Abstract
Approximately 1% of the population suffers from rheumatoid arthritis (RA) worldwide (0.45% in Poland). The therapy consists of the use of disease-modifying antirheumatic drugs (DMARDs). Biologics are used in the form of the drug programme. Analysis of the NHF database demonstrated the sequence of conversion between drugs and time spent in a single treatment. In 2009, the patients would start the following treatments: adalimumab 5.8%; etanercept 14.4%; infliximab 23.1%; leflunomide 53.6%; rituximab 3%. After the first year 16% of patients changed therapy or abstained, and in the second year this situation affected 65% of patients. The following percentages maintained the same treatment in the last 6 years: infliximab 4%; adalimumab 15%; etanercept 21%; leflunomide on prescription was continued by 70%. Patients remain too long on the same therapy when it is inefficient. Achieving remission or low disease activity (DAS28 < 2.6) should take place within 6 months of starting therapy.
Keywords: biological drug, rheumatoid arthritis, sequentiality of treatment
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease of unknown aetiology leading to injuries of joint structure and tissue surrounding the joints. Available data indicate that worldwide 1% of the population suffers from it (from 0.5% to 2%; 0.45% in Poland) [1, 2]. The studies indicate that the longevity of RA patients compared to the general population is 3 years shorter in the case of women and 7 years shorter in the case of men [3].
The diagnosis was established based on the criteria established by the American College of Rheumatology (ACR) in 1987, based on the clinical picture of the advanced stage of the disease, currently the common criteria of the European League Against Rheumatism (EULAR) and the ACR, which enable the diagnosis of its early forms [4]. The basis for the treatment consists of classical synthetic drugs which modify the course of the disease (methotrexate and leflunomide, in some cases sulfasalazine). In the case of their failure, the use of biological drugs which modify the course of the disease is recommended [5], adding them to the synthetic drugs, preferably methotrexate.
Due to the very high price of biological drugs, this therapy in Poland is financed as part of a special procedure, called the drug programme (previously called the treatment programme). The programme indicates the method of patient therapy using specific molecules (drugs) with precisely established programme admittance and discharge criteria established first pursuant to the NICE recommendations of 2002, and currently to the latest EULAR recommendations [6, 7]. The goal of the analysis is to establish the process of biological drug changes in RA patients which were admitted to the programme therapy in 2009.
The report databases of the public payer (National Health Fund) were analysed in order to identify a separate population of patients with rheumatoid arthritis (RA) who began their therapy under the drug therapy programme in 2009. The type of drug administered (active particle) to a given patient in the report data provided by healthcare providers was verified at yearly intervals (at the beginning of 2009 and subsequently at the beginning of each year, and at the beginning and at the end of 2014). During the period considered (2009–2014) the following drugs were financed under the drug therapy programme: leflunomide (LEFL) (which was next moved in 2010 to the segment of pharmacies open to the public and became available on a medicinal prescription), adalimumab (ADAL), etanercept (ETAN), infliximab (INFL), rituximab (RITU), certolizumab (CERT), and golimumab (GOLI), which were successively included in the therapy. Based on the patient’s individual identification number (the PESEL number), therapy lines with the drugs used in therapy and the switches between types of therapy were determined for individual patients.
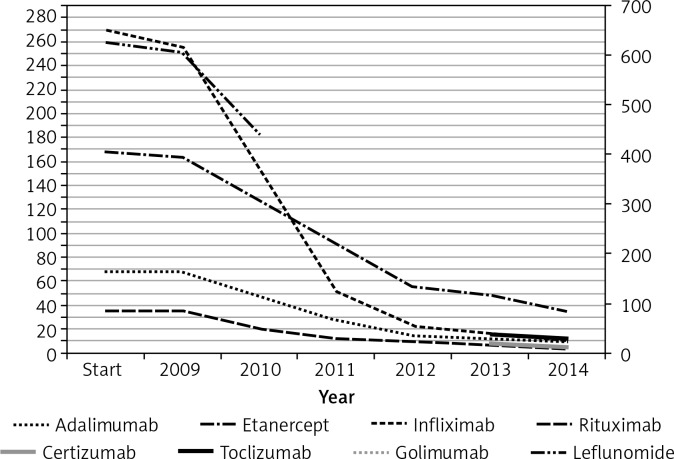
The analysis resulted in report data for the RA drug programme therapy concerning 1167 patients who started the therapy in 2009 with drugs available at the time. Sixty-eight (5.8%) patients started therapy with adalimumab; 168 (14.4%) with etanercept; 270 (23.1%) with infliximab; 35 (3%) with rituximab; 53.6% (626) with leflunomide. During the first year the therapy with the same molecule was continued by: 80.9% of patients for adalimumab; 85.4% for etanercept; 84.1% for infliximab; 74.9% for leflunomide (Figure 1).
Figure 1.
Number of patients who continue the therapy with the same drug by individual years.
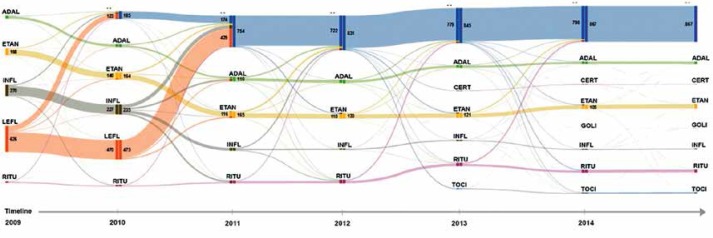
The highest frequency of therapy changes occurred for infliximab treatment; the number of patients who changed to another therapy was after the 1st year 5%, after the 2nd year 32%, after the 3rd year 80%, after the 4th year 91%, after the 5th year 94%, and after the 6th year 96% (average slope of patient loss curve over 6 years was 48%). Four percent of the patients included in the treatment remained on therapy using the same molecule. Adalimumab had the second highest frequency of therapy changes, with the following patient loss over successive years of therapy: 1st year 0%; 2nd year 31%; 3rd year 59%; 4th year 78%; 5th year 82%; 6th year 85%. The continuation of adalimumab therapy after 6 years was observed in 15% of patients (average slope of patient loss curve over 6 years was 41%). Etanercept therapy showed the lowest variability: 1st year 2%; 2nd year 24%; 3rd year 36%; 4th year 67%; 5th year 71%; 6th year 79%. The continuation of therapy after 6 years applied to 21% of patients (average slope of patient loss curve over 6 years was 41%). The analysis of patient flows between therapies is presented in the figure (Figure 2). The tendency of changes of therapy and the large percentage of patients who end their therapy (understood as lack of continuation of therapy within the drug programme) can be observed.
Figure 2.
Sankey diagrams for changes of therapy using individual drugs for patients admitted for treatment in 2009 (for the period of 2009 to 2014)
ADAL – adalimumab, ETAN – etanercept, INFL – infliximab, LEFL – leflunomide, RITU – rituximab, TOCI – tocilizumab, GOLI – golimumab, CERT – certolizumab.
Out of 1167 patients who started therapy in 2009, 186 persons (approx. 16%) stopped/halted the therapy after the first year. During the next 2 years the number of stopped/interrupted therapies was already 754 persons (approx. 65%). From this population which had finished/halted the therapy some patients returned to therapy with the same or a different molecule during the third year of observation. Legislative changes and moving leflunomide to the group of reimbursed pharmacy drugs has forced the end of this part of the programme, yet among the 626 patients treated with this molecule 441 continued therapy under out-patient care, purchasing the drug in the pharmacy.
In the current treatment recommendations established by EULAR the therapeutic goal of remission (or low activity of the disease in each patient) after 6 months of treatment (DAS28 below 2.6) was established. If the goal is not reached, the therapy should be modified regardless of the drug used. The anchor drug is methotrexate used in a dosage of 25–30 mg/week for a period of at least 2 months, and if its use fails it should be supplemented by another classical synthetic or biological drug which modifies the course of the disease. The treatment should be modified until the therapeutic goal is reached [8], and only then may it be continued. Effectiveness of biological drugs in reaching this goal is 24.2% for etanercept monotherapy and 49.4% for etanercept with methotrexate [9], 26% for adalimumab monotherapy and 43% in combination with methotrexate, 33% (in week 52) for the combination of infliximab with methotrexate [10] and 20% for the combination of rituximab with methotrexate [11]. The analysis of data shows that most therapies are continued for a period exceeding 1 year, which is in contravention of the new EULAR recommendations. The recommendations clearly state that the therapeutic goal should be achieved within 6 months or the type of therapy should be changed, which leads to the conclusion that 50–70% of patients should have their therapy changed earlier than after 1 year (after the first 6 months).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Silman AJ, Hochberg MC. Epidemiology of the rheumatic diseases, rheumatoid arthritis. New York: Oxford Univerity Press,; 2001. pp. 31–71. [Google Scholar]
- 2.Iltchev P, Śliwczyński A, Czeleko T, et al. Rheumatoid arthritis morbidity rate in Poland in the years 2008-2012 in rural and urban areas. Ann Agricult Envirom Med Lublin. 2016;23:2. doi: 10.5604/12321966.1203904. in print. [DOI] [PubMed] [Google Scholar]
- 3.Filipowicz-Sosnowska A, Stanisławska-Biernat E, Zubrzycka-Sienkiewicz A. Rheumatoid arthritis [Polish] Reumatologia. 2004;42(Suppl):8–13. [Google Scholar]
- 4.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 5.Lis K, Kuzawińska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors – state of knowledge. Arch Med Sci. 2014;10:1175–85. doi: 10.5114/aoms.2014.47827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Clinical Excellence . Guidance on the use of etanercept and infliximab for the treatment of rheumatoid arthritis, Technology Appraisal No 36. 2002. Mar, [Google Scholar]
- 7.Description of drug programs – appendix B33. in http://www.mz.gov.pl/leki/refundacja/lista-lekow-refundowanych-obwieszczenia-ministra-zdrowia, accessed on 26.10.2015.
- 8.Smolen J, Landewé R, Breedveld F, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klareskog L, van der Heijde D, de Jager JP, et al. TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 10.St Clair EW, van de Heijde D, Smolen JS, et al. Active-Controlled Study of Patients Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset Study Group. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis. Arthritis Rheum. 2004;50:3432–43. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 11.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment. Arthritis Rheum. 2006;54:1390–14. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]