Abstract
Introduction
Osteoarthritis is an inflammatory disorder associated with oxidative stress and apoptosis leading to cartilage destruction and impairment of cartilage formation. In the present study, we studied the protective effect of lutein against monosodium iodoacetate (MIA)-induced osteoarthritis in primary chondrocyte cells.
Material and methods
Oxidative stress was determined through testing antioxidant status, reactive oxygen species levels and lipid peroxide content. Also, Nrf2 expression and its downstream target genes HO-1 and NQO-1 were determined. Inflammation was analyzed through NF-κB, COX-2 and pro-inflammatory cytokines (IL-6, TNF-α, IL-1β). In addition, the effects of MIA and lutein on mitochondrial membrane potential and caspase-3 levels were analyzed.
Results
The results showed that lutein treatment significantly increased the cell viability of chondrocytes and offered significant cytoprotection by enhancing the antioxidant defense mechanisms and reducing oxidative stress (reactive oxygen species and lipid peroxidation). Lutein treatment showed anti-inflammatory effects by downregulating inflammatory proteins (NF-κB, COX-2) and pro-inflammatory cytokines (IL-6, TNF-α, IL-1β). Lutein reduced MIA-induced apoptosis through maintaining mitochondrial membrane potential and downregulating caspase-3 activity.
Conclusions
The present study shows significant cytoprotection offered by lutein against MIA-induced oxidative stress, inflammation and apoptosis by the modulatory effect of NF-κB and Nrf2 activation.
Keywords: oxidative stress, reactive oxygen species, lutein, osteoarthritis, inflammation
Introduction
Osteoarthritis (OA) is identified as the most common musculoskeletal disorder with deregulation in the inflammatory pathway and apoptosis. The degenerative joint disease is associated with loss of cartilage function which mainly occurs as an imbalance between anabolic and catabolic pathways of matrix formation, with more pronounced catabolism of cartilage [1]. The associated risk factors of osteoarthritis include age, genetic predisposition, injury, obesity, etc. An important contributor to cartilage function is chondrocytes, cells which are involved in production and formation of matrix proteins. Terminally differentiated chondrocytes undergo apoptosis with ultimate matrix calcification and vascular invasion of cartilage. Thus, the principle functional loss of chondrocytes is the major event in osteoarthritis. Oxidative stress and activation of inflammation has been known to be an important mediator in the development of osteoarthritis [2, 3]. Thus, an effective protective strategy could be established by targeting oxidative stress and apoptosis, thereby improving the functional status of chondrocytes. Recently, there has been much focus on natural compounds with potent antioxidant properties, by which they target redox signaling and ameliorate negative effects leading to disease status [4, 5]. In the present study, we aimed to study the potential role of lutein against monosodium iodoacetate (MIA) induced osteoarthritis. Lutein (C40H56O2) is a tetraterpenoid that has been identified as a potent antioxidant. Rich sources are dark green leafy vegetables, eggs and fruits. The hydroxyl group of lutein scavenges reactive oxygen species and exerts protection against oxidative stress and inflammatory diseases [6–8]. Lutein suppresses arthrosclerosis by reducing oxidative stress and inflammation [9]. Anti-inflammatory effects of lutein in lipopolysaccharide (LPS)-induced inflammation in macrophages and skin inflammation were mediated through downregulating inflammatory proteins and cytokine expression [9, 10]. Furthermore, the protective role of lutein against ischemic injury in the small intestine and kidney [11, 12] has been reported. Lutein’s protection against paracetamol, carbon tetrachloride and ethanol induced liver damage is mediated through its antioxidant effects [13]. In this study, we aimed to analyze the action of lutein against osteoarthritis by evaluating oxidative stress and inflammatory mechanisms.
Material and methods
Chemicals
Lutein, minimum essential Eagle’s medium, fetal bovine serum (FBS), trypsin, penicillin, streptomycin, DCF-DA (dichloro-dihydro-fluorescein diacetate), and DiOC6 (3,3’- dihexyloxacarbocyanine iodide) were purchased from Sigma Aldrich Chemicals Private Limited, USA. Primary monoclonal antibodies (NF-κB, Nrf2, HO-1, COX-2, NQO1) and secondary antibodies were purchased from Cell Signaling Technology, USA. ELISA kits (IL-6, IL-1β, TNF-α and caspase-3) were procured from Abcam, USA.
Cell culture and treatment schedule
Primary rat chondrocytes were used for the present study. The isolation and cell culture were carried out as described previously [14]. All the protocols were approved by the animal ethics committee at the Department of Orthopedics, Xinxiang Central Hospital, Xinxiang, China. Five-week-old Sprague-Dawley rats were used for the present study. The articular cartilage from the femoral heads of rats were excised and digested in trypsin-EDTA for 20 min followed by collagenase II treatment for 3 h. The primary chondrocytes were cultured in minimum essential Eagle’s medium supplemented with 10% FBS and antibiotics. The chondrocytes at 3–5 passages were used for all the experiments. The cytotoxic concentration of MIA was determined and the protective dose of lutein was identified by pre-treating the cells with different concentrations of lutein followed by MIA. The shortlisted concentrations of MIA and lutein were used for further studies.
Briefly, articular cartilages of femoral heads were harvested from the 4-week-old male Sprague-Dawley rats. The cartilage pieces were digested with trypsin–EDTA and 0.2% Collagenase II (Sigma, St. Louis, MO) for 15 min and 3 h, respectively. The chondrocytes were cultured in Mini-mum essential Eagle’s medium (MEM, Gibco, New York, NY) at 378°C in 5% CO2. For all experiments, the cells of early passages (primary to 3) were used.
Cell viability
Cell viability was assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Briefly, cells (1 × 105) were seeded and treated with different concentrations of MIA (2, 4, 6, 8 10 μM) for 24 and 48 h. The IC50 value was determined. Further, cytoprotective concentration was determined by treating with lutein (0.5, 1, 5 and 10 μM) for 24 h followed by MIA treatment. After the complete treatment schedule, cells were incubated with MTT (5 mg/ml) and resulting formazan crystals were dissolved in DMSO (dimethyl sulfoxide) and the absorbance was read at 570 nm. Cell viability was calculated by comparing the values to that of control cells [15].
Intracellular reactive oxygen species (ROS) generation
For ROS determination, the cells (1 × 105) were treated with lutein for 24 h, followed by DCF-DA for 30 min. After that the cells were washed in PBS and treated with MIA for 48 h. The cells were trypsinized and suspended in PBS and ROS levels were measured fluorimetrically (excitation wavelength 480 nm, emission wavelength 520 nm). The results were expressed as % DCF fluorescence per mg of protein samples compared to the control group [16].
Lipid peroxidation
After treatment, cells were incubated in 8% sodium dodecyl sulfate (SDS); 0.8% thiobarbituric acid (TBA) in 20% acetic acid and heated for 1 h at 90°C. A butanol/pyridine mixture was added and it was shaken vigorously, then centrifuged at 4000 rpm for 10 min, and the organic layer was read at 532 nm. The lipid peroxide content was expressed as n moles of TBA reactants/mg of protein [17].
Antioxidant enzyme activities
Superoxide dismutase (SOD) activity: The SOD activity was determined as described by Sun et al. [18]. The assay is based on reduction of nitro blue tetrazolium (NBT). 1 U of SOD activity = amount required for 50% inhibition of nitro blue tetrazolium chloride (NBT) reduction. The SOD activity is expressed as U/mg of protein. Catalase (CAT) activity: The activity was determined according to the method described by Aebi (1974) [19]. The reaction mixture contained tissue homogenate and 30 mM H2O2 in a 50 mM phosphate buffer (pH 7.0). The activity was estimated by the decrease in absorbance of H2O2 at 240 nm. Glutathione-S-transferase (GST) activity: The reaction between 1-chloro-2,4-dinitrobenzene (CDNB) and reduced glutathione results in formation of dinitrophenylthioether, which is measured at 340 nm [20]. 1 U = amount of enzyme producing 1 mmol of CDNB-GSH conjugate/min. Glutathione peroxidase (GPx) activity: The GPx activity was determined as described by Paglia and Valentine (1967) [21]. The oxidized glutathione (GSSG) is reduced by glutathione reductase and NADPH (nicotinamide adenine dinucleotide phosphate). The oxidation of NADPH to NADP+ is measured by the decrease in absorbance at 340 nm. GPx activity is expressed as U/mg of protein.
Mitochondrial membrane potential (Δψm)
The cells (1 × 105) were treated with lutein for 24 h followed by MIA. After treatment, the cells were washed with PBS and treated with DiOC6 (3) and incubated for 1 h. The fluorescence intensity was measured (excitation wavelength of 488 nm and emission wavelength at 500 nm). The mitochondrial membrane potential was calculated per mg of protein, while the control was set to 100%.
ELISA: IL-6, TNF-α, IL-1β and caspase-3 levels
After the treatment schedule mentioned above, the supernatant was analyzed for interleukins levels of TNF-α, IL-1β and IL-6 levels using ELISA kits (Sigma). The levels of interleukins were expressed as pg/ml. The cellular protein was analyzed for caspase-3 activity. Results were expressed as RU/mg of protein. The absorbance was measured using an ELISA reader (MTP-800 Microplate reader; Corona Electric, Tokyo, Japan).
Western blot
After treatment, the cells were treated with RIPA lysis buffer for isolation of whole cell protein extract. The nuclear extract was isolated for determining the expression of NF-κB and Nrf2. The samples (50 μg protein) were separated on 10% SDS-PAGE gels and transferred onto polyvinylidene fluoride (PVDF) membranes. After protein transfer, non-specific sites were blocked with 5% nonfat dried milk for 1 h at room temperature. The membrane was washed with TBST and incubated with primary mouse monoclonal antibodies against NF-κB, COX-2, Nrf2, HO-1 and NQO1 (1 : 1000) at 4°C overnight. Followed by TBST wash, the membrane was incubated for 1 h with secondary peroxidase-conjugated goat anti-mouse or -rabbit IgG (1 : 5000–1 : 10,000). The bands were visualized with an enhanced chemiluminescence (ECL) system according to the manufacturer’s instructions. Densitometric analyses of the western blot bands were performed using Image J software (GE Healthcare Life Sciences).
Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. All the experiments were performed thrice in triplicate to ensure reproducibility.
Results
Protective effect of lutein against MIA-induced cytotoxicity
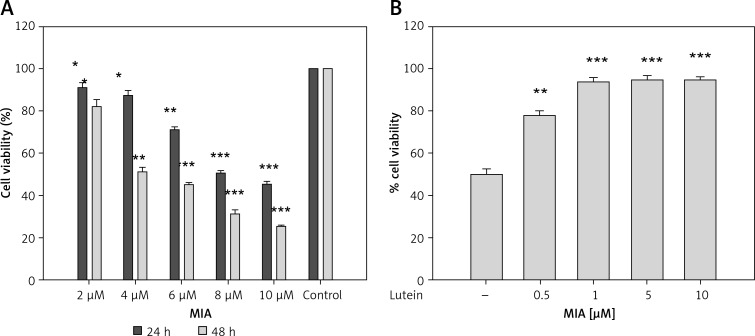
In order to identify the cytotoxic concentration of MIA, the MTT assay was performed. Monosodium iodoacetate caused dose-dependent cell death compared to control cells. The results show that MIA at a concentration of 4 μM for 48 h showed the IC50 value. Further, the cytoprotective effect of lutein was tested at this dose. Lutein was pre-treated for 24 h at different concentrations followed by MIA treatment. Cells treated with 1 μM of lutein showed significant cytoprotection and increased the cell viability to 95%. Cells treated with lutein alone did not show any cytotoxic effect at any of the tested dosages (Figures 1 A, B). Human plasma levels of lutein have been demonstrated to be in the range of 0.25–0.85 μM [22]; thus the concentration of 1 μM lutein was used for further studies.
Figure 1.
Effect of MIA and lutein against primary chondrocyte cells. Cells were treated with MIA for 24 and 48 h. IC50 concentration was used to determine the protective effect of lutein against MIA-induced cell death. Cell viability was measured using MTT assay. Results were expressed as % cell viability compared to control. Results are given as the mean ± SEM. A – *p < 0.05, **p < 0.01, ***p < 0.001, when compared to control group. B – **p < 0.01, ***p < 0.001 when compared to MIA treatment (One-way ANOVA followed by Tukey’s multiple comparison). NS – non-significant compared to control group
Lutein protected against MIA-induced oxidative stress
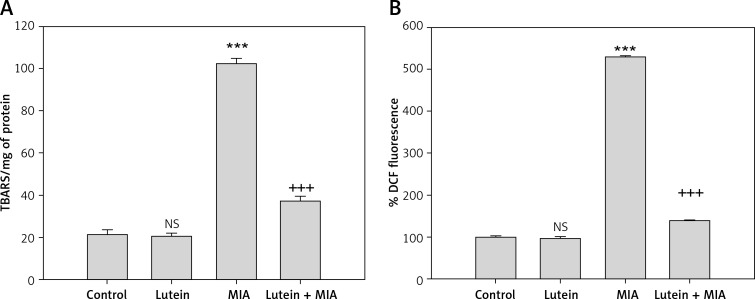
The results showed that MIA treatment resulted in a significant increase in oxidative stress status which was reflected in increased ROS and lipid peroxide content compared to control cells. Lutein pre-treatment followed by MIA treatment significantly reduced the ROS and lipid peroxide content compared to MIA-treated cells (Figures 2 A, B).
Figure 2.
Lutein reduces MIA-induced oxidative stress. A – The results are expressed as nanomoles of TBARS formed/mg of protein. B – The results are expressed as % DCF fluorescence when compared to control. Results are given as the mean ± SEM. ***p < 0.001, when compared to control group. +++p < 0.001, when compared to MIA treatment. NS – non-significant compared to control group (One-way ANOVA followed by Tukey’s multiple comparison)
Lutein protected MIA-induced toxicity through Nrf2 upregulation
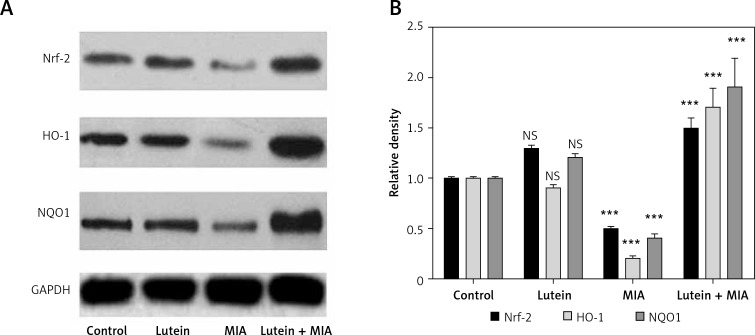
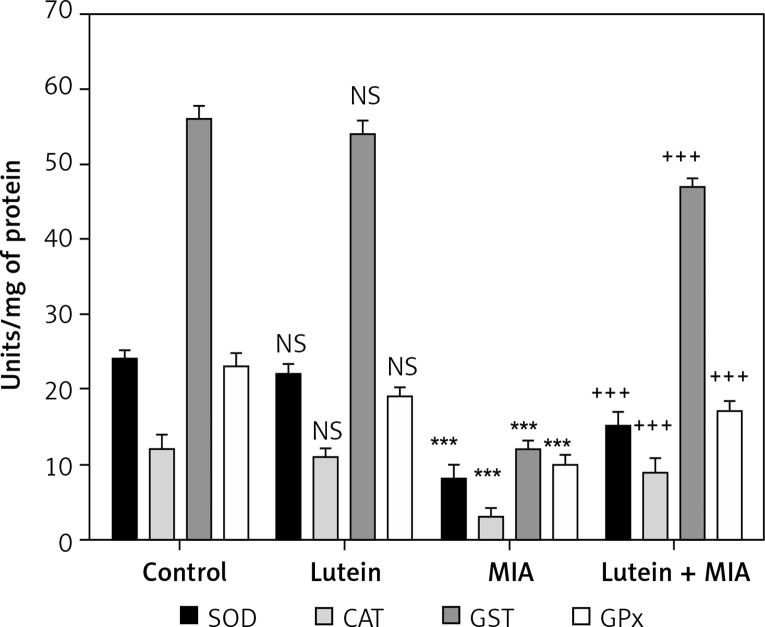
Nrf2 is a transcription factor involved in regulating redox homeostasis by upregulating antioxidant status. In the present study, we found that MIA treatment showed significant downregulation of Nrf2 and its downstream target antioxidant genes HO-1 and NQO-1. Furthermore, various antioxidant enzymes involved in balancing redox homeostasis such as SOD, CAT, GST and GPx were significantly downregulated compared to the control cells. Treatment with lutein showed cytoprotection by significant upregulation of Nrf2, NQO-1 and HO-I. Moreover, there was a concomitant increase in the antioxidant status compared to MIA-treated cells (Figures 3, 4).
Figure 3.
Lutein improves antioxidant mechanism: upregulation of Nrf2, HO-1 and NQO1 expression. Protein expression was analyzed through western blot analysis. Results were expressed as mean ± SEM. Densitometric analysis show significant downregulation of Nrf2, HO-1 and NQO1 expression in MIA treatment. Lutein treatment upregulated the protein expression compared to MIA treatment. ***p < 0.001, when compared to control group. +++p < 0.001, when compared to MIA treatment. NS – non-significant compared to control group
Figure 4.
Lutein enhances antioxidant enzyme activities. Results are given as the mean ± SEM. SOD, CAT, GST and GPx expressed in units/mg protein. ***p < 0.001 when compared to control group. +++p < 0.001 when compared to MIA treatment. NS–non-significant compared to control group (One-way ANOVA followed by Tukey’s multiple comparison)
Lutein protected against MIA-induced inflammation
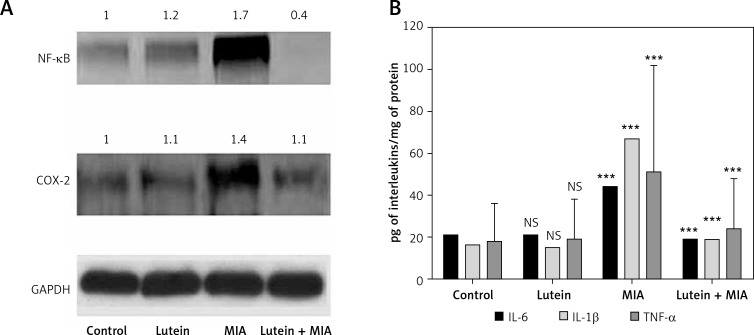
Oxidative stress induces activation of inflammatory responses through NF-κB. In the present study, MIA showed significant upregulation of NF-κB and the downstream inflammatory gene COX-2 compared to the control. However, lutein pre-treatment significantly downregulated the protein levels of NF-κB and COX-2 compared to MIA-treated cells and exerted an anti-inflammatory effect (Figure 5 A). In order to determine role of lutein and MIA on inflammatory cytokines, the levels of IL-6, IL-1β and TNF-α were determined. Cells treated with MIA showed significant upregulation of inflammatory cytokines compared to the control cells. However, treatment with lutein reduced the levels of cytokines compared to those of MIA-treated cells (Figure 5 B).
Figure 5.
Lutein shows anti-inflammatory effect: downregulation of NF-κB and COX-2 and inflammatory cytokines. A – Protein expression of NF-κB and COX-2 was analyzed through western blot analysis. Results were expressed as mean ± SEM. Densitometric analysis shows significant upregulation of NF-κb and COX-2 expression in MIA treatment group. Lutein pre-treatment downregulated the inflammatory proteins compared to MIA treatment. B – MIA treatment showed significant upregulation of IL-6, IL-1β and TNF-α in the MIA treatment group. Lutein pre-treatment downregulated the inflammatory cytokines compared to MIA treatment. ***p < 0.001 when compared to control group. +++p < 0.001 when compared to MIA treatment. NS – non-significant compared to control group
Lutein prevented MIA-induced apoptosis
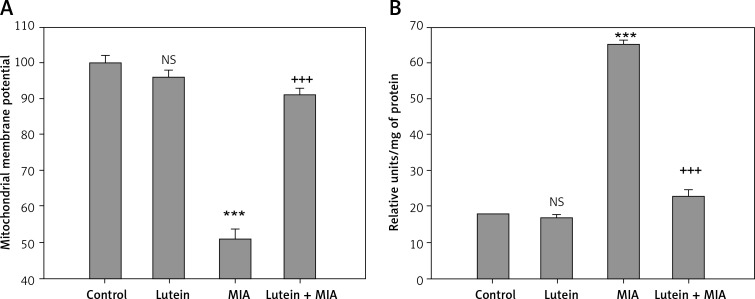
The results showed that MIA treatment resulted in a significant loss of mitochondrial membrane potential with upregulation of caspase-3 activation compared to that of control cells. Treatment with lutein showed significant restoration of membrane potential with downregulation of caspase-3 activation compared to that of MIA-treated cells (Figures 6 A, B).
Figure 6.
Lutein inhibits apoptosis. Results are given as the mean ± SEM. Lutein pre-treatment maintained the mitochondrial membrane potential (A) and downregulated caspase-3 activity (B) compared to MIA treatment. ***P < 0.001 when compared to control group. +++P < 0.001 when compared to MIA treatment. NS – non-significant compared to control group (One-way ANOVA followed by Tukey’s multiple comparison)
Discussion
Osteoarthritis involves abnormal function of chondrocytes with deregulation in matrix proteins and cartilage structure. Until now, reports suggest that apoptosis and initial activation of oxidative stress are important contributors. In the present study, we aimed to identify the potential role of lutein in prevention of MIA-induced osteoarthritis through studying ROS generation, the Nrf2 pathway of antioxidant mechanisms, inflammation and apoptosis.
A cellular toxicity study revealed that MIA caused significant dose-dependent cell death when compared to the control group. The MIA is an established model for osteoarthritis and it results in substantial cartilage degradation through chondrocyte apoptosis in vivo and in vitro [23, 24]. In the present study, MIA treatment resulted in significant upregulation of cellular oxidant status through ROS levels, lipid peroxide content and a concomitant decrease in antioxidant status. Free radical mediated chondrocyte cell death and calcification have been reported to involve ROS, H2O2 and nitric oxide [14, 25–27]. Since Nrf2 is a prime regulator of redox status, its role in osteoarthritis is not well explored. In the present study, lutein treatment of chondrocyte cells showed significant protection against MIA-induced oxidative stress events which could be attributed to its potential to activate Nrf2. Nrf2, nuclear factor erythroid 2-related factor 2, is an important transcription factor which regulates the redox imbalance through upregulation of ARE-responsive antioxidant enzymes. Under oxidative stress, Nrf2 functions by cytoprotective activity, but under extremes of redox conditions leads to Nrf2 downregulation, favoring changes in normal cellular processes. Along with significant upregulation of Nrf2, lutein treatment showed increased expression of downstream genes HO-1 and NQO-1. Altogether, lutein reduces oxidative stress through upregulation of Nrf2 and the cytoprotective antioxidant system. Increased antioxidant effects have been documented in vitro and in vivo [28], and protection against LPS-induced uveitis was mediated through its antioxidant property [29]. Attenuation of neuroinflammation in micro-glia cells by lutein was mediated through inhibition of NF-κB activation and activation of Nrf2 levels [30]. Lutein prevented arsenic-induced reproductive toxicity through enhancing Nrf2 levels and their target proteins HO-1, NQO1 and GST [31].
Inflammation and onset of osteoarthritis are closely linked. In addition, oxidative stress and activation of NF-κB are well established. Nuclear factor κB is sequestered in the cytoplasm, whereas under oxidative stress it translocates into the nucleus and induces various inflammatory proteins such as COX-2 and iNOS [32].The redox-sensitive transcription factor is activated under enhanced ROS levels, and in the present study there was significant upregulation of NF-κB and COX-2 levels during MIA treatment. Inflammatory activation and release of pro-inflammatory cytokines destroy the cartilage matrix; however, ROS levels further enhance the catabolic process with negative regulation in formation of cartilage structure [33, 34]. In order to achieve a balance between these 2 metabolic processes, redox status and inflammation must be effectively downregulated. In the present study, lutein treatment showed significant downregulation in inflammatory proteins (NF-κB and COX-2) and release of pro-inflammatory cytokines. Endotoxin-induced uveitis was suppressed by lutein by its anti-inflammatory effects through suppression of activation of NF-κB, NO, TNF-α, IL-6, and PGE2 expression [35]. Oxidative stress induced inflammation in gastric epithelial cells was effectively inhibited by reducing ROS levels, inhibition of NF-κB activation and IL-8 expression [36]. Lutein prevented linoleic acid induced inflammatory gene expression and protected retinal pigment epithelial cells through inhibition of NF-κB activation [37].
In conclusion, inflammatory cytokines have a prominent role in chondrocyte mitochondrial dysfunction and cartilage destruction through redox species. Mitochondria regulate apoptosis, whereas loss of membrane potential releases apoptotic factors and induces caspase activation. Caspase-3 is a key protein involved in apoptosis and executes cell death [38]. In the present study, treatment with lutein significantly regulated MIA-induced loss of membrane potential and caspase-3 activation and prevented cell death in chondrocytes. Lutein inhibited mammary tumor through inhibition of apoptosis and angiogenesis through increased expression of the pro-apoptotic genes p53 and Bax, and decreased expression of the anti-apoptotic gene Bcl-2 [39]. Lutein along with zeaxanthin and DHA increased photoreceptor survival and differentiation by reducing oxidative stress-induced apoptosis [40]. The present study shows the promising role of lutein in protection against osteoarthritis by modulation of oxidative stress and apoptosis through Nrf2 and NF-κB expression.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Busija L, Bridgett L, Williams SR, et al. Osteoarthritis. Best Pract Res Clin Rheumatol. 2010;24:757–68. doi: 10.1016/j.berh.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthr Cartilage. 1998;6:374–6. doi: 10.1053/joca.1998.0140. [DOI] [PubMed] [Google Scholar]
- 3.Blanco FJ, Lopez-Armada MJ, Maneiro E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion. 2004;4:715–28. doi: 10.1016/j.mito.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Huang R, Zhong T, Wu H. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch Med Sci. 2015;11:427–32. doi: 10.5114/aoms.2015.50975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kertmen H, Gürer B, Yilmaz ER, et al. Antioxidant and antiapoptotic effects of darbepoetin-alpha against traumatic brain injury in rats. Arch Med Sci. 2015;11:1119–28.. doi: 10.5114/aoms.2015.54869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J Am Diet Assoc. 1993;93:284–96. doi: 10.1016/0002-8223(93)91553-3. [DOI] [PubMed] [Google Scholar]
- 7.Sommerburg O, Keunen JE, Bird AC, van Kuijk FJ. Fruits and vegetables that are sources for lutein and zeaxanthin: the macular pigment in human eyes. Br J Ophthalmol. 1998;82:907–10.. doi: 10.1136/bjo.82.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JE, Leite JO, DeOgburn R, Smyth JA, Clark RM, Fernandez ML. A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J Nutr. 2011;141:1458–63. doi: 10.3945/jn.111.141630. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Na HJ, Kim CK, et al. The non-provitamin A carotenoid lutein inhibits NF-kappaB-dependent gene expression through redox-based regulation of the phosphatidylinositol 3-kinase/PTEN/Akt and NF-kappaB-inducing kinase pathways: role of H(2)O(2) in NF-kappaB activation. Free Radic Biol Med. 2008;45:885–96. doi: 10.1016/j.freeradbiomed.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Oh J, Kim JH, Park JG, et al. Radical scavenging activity-based and AP-1-targeted anti-inflammatory effects of lutein in macrophage-like and skin keratinocytic cells. Mediators Inflamm. 2013;2013:787042. doi: 10.1155/2013/787042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu ZG, Qi ZC, Liu WL, Wang WZ. Lutein protects against ischemia/reperfusion injury in rat kidneys. Mol Med Rep. 2015;11:2179–84. doi: 10.3892/mmr.2014.2982. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Kobayashi M, Itagaki S, et al. Protective effect of lutein after ischemia-reperfusion in the small intestine. Food Chem. 2011;127:893–8. doi: 10.1016/j.foodchem.2011.01.096. [DOI] [PubMed] [Google Scholar]
- 13.Sindhu ER, Firdous AP, Preethi KC, Kuttan R. Carotenoid lutein protects rats from paracetamol- carbon tetrachloride- and ethanol-induced hepatic damage. J Pharm Pharmacol. 2010;62:1054–60. doi: 10.1111/j.2042-7158.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Li L, Geng C, et al. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J Orthop Res. 2013;31:364–9. doi: 10.1002/jor.22250. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Royall JA, Ischiropoulos H. Evaluation of 27-dichlorofluorescein and dihydrorhodamine 123 a fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophy. 1993;302:348–55. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbitturic acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Oberley LW, Ying L. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 19.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:1–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 20.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 22.Siems W, Grune T. Clinical use of carotenoids. In: Grune T, editor. Free radicals and diseases: gene expression, cellular metabolism and pathophysiology. IOS Press: NATO Science Series; 2005. p. 181. [Google Scholar]
- 23.Wu WJ, Xu XX, Dai Y, Xia L. Therapeutic effect of the saponin fraction from Clematis chinensis Osbeck roots on osteoarthritis induced by monosodium iodoacetate through protecting articular cartilage. Phytother Res. 2010;24:538–46. doi: 10.1002/ptr.2977. [DOI] [PubMed] [Google Scholar]
- 24.Peters HC, Otto TJ, Enders JT, Jin W, Moed BR, Zhang Z. The protective role of the pericellular matrix in chondrocyte apoptosis. Tissue Eng Part A. 2011;17:2017–24. doi: 10.1089/ten.TEA.2010.0601. [DOI] [PubMed] [Google Scholar]
- 25.Hiran TS, Moulton PJ, Hancock JT. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic Biol Med. 1997;23:736–43. doi: 10.1016/s0891-5849(97)00054-3. [DOI] [PubMed] [Google Scholar]
- 26.Horton WE, Feng L, Adans C. Chondrocyte apoptosis in development aging and disease. Matrix Biol. 1998;17:107–15. doi: 10.1016/s0945-053x(98)90024-5. [DOI] [PubMed] [Google Scholar]
- 27.Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643–54. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Sindhu ER, Preethi KC, Kuttan R. Antioxidant activity of carotenoid lutein in vitro and in vivo. Indian J Exp Biol. 2010;48:843–8. [PubMed] [Google Scholar]
- 29.He RR, Tsoi B, Lan F, Yao N, Yao XS, Kurihara H. Antioxidant properties of lutein contribute to the protection against lipopolysaccharide-induced uveitis in mice. Chin Med. 2011;6:38. doi: 10.1186/1749-8546-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W, Li Y, Wu Y, Zhang Y, Wang Z, Liu X. Lutein suppresses inflammatory responses through Nrf2 activation and NF-kappaB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol Nutr Food Res. 2015;59:1663–73. doi: 10.1002/mnfr.201500109. [DOI] [PubMed] [Google Scholar]
- 31.Li SG, Xu SZ, Niu Q, et al. Lutein alleviates arsenic-induced reproductive toxicity in male mice via Nrf2 signaling. Hum Exp Toxicol. 2015:0960327115595682. doi: 10.1177/0960327115595682. [DOI] [PubMed] [Google Scholar]
- 32.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 33.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 34.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 35.Jin XH, Ohgami K, Shiratori K, et al. Inhibitory effects of lutein on endotoxin-induced uveitis in Lewis rats. Invest Ophthalmol Vis Sci. 2006;47:2562–8. doi: 10.1167/iovs.05-1429. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y, Seo JH, Kim H. Beta-carotene and lutein inhibit hydrogen peroxide-induced activation of NF-kappaB and IL-8 expression in gastric epithelial AGS cells. J Nutr Sci Vitaminol (Tokyo) 2011;57:216–23. doi: 10.3177/jnsv.57.216. [DOI] [PubMed] [Google Scholar]
- 37.Fang IM, Yang CH, Yang CM, Chen MS. Comparative effects of fatty acids on proinflammatory gene cyclooxygenase 2 and inducible nitric oxide synthase expression in retinal pigment epithelial cells. Mol Nutr Food Res. 2009;53:739–50. doi: 10.1002/mnfr.200800220. [DOI] [PubMed] [Google Scholar]
- 38.Orth K, O’Rourke K, Salvesen GS, Dixit VM. Molecular ordering of apoptotic mammalian CED-3/ICE-like proteases. J Biol Chem. 1996;271:20977–80. doi: 10.1074/jbc.271.35.20977. [DOI] [PubMed] [Google Scholar]
- 39.Chew BP, Brown CM, Park JS, Mixter PF. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res. 2003;23:3333–9. [PubMed] [Google Scholar]
- 40.Chucair AJ, Rotstein NP, Sangiovanni JP, During A, Chew EY, Politi LE. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:5168–77. doi: 10.1167/iovs.07-0037. [DOI] [PubMed] [Google Scholar]