Graphical abstract
Abbreviations: NO, nitric oxide; TNF, tumor necrosis factor; IFN, interferon; LPS, lipopolysaccharide; NOS2, nitric oxide synthase type 2; DMSO, dimethylsulfoxide; IL, interleukin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; AP-1, activator protein 1; STAT, signal transducer and activator of transcription; PAMP, pathogen-associated molecular pattern; ER, estrogen receptor
Keywords: Endosulfan, Macrophage, Inflammation, TNF, Nitric oxide
Highlights
-
•
Non-cytotoxic concentrations of endosulfan suppressed NO production.
-
•
Suppression of NO was a more sensitive endpoint than suppression of TNF.
-
•
Endosulfan alpha had greater cytotoxic potency than endosulfan beta.
Abstract
Endosulfan is an organochlorine insecticide comprised of two isomers: endosulfan-α and endosulfan-β. Endosulfan exposure has been shown to elevate some inflammatory factors, such as nitric oxide (NO) and tumor necrosis factor (TNF), in animals or cultures of animal cells. Because the two endosulfan isomers can vary in their biological activities, the goal of this study was to determine if individual endosulfan isomers differentially impact production of NO or TNF by the mouse macrophage cell RAW 264.7 at non-cytotoxic levels. We found elevated TNF with exposure to endosulfan-α (not endosulfan-β), but only at concentrations that were cytotoxic (≥100 μM), whereas neither endosulfan isomer altered baseline levels of NO at any concentration up to 300 μM. In interferon (IFN)-γ-activated cultures, NO levels were significantly suppressed by either endosulfan isomer at 10 μM (the lowest concentration examined), whereas only endosulfan-β significantly lowered TNF levels at non-cytotoxic concentrations. In lipopolysaccharide (LPS)-activated cultures, both endosulfan isomers significantly reduced NO, but not TNF, at non-cytotoxic concentrations. These results suggest that the endosulfan isomers have some capacity to alter inflammatory responses differentially, particularly with IFN-γ stimulation.
1. Introduction
Endosulfan is an organochlorine insecticide of the cyclodiene group that typically contains two isomers: endosulfan-α (endosulfan I) and endosulfan-β (endosulfan II). Adverse effects have been reported in non-target species including neurological toxicity, endocrine disruption, altered immune and liver function, abnormal development, and others [1]. The two endosulfan isomers can vary in their biological activities. For example: endosulfan-α, but not endosulfan-β, can bind and activate the pregnane-xenobiotic receptor [2], endosulfan-α is more potent than endosulfan-β as a disrupter of lipid bilayer organization [3], only endosulfan-α stimulates proliferation of uterin leiomyoma cells even though both isomers are agonists of the estrogen receptor [4], and endosulfan-β has greater genotoxicity than endosulfan-α [5]. Because of the potential differences in biological effect, and because of the different proportions of each isomer that may be present in endosulfan samples or residues, assessing the impacts of the individual endosulfan isomers can aid in establishing the potential risks of exposure.
Immunity to infection often relies heavily on macrophages. These cells are capable of phagocytosis, production of cytokines, chemokines, reactive oxygen/nitrogen species, and other defensive factors. Inflammation and destruction of some microbes depends upon the proper function of macrophages. Endosulfan has been previously shown to modify some macrophage functions including the production of tumor necrosis factor (TNF), inducible nitric oxide synthase (NOS2), and nitric oxide (NO) [[6], [7], [8]]. These findings suggest that endosulfan has the potential to alter inflammation and/or disease resistance. Because previous studies only examined mixtures of the endosulfan isomers, it is unclear if the reported effects were due to one or both of the endosulfan isomers. The goal of this study was to determine if individual endosulfan isomers impact NO or TNF production by mouse macrophages at non-cytotoxic levels using the RAW 264.7 cell line. We found that either isomer of endosulfan was capable of significantly lowering NO or TNF levels in IFN-γ-stimulated cultures. Suppression of NO occurred with 10 μM of either endosulfan isomer and was the most sensitive endpoint measured. Exposure to either endosulfan isomer also caused significant reduction of NO levels, but not TNF levels, following LPS stimulation.
2. Material and methods
2.1. Cell culture and chemical exposures
Mouse RAW 264.7 cells (ATCC) were cultured at 37 °C in humidified air plus 5% CO2 in medium comprised of DMEM (Life Technologies, cat #11965-092) plus 10% heat-inactivated fetal bovine serum (Hyclone), penicillin/streptomycin (100 U/100 μg per mL), and sodium pyruvate (1 mM). Endosulfan-α and endosulfan-β (ChemService) were dissolved separately in dimethylsulfoxide (DMSO) for stock solutions at 100 mM which were stored at −20 °C until needed. Dilutions of endosulfan in DMSO were prepared fresh for each experiment such that addition of endosulfan to culture medium resulted in a uniform final DMSO concentration of 0.1% (v/v) across exposure levels. Within each experiment, cultures contained medium, DMSO, or endosulfan at concentrations of 10, 33, 100 or 300 μM. Lipopolysaccharide (LPS, E. coli 055:B5) and/or mouse interferon gamma (IFN-γ) were added to some cultures at 100 ng/mL or 6 ng/mL, respectively. Exposure to endosulfan, LPS and/or IFN-γ occurred simultaneously and for a duration of 24 h with a minimum of two replicate cultures for each condition.
2.2. Cytotoxicity assays
The reductive metabolism of cells, an indicator of cytotoxicity, was measured using a WST-1 cell cytotoxicity kit using the manufacturer's protocol (G-Biosciences). Briefly, 4.2 × 104 RAW 264.7 cells were added to wells of 96-well tissue culture plates and allowed to adhere overnight. The medium in each well was then replaced with 100 μL of medium containing medium only, DMSO, or endosulfan. One μL of LPS and/or 1.0 μL of IFN-γ was added to some wells. All plates were incubated for 24 h. WST-1 reagent was then added to each well followed by incubation for an additional three hours after which the optical density (OD) at 430 nm was measured for each well and the percent cytotoxicity was calculated. Percent cytotoxicity for each culture was calculated as 100× [1 − (Test − Blank) ÷ (Control − Blank)], where Test is the OD for any well containing cells, Control is the OD for cells cultured in medium only, and Blank is the OD for medium without cells. To reveal the effects of endosulfan exposure that were additive or interactive with those caused by LPS and/or IFN-γ exposure, the percent cytotoxicity for a culture containing DMSO (including those containing endosulfan) was normalized by subtracting the cytotoxicity of control cultures that did not contain DMSO as follows: DMSOX – ControlX where X indicates that cultures contained either medium, LPS, IFN-γ, or both LPS and IFN-γ, as appropriate.
2.3. Nitrite assays and ELISAs
Nitrite (the degradation product of nitric oxide) and cytokine levels were assessed in culture supernatant. Briefly, 2.5 × 105 RAW 264.7 cells were added to wells of 24-well tissue culture plates and allowed to adhere overnight. The medium in each well was then replaced with 1.0 mL of medium containing medium only, DMSO, or endosulfan. Ten μL of LPS and/or 10 μL IFN-γ was added to some wells. All plates were incubated for 24 h after which supernatants were harvested and stored at −20 °C until assayed. Nitrite levels were assessed by the Greiss reaction as described previously [9]. Mouse TNF and IL-6 levels were assessed using OptEIA™ kits (BD Biosciences) and procedures provided by the manufacturer.
2.4. Statistics
Statistical analyses were performed using the GLM procedure of SAS for Windows version 9.3 (SAS Institute Inc.). Analysis of variance (ANOVA) was used for data sets containing multiple sources of variance such as experimental trial, endosulfan treatment, LPS treatment, and IFN-γ treatment. Post-hoc all-pairwise t-tests were used to analyze significant differences with no lower stringency than Fisher LSD. Differences between means were considered significant at p ≤ 0.05.
3. Results
3.1. Cytotoxicity
The influence of IFN-γ and LPS exposure (without DMSO or endosulfan) on reductive metabolism by RAW 264.7 cells is shown in Fig. 1A. Exposure to either IFN-γ or LPS alone enhanced formazan production (approximately −13.5% cytotoxicity overall), whereas cytotoxicity increased by 25% (to 12%) when cells were exposed to IFN-γ and LPS together (untreated control values were excluded from the statistical model).
Fig. 1.
Cytotoxicity of endosulfan-α or endosulfan-β in the presence of IFN-γ and/or LPS. Data are represented as means ± SEM. Panel A: Cells were cultured in medium alone or with activating supplements as shown. The data are presented as relative to medium control for nine independent experiments. Means with different letters are significantly different (p ≤ 0.05). Panels B & C: Cells were cultured in medium containing DMSO (vehicle) or endosulfan isomer, and some cultures were stimulated with IFN-γ, LPS, or both LPS and IFN-γ as shown. The data were normalized as described in Methods to reveal the effects of endosulfan that were additive or interactive with those caused by LPS and/or IFN-γ exposure. The data represent means ± SEM for four independent experiments per panel. For hypothesis testing, data within each endosulfan treatment level (stimulated or not) were pooled, and different letters indicate pooled means that are significantly different (p ≤ 0.05).
The normalized cytotoxicities resulting from endosulfan exposures are shown in Fig. 1B and C. No significant interactions between endosulfans and IFN-γ or LPS were evident. Therefore, data within each concentration group (for each isomer) were pooled prior to statistically testing for differences across endosulfan concentrations. When compared to DMSO controls, endosulfan-α was found to cause significant and concentration-dependent cytotoxicity at concentrations of 100 μM and higher (Fig. 1B). In contrast, endosulfan-β exposure significantly enhanced formazan production (negative cytotoxicity) at low concentrations (10–33 μM). Relative to those low concentrations, 300 μM endosulfan-β caused significantly increased cytotoxicity that was not different from controls.
3.2. Nitric oxide production
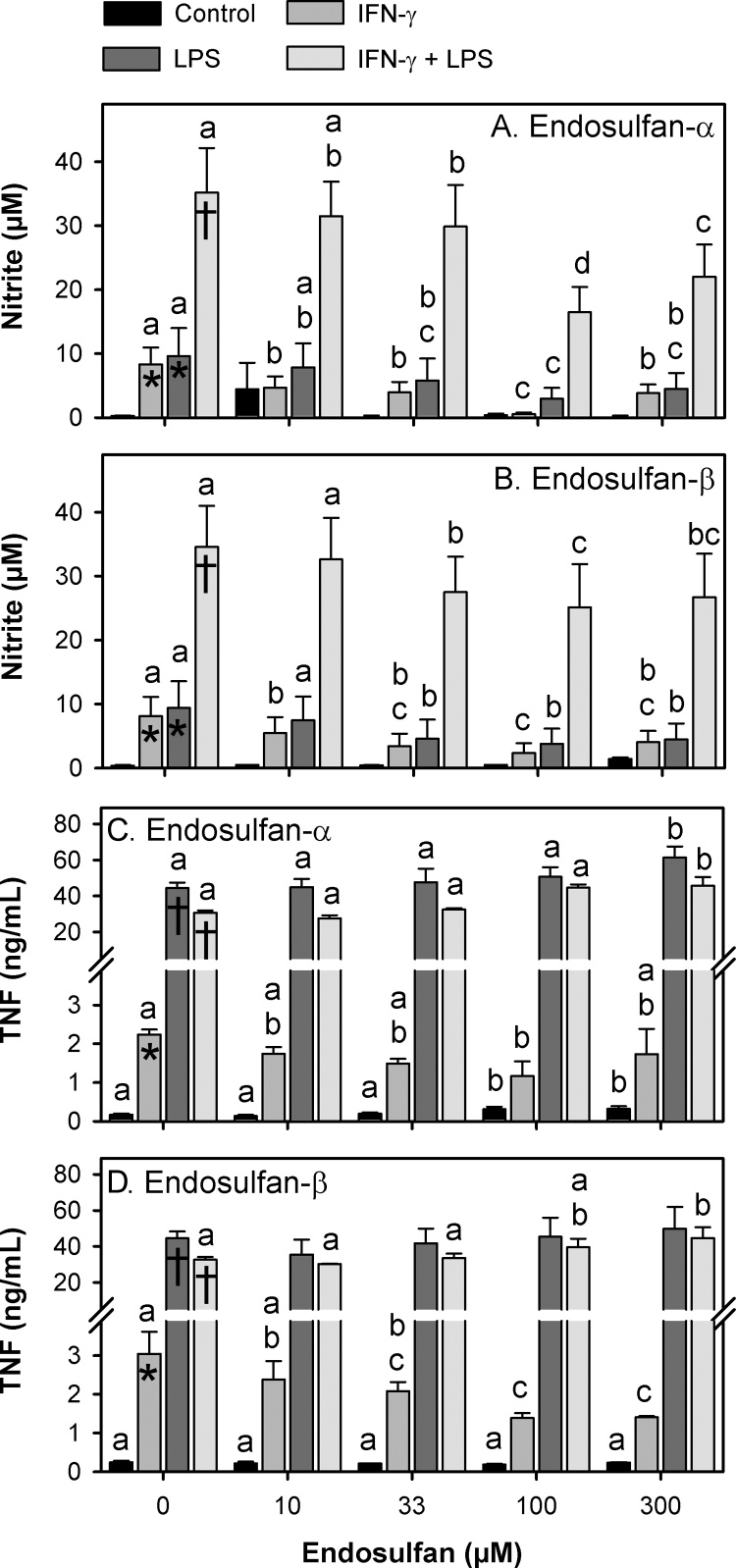
In the absence of activation with IFN-γ or LPS, NO production in control cultures (DMSO) was very low with nitrite levels reaching approximately 0.4 μM after 24 h (Fig. 2A and B). In the presence of either IFN-γ or LPS, the nitrite levels of control cultures were significantly increased by approximately 23-fold to an average of 8.8 μM. In the presence of both IFN-γ and LPS, nitrite levels were further significantly increased over 100-fold to approximately 35 μM. In the absence of stimulation, NO production was unchanged by endosulfan exposure. In contrast, both endosulfan isomers significantly reduced IFN-γ-stimulated NO production beginning at the non-cytotoxic 10 μM concentration and reaching a maximal effect at 100 μM (93% and 70% reduction, respectively). A similar but less potent impact of the different endosulfan isomers on NO production was observed in LPS-treated cultures and in cultures containing both IFN-γ and LPS.
Fig. 2.
Nitrite and TNF levels after exposure to endosulfan-α or endosulfan-β. Data are represented as means ± SEM for four (nitrite) or three (TNF) independent experiments per panel. Panels A & C: Endosulfan-α exposures. Panels B & D: Endosulfan-β exposures. Means that were significantly different (p ≤ 0.05) within vehicle only groups (no endosulfan) are indicated with an asterisk (*) if different from control cultures or with a dagger (†) if different from both control and IFN-γ exposed cultures. Means that were significantly different (p ≤ 0.05) across endosulfan exposure levels but within activation level (IFN-γ only, LPS only, both, or neither) are indicated with different letters.
3.3. TNF production
In the absence of activation with IFN-γ or LPS, low levels of TNF were detected (∼200 pg/mL) in control cultures (DMSO) (Fig. 2C and D). IFN-γ alone significantly increased TNF levels approximately 13-fold on average, and LPS alone significantly increased TNF levels more than 200-fold. When combined with LPS, IFN-γ stimulation did not significantly elevate TNF levels beyond that seen with LPS alone.
Endosulfan-α exposure significantly altered TNF levels in both stimulated and unstimulated cultures, but only at concentrations that were significantly cytotoxic (100 μM and higher). It is unclear if these changes in TNF production were a cause or a consequence of that cytotoxicity. Endosulfan-β exposure did not alter unstimulated TNF production, but it did significantly reduce TNF production at the non-cytotoxic concentration of 33 μM and at higher concentrations. In contrast, endosulfan-β significantly increased TNF production in double-stimulated (IFN-γ + LPS) cultures, but only at the highest and potentially cytotoxic concentration of 300 μM.
4. Discussion
The goal of this study was to determine if non-cytotoxic levels of individual endosulfan isomers impact NO or TNF production by mouse macrophages using the RAW 264.7 cell line. We first determined what concentrations were cytotoxic using a tetrazolium reduction assay (Fig. 1). Endosulfan-α exposure caused significant cytotoxicity at concentrations of 100 μM and higher. In contrast, endosulfan-β exposure at 10–33 μM caused significant enhancement of viability, although this effect declined significantly with increasing concentration such that cell viability at 300 μM was not different from that in control cultures. These results suggest that endosulfan-α has a higher cytotoxic potency than endosulfan-β in RAW 264.7 cells. Opposite cytotoxic potencies were found for these endosulfan isomers by Enhui et al. [14] who used a tetrazolium reduction assay to show endosulfan-β to be more cytotoxic than endosulfan-α in a human neuroblastoma cell line [10]. These results suggest that the toxicity of endosulfan isomers varies with the cell type examined. Technical grade endosulfan products, or other mixtures of α and β endosulfans, have been examined extensively for cytotoxic potential [[7], [8], [10], [11], [12], [13], [14], [15], [16], [17], [18]]. Not surprisingly, these studies have shown that endosulfan exposure in vitro causes loss of cell viability that increases in severity as the concentration and duration of exposure increases. A proposed mechanism for endosulfan-induced cytotoxicity involves oxidative stress and mitochondrial dysfunction leading to apoptosis [[1], [6], [7], [8], [15], [19], [20], [21], [22], [23]].
In vivo studies have shown that endosulfan exposure can increase the level or activity of a number of inflammatory factors in animals. For example, inducible nitric oxide synthase was elevated by endosulfan exposure in rat and frog tissues [[12], [24], [25]], and increased expression of inflammatory cytokines, including TNF, IL-1 and IL-6, have been reported in rats and mice [[26], [27], [28]]. In many tissues, macrophages are a principle source of inflammatory cytokines and NO. An in vitro study by Han et al. [7] using mouse-derived RAW 264.7 macrophages showed that 24 h exposures to non-cytotoxic concentrations of endosulfan (up to 10 μM) caused significantly elevated levels of activated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and higher levels of NO, TNF and IL-6 [7]. A follow-up study by Kim et al. [8] showed that non-cytotoxic concentrations of endosulfan caused significantly elevated levels of reactive oxygen species which they suggested as a driving mechanism for the coincidently increased levels of activated NF-κB, activator protein 1 (AP-1) and other inflammation-associated transcription factors [8]. Using RAW 264.7 cells, we found that individual α or β isomers of endosulfan caused no significant changes in NO levels after 24 h of exposure, even at cytotoxic concentrations. However, TNF levels were increased by endosulfan-α exposure (not by endosulfan-β), but only at concentrations that were cytotoxic. Because endosulfan-induced cytotoxicity is associated with both oxidative stress [[1], [6], [7], [8], [15], [19], [20], [21], [22], [23]] and activation of transcription factors that upregulate TNF expression (e.g., NF-κB, AP-1) [[8], [29]], our results suggest that endosulfan-α, but not endosulfan-β, may be the primary cause of oxidative stress leading to TNF production. Given that both TNF and oxidative stress can activate NF-κB to upregulate NOS2 expression [30], it is unclear why cytotoxic concentrations of endosulfan-α failed to increase NO levels. In the absence of other stimulating factors, mixtures of endosulfan isomers may have greater potency for activating macrophages than do individual isomers alone. Interestingly, Ayub et al. [6] found no increase in NO or TNF from rat peritoneal macrophages exposed to endosulfan at concentrations up to approximately 50 μM, an exposure level that caused no detectable lipid peroxidation [6]. Their results suggest that rat macrophages may be less sensitive to endosulfan than mouse macrophages.
Macrophages can be stimulated to upregulate NO or cytokine production by exposure to a large number of pathogen-associated molecular patterns (PAMPs) or endogenously-produced factors following infection or tissue damage. NOS2 is predominantly regulated at the level of expression and, in the mouse, that regulation is primarily via two pathways: 1) NF-κB which can be activated following exposure to a host of factors (e.g., LPS and other PAMPs, TNF, reactive oxygen species), and 2) interferon regulatory factor (IRF)-1 and/or signal transducer and activator of transcription (STAT)-1, both of which can be activated following stimulation with IFN-γ [[30], [31], [32], [33]]. Optimal expression of NOS2 requires activation of both NF-κB and IRF-1/STAT-1 [30], as suggested by the synergistic increase in nitrite levels observed with combined IFN-γ and LPS stimulation (Fig. 2A & B). Suppression of NOS2 expression can occur through the activation of several other transcription factors including the classical glucocorticoid receptor, peroxisome proliferator-activated receptors, and the estrogen receptor-α (ER-α), all of which can reduce NF-κB activity [30]. In this study, we exposed RAW 264.7 cells to IFN-γ and/or LPS to determine which paths to NOS2 upregulation could be influenced by individual endosulfan isomers. We found that non-cytotoxic concentrations of either endosulfan isomer were capable of suppressing NO production stimulated by IFN-γ or LPS. Given that both endosulfans are agonists of the estrogen receptor [4], and ER-α is known to be expressed in RAW cells [[34], [35]], suppressed NO production by non-cytotoxic concentrations of endosulfan could be explained by activation of ER-α receptors and suppression of NF-κB in this model. A similar mechanism could account for the endosulfan-suppressed TNF levels observed after IFN-γ stimulation [36].
Taken together, the results shown here support the suggestion of differential potencies for endosulfan isomers on macrophage function, particularly cytotoxicity. At non-cytotoxic concentrations, the two endosulfan isomers had similar suppressive impact on IFN-γ/LPS-stimulated production of NO. Suppression of TNF production was apparent only with non-cytotoxic levels of endosulfan-β combined with IFN-γ stimulation.
Conflicts of interest
None declared.
Fundings
This work was supported by the University of Northern Colorado and U.S. National Science Foundation [grant number 0622421].
Contributor Information
Alexander I. Terry, Email: Alex.terry@hypoxygen.com.
Sandra Benitez-Kruidenier, Email: skruidenier@clinica.org.
Gregory K. DeKrey, Email: gregory.dekrey@unco.edu.
References
- 1.Agency for Toxic Substances and Disease Registry (ATSDR) Public Health Service; Atlanta, GA: 2015. Toxicological Profile for Endosulfan, U.S. Public Health Services.https://www.atsdr.cdc.gov/toxprofiles/TP.asp?id=609&tid=113 [PubMed] [Google Scholar]
- 2.Casabar R.C., Das P.C., DeKrey G.K., Gardiner C.S., Cao Y., Rose R.L., Wallace A.D. Endosulfan induces CYP2B6 and CYP3A4 by activating the pregnane X receptor. Toxicol. Appl. Pharmacol. 2010;245 doi: 10.1016/j.taap.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Videira R.A., Antunes-Madeira M.C., Madeira V.M. Differential effects induced by alpha – and beta-endosulfan in lipid bilayer organization are reflected in proton permeability. Biochim. Biophys. Acta. 2002;1564:140–148. doi: 10.1016/s0005-2736(02)00441-8. PMID: 12101006. [DOI] [PubMed] [Google Scholar]
- 4.Hodges L.C., Bergerson J.S., Hunter D.S., Walker C.L. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol. Sci. 2000;54:355–364. doi: 10.1093/toxsci/54.2.355. PMID: 10774817. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y., Morimoto K., Takeshita T., Takeuchi T., Saito T. Genotoxic effects of alpha-endosulfan and beta-endosulfan on human HepG2 cells. Environ. Health Perspect. 2000;108:559–561. doi: 10.1289/ehp.00108559. PMID: 10856031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayub S., Verma J., Das N. Effect of endosulfan and malathion on lipid peroxidation, nitrite and TNF-alpha release by rat peritoneal macrophages. Int. Immunopharmacol. 2003;3 doi: 10.1016/j.intimp.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Han E.H., Hwang Y.P., Kim H.G., Jeong H.G. Inflammatory effect of endosulfan via NF-kappaB activation in macrophages. Biochem. Biophys. Res. Commun. 2007;355 doi: 10.1016/j.bbrc.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 8.Kim H.G., Kim Y.R., Park J.H., Khanal T., Choi J.H., Do M.T., Jin S.W., Han E.H., Chung Y.H., Jeong H.G. Endosulfan induces COX-2 expression via NADPH oxidase and the ROS, MAPK, and Akt pathways. Arch. Toxicol. 2015;89 doi: 10.1007/s00204-014-1359-7. [DOI] [PubMed] [Google Scholar]
- 9.Urioste S., Hall L.R., Telford S.R., 3rd, Titus R.G. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin-dependent mechanism. J. Exp. Med. 1994;180:1077–1085. doi: 10.1084/jem.180.3.1077. PMCID: PMC2191645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enhui Z., Na C., MengYun L., Jia L., Dan L., Yongsheng Y., Ying Z., DeFu H. Isomers and their metabolites of endosulfan induced cytotoxicity and oxidative damage in SH-SY5Y cells. Environ. Toxicol. 2016;31 doi: 10.1002/tox.22066. [DOI] [PubMed] [Google Scholar]
- 11.Sultana Shaik A., Shaik A.P., Jamil K., Alsaeed A.H. Evaluation of cytotoxicity and genotoxicity of pesticide mixtures on lymphocytes. Toxicol. Mech Methods. 2016;26 doi: 10.1080/15376516.2016.1218577. [DOI] [PubMed] [Google Scholar]
- 12.Dhouib M., Lugnier A. Induction of nitric oxide synthase by chlorinated pesticides (p, p’-DDT, chlordane, endosulfan) in rat liver. Cent. Eur. J. Public Health. 1996;4(Suppl. 48) PMID: 9167060. [PubMed] [Google Scholar]
- 13.Wan M.T., Kuo J.N., Buday C., Schroeder G., Van Aggelen G., Pasternak J. Toxicity of alpha-, beta-, (alpha + beta)-endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchus kisutch, and biological implications in streams. Environ. Toxicol. Chem. 2005;24:1146–1154. doi: 10.1897/04-300r1.1. PMID: 16110993. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Wei J., Ren L., Zhang J., Yang M., Jing L., Wang J., Sun Z., Zhou X. Endosulfan inducing apoptosis and necroptosis through activation RIPK signaling pathway in human umbilical vascular endothelial cells. Environ. Sci. Pollut. Res. Int. 2017;24 doi: 10.1007/s11356-016-7652-7. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed T., Banerjee B.D. HSP27 modulates survival signaling in endosulfan-exposed human peripheral blood mononuclear cells treated with curcumin. Hum. Exp. Toxicol. 2016;35 doi: 10.1177/0960327115597986. [DOI] [PubMed] [Google Scholar]
- 16.Lacher S.E., Skagen K., Veit J., Dalton R., Woodahl E.L. P-Glycoprotein transport of neurotoxic pesticides. J. Pharmacol. Exp. Ther. 2015;355 doi: 10.1124/jpet.115.226373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleem U., Ejaz S., Ashraf M., Omer M.O., Altaf I., Batool Z., Fatima R., Afzal M. Mutagenic and cytotoxic potential of Endosulfan and Lambda-cyhalothrin – in vitro study describing individual and combined effects of pesticides. J. Environ. Sci. (China) 2014;26 doi: 10.1016/j.jes.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Nawaz A., Razpotnik A., Rouimi P., de Sousa G., Cravedi J.P., Rahmani R. Cellular impact of combinations of endosulfan, atrazine, and chlorpyrifos on human primary hepatocytes and HepaRG cells after short and chronic exposures. Cell Biol. Toxicol. 2014;30 doi: 10.1007/s10565-013-9266-x. [DOI] [PubMed] [Google Scholar]
- 19.Hincal F., Gurbay A., Giray B. Induction of lipid peroxidation and alteration of glutathione redox status by endosulfan. Biol. Trace Elem. Res. 1995;47 doi: 10.1007/BF02790133. [DOI] [PubMed] [Google Scholar]
- 20.Kannan K., Holcombe R.F., Jain S.K., Alvarez-Hernandez X., Chervenak R., Wolf R.E., Glass J. Evidence for the induction of apoptosis by endosulfan in a human T-cell leukemic line. Mol. Cell. Biochem. 2000;205:53–66. doi: 10.1023/a:1007080910396. PMID: 10821422. [DOI] [PubMed] [Google Scholar]
- 21.Dorval J., Hontela A. Role of glutathione redox cycle and catalase in defense against oxidative stress induced by endosulfan in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) Toxicol. Appl. Pharmacol. 2003;192(2):191–200. doi: 10.1016/s0041-008x(03)00281-3. PMID: 14550752. [DOI] [PubMed] [Google Scholar]
- 22.Akbar S.M., Sreeramulu K., Sharma H.C. Tryptophan fluorescence quenching as a binding assay to monitor protein conformation changes in the membrane of intact mitochondria. J. Bioenerg. Biomembr. 2016;48 doi: 10.1007/s10863-016-9653-0. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh R., Siddharth M., Singh N., Kare P.K., Banerjee B.D., Wadhwa N., Tripathi A.K. Organochlorine pesticide-mediated induction of NADPH oxidase and nitric-oxide synthase in endothelial cell. J. Clin. Diagn. Res. 2017;11 doi: 10.7860/JCDR/2017/25276.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caride A., Lafuente A., Cabaleiro T. Endosulfan effects on pituitary hormone and both nitrosative and oxidative stress in pubertal male rats. Toxicol. Lett. 2010;197 doi: 10.1016/j.toxlet.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Bernabo I., Guardia A., La Russa D., Madeo G., Tripepi S., Brunelli E. Exposure and post-exposure effects of endosulfan on Bufo bufo tadpoles: morpho-histological and ultrastructural study on epidermis and iNOS localization. Aquat. Toxicol. 2013:142–143. doi: 10.1016/j.aquatox.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Omurtag G.Z., Tozan A., Sehirli A.O., Sener G. Melatonin protects against endosulfan-induced oxidative tissue damage in rats. J. Pineal Res. 2008;44 doi: 10.1111/j.1600-079X.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 27.Jang T.C., Jang J.H., Lee K.W. Mechanism of acute endosulfan intoxication-induced neurotoxicity in Sprague-Dawley rats. Arh. Hig. Rada Toksikol. 2016;67 doi: 10.1515/aiht-2016-67-2702. [DOI] [PubMed] [Google Scholar]
- 28.Tellez-Banuelos M.C., Haramati J., Franco-Topete K., Peregrina-Sandoval J., Franco-Topete R., Zaitseva G.P. Chronic exposure to endosulfan induces inflammation in murine colon via beta-catenin expression and IL-6 production. J. Immunotoxicol. 2016;13 doi: 10.1080/1547691X.2016.1206998. [DOI] [PubMed] [Google Scholar]
- 29.Falvo J.V., Tsytsykova A.V., Goldfeld A.E. Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 2010;11 doi: 10.1159/000289196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23 doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Weisz L. Regulation of the mouse inducible-type nitric oxide synthase gene promoter by interferon-gamma, bacterial lipopolysaccharide and NG-monomethyl-L-arginine. Biochem. J. 1996;316(Pt. 1):209–215. doi: 10.1042/bj3160209. PMCID: PMC1217324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleinert H., Schwarz P.M., Forstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol. Chem. 2003;384 doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 33.Matsuura M., Saito S., Hirai Y., Okamura H. A pathway through interferon-gamma is the main pathway for induction of nitric oxide upon stimulation with bacterial lipopolysaccharide in mouse peritoneal cells. Eur. J. Biochem. 2003;270:4016–4025. doi: 10.1046/j.1432-1033.2003.03792.x. PMID: 14511384. [DOI] [PubMed] [Google Scholar]
- 34.Villa A., Rizzi N., Vegeto E., Ciana P., Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015;5 doi: 10.1038/srep15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan G.W., Gao X.M., Wang H., Zhu Y., Zhang J., Hu L.M., Su Y.F., Kang L.Y., Zhang B.L. The anti-inflammatory activities of Tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J. Steroid Biochem. Mol. Biol. 2009;113 doi: 10.1016/j.jsbmb.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Cvoro A., Tzagarakis-Foster C., Tatomer D., Paruthiyil S., Fox M.S., Leitman D.C. Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Mol. Cell. 2006;21 doi: 10.1016/j.molcel.2006.01.014. [DOI] [PubMed] [Google Scholar]