Abstract
The maize (Zea mays L.) Opaque2 (O2) protein is an endosperm-specific transcriptional activator whose DNA-binding activity is regulated diurnally by a phosphorylation/dephosphorylation mechanism. We show that the O2 transcript undergoes pronounced oscillations during the day-night cycle. The highest level of the O2 message is present at midday and the lowest level at midnight. The level of O2 transcript follows a diurnal rhythm that appears controlled by the circadian clock. Two different endosperm-expressed DNA-binding proteins, PBF (prolamin box-binding factor) and OHP1 (O2-heterodimerizing protein 1), were also analyzed. While the PBF message levels oscillate diurnally, the steady-state levels of OHP1 transcript were constant through the day and night. We present data showing that the seed is not directly involved in the perception of the light signal, but presumably responds to diurnal fluxes of nutrients into the endosperm. Moreover, we show that the O2 protein is not involved in the regulation of its own transcript levels. These data indicate that O2 activity is down-regulated at night by both a reduction in O2 transcript and by hyperphosphorylation of residual O2 protein, and suggest that regulatory gene activity during endosperm development may be acutely sensitive to a diurnal signal(s) emanating from the plant and passing into the developing seeds.
The maize (Zea mays L.) O2 b-ZIP (basic leucine zipper) transcriptional activator controls the expression of certain members of the zein seed storage protein gene family. Mutations in o2 result in a reduction of zein gene expression, with the 22-kD zein class being the most severely affected (for review, see Schmidt, 1993; Aukerman and Schmidt, 1994; Müller et al., 1995). The O2 protein recognizes an imperfect palindromic sequence 5′-TCCACGTAGA-3′ (O2-box) present in the promoter of the 22-kD zein genes (Schmidt et al., 1992). A second maize b-ZIP protein, OHP1, has been identified that can also bind to the O2-box, either as a homodimer or in a heterodimeric complex with O2 (Pysh et al., 1993).
PBF is another protein that was recently shown to specifically bind the 22-kD zein gene promoter and interact in vitro with O2. This endosperm-specific trans-acting factor binds with high affinity to the prolamin box (P-box), a highly conserved 7-bp sequence element (5′-TGTAAAG-3′) found in the promoters of many cereal seed storage protein genes (Vicente-Carbajosa et al., 1997).
In a previous study we demonstrated that the O2 protein is multiphosphorylated in vivo and that phosphorylation is crucial for the regulation of the O2 DNA-binding activity (Ciceri et al., 1997). In fact, only the unphosphorylated and hypophosphorylated forms of O2 bind the target DNA sequence with high affinity, and the hyperphosphorylated forms only bind after in vitro enzymatic dephosphorylation. In addition, we showed that the O2 phosphorylation pattern changes diurnally: unphosphorylated and hypophosphorylated forms accumulate by day and hyperphosphorylated forms at night (Ciceri et al., 1997). This pattern suggests a temporal regulation of the O2 activity that might be important in coordinating the expression of O2-regulated genes with different times of energy supply.
Many cellular processes function with a daily rhythmicity. These rhythms are innately generated by an endogenous oscillator, the biological clock. This clock operates widely in eukaryotes and prokaryotes (for review, see Iwasaki and Thomas, 1997; Kreps and Kay, 1997; Millar and Kay, 1997; Young, 1998). Diurnal and circadian regulations of gene expression are very common in plants and are necessary for the proper temporal regulation of many physiological and biochemical processes such as stomatal aperture, leaf movement, ion uptake, nitrogen assimilation, carbon fixation, and photosynthesis (for review, see Millar and Kay, 1997; Kreps and Kay, 1997). Despite the above-mentioned examples, little is known about diurnal and circadian control of gene expression during seed development.
In the present study, we provide evidence that the steady-state level of the O2 transcript is also subject to diurnal changes. In fact, transcription of O2 follows a diurnal rhythm that seems to be regulated by the circadian clock. The highest level of the O2 transcript is present at midday and the lowest level at midnight. We also observe similar diurnal changes in the steady-state levels of the PBF message. In contrast, steady-state levels of OHP1 transcript were constant through the day and night. The changes in the steady-state levels of the O2 message were present even when the ear was covered with aluminum foil, suggesting that the seed is not directly involved in the perception of the light signal, but probably is responding to diurnal fluxes of metabolites into the endosperm. The diurnal changes in the O2 transcript levels are also detectable in the opaque2 Truncated (o2T) mutant allele that codes for a truncated polypeptide lacking the b-ZIP domain (Lazzari et al., 1995), suggesting that the O2 protein is not involved in controlling the day-night oscillations of its own transcript. These data indicate that O2 activity is down-regulated at night by both a reduction in O2 steady-state transcript levels and hyperphosphorylation of O2 protein.
MATERIALS AND METHODS
Plant Growth Conditions
Maize (Zea mays L.) plants harboring either the wild-type O2w1 or the o2R null-transcript allele (Schimdt et al., 1987; Bernard et al., 1994) in the Oh43 genetic background were grown during the summer under greenhouse conditions (30°C day/20°C night, 70%–80% RH, and 14 h of light). Maize plants harboring either the wild-type O2w1 or the o2T allele (Lazzari et al., 1995) in the W64A genetic background were grown in a phytotron under conditions reported previously (Ciceri et al., 1997) and used only in the experiment reported in Figure 5.
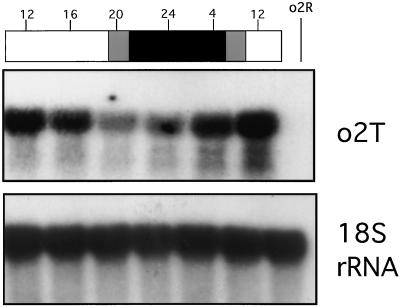
Figure 5.
Northern analysis showing that the O2 protein does not control the day-night regulation of its own transcription. Plants homozygous for the o2T allele were phytotron-grown and self-pollinated. At the indicated time points (shown on the top of the bars as a 24-h clock) 15-DAP kernels were harvested and the o2T message detected by northern blot using the O2 probe. Hybridization with the 18S rDNA probe was also carried out as control of loading. White and black bars indicate light and dark periods, respectively. Gray bars indicate dimlight. Lane o2R is as indicated in Figure 1.
For the experiment shown in Figure 4, greenhouse-grown plants were transferred at midnight into a dark chamber with a constant temperature of 20°C for 24 h.
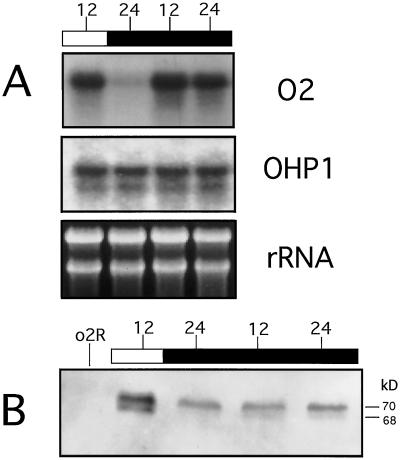
Figure 4.
The day-night oscillations in the O2 message level and O2 phosphorylation pattern continued when the plants were shifted to constant darkness. A, Wild-type plants grown in the greenhouse under normal light-dark and temperature conditions were transferred at midnight to a dark chamber with a constant temperature of 20°C for 24 h. White and black bars indicate light and dark periods, respectively. At the indicated time points (shown on the top of the bars as a 24-h clock), 15-DAP seeds were harvested from these plants prior to transfer to constant darkness (lanes 12 light and 24 dark) and after transfer to darkness (lanes 12 dark and 24 dark). The O2 and OHP1 expression were analyzed by northern blot. rRNA stained with ethidium bromide was used as a loading control. B, Twenty micrograms of total protein extracts per sample, obtained from the same kernels used for the northern-blot analysis indicated above, were analyzed as indicated in Figure 2A. Apparent sizes are indicated on the right in kD. Lane o2R is as indicated in Figure 1.
For RNA and protein analyses, kernels were harvested, immediately frozen in liquid nitrogen, and stored at −80°C until use.
RNA Isolation and Northern Analysis
Total RNA was isolated from endosperm tissue harvested at several developmental stages (from 11–25 DAP) as described by Cone et al. (1986). Five micrograms of total RNA from each sample were separated by electrophoresis on a 1.5% (w/v) agarose-formaldehyde gel, stained, and blotted overnight as described in Pysh et al. (1993). Conditions for prehybridization, hybridization, and washes were as described previously (Schmidt et al., 1987). The following gel-purified restriction fragments were used as probes: the entire O2 cDNA (Schmidt et al., 1990), a 720-bp XbaI-SpeI fragment from the PBF cDNA (Vicente-Carbajosa et al., 1997), and the entire OHP1 cDNA (Pysh et al., 1993). A flax rDNA clone (Bernard et al., 1994) was used as a probe for the 18S rRNA. Probes were labeled using random priming and [32P]dATP.
Protein Isolation and Western Analysis
Total protein extracts were obtained from endosperm tissue harvested at several developmental stages (from 11–15 DAP) according to the procedure of Bernard et al. (1994). The protein concentration was determined as described by Bernard et al. (1994). SDS-PAGE, Ponceau S stain, and immunoblotting were performed as reported in Bernard et al. (1994) and Ciceri et al. (1997).
RESULTS
The Steady-State Level of the O2 and PBF Messages Changes Diurnally
In a previous paper (Ciceri et al., 1997), we showed that the DNA-binding activity of the O2 protein is regulated by phosphorylation and that the pattern of the O2 phosphorylation is subject to diurnal changes. This indicates that the activity of the O2 transcriptional activator is regulated diurnally by a post-translational mechanism.
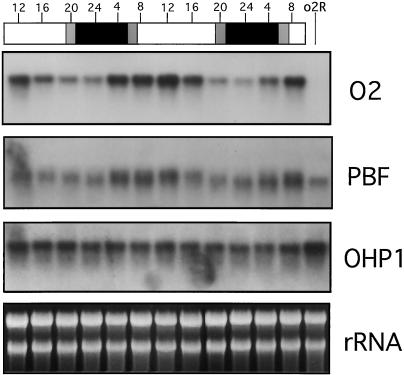
To monitor possible diurnal mRNA oscillations, the steady-state level of O2, PBF, and OHP1 transcripts were followed in greenhouse-grown plants. Kernels were harvested every 4 h during a 48-h period. As shown in Figure 1, we detect strong fluctuations in the abundance of the O2 and PBF transcripts by northern analysis. These oscillations occur in the same phase for both O2 and PBF messages: maximal expression was observed at midday and minimal expression at midnight. On the other hand, the steady-state level of the OHP1 transcript remains constant during the day-night cycle. Interestingly, the amount of the O2 and PBF messages increased a few hours before sunrise (Fig. 1, samples harvested at time point 4), when the plants were still in the dark. As reported for the transcription of other genes under the control of the biological clock (for review, see Kreps and Kay, 1997), this “anticipation” of the dark-to-light transition suggests circadian regulation of O2 and PBF transcription.
Figure 1.
Results of northern analysis demonstrating diurnal changes in O2 and PBF messages. Kernels were harvested every 4 h for a 48-h period (from 11–13 DAP) from greenhouse-grown wild-type plants. Total RNA was extracted from endosperm tissue and 5 μg from each sample was separated by electrophoresis on a 1.5% (w/v) agarose-formaldehyde gel, stained, and blotted. The same RNA gel blot was first hybridized with O2, then sequentially stripped and reprobed with PBF followed by OHP1. rRNA stained with ethidium bromide is used as loading control. White and black bars indicate light and dark periods, respectively. Gray bars indicate dawn and dusk. The time at which endosperm samples were collected is indicated on the top of the bars as a 24-h clock. The mutant o2R allele is a null transcript and serves as a negative control for the O2 probe. The o2R kernels were harvested at midday from a 15-DAP ear.
These data indicate that the steady-state level of O2 and PBF transcripts follows a diurnal rhythm with higher level of expression during the day.
The O2 Phosphorylation Pattern Changes during the Day-Night Cycle
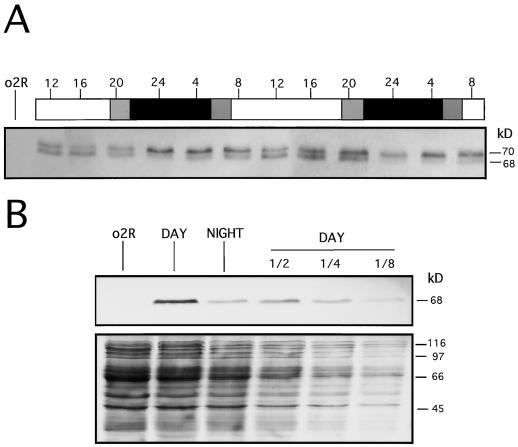
In an earlier analysis conducted on phytotron-grown plants, we showed that the day-night cycle determines a periodic oscillation in O2 phosphorylation (Ciceri et al., 1997). To reconfirm the diurnal changes of the O2 phosphorylation pattern in the population of greenhouse-grown plants used for the northern analysis in Figure 1, we performed a western analysis for O2 protein using the same seeds utilized for the RNA analysis. The results of this experiment are reported in Figure 2A. The relative abundance of the various O2 isoforms changes dramatically during the day-night cycle. In fact, during the dark period the intensity of the 70-kD band containing the inactive DNA-binding isoforms (hyperphosphorylated forms) progressively increases relative to the 68-kD band. To determine if the nocturnal reduction in the steady-state level of the O2 mRNA leads to a parallel decrease in the total amount of the O2 protein, we carried out a western analysis using total protein extracts obtained from a midday and a midnight sample (Fig. 2B). To facilitate the quantification of the O2 protein, we increased the concentration of the SDS-PAGE gel from 10% to 15% so that the O2 doublet would migrate as a single band. We also loaded a series of dilutions (1/2, 1/4, and 1/8) of the day sample so as to better quantify the difference between the amount of O2 protein present in the night versus the day sample. The results indicate that the night sample contains only one-quarter to one-half of the total O2 protein present during the day. Therefore, the observed diurnal fluctuations in the steady-state levels of O2 mRNA are paralleled by diurnal changes in the O2 phosphorylation pattern and reduction in the total amount of the O2 protein. High levels of O2 mRNA correlate with a high DNA-binding activity during the day, whereas the shift to a DNA-binding inactive form of the protein at night correlates with reduction in steady-state levels of the O2 message.
Figure 2.
Results of western analysis showing diurnal changes in O2 phosphorylation (A) and accumulation (B). A, Wild-type kernels (from 11–13 DAP) were harvested as indicated in Figure 1. Total protein extracts were made from endosperm tissue, and 20 μg from each sample were electrophoresed into a 10% (w/v) SDS-PAGE gel, blotted, and immunodetected using a polyclonal anti-O2 antiserum. Apparent sizes are indicated at right in kD. The 70-kD band contains the inactive DNA-binding isoforms (hyperphosphorylated forms), whereas the 68-kD band includes the active DNA-binding isoforms (unphophosphorylated and hypophosphorylated forms). White and black bars indicate light and dark periods, respectively. Gray bars indicate dawn and dusk. The time at which endosperm samples were collected is indicated on the top of the bars as a 24-h clock. Lane o2R is as indicated in Figure 1. B, Total protein extracts were prepared from o2R (15 DAP) and wild-type kernels (15 DAP) harvested at midday (DAY) or at midnight (NIGHT). After separation by 15% (w/v) SDS-PAGE gel and electroblotting, the O2 protein was visualized using a polyclonal anti-O2 antiserum (upper panel). Approximately equal amounts of total protein extract (40 μg) from o2R, DAY, and NIGHT samples were loaded adjacent to a serial dilution (1/2, 1/4, and 1/8) of the DAY sample. The staining of the membrane used for the immunodetection with Ponceau S serves as loading control (lower panel).
The Maize Seed Is Not Directly Involved in the Perception of the Light Signal
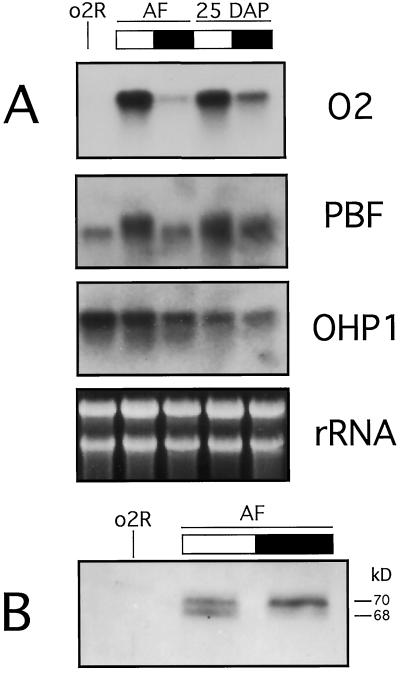
To understand if the seed is directly involved in the perception of the light signal, we covered a fertilized ear with aluminum foil 3 d prior to the day of the harvest. This effectively imposed a period of 72 h of darkness to the developing seeds only. Figure 3A (lanes AF) shows the results obtained by northern analysis. The diurnal oscillations in the O2 and PBF messages were still present with the same amplitude as was observed when the ears were developing uncovered (see Fig. 1). Moreover, the steady-state level of the OHP1 transcript remains constant as previously observed (see Fig. 1). As shown in Figure 3B, the day-night oscillations in the phosphorylation pattern of O2 are also still detectable. These results indicate that the direct perception of the light by the seed is not critical for the diurnal changes observed in O2 transcription and the O2 phosphorylation pattern.
Figure 3.
Northern and western analyses demonstrating that the seed is not directly involved in the perception of the light signal. A, From greenhouse-grown wild-type plants, total RNA was extracted from 15-DAP seeds obtained from an ear covered for 3 d with aluminum foil (AF) or from kernels obtained from a 25-DAP ear. Northern-blot analysis was carried out using the probes indicated on the right. rRNA stained with ethidium bromide is used as control of loading. Seeds were harvested at midday (white bar) or at midnight (black bar). B, Twenty micrograms of whole cell protein extracts per lane, made from the same seeds used for the RNA analysis of the aluminum foil experiment, were separated by 10% (w/v) SDS-PAGE and analyzed by western blot using a polyclonal O2 antibody. Seeds were harvested at midday (white bar) or at midnight (black bar). Apparent sizes are indicated at right in kD. Lane o2R is as indicated in Figure 1.
The results showed in Figure 1 were obtained using seeds at early stages of development (from 11–13 DAP). To investigate if the day-night oscillations observed in the O2 and PBF transcripts at 11 to 13 DAP were also present in a later stage of endosperm development, we carried out northern analysis using seeds harvested at 25 DAP (Fig. 3A). The results of this experiment showed that the same diurnal oscillation observed for O2 and PBF messages are also detectable at a late stage of endosperm development. This experiment suggests that the day-night fluctuations detected in the steady-state levels of O2 and PBF transcripts can play an important role in the activity of these two storage protein regulatory genes during the most crucial period of endosperm development.
The Diurnal Oscillations in the O2 Transcript and O2 Phosphorylation Pattern Seem to Be Controlled by the Biological Clock
To try to discriminate between diurnal (light-regulated) and endogenous circadian regulation of O2 mRNA accumulation, greenhouse-grown plants were transferred at midnight into a dark chamber with a constant temperature of 20°C for 24 h. As shown in Figure 4A, even when the plants were kept in complete darkness, the amount of the steady-state level of the O2 message increased (time point 12, black bar) during the time period that would have corresponded to the midday period. In the dark, the day-night oscillation of the O2 message was still detectable after 24 h, although its amplitude is significantly decreased relative to that observed under normal day-night conditions (time point 24, black bar). Under these conditions no differences in the OHP1 transcript profile were detected relative to that observed under standard greenhouse conditions.
To determine if the rhythm in O2 phosphorylation was continuing in total darkness, whole-cell protein extracts prepared from the same seeds used in Figure 4A were analyzed by western analysis using an O2 antibody (Fig. 4B). In parallel to what was observed for the O2 transcript, the day-night changes in the O2 phosphorylation pattern are still detectable after 24 h in the dark. However, it appears that the dephosphorylation process occurs in a way that is less efficient than that observed under normal day-night conditions.
The O2 Protein Does Not Control the Day-Night Regulation of Its Own Transcription
As previously reported, the O2 protein can bind its own promoter and it has been suggested that this interaction may have a regulatory role in modulating its own transcription (Lohmer et al., 1991). To test if the O2 protein was responsible for the day-night oscillations of its own transcript, we performed a day-night experiment using plants carrying the o2T allele. This allele codes for a mutant o2 protein that is cytoplasmically localized and lacks the whole b-ZIP domain (Bernard et al., 1994; Lazzari et al., 1995). In the case of O2, the basic domain has a double function of sequence-specific DNA-binding and nuclear localization (Varagona and Raikhel, 1994). As shown in Figure 5, the day-night fluctuation of the o2T transcript follows the same pattern already observed for the normal O2 line (see Fig. 1) and for the wild-type isogenic line (data not shown).
These data demonstrate that the O2 protein is not directly involved in the day-night regulation of its own transcript levels.
DISCUSSION
Our previous research demonstrated that O2 DNA-binding activity is controlled through a phosphorylation/dephosphorylation mechanism that follows the day-night cycle. In particular, active DNA-binding isoforms (unphosphorylated and hypophosphorylated) accumulate during the day, and inactive DNA-binding isoforms (hyperphosphorylated) during the night (Ciceri et al., 1997 and Fig. 2). In the present study we have shown that the steady-state level of the O2 transcript is also subject to diurnal changes. In fact, the steady-state amount of O2 message is highest around midday and lowest at about midnight (Fig. 1). In addition to O2 we monitored the transcript levels of two other genes encoding DNA-binding proteins that recognize the zein promoter, PBF and OHP1. The first undergoes the same pronounced day-night oscillation observed for O2, whereas the second remains constant (Fig. 1).
The fact that OHP1 message levels appear invariable indicates that the oscillation in the levels of O2 and PBF transcript is a specific effect and not some general change in endosperm transcription or mRNA stability during the day and night. That the effect is specific rather than general is further supported by our observation that the phases of the O2 and PBF message oscillations are not coincident under some conditions of growth. The experiments reported here were conducted in our greenhouse under long-day conditions (summer). When these experiments were repeated in our greenhouse at other times of the year, when significant differences in day length but not temperature were noted, the O2 transcript levels always fluctuated predictably. However, under short-day conditions (winter) the day-night fluctuations observed for the PBF message were shifted in phase relative to O2. The reasons for this uncoupling in their phases under short days is not understood. The OHP1 transcript was never observed to cycle under any conditions analyzed (data not shown).
The day-night oscillation observed for the steady-state levels of O2 and PBF transcripts is due either to transcriptional regulation involving specific motifs within the promoter region or to a differential (day-night) post-transcriptional stability of these messages. In most of the cases where a day-night modulation in mRNA has been reported, the regulation occurs at the transcriptional level (for review, see Kreps and Kay, 1997). Although we have not specifically demonstrated this for the regulatory genes analyzed in this report, our results clearly show pronounced changes in the levels of O2 and PBF messages between day and night. Not only does the O2 message decrease at night, but, under the same conditions, the residual O2 protein becomes hyperphosphorylated (non-DNA-binding). Apparently, down regulation of O2 activity at night is important enough in endosperm development that two mechanisms have evolved to ensure its occurrence: a reduction in O2 message, leading to a parallel decrease in O2 protein, and the inactivation of any remaining O2 protein through hyperphosphorylation and subsequent inhibition of DNA binding.
Because of the documented role of O2 in zein gene regulation, one might expect to observe parallel diurnal changes in 22-kD zein transcript levels. The steady-state level of 22-kD zein messages was tested by northern analysis during the day-night cycle to determine if the nocturnal down-regulation of O2 activity has any impact on the level of accumulation of zein transcripts. However, no significant day-night differences in steady-state message levels were detected (P. Ciceri, A. Viotti, and R.J. Schmidt, data not shown). We presume this to be a consequence of the long half-life of the zein messages (Plotnikov and Bakaldina, 1996). In addition, we performed run-on transcription experiments using seeds harvested during several time points in the day-night cycle. Under our experimental conditions, we were not able to observe any in vitro transcription for the 22-kD zein genes even though other run-on transcripts (i.e. ubiquitin and tubulin) were detected (data not shown).
Although the long half-life and relative abundance of 22-kD zein messages precluded our ability to detect any diurnal changes in the levels of zein transcript, it is still possible that the diurnal changes in O2 activity are important in keeping the levels of 22-kD zein transcripts within some defined range relative to other messages. Clearly, O2 is essential for high levels of zein gene expression, as evidenced by the effect of o2 mutations on zein mRNA accumulation. Possibly, without diurnal changes in O2 activity, zein message would accumulate to levels that would become deleterious to other aspects of endosperm development. Transgenic maize constitutively synthesizing O2 in the endosperm, in a form not subject to inhibition of DNA-binding by hyperphosphorylation, would allow us to test this hypothesis. Alternatively, these diurnal changes in O2 activity may be inconsequential to the process of seed storage protein gene expression but important to some other aspect of O2 activity in maize endosperm. Several endosperm-expressed genes are known to be influenced by O2, such as those encoding the cytosolic form of the pyruvate orthophosphate dikinase-1, the Lys-ketoglutarate reductase, and the acetohydroxy acid synthase (Brochetto-Braga et al., 1992; Maddaloni et al., 1996; Damerval and Le Guilloux, 1998).
The “anticipation” of the dark-light transition observed for the O2 message (Fig. 1, time point 4) and the rise in O2 message levels at midday, even when plants are kept in the dark (Fig. 4A), are both responses that would be predicted if O2 transcription were under control of the circadian clock. Using plants grown in a phytotron under constant light and temperature regime, preliminary data support the involvement of the circadian clock in the regulation of O2 transcription (A. Genga and A. Viotti, unpublished results). Even though we failed to observe an obvious “anticipation” in dephosphorylation in pre-dawn samples (Fig. 2A, time point 4), the results of the constant dark experiment (Fig. 4B) suggest that the changes in the O2 phosphorylation pattern have a circadian basis. These results indicate that the steady-state level of the O2 transcript and the O2 phosphorylation are controlled by a common circadian mechanism.
It has been shown that the O2 protein is able to bind its own promoter and weakly activate the transcription of a downstream reporter gene in tobacco mesophyll protoplast cells (Lohmer et al., 1991). From these data it was suggested that O2 expression could be modulated by the O2 protein itself. However, our data do not support this suggestion. When we monitored the mRNA levels of the o2T allele that codes for a truncated protein lacking the whole b-ZIP domain (Lazzari et al., 1995), the same diurnal changes observed for wild-type O2 message were observed for the o2T mutant. These data demonstrate that the O2 protein is not directly involved in the day-night oscillations of its own message levels (Fig. 5). Furthermore, this result suggests that O2 has no effect on its own transcription. This is demonstrated by the fact that the o2T message accumulates normally in spite of the absence in the nucleus of a DNA-binding competent form of the O2 protein. Our conclusion that the O2 protein has no effect on its own transcription is supported by similar observations on other o2 mutant alleles (Aukerman et al., 1991; Aukerman and Schmidt, 1993; Bernard et al., 1994).
The day-night oscillations in the O2 and PBF transcript levels occured even when the developing seeds were kept in the dark by covering the fertilized ear with aluminum foil (Fig. 3A). These results demonstrate that the seed is not directly involved in the perception of the light signal. It is possible that these day-night oscillations are controlled by diurnal fluxes of metabolites (e.g. Suc and/or amino acids) transported from the photosynthetic tissues to the developing seeds. It has been shown that carbohydrates can modulate the expression of several plant genes involved in different metabolic pathways, including photosynthesis, remobilization of starch, lipid and protein, Suc metabolism, storage protein biosynthesis, respiration, and defense against pathogens (for review, see Koch, 1996; Jang and Sheen, 1997). However, the molecular mechanism of sugar feedback regulation in plants is still poorly understood.
Interestingly, analysis of the O2 promoter sequence has revealed the presence of motifs recently shown to be crucial for Glc-regulated transcription of a rice α-amylase gene (Lu et al., 1998). That the O2 promoter might be responding to changes in carbon flux into the endosperm is supported by preliminary experiments carried out on isogenic maize lines carrying different mutations affecting starch biosynthesis and accumulation. With such mutants we have observed that the amplitude of the day-night oscillation in the O2 transcript can vary depending on the different amounts of free Suc present in the endosperm (P. Ciceri, A. Viotti, and R.J. Schmidt, unpublished data). This indicates that a dual control mechanism based on a biological clock and metabolic signals might in concert modulate O2 activity. Although perception of light directly by the seed does not appear to be involved in controlling the day-night oscillations of the O2 transcript, the O2 promoter does contain the same highly conserved cis-elements recognized by the trans-acting factors CCA1 and CA-1 (Sun et al., 1993; Wang et al., 1997). The CCA1 regulatory protein was recently demonstrated to play a central role in circadian-regulated gene expression (Green and Tobin, 1999). Future studies will be directed at identifying the factors that are involved in the day-night control of O2 transcription and phosphorylation and elucidating their role in endosperm biosynthesis and development.
ACKNOWLEDGMENTS
We thank Jesús Vicente-Carbajosa, Stephen P. Moose, Hank W. Bass, and Alyson M. Mack for very stimulating discussions.
Footnotes
This work was supported by the National Institutes of Health (grant no. GM41286 to R.J.S.) and by a grant from Ministero per le Politiche Agricole (Piano Nazionale Biotecnologie Vegetali) to A.V.
LITERATURE CITED
- Aukerman MJ, Schmidt RJ. A 168 bp derivative of Suppressor-mutator/Enhancer is responsible for the maize o2–23 mutation. Plant Mol Biol. 1993;21:355–362. doi: 10.1007/BF00019950. [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Schmidt RJ. Regulation of α-zein expression during maize endosperm development. In: Nover L, editor. Plant Promoters and Transcription Factors. Berlin-Heidelberg: Springer-Verlag; 1994. pp. 209–233. [Google Scholar]
- Aukerman MJ, Schmidt RJ, Burr B, Burr FA. An arginine to lysine substitution in the bZIP domain of an opaque-2 mutant in maize abolishes specific DNA binding. Genes Dev. 1991;5:310–320. doi: 10.1101/gad.5.2.310. [DOI] [PubMed] [Google Scholar]
- Bernard L, Ciceri P, Viotti A. Molecular analysis of wild-type and mutant alleles at the opaque-2 regulatory locus of maize reveals different mutations and types of O2 products. Plant Mol Biol. 1994;24:949–959. doi: 10.1007/BF00014448. [DOI] [PubMed] [Google Scholar]
- Brochetto-Braga MR, Leite A, Arruda P. Partial purification and characterization of lysine-ketoglutarate reductase in normal and opaque-2 maize endosperms. Plant Physiol. 1992;98:1139–1147. doi: 10.1104/pp.98.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri P, Gianazza E, Lazzari B, Lippoli G, Genga A, Hoscheck G, Schmidt RJ, Viotti A. Phosphorylation of Opaque2 changes diurnally and impacts its DNA binding activity. Plant Cell. 1997;9:97–108. doi: 10.1105/tpc.9.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone KC, Burr FA, Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proc Natl Acad Sci USA. 1986;83:9631–9635. doi: 10.1073/pnas.83.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerval C, Le Guilloux M. Characterization of novel proteins affected by the o2 mutation and expressed during maize endosperm development. Mol Gen Genet. 1998;257:354–361. doi: 10.1007/s004380050657. [DOI] [PubMed] [Google Scholar]
- Green RM, Tobin EM. Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proc Natl Acad Sci USA. 1999;96:4176–4179. doi: 10.1073/pnas.96.7.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Thomas JH. Genetics in rhythm. Trends Genet. 1997;13:111–115. doi: 10.1016/s0168-9525(97)01059-7. [DOI] [PubMed] [Google Scholar]
- Jang J-C, Sheen J. Sugar sensing in higher plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Kreps JA, Kay SA. Coordination of plant metabolism and development by the circadian clock. Plant Cell. 1997;9:1235–1244. doi: 10.1105/tpc.9.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari B, Ciceri P, Carzaniga R, Genga A, Faoro F, Viotti A. Molecular characterization of o2T: a mutant allele at the opaque-2 locus. Maize Genet Coop Newslett. 1995;69:102. [Google Scholar]
- Lohmer S, Maddaloni M, Motto M, Di Fonzo N, Hartings H, Salamini F, Thompson RD. The maize regulatory locus Opaque-2 encodes a DNA-binding protein which activates the transcription of the b-32 gene. EMBO J. 1991;10:617–624. doi: 10.1002/j.1460-2075.1991.tb07989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C-A, Lim E-K, Yu S-M. Sugar response sequence in the promoter of a rice α-amylase gene serves as a transcriptional enhancer. J Biol Chem. 1998;273:10120–10131. doi: 10.1074/jbc.273.17.10120. [DOI] [PubMed] [Google Scholar]
- Maddaloni M, Donini G, Balconi C, Rizzi E, Gallusci P, Forlani F, Lohmer S, Thompson RD, Salamini F, Motto M. The transcriptional activator Opaque-2 controls the expression of cytosolic form of pyruvate orthophosphate dikinase-1 in maize endosperms. Mol Gen Genet. 1996;250:647–654. doi: 10.1007/BF02174452. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Kay SA. The genetics of phototransduction and circadian rhythms in Arabidopsis. BioEssays. 1997;19:209–214. doi: 10.1002/bies.950190306. [DOI] [PubMed] [Google Scholar]
- Müller M, Muth JR, Gallusci P, Knudsen S, Maddaloni M, Motto M, Schmitz D, Sørensen MB, Salamini F, Von Wettstein D, Thompson RD. Regulation of storage protein synthesis in cereal seeds: developmental and nutritional aspects. J Plant Physiol. 1995;145:606–613. [Google Scholar]
- Plotnikov VK, Bakaldina NB. Differential stability of zein mRNA in developing corn kernel. Plant Mol Biol. 1996;31:507–515. doi: 10.1007/BF00042224. [DOI] [PubMed] [Google Scholar]
- Pysh LD, Aukerman MJ, Schmidt RJ. OHP1: a maize basic domain/leucine zipper protein that interacts with Opaque-2. Plant Cell. 1993;5:227–236. doi: 10.1105/tpc.5.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ. Opaque-2 and zein gene expression. In: Verma DPS, editor. Control of Plant Gene Expression. Boca Raton, FL: CRC Press; 1993. pp. 337–355. [Google Scholar]
- Schmidt RJ, Burr FA, Aukerman MJ, Burr B. Maize regulatory gene opaque-2 encodes a protein with a “leucine-zipper” motif that binds to zein DNA. Proc Natl Acad Sci USA. 1990;87:46–50. doi: 10.1073/pnas.87.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Burr FA, Burr B. Transposon tagging and molecular analysis of the maize regulatory locus opaque-2. Science. 1987;238:960–963. doi: 10.1126/science.2823388. [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Ketudat M, Aukerman MJ, Hoschek G. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell. 1992;4:689–700. doi: 10.1105/tpc.4.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Doxsee RA, Harel E, Tobin EM. CA-1, a novel phosphoprotein, interacts with the promoter of the cab140 gene in Arabidopsis and is undetectable in det1 mutant seedlings. Plant Cell. 1993;5:109–121. doi: 10.1105/tpc.5.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagona MJ, Raikhel NV. The basic domain in the bZIP regulatory protein Opaque-2 serves two independent functions: DNA binding and nuclear localization. Plant J. 1994;5:207–214. doi: 10.1046/j.1365-313x.1994.05020207.x. [DOI] [PubMed] [Google Scholar]
- Vicente-Carbajosa J, Moose SP, Parsons RL, Schmidt RJ. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque-2. Proc Natl Acad Sci USA. 1997;94:7685–7690. doi: 10.1073/pnas.94.14.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MW. The molecular control of circadian behavioral rhythms and their entrainment in Drosophila. Annu Rev Biochem. 1998;67:135–152. doi: 10.1146/annurev.biochem.67.1.135. [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Kenigsbuch D, Sun L, Harel E, Ong MS, Tobin EM. A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell. 1997;9:491–507. doi: 10.1105/tpc.9.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]