the safety, pharmacokinetics, and pharmacodynamics of this compound for a future therapeutic trial.
2. Materials and Methods
2.1. Regulatory Approval
Subjects were evaluated under NIH protocol 12-HG-0207 “Phase I Clinical Trial of ManNAc in Patients with GNE Myopathy or Hereditary Inclusion Body Myopathy” (ClinicalTrials.gov Identifier NCT01634750). The protocol was reviewed and approved by the National Human Genome Research Institute (NHGRI) Institutional Review Board (IRB). Subjects gave written, informed consent and were treated with ManNAc under FDA Investigational New Drug 078091.
2.2. Study Population
The study enrolled subjects of either gender, between 18 and 70 years of age, with a diagnosis of GNE myopathy confirmed by the identification bialellic GNE mutations. Subjects were required to avoid receiving ManNAc, sialic acid, IVIG, or other supplements containing sialic acid (e.g., St John’s wort, sialyllactose) 30 days before randomization and for the duration of the trial. Women of reproductive age were required to use an effective method of contraception for the duration of the trial. Exclusion criteria included: severe disease manifestations or presence of conditions that would interfere with the ability to comply with the requirements of the protocol, hepatic or renal laboratory parameters greater than 3 times the upper limit of normal, a QT corrected using Bazett’s formula (QTcB) >450 msec in males or QTcB >470 msec in females, anemia (defined as two standard deviations below normal for age and gender), clinically significant cardiovascular, pulmonary, hepatic, renal, hematological, metabolic, or gastrointestinal disease, or a condition that requires immediate surgical intervention, pregnancy or breastfeeding at any time during the study, investigational drug, investigational device, or approved therapy for investigational use within 4 weeks of initial screening, a history of hypersensitivity to ManNAc or a condition that places the subject at increased risk for adverse effects.
2.3. Study Design
This was a Phase 1, randomized, placebo-controlled, double-blind, single-site, escalating single-dose study with the primary objectives of evaluating the safety, tolerability and pharmacokinetics (PK) of orally administered ManNAc in subjects with GNE myopathy. All subjects were evaluated at the NIH Clinical Center. Cohort A included 6 subjects randomized in a 2:1 ratio to receive either a single oral dose of 3 g ManNAc (4 subjects) or placebo (mannitol) (2 subjects). Cohorts B and C each included 8 subjects randomized in a 3:1 ratio to receive either ManNAc (6 subjects) or placebo (mannitol) (2 subjects), in 6 g (Cohort B) or 10 g (Cohort C) single oral single doses. The study design is typical for a Phase 1, single ascending dose (SAD) study. The starting dose was based on a 90-day nonclinical Good Laboratory Practice (GLP) toxicology study in rats (most sensitive species), which included doses up to 12 g/kg/day. Based on the no observable adverse effect level (NOAEL) and a 10-fold safety margin, the starting dose was set at 3 g ManNAc and the highest dose at 10 g.
2.4. Drug Product
ManNAc (N-acetyl-D-mannosamine monohydrate) is the active drug product; mannitol was chosen as the placebo because it has similar taste and color as ManNAc, it is not involved in the sialic acid synthesis pathway and cannot be converted to ManNAc. New Zealand Pharmaceuticals Ltd (NZP) was the Good Manufacturing Practice (GMP) manufacturer of the ManNAc drug substance. Storage conditions for the study drug were 2 to 8 °C. ManNAc was supplied as a powder-in-bottle at doses of 3 g, 6 g and 10 g. Mannitol was provided as powder-in-bottle at a dose of 5 g. ManNAc or placebo were reconstituted with 200 mL of sterile water for irrigation for delivery as an oral liquid at the National Institutes of Health (NIH) Pharmacy just prior to administration. After ingestion, the bottle was rinsed with 100 mL water for irrigation.
2.5. Procedures
Subjects were screened 1 to 30 days prior to dosing. After eligibility was determined and informed consent obtained, the following was obtained at baseline: medical history, demographics, concomitant medication, vital signs, physical examination, 12-lead electrocardiogram (ECG), pregnancy test, and blood and urine samples for clinical laboratory tests and research.
After dosing, subjects remained at the study site for observation until completion of study related activities on Day 3 (48 hours after dosing). A follow-up phone contact on Day 8 assessed for adverse events (AEs).
2.6. Safety
Safety was assessed by physical examinations, body weight, vital signs, ECGs and clinical laboratory tests. The severity of AEs was graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.3. Vital signs were collected at baseline, pre-dose, and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, and 48 h after study drug administration and included temperature, respiration, and orthostatic blood pressure and heart rate (blood pressure and heart rate were measured after the subject had been supine for 5 min followed by repeat measurement after standing or sitting for 1 minute). ECGs were performed before dosing, and at approximately 0.5, 2, 4, and 48 h after dosing; QT interval was corrected using Bazetts’s formula (QTcB = QT/RR0.5). Clinical laboratory tests were performed at baseline, 24 and 48 h after dosing, including complete blood count, urinalysis and serum albumin, alkaline phosphatase, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), calcium, chloride (Cl), carbon dioxide (CO2), creatinine, creatine kinase (CK), glucose, inorganic phosphorus, lactate dehydrogenase (LD), magnesium (Mg), potassium (K), sodium (Na), total bilirubin, direct bilirubin, total protein, and uric acid. Because of muscle wasting characteristic of GNE myopathy, glomerular filtration rate was estimated (eGFR) from serum cystatin C utilizing the CKD-EPI cystatin C equation [23].
A Safety Review Committee reviewed all available safety data for a completed cohort of subjects in a blinded fashion prior to making a decision to escalate to the next higher dose level. The safety information reviewed included AEs, physical exams, ECGs, vital signs, and clinical laboratories data. Safety data were evaluated pre- and post-dosing through at least Day 8 before the next dose level was administered.
2.7. Pharmacokinetic Evaluations
Blood for pharmacokinetic analysis was collected into potassium (K2) EDTA tubes pre-dose and at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 16, 24, and 48 h after study drug administration. Following blood collection, the samples were held on wet ice and centrifuged (8 °C for 10 min at ~1500 g) within 30 min to obtain plasma samples. Each plasma sample was split into three aliquots in cryogenic vials. Plasma samples were frozen immediately at −80°C. Samples were shipped overnight on dry ice to the analytical lab (Alliance Pharma, PA, USA) for drug concentration measurement. Samples were analyzed using validated bioanalytical methods for the quantitative analysis of ManNAc and free (unconjugated) Neu5Ac (sialic acid) in human plasma using liquid chromatography-mass spectrometry (LC-MS/MS) [24]. The established lower limit of quantification (LLOQ) was 10 and 25 ng/mL for ManNAc and Neu5Ac, respectively. These two methods demonstrated good sensitivity, selectivity, linearity, accuracy, and precision as well as assay ruggedness; they were validated using a “fit-for-purpose” approach.
Pharmacokinetic (PK) parameters were calculated using noncompartmental method with Phoenix WinNonLin, Version 6.2.0 (Certara, St. Louis, MO, USA). Concentration-time graphics were prepared with either SAS Version 9.2 or SigmaPlot Version 12.0. For both ManNAc and unconjugated free Neu5Ac concentration data the following PK parameters were evaluated: maximum observed plasma concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration versus time curve from time 0 (pre-dose) to time infinity (AUCinf), AUC from time 0 to time of last measurable plasma concentration (AUClast), percentage of the AUC that is extrapolated beyond the last measurable concentration over AUCinf (%AUCextr), apparent systemic clearance (CL/F), terminal-phase half-life (t½) and mean residence time (MRT). Similar parameters were determined for Neu5Ac, except CL/F which was not calculated.
A simulation was conducted to predict plasma concentrations of ManNAc and Neu5Ac after multiple dosing of ManNAc at 6 g twice daily, based on the single dose study results after oral administration of 6 g ManNAc. The simulated concentration-time data were generated with Phoenix WinNonLin (Version 6.2.0), assuming a linear kinetics after a multiple-dose treatment.
3. Results
A total of 22 subjects (10 male and 12 female) were enrolled in the study. The mean age of the study population was 43 years (range 30-65 years), and mean weight was 86 kg (range 49-115 kg) with a mean BMI of 28.4 kg/m2 (range 21-40 kg/m2). All subjects had normal renal function with a mean eGFR of 118 mL/min. Mean creatine kinase at baseline was 230 (range: 80-538 U/L). Sixteen subjects received ManNAc (4 in cohort A and 6 in cohorts B and C), and 6 subjects received placebo. The mean age of subjects in cohorts A, B and C was 40.8 (±11.6), 46.8 (±11.8) and 42.7 (±8.4) years, respectively; mean weight was 77.1 (±7.6), 98.6 (±14.2) and 79.5 (±22.5) kg, respectively. Fifteen subjects (68%) were compound heterozygotes for missense mutations in the UDP-GlcNAc 2-epimerase and ManNAc kinase domains of the GNE gene. Six patients had two kinase missense mutations, three of which were randomized to placebo and one on each of the cohorts. Only one patient had two epimerase mutations in the GNE and was randomized to cohort B (Table 1).
Table 1.
Subject Characteristics
| Subject | Treatment Group | Age (years) | Gender | Weight (kg) | BMI (kg/m2) | GNE Mutation Allele 1 / Allele 2 | GNE Domain1 |
|---|---|---|---|---|---|---|---|
| PH100001 | ManNAc 3 g | 54 | Male | 83.7 | 30 | c.1225G>T (p.Asp409Tyr)/c.1909+5G>A (splice) | ep/kin |
| PH100002 | ManNAc 3 g | 47 | Female | 71.2 | 26.3 | c.2228T>C (p.Met743Thr)/c.2228T>C (p.Met743Thr) | kin/kin |
| PH100004 | ManNAc 3 g | 30 | Female | 69.8 | 25.5 | c.740T>C (p.Val247Ala)/c.1985C>T (p.Ala662Val) | ep/kin |
| PH100006 | ManNAc 3 g | 32 | Male | 83.7 | 24.5 | c.1225G>T (p.Asp409Tyr)/c.1985C>T (p.Ala662Val) | ep/kin |
| PH1000082 | ManNAc 3 g | 51 | Female | 97.3 | 32.5 | c.1225G>T (p.Asp409Tyr)/c.2036C>T (p.Ala679Val) | ep/kin |
| PH100009 | ManNAc 6 g | 44 | Female | 76.7 | 25.6 | c.1225G>T (p.Asp409Tyr)/c.2036C>T (p.Ala679Val) | ep/kin |
| PH100010 | ManNAc 6 g | 52 | Male | 95.0 | 28.1 | c.731A>T (p.Asp244Val)/c.2179G>A (p.Val727Met) | ep/kin |
| PH100016 | ManNAc 6 g | 32 | Male | 113.8 | 29.4 | c.1225G>T (p.Asp409Tyr)/c.2036C>T (p.Ala679Val) | ep/kin |
| PH100017 | ManNAc 6 g | 37 | Male | 94.0 | 27.2 | c.497G>T (p.Gly166Val)/c.829C>T (p.Arg277Trp) | ep/ep |
| PH100018 | ManNAc 6 g | 65 | Male | 114.9 | 39.8 | c.2228T>C (p.Met743Thr)/c.2228T>C (p.Met743Thr) | kin/kin |
| PH100019 | ManNAc 10 g | 50 | Female | 69.5 | 26 | c.983T>C (p.Met328Thr)/c.2179G>A (p.Val727Met) | ep/kin |
| PH100020 | ManNAc 10 g | 32 | Male | 86.8 | 27.4 | c.559T>C (p.Tyr187His)/c.2179G>A (p.Val727Met) | ep/kin |
| PH100022 | ManNAc 10 g | 35 | Female | 59.3 | 23.5 | c.1985C>T (p.Ala662Val)/c.1985C>T (p.Ala662Val) | kin/kin |
| PH100023 | ManNAc 10 g | 39 | Female | 53.6 | 21.6 | c.478C>T (p.Arg160*)/c.2179G>A (p.Val727Met) | ep/kin |
| PH100025 | ManNAc 10 g | 49 | Male | 111.9 | 29.3 | c.1225G>T (p.Asp409Tyr)/c.2036C>T (p.Ala679Val) | ep/kin |
| PH1000262 | ManNAc 10 g | 51 | Female | 95.6 | 31.2 | c.1225G>T (p.Asp409Tyr)/c.2036C>T (p.Ala679Val) | ep/kin |
| PH100003 | Placebo | 53 | Female | 55.4 | 21.1 | c.986T>C (p.Ile329Thr)/c.2179G>A (p.Val727Met) | ep/kin |
| PH100007 | Placebo | 49 | Male | 111.8 | 28.9 | c.1225G>T (p.Asp409Tyr)/c.2036C>T (p.Ala679Val) | ep/kin |
| PH100014 | Placebo | 47 | Male | 109.0 | 31.5 | c.1225G>T (p.Asp409Tyr)/c.2036C>T (p.Ala679Val) | ep/kin |
| PH1000152 | Placebo | 36 | Female | 96.3 | 36.7 | c.2228T>C (p.Met743Thr)/c.2228T>C (p.Met743Thr) | kin/kin |
| PH100021 | Placebo | 30 | Female | 49.3 | 21.6 | c.1618C>T (p.His540Tyr)/c.1769G>C (p.Gly590Ala) | kin/kin |
| PH1000242 | Placebo | 36 | Female | 95.3 | 36.8 | c.2228T>C (p.Met743Thr)/c.2228T>C (p.Met743Thr) | kin/kin |
ep = UDP-GlcNAc 2-epimerase enzymatic domain; kin = ManNAc kinase enzymatic domain.
PH100008 (PH100026) and PH100015 (PH100024) participated in two dosing cohorts with more than 8 weeks washout time in between their two single doses.
3.1. Safety and Tolerability
All subjects completed study treatment. Oral ManNAc and the placebo, oral mannitol, were well tolerated with no serious or severe adverse events reported. All adverse events observed in the study were either CTCAE Grade 1 (mild; asymptomatic or mild symptoms) or Grade 2 (Moderate; minimal, local or noninvasive intervention indicated). Adverse events observed at singles doses of 3 or 6 g of ManNAc, were comparable with those observed in the placebo group (Table 2). However, 50% of subjects who received ManNAc at a dose of 10 g had a single, self-resolving episode of diarrhea and nausea (Grade 1) shortly after ingestion of ManNAc, which was not associated with abdominal pain or other gastrointestinal symptoms. Diarrhea and nausea were not observed at either doses of 3 or 6 g or in the placebo group. These gastrointestinal symptoms were attributed to unabsorbed ManNAc in the gastrointestinal tract, and may suggest saturation in the absorption of ManNAc at a single dose of 10 g. Other adverse events such as increased liver function tests were seen at higher frequency on subjects receiving a single dose of 10 g. Headache was seen in all cohorts, including the placebo group, and presented in fasting subjects and resolved by food intake. Therefore it was considered to be possibly or unlikely related to the study drug.
Table 2.
Adverse Events by Treatment Group
| Treatment Group
|
|||||
|---|---|---|---|---|---|
| 3 g | 6 g | 10 g | All ManNAc | Placebo | |
|
| |||||
| Number of Subjects | 4 | 6 | 6 | 16 | 6 |
|
| |||||
| Adverse Events by SOC | |||||
| Nervous system disorders | 3 (75.0%) | 1 (16.7%) | 3 (50.0%) | 7 (43.8%) | 2 (33.3%) |
| Headache* | 3 (75.0%) | 1 (16.7%) | 2 (33.3%) | 6 (37.5%) | 2 (33.3%) |
| Dizziness* | 1 (25.0%) | 0 | 1 (16.7%) | 2 (12.5%) | 0 |
| Blood and lymphatic system disorders | 1 (25.0%) | 1 (16.7%) | 2 (33.3%) | 4 (25.0%) | 1 (16.7%) |
| Anemia | 1 (25.0%) | 1 (16.7%) | 1 (16.7%) | 3 (18.8%) | 1 (16.7%) |
| Hypocalcemia | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| General disorders | 2 (50.0%) | 0 | 1 (16.7%) | 3 (18.8%) | 2 (33.3%) |
| Fatigue | 2 (50.0%) | 0 | 0 | 2 (12.5%) | 2 (33.3%) |
| Pain left shoulder | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 1 (16.7%) | 2 (33.3%) | 3 (18.8%) | 1 (16.7%) |
| Increased CPK | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 1 (16.7%) |
| Back Pain | 0 | 1 (16.7%) | 0 | 1 (6.3%) | 0 |
| Increased LDH | 0 | 0 | 0 | 0 | 1 (16.7%) |
| Hip pain | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Gastrointestinal disorders | 0 | 0 | 3 (50.0%) | 3 (18.8%) | 0 |
| Diarrhea* | 0 | 0 | 3 (50.0%) | 3 (18.8%) | 0 |
| Nausea* | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Hepatobiliary disorders | 0 | 0 | 2 (33.3%) | 2 (12.5%) | 1 (16.7%) |
| Increased AST* | 0 | 0 | 2 (33.3%) | 2 (12.5%) | 1 (16.7%) |
| Increased ALT* | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 1 (16.7%) |
| Increased GGT* | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Immune system disorders | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 2 (33.3%) |
| Allergic Rhinitis | 0 | 0 | 0 | 0 | 1 (16.7%) |
| Increased ESR | 0 | 0 | 0 | 0 | 1 (16.7%) |
| Increased eosinophils | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Infections and infestations | 0 | 0 | 2 (33.3%) | 2 (12.5%) | 0 |
| Bacterial pharyngitis | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Upper Respiratory Infection | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Skin and subcutaneous tissue disorders | 0 | 0 | 2 (33.3%) | 2 (12.5%) | 0 |
| Palmar rash and tenderness bilaterally | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Rash | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Injury, poisoning and procedural complications | 0 | 0 | 0 | 0 | 1 (16.7%) |
| Injection site reaction | 0 | 0 | 0 | 0 | 1 (16.7%) |
| Metabolism and nutrition disorders | 0 | 1 (16.7%) | 0 | 1 (6.3%) | 0 |
| Hyperglycemia* | 0 | 1 (16.7%) | 0 | 1 (6.3%) | 0 |
| Vascular disorders | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
| Hypotension | 0 | 0 | 1 (16.7%) | 1 (6.3%) | 0 |
Abbreviations: SOC: System Organ Class
Note: Events are sorted in decreasing frequency by percent of overall subjects with the event by SOC and verbatim terms within SOC.
Denotes AEs identified to be possibly or probably related to study drug administration. Other AEs were considered to be unlikely or not related.
No other clinically relevant changes in clinical laboratory tests or vital signs, including orthostatic heart rate or blood pressure, or laboratory values were observed. There was a 6 ms increase in the mean baseline corrected QTcB interval for subjects receiving ManNAc that was not clinically significant. No other clinically relevant changes in the electrocardio[25] were noted.
3.2. Single Dose Pharmacokinetics of ManNAc
The mean pre-dose plasma ManNAc concentration was 53.4 ±12.0 ng/mL in GNE myopathy subjects (n=22). Plasma ManNAc concentrations did not increase in GNE myopathy subjects who received mannitol as placebo (n=6); the mean plasma ManNAc concentration in these subjects was 63.4 ±33.4 ng/mL. The plasma concentration-time profiles of ManNAc in placebo or ManNAc treated subjects are shown in Figure 2. For subjects treated with ManNAc, the “baseline adjusted” plasma concentrations of ManNAc were calculated by subtracting pre-dose level of ManNAc from the total measured ManNAc concentrations at each time point. The mean “baseline adjusted” plasma concentration-time profiles of ManNAc are presented in Figure 3.
Figure 2. Plasma Concentration-Time Profiles of ManNAc and Neu5Ac after Oral Administration of a Single Dose of 3, 6 or 10 g of ManNAc to Fasted GNE Myopathy Subjects.
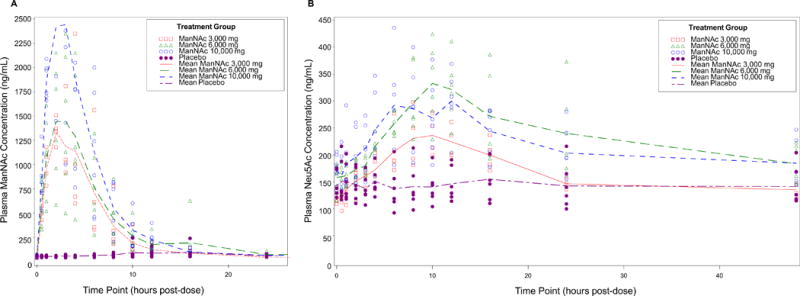
Mean cohort and individual subject plasma concentrations (ng/mL) of ManNAc (A) and Neu5Ac (B).
Figure 3. Mean Adjusted Plasma Concentration-Time Profiles.
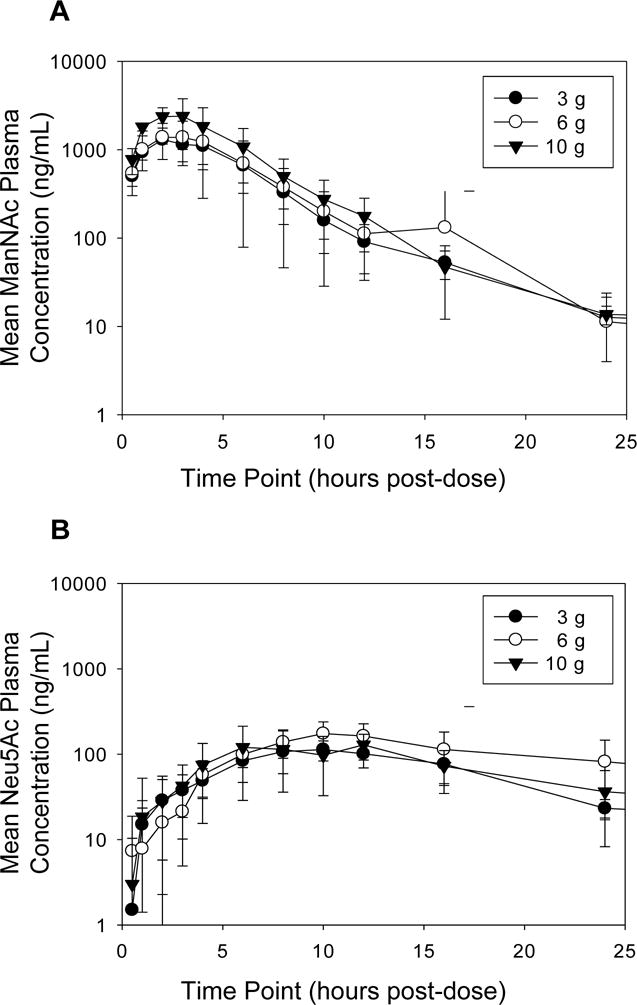
Mean (± SD) plasma concentrations of ManNAc (A) and Neu5Ac (B) after a single oral dose of ManNAc administered to fasted GNE myopathy subjects with baseline adjustment. Baseline adjustment was made by subtracting ManNAc or Neu5Ac concentration at time t=0 h for each subject.
Following single oral dose of 3, 6 and 10 g ManNAc, the systemic exposures (Cmax and AUCinf) of ManNAc in fasted subjects with GNE myopathy were determined with the measured plasma drug concentrations. The geometric mean of Cmax was 1502, 1407, and 2638 ng/mL, respectively, for the treatment of 3, 6 and 10 g ManNAc. The corresponding mean (±SD) AUCinf value was 10715±3607, 12764±3711, and 16761±5425 ng.h/mL, respectively, for the treatment groups of 3, 6 and 10 g ManNAc. These data suggest that the systemic exposure of oral ManNAc increased with dose in a less than dose-proportional manner over the dose range of 3 to 10 g.
The PK parameters of ManNAc were also calculated with baseline adjusted drug concentrations by subtracting ManNAc concentration at t=0 for each subject and are shown in Table 3. The baseline adjusted ManNAc concentrations are more reflective of the drug treatment. Similarly to the measured ManNAc concentrations, Cmax and AUCinf of baseline adjusted ManNAc increased less than dose-proportionally. When the dose was increased from 3 to 10 g (3.3X), the mean Cmax and AUCinf increased 1.8 and 1.7 fold, respectively. ManNAc was rapidly absorbed and the median Tmax was 2.0 to 2.5 h. The median ManNAc t1/2 after a single dose ranged from 2.2 to 2.6 h. Mean apparent clearance (CL/F) ranged from 418 to 827 L/h (Table 3).
Table 3.
Plasma ManNAc and Neu5Ac PK Parameters after a Single Dose of Oral ManNAc to Fasted GNE Myopathy Subjects (Baseline adjusted concentrations were used in the PK calculation).
| Dose | Tmax1 | Cmax2 | Cmax Ratio | Mean AUCinf3 | AUCinf Ratio | CL/F3 | t1/21 | MRT∞3 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (h) | (ng/mL) | Dose Compared | Ratio | (ng.h/mL) | Dose Compared | Ratio | (L/h) | (h) | (h) |
| Plasma ManNAc | ||||||||||
| 3 | 2.0 | 1451 | 6/3 g | 0.9 | 8244±4090 | 6/3 g | 1.2 | 418±147 | 2.2 | 5.6±0.8 |
| 6 | 2.5 | 1336 | 10/6 g | 1.9 | 9552±3441 | 10/6 g | 1.5 | 691±215 | 2.6 | 6.4±2.3 |
| 10 | 2.0 | 2585 | 10/3 g | 1.8 | 14161±5793 | 10/3 g | 1.7 | 827±375 | 2.4 | 5.3±0.5 |
| Plasma Unconjugated Free Neu5Ac | ||||||||||
| 3 | 10.0 | 115 | 6/3 g | 1.6 | 2147±405 | 6/3 g | 1.9 | ND | 5.5 | 17.9±3.1 |
| 6 | 11.0 | 181 | 10/6 g | 0.8 | 4171±1785 | 10/6 g | 0.7 | ND | 7.8 | 22.9±5.5 |
| 10 | 8.0 | 143 | 10/3 g | 1.2 | 3007±1162 | 10/3 g | 1.4 | ND | 7.2 | 19.2±4.5 |
Median
Geometric Mean
PK calculations (mean ± SD) were conducted with baseline adjustment by subtracting ManNAc or Neu5Ac concentrations at t=0 for each subject.
Abbreviations: Cmax: maximum observed plasma concentration, Tmax: time to Cmax, AUCinf: area under the plasma concentration versus time curve from time 0 (pre-dose) to time infinity, CL/F: apparent systemic clearance, t½: terminal-phase half-life, MRT: mean residence time, ND: not determined.
3.3. Pharmacodynamics
The mean pre-dose (unconjugated) free Neu5Ac concentration in plasma of GNE myopathy subjects was 152 ±29.0 ng/mL (n=22). Plasma concentrations of free Neu5Ac did not increase in GNE myopathy subjects who received mannitol as placebo (n=6); the mean plasma Neu5Ac after administration of placebo was 145 ±32.3 ng/mL.
Following a single oral dose of ManNAc at 3, 6 and 10 g, the plasma concentrations of Neu5Ac displayed a slow increase to reach a median Tmax of 10.0, 11.0 and 8.0 h, respectively, for the 3, 6, and 10 g doses, followed by a slow decline of plasma Neu5Ac concentrations. When unconjugated free Neu5Ac concentrations were measured, the geometric mean of Cmax of Neu5Ac was 243, 350 and 329 ng/mL, respectively, for the oral treatment groups of 3, 6 and 10 g ManNAc. The AUCinf for unconjugated free Neu5Ac couldn’t be calculated due to the slow decline of the Neu5Ac curve (not back to baseline) over the sample collection period of 48 h (%AUCextr at the terminal phase > 30%). The mean AUC48hr for unconjugated free Neu5Ac was 7942±625, 11322±2320, and 10562±2152 ng.h/mL, respectively, for the treatment groups of 3, 6 and 10 g.
PK parameters for Neu5Ac were also calculated with baseline adjusted concentrations by subtracting Neu5Ac concentration at t=0 for each subject and are shown in Table 3. Again, there was a sustained increase in plasma Neu5Ac with a median Tmax of 8-11 h over the dose range of doses, which was approximately 6-8 h after the Tmax of ManNAc (Fig. 2A, Table 3). The prolonged Tmax for Neu5Ac suggest that the apprearance of Neu5Ac in the systemic circulation could be formation and/or cellular membrane tranport rate-limited. The Cmax and AUCinf of Neu5Ac increased approximately dose-proportionally from 3 to 6 g of ManNAc, but did not increase further upon administration of 10 g of ManNAc (Table 3). The relative slow formation/celluar membrane transport followed by a slow elimination resulted a sustained and flatter plasma concentration curves for Neu5Ac.
The increase in plasma Neu5Ac was comparable across genotypes, including those subjects with two kinase domain missense mutations of the GNE gene. There was one subject with two kinase mutations on each cohort. Subjects Ph100002, Ph100018 and Ph100022 received 3, 6 and 10 g, respectively (Table 1). The mean Cmax for Neu5Ac with baseline adjustment observed on subjects Ph100002, Ph100018 and Ph100022 were 91 ng/mL (3 g cohort mean: 115 ng/mL), 197 ng/mL (6 g cohort mean: 181 ng/mL) and 270 ng/mL (10 g cohort mean: 143 ng/mL), respectively.
3.4. Simulated Concentration-Time Profiles
Simulated plasma concentration-time profiles of ManNAc and Neu5Ac after 6 g of ManNAc twice daily (BID) (total 12 g per day) were generated to estimate the time to reach steady-state after multiple doses. No significant accumulation of ManNAc is predicted following BID dosing of 6 g of ManNAc that would lead to toxic levels. Furthermore, due to the longer half-life of Neu5Ac the simulations predict appropriate levels of plasma Neu5Ac following this dosing regimen (Fig. 4).
Figure 4. Simulated Concentration-Time Profiles.
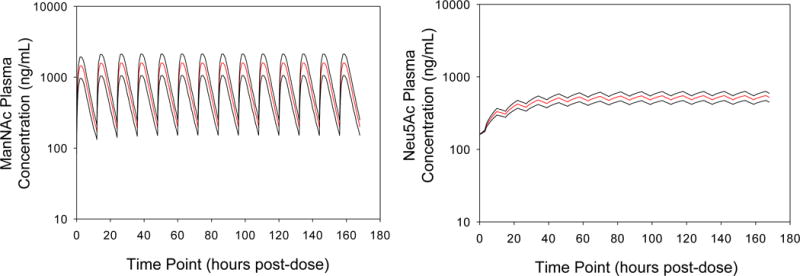
Simulated mean plasma ManNAc (A) and Neu5Ac (B) concentrations after oral administration of 6 g of ManNAc twice daily to subjects with GNE myopathy [Mean (red line) ±SD (black lines)].
4. Discussion
The proximal pursuit of ManNAc therapy for GNE myopathy began with mouse studies. A transgenic GNE myopathy mouse model (on a Gne null background), mimicking a founder mutation in Japanese patients (p. Asp207Val) recapitulated the muscle phenotype of GNE myopathy, developing progressive muscle weakness at 10 weeks and by 40 weeks showing significant changes in muscle pathology and biochemistry similar to those of GNE myopathy patients. Prophylactic continuous oral ManNAc treatment of these mice starting at week 5 resulted in prevention of muscle weakness and atrophy, reduced or absent rimmed vacuoles and intracellular amyloid inclusions, improved survival, and improved sialylation of muscle membrane-bound glycans in muscle and other tissues [17]. A knock-in mouse model, mimicking a founder mutation in Persian-Jewish patients (p.Met743Thr) showed marked muscle hyposialylation at ~4-6 months of age. Therapeutic oral treatment of these mice with ManNAc starting at ~6 months of age, normalized muscle sialylation after 12 weeks of therapy [18]. This therapeutic potential of ManNAc is promising for patients with GNE myopathy, since they are diagnosed after development of (significant) symptoms; most patients experience a delayed diagnosis of ~10 years after onset of symptoms [1, 3].
Oral supplementation with not only ManNAc, but also Neu5Ac to Gne-deficient mice appeared to restore sialylation [18] and prevent muscle weakness [17]. While clinical trials for Neu5Ac (Ace-ER) in GNE myopathy patients are ongoing [25], we favored the development of ManNAc for patients with GNE myopathy after review of preclinical studies [26, 27]. ManNAc (MW 221.2 Da) is a naturally occurring uncharged monosaccharide, while Neu5Ac (MW 309.3 Da) is a negatively charged acidic monosaccharide. ManNAc is the only neutral molecule in sialic acid biosynthesis (Fig. 1), so it crosses membranes more easily than other pathway intermediates and is expected to readily reach the intracellular space, as confirmed by several in vitro studies [28, 29]. Such assays used radioactive tracers to demonstrate that the cellular uptake of ManNAc occurs though passive diffusion, this low-energy process is linear and nonsaturable (up to 20 mM) and is not influenced by the concentrations of glucose, serum, amino acids, vitamins, or glutamine in the culture media [30]. The negatively charged Neu5Ac is taken up by cells through an active transport endocytic pathway (micropinocytosis), which makes uptake by cells in culture inefficient [29, 31]. In addition, accumulation of acidic Neu5Ac in cell culture medium or cell cytoplasm alters extracellular and/or intracellular pH; acidic pH negatively influences cell metabolism and proliferation [32].
Oral Neu5Ac is almost totally absorbed in the gastrointestinal tract of rats [33], and is mainly excreted in its free form via the kidneys (80%), while the remaining 20% is found in the feces. GNE myopathy mouse pharmacokinetic studies indicated that plasma Neu5Ac peaks within 30 min administration and decreases rapidly over the next 8 h [17]. This significant first-pass effect of Neu5Ac and inefficient uptake in cells may require administration of high concentrations or a different oral formulation to obtain sufficient cellular uptake in mammals.
In addition, biochemists have used ManNAc to supplement culture medium and increase cellular sialic acid production in vitro. Thomas et al [34] demonstrated that human fibroblast cultures, incubated with ManNAc (10-20 mM), expanded their intracellular free (unbound) Neu5Ac in a dose-dependent manner. This expansion could be reversed; upon the removal of extracellular ManNAc, free sialic acid levels decreased >80% over a 96-h period. Similarly, in cellular studies, radiolabeled ManNAc is commonly used to identify sialoglycoproteins [35, 36]. In multiple other studies and production processes, ManNAc is added to culture medium to allow for full sialylation and production of recombinant sialylated proteins [37, 38].
With respect to the use of ManNAc in humans, there is only one previous report that describes 6 healthy subjects receiving single oral doses of ManNAc. Specifically, two subjects received single 5 g oral doses and four subjects received single 10 g oral doses. ManNAc was said to have a “pleasant bland sweetish” taste, and no side effects were reported [39].
In this first-in-human study, we found that administration of oral ManNAc to subjects with GNE myopathy at single doses of 3 or 6 g was safe and well tolerated. A single dose of 10 g was considered the Maximum Tolerated Dose (MTD). Single doses of 10 g in GNE myopathy subjects were associated with nausea and a single episode of diarrhea shortly after ingestion of ManNAc and that resolved within hours, suggestive of unabsorbed ManNAc. These gastrointestinal adverse events were not reported with administration of 3 or 6 g of ManNAc or in the placebo group. No trends or clinically meaningful changes were seen in serial clinical laboratory tests and orthostatic vital signs in subjects receiving ManNAc at doses of 3 and 6 g compared to placebo. Elevated AST was seen at a higher frequency than placebo in subjects who received 10 g. Although this study was not powered to exclude an effect on the QTc interval, we observed that ManNAc did not cause a mean QTc interval prolongation exceeding 10 ms [40] and there were no clinically significant changes in serial electrocardiograms.
Mannitol was chosen as the placebo as it was not expected to act as an intermediate in the sialic acid biosynthesis pathway, unlike other possible placebos like glucose or fructose. Indeed, subjects receiving mannitol as placebo had no increase in the plasma concentrations of ManNAc or Neu5Ac. Mannitol performed as a suitable placebo, with similar color and taste to ManNAc, resulting in no unblinding issues.
Oral ManNAc was rapidly absorbed into the plasma (Tmax ca. 2 – 2.5 h) and exhibited a short half-life (2.4 h) when administered to fasting subjects with GNE myopathy. Our data suggest that the absorption of ManNAc may not increase in a dose-dependent manner. In the light of gastrointestinal adverse events likely due to unabsorbed drug at the 10 g dose, further studies should evaluate the oral bioavailability of ManNAc at different dose levels and the effect of food on absorption.
Following oral administration of ManNAc, free unconjugated plasma Neu5Ac increased significantly above the pre-dose levels. Plasma Neu5Ac remained elevated above baseline for more than 48 h after singles doses of ManNAc of 6 and 10 g. These findings strongly suggest that orally-administered ManNAc is effectively used as a substrate for sustained intracellular Neu5Ac biosynthesis in subjects with GNE myopathy. The increase in plasma Neu5Ac was comparable in all GNE genotypes, including subjects homozygous for GNE ManNAc-kinase enzymatic domain mutations. This provides further evidence that kinases other than ManNAc kinase can phosphorylate ManNAc; a previous study showed that GlcNAc kinase can perform this conversion (Fig. 1) [14].
Future dosing regimens can be based on the safety and PK findings of this study. The AUC of Neu5Ac increased dose-proportionally when the dose of ManNAc was increased from 3 to 6 g, but less than dose-proportionally with 10 g of ManNAc (Table 3). This suggests that the transport of ManNAc into the cell or enzymatic activities may become saturated or are controlled by intracellular Neu5Ac concentrations (i.e. acidity, negative charge) at ManNAc (substrate)/Neu5Ac (product) concentrations reached after a single dose of 6 g. Simulated plasma concentration-time profiles of ManNAc and Neu5Ac suggest that ManNAc at 6 g twice daily would be an appropriate dose regimen for a multiple dose study. It was estimated that steady-state plasma Neu5Ac concentration would be reached and maintained as desired, due to its longer half-life, while ManNAc would not accumulate due to its short half-life. A dosing regimen of 6 g twice daily, for a total of 12 g per day, is also supported by rat and dog toxicokinetic studies.
5. Conclusions
Oral ManNAc at single doses of 3 and 6 g are safe and well-tolerated. Oral administration of ManNAc leads to sustained and significantly increased circulating levels of free Neu5Ac (sialic acid) in GNE myopathy subjects, including those homozygous for mutations in the kinase enzymatic activity domain of GNE. Safety and PK data suggest that oral ManNAc at doses of 6 g BID would be an appropriate dosing regimen in future human trials of oral ManNAc in GNE myopathy subjects. Subsequent multiple dose efficacy trials with oral ManNAc will determine whether the increased free Neu5Ac concentrations seen in this Phase 1 trial translate to increased sialylation of muscle glycans.
Acknowledgments
The authors would like to thank the participating patients with GNE myopathy and their families. We also thank Asma Idriss and Patricia Pullen of the Clinical Trial Database (CTDB) for database support; New Zealand Pharmaceuticals for providing the drug product; Robert Giannini of Irysis for drug packaging; Neely Gal-Edd for project coordination; Karl John DeDios M.D., Chevalia Robinson R.N., Casey Mulholland Moore R.N. and the 5NW staff at the NIH Clinical Center for excellent patient care. In addition, we thank Alliance Pharma and Leidos for providing bioanalytical supports for ManNAc and Neu5Ac concentration measurements.
This project was funded by the intramural programs of the National Human Genome Research Institute (NHGRI) and the Therapeutics for Rare and Neglected Diseases (TRND) of the National Center of Advancing Translational Sciences, National Institutes of Health, Bethesda, Maryland. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Celeste FV, Vilboux T, Ciccone C, de Dios JK, Malicdan MC, Leoyklang P, McKew JC, Gahl WA, Carrillo-Carrasco N, Huizing M. Mutation update for GNE gene variants associated with GNE myopathy. Hum Mutat. 2014;35:915–926. doi: 10.1002/humu.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishino I, Carrillo-Carrasco N, Argov Z. GNE myopathy: current update and future therapy. J Neurol Neurosurg Psychiatry. 2015;86:385–392. doi: 10.1136/jnnp-2013-307051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huizing M, Malicdan MV, Krasnewich DM, Manoli I, Carrillo-Carrasco N. GNE Myopathy. In: Scriver CR, Childs B, Sly WS, Valle D, Beaudet AL, Vogelstein B, Kinsler KW, editors. Scriver’s Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2014. http://www.ommbid.com/ [Google Scholar]
- 4.Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardini G, Shmilevich I, Friedmann A, Karpati G, Bradley WG, Baumbach L, Lancet D, Asher EB, Beckmann JS, Argov Z, Mitrani-Rosenbaum S. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- 5.Stasche R, Hinderlich S, Weise C, Effertz K, Lucka L, Moormann P, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Molecular cloning and functional expression of UDP-N-acetyl-glucosamine 2-epimerase/N-acetylmannosamine kinase. J Biol Chem. 1997;272:24319–24324. doi: 10.1074/jbc.272.39.24319. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol. 2012;94:887–905. doi: 10.1007/s00253-012-4040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhide GP, Colley KJ. Sialylation of N-glycans: mechanism, cellular compartmentalization and function. Histochem Cell Biol. 2017;147:149–174. doi: 10.1007/s00418-016-1520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornfeld S, Kornfeld R, Neufeld EF, O’Brien PJ. The Feedback Control of Sugar Nucleotide Biosynthesis in Liver. Proc Natl Acad Sci U S A. 1964;52:371–379. doi: 10.1073/pnas.52.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seppala R, Lehto VP, Gahl WA. Mutations in the human UDP-N-acetylglucosamine 2- epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am J Hum Genet. 1999;64:1563–1569. doi: 10.1086/302411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noguchi S, Keira Y, Murayama K, Ogawa M, Fujita M, Kawahara G, Oya Y, Imazawa M, Goto Y, Hayashi YK, Nonaka I, Nishino I. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J Biol Chem. 2004;279:11402–11407. doi: 10.1074/jbc.M313171200. [DOI] [PubMed] [Google Scholar]
- 12.Sparks SE, Ciccone C, Lalor M, Orvisky E, Klootwijk R, Savelkoul PJ, Dalakas MC, Krasnewich DM, Gahl WA, Huizing M. Use of a cell-free system to determine UDP-N-acetylglucosamine 2-epimerase and N-acetylmannosamine kinase activities in human hereditary inclusion body myopathy. Glycobiology. 2005;15:1102–1110. doi: 10.1093/glycob/cwi100. [DOI] [PubMed] [Google Scholar]
- 13.Penner J, Mantey LR, Elgavish S, Ghaderi D, Cirak S, Berger M, Krause S, Lucka L, Voit T, Mitrani-Rosenbaum S, Hinderlich S. Influence of UDP-GlcNAc 2-epimerase/ManNAc kinase mutant proteins on hereditary inclusion body myopathy. Biochemistry. 2006;45:2968–2977. doi: 10.1021/bi0522504. [DOI] [PubMed] [Google Scholar]
- 14.Hinderlich S, Berger M, Keppler OT, Pawlita M, Reutter W. Biosynthesis of N-acetylneuraminic acid in cells lacking UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. Biol Chem. 2001;382:291–297. doi: 10.1515/BC.2001.036. [DOI] [PubMed] [Google Scholar]
- 15.Salama I, Hinderlich S, Shlomai Z, Eisenberg I, Krause S, Yarema K, Argov Z, Lochmuller H, Reutter W, Dabby R, Sadeh M, Ben-Bassat H, Mitrani-Rosenbaum S. No overall hyposialylation in hereditary inclusion body myopathy myoblasts carrying the homozygous M712T GNE mutation. Biochem Biophys Res Commun. 2005;328:221–226. doi: 10.1016/j.bbrc.2004.12.157. [DOI] [PubMed] [Google Scholar]
- 16.Tajima Y, Uyama E, Go S, Sato C, Tao N, Kotani M, Hino H, Suzuki A, Sanai Y, Kitajima K, Sakuraba H. Distal myopathy with rimmed vacuoles: impaired O-glycan formation in muscular glycoproteins. Am J Pathol. 2005;166:1121–1130. doi: 10.1016/S0002-9440(10)62332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009;15:690–695. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- 18.Niethamer TK, Yardeni T, Leoyklang P, Ciccone C, Astiz-Martinez A, Jacobs K, Dorward HM, Zerfas PM, Gahl WA, Huizing M. Oral monosaccharide therapies to reverse renal and muscle hyposialylation in a mouse model of GNE myopathy. Mol Genet Metab. 2012;107:748–755. doi: 10.1016/j.ymgme.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yonekawa T, Malicdan MC, Cho A, Hayashi YK, Nonaka I, Mine T, Yamamoto T, Nishino I, Noguchi S. Sialyllactose ameliorates myopathic phenotypes in symptomatic GNE myopathy model mice. Brain. 2014;137:2670–2679. doi: 10.1093/brain/awu210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, Darvish D, Starost MF, Zerfas PM, Hoffmann VJ, Hoogstraten-Miller S, Krasnewich DM, Gahl WA, Huizing M. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117:1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keppler OT, Hinderlich S, Langner J, Schwartz-Albiez R, Reutter W, Pawlita M. UDP-GlcNAc 2-epimerase: a regulator of cell surface sialylation. Science. 1999;284:1372–1376. doi: 10.1126/science.284.5418.1372. [DOI] [PubMed] [Google Scholar]
- 22.Schwarzkopf M, Knobeloch KP, Rohde E, Hinderlich S, Wiechens N, Lucka L, Horak I, Reutter W, Horstkorte R. Sialylation is essential for early development in mice. Proc Natl Acad Sci U S A. 2002;99:5267–5270. doi: 10.1073/pnas.072066199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, C.-E. Investigators Estimating glomerular filtration rate from serum creatinine and cystatin. C N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Xu X, Fang M, Zhang M, Li Y, Gillespie B, Yorke S, Yang N, McKew JC, Gahl WA, Huizing M, Carrillo-Carrasco N, Wang AQ. Quantitative hydrophilic interaction chromatography-mass spectrometry analysis of N-acetylneuraminic acid and N-acetylmannosamine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1000:105–111. doi: 10.1016/j.jchromb.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Argov Z, Caraco Y, Lau H, Pestronk A, Shieh PB, Skrinar A, Koutsoukos T, Ahmed R, Martinisi J, Kakkis E. Aceneuramic Acid Extended Release Administration Maintains Upper Limb Muscle Strength in a 48-week Study of Subjects with GNE Myopathy: Results from a Phase 2, Randomized, Controlled Study. J Neuromuscul Dis. 2016;3:49–66. doi: 10.3233/JND-159900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009;15:690–695. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- 27.Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, Darvish D, Starost MF, Zerfas PM, Hoffmann VJ, Hoogstraten-Miller S, Krasnewich DM, Gahl WA, Huizing M. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117:1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argov Z, Mitrani-Rosenbaum S. The hereditary inclusion body myopathy enigma and its future therapy. Neurotherapeutics. 2008;5:633–637. doi: 10.1016/j.nurt.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 30.Diaz S, Varki A. Metabolic labeling of sialic acids in tissue culture cell lines: methods to identify substituted and modified radioactive neuraminic acids. Anal Biochem. 1985;150:32–46. doi: 10.1016/0003-2697(85)90438-5. [DOI] [PubMed] [Google Scholar]
- 31.Hirschberg CB, Goodman SR, Green C. Sialic acid uptake by fibroblasts. Biochemistry. 1976;15:3591–3599. doi: 10.1021/bi00661a029. [DOI] [PubMed] [Google Scholar]
- 32.Hynes J, O’Riordan TC, Zhdanov AV, Uray G, Will Y, Papkovsky DB. In vitro analysis of cell metabolism using a long-decay pH-sensitive lanthanide probe and extracellular acidification assay. Anal Biochem. 2009;390:21–28. doi: 10.1016/j.ab.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Witt W, von Nicolai H, Zilliken F. Uptake and distribution of orally applied N-acetyl-(14C)neuraminosyl-lactose and N-acetyl-(14C)neuraminic acid in the organs of newborn rats. Nutr Metab. 1979;23:51–61. doi: 10.1159/000176241. [DOI] [PubMed] [Google Scholar]
- 34.Thomas GH, Scocca J, Miller CS, Reynolds LW. Accumulation of N-acetylneuraminic acid (sialic acid) in human fibroblasts cultured in the presence of N-acetylmannosamine. Biochim Biophys Acta. 1985;846:37–43. doi: 10.1016/0167-4889(85)90107-7. [DOI] [PubMed] [Google Scholar]
- 35.Charter NW, Mahal LK, Koshland DE, Jr, Bertozzi CR. Differential effects of unnatural sialic acids on the polysialylation of the neural cell adhesion molecule and neuronal behavior. J Biol Chem. 2002;277:9255–9261. doi: 10.1074/jbc.M111619200. [DOI] [PubMed] [Google Scholar]
- 36.Tian Y, Almaraz RT, Choi CH, Li QK, Saeui C, Li D, Shah P, Bhattacharya R, Yarema KJ, Zhang H. Identification of sialylated glycoproteins from metabolically oligosaccharide engineered pancreatic cells. Clin Proteomics. 2015;12:11. doi: 10.1186/s12014-015-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu X, Wang DI. Improvement of interferon-gamma sialylation in Chinese hamster ovary cell culture by feeding of N-acetylmannosamine. Biotechnol Bioeng. 1998;58:642–648. [PubMed] [Google Scholar]
- 38.Bork K, Reutter W, Gerardy-Schahn R, Horstkorte R. The intracellular concentration of sialic acid regulates the polysialylation of the neural cell adhesion molecule. FEBS Lett. 2005;579:5079–5083. doi: 10.1016/j.febslet.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Amir SM, Barker SA, Butt WR, Crooke AC, Davies AG. Administration of N-acetyl-D-mannosamine to mammals. Nature. 1966;211:976–977. doi: 10.1038/211976a0. [DOI] [PubMed] [Google Scholar]
- 40.Nachimuthu S, Assar MD, Schussler JM. Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf. 2012;3:241–253. doi: 10.1177/2042098612454283. [DOI] [PMC free article] [PubMed] [Google Scholar]


