Abstract
Over the last decade, several steps forward in the treatment of patients with stage IV non-small cell lung cancer (NCSLC) were made. Examples are the use of pemetrexed, pemetrexed maintenance therapy, or bevacizumab for patients with nonsquamous NSCLC. A big leap forward was the use of tyrosine kinase inhibitors in patients selected on the basis of an activating oncogene, such as epidermal growth factor receptor (EGFR) activating mutations or anaplastic lymphoma kinase (ALK) translocations. However, all of these achievements could not be translated into survival benefits when studied in randomized controlled trials in patients with nonmetastatic NSCLC. Aside from chemotherapy and targeted therapy, immunotherapy has become the third pillar in the treatment armamentarium of advanced NSCLC. Antigen-specific immunotherapy (cancer vaccination) has been disappointing in large phase III clinical trials in stages I–III NSCLC. Based on the recent breakthroughs with immune checkpoint inhibitor immunotherapy in metastatic NSCLC, much hope currently rests on the use of this approach in patients with stage I–III NSCLC as well. Here we give a brief overview of how most new therapeutic approaches for advanced NSCLC failed in other stages, and then elaborate on the role of immunotherapy in patients with stage I–III NSCLC.
Keywords: adjuvant therapy, early stage, immune checkpoint inhibitors, locally advanced stage, neoadjuvant therapy, non-small cell lung cancer, review, targeted therapy
Introduction
Non-small cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. The prospects of patients with NSCLC are highly dependent on the stage of their disease. The most recent TNM classification makes a distinction between four stages. Patients with stage I NSCLC have a tumor of limited size and no metastatic lymph nodes, while those with stage II disease have a tumor of limited size and metastatic lymph nodes in either intrapulmonary or ipsilateral hilar positions. Patients with stage III NSCLC have a tumor up to a larger size or metastatic lymph nodes in the mediastinum. Finally, those with stage IV disease have distant metastases outside of the ipsilateral lung and locoregional lymph nodes.1
Based on the potential role of surgery in the treatment, the term ‘early stage’ is often used for patients with stage I and stage II disease, and for selected patients with stage IIIA disease with feasibility of complete tumor resection. Most patients with stage III disease have an ‘intermediate stage’, will not have surgery and are typically treated with a combination of chemotherapy and radiotherapy. Patients with stage III disease in whom radical radiotherapy is not feasible because of the tumor volume in the thorax, and those with stage IV disease have ‘advanced stage’. The expected 5-year survival ranges from 92% to 36% for early stage, from 36% to 13% for unresectable stage III, and from 10% to 0% for advanced stage (Figure 1).1
Figure 1.
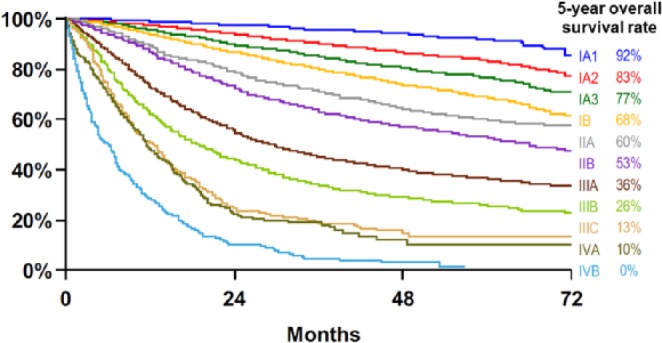
TNM clinical stages and 5-year overall survival rates of patients with non-small cell lung cancer (adapted from Goldstraw and colleagues1).
Over the last decade, major progress has been made in systemic therapies to improve the length of good-quality survival in patients with advanced NSCLC. Examples are histology-based chemotherapy, with a choice of platinum-pemetrexed2 and pemetrexed maintenance therapy,3 or the addition of bevacizumab to carboplatin-paclitaxel for non-squamous NSCLC.4 Furthermore, tyrosine kinase inhibitor (TKI) therapy has brought major progress in the treatment of patients selected with a molecular marker for their oncogene addiction. Most common examples are activating epidermal growth factor receptor (EGFR) mutations that are effectively targeted by gefitinib,5 erlotinib,6 afatinib7 or osimertinib,8 and anaplastic lymphoma kinase (ALK) translocations that can be targeted with inhibitors such as crizotinib,9 ceritinib10 or alectinib.11 Last but not least, checkpoint inhibition immunotherapy has been proven to be a major breakthrough, both in patients with pretreated12–14 and treatment-naïve advanced NSCLC.15
Most of these new therapeutic approaches for advanced NSCLC have been or are being studied in the context of nonmetastatic NSCLC, though often with disappointing results. In this contribution, we will give a brief overview of how new systemic therapies were tested in patients with stage I–III NSCLC, with emphasis on recent data and ongoing trials with immunotherapy.
Early stage NSCLC
Chemotherapy and antiangiogenic agents
In 2004, the large International Adjuvant Lung cancer Trial (IALT) randomly assigned 1867 patients with completely resected stage I–III NSCLC to three or four cycles of adjuvant cisplatin-based chemotherapy or observation.16 This positive study established postoperative cisplatin-based chemotherapy for patients with stage II or III resected NSCLC.17
The progress in patients with advanced nonsquamous NSCLC in stage IV was also assessed in the adjuvant setting. Cisplatin–pemetrexed was compared to cisplatin–vinorelbine in the phase II randomized TREAT study.18 While the tolerability and drug delivery of pemetrexed-based chemotherapy was significantly better, the 3-year follow-up report could not demonstrate a survival benefit.19 However, this phase II study was not powered for that purpose. A large phase III study on this question is ongoing (JIPANG trial UMIN000006737).
The addition of bevacizumab to standard cisplatin-based adjuvant chemotherapy was studied in the Eastern Cooperative Oncology Group (ECOG) 1505 study.20 This did not result in any benefit in overall survival (OS).
Hence, so far, no improvements have been made in standard adjuvant chemotherapy since the 2004 IALT trial. However, the results of the JIPANG trial are being awaited to learn whether adjuvant pemetrexed doublet will improve survival in resected stage II–III nonsquamous NSCLC.
Targeted agents
The efficacy of TKIs in molecularly driven stage IV NSCLC was the starting point for adjuvant trials. In 2013, the first randomized controlled trial of gefitinib versus placebo in patients with completely resected NSCLC unselected for EGFR status was published.21 There was no significant difference in OS or disease-free survival (DFS) between both arms. Instead, a detrimental effect of adjuvant TKI may even be perceived from the data published, given the (although nonsignificantly) shorter OS and DFS in the gefitinib arm, both in the EGFR wild-type and mutated subgroups. The results of the RADIANT trial came 2 years later.22 In that study, 973 patients with completely resected IB–IIIA NSCLC with expression of EGFR (by immunohistochemistry staining) or EGFR amplification (by fluorescence in situ hybridization) were randomly assigned to erlotinib or placebo for 2 years. There was no statistically significant difference in DFS. Among the 161 patients (16.5%) with a tumor having an EGFR activating mutation, DFS favored erlotinib [hazard ratio (HR) 0.61; 95% confidence interval (CI) 0.38–0.98], but this was not statistically significant because of the hierarchical testing procedure. Rash and diarrhea were common adverse events of erlotinib, of grade 3 or more in 22.3% and 6.2% of patients, respectively.
More recently, a Chinese group presented preliminary data of the phase III trial of 220 patients with resected stage II–IIIA (N1–N2) NSCLC with activating EGFR mutation.23 Patients were randomly assigned to cisplatin plus vinorelbine for four cycles or gefitinib. DFS was significantly longer with gefitinib (28.7 versus 18 months; HR 0.60; p = 0.005).
These first preliminary results in the adjuvant setting need to be handled with care, as there were several limitations of this study. First, this was not a standard ‘early stage’ study, as 64% of the patients had stage IIIA disease (without information on heterogeneity of N2: single level? multi-level? bulky? …). There was no preoperative positron emission tomography or computed tomography staging, so some patients may have had undetected stage IV disease. Probably for that reason, the DFS curves merged after 3 years at a disappointingly 3-year DFS rate of about 30% in both groups. Further follow up for OS is needed, but is unlikely to be significantly different.
Therefore, at the present time, there is no indication for adjuvant TKI therapy in early stage NSCLC.
Antigen-specific immunotherapy
The MAGRIT (MAGE-A3 as Adjuvant Non-Small Cell Lung Cancer Immunotherapy) trial is one of the largest phase III therapeutic trials in NSCLC ever performed. Melanoma-associated antigen (MAGE)-A3 was an interesting target as it is almost exclusively expressed on tumor cells and not in normal tissues (except in male germ-line cells, which do not present the antigen). The MAGE-A3 vaccine was a recombinant protein antigen-based vaccine containing the recombinant fusion protein (MAGE-A3 and protein D of Haemophilus Influenzae) in combination with an immune response enhancing adjuvant. Clear responses to this compound had been noted in early experience in patients with metastatic melanoma.24 The adjuvant setting in patients with completely resected NSCLC, for which the aim is to eliminate sparse tumor cells remaining after surgery, was judged to be the ideal setting because no progress had been made there since the implementation of adjuvant cisplatin-based chemotherapy, which is a treatment hard to tolerate for some postoperative patients.
For NSCLC, the proof of concept study was a double-blind, placebo-controlled, randomized phase II trial.25 Patients with completely resected MAGE-A3-positive stage IB-II NSCLC were randomly assigned to either placebo (n = 60) or the MAGE-A3 vaccine (n = 122) for five administrations every 3 weeks followed by eight administrations every 3 months. No adjuvant chemotherapy was given, as this therapy was not yet established in the study interval. Disease-free interval (DFI) was the primary endpoint. After a median postresection period of 70 months, there was a trend in favor of MAGE-A3, with a HR for DFI of 0.78 (95% CI 0.49–1.24). No significant toxicity was observed, resulting in very high therapy compliance. Furthermore, a possible gene signature (GS), predictive of clinical activity of the MAGE-A3 vaccine in previous metastatic melanoma experience,24 could be validated in early stage NSCLC.26
This led to the large double-blind, randomized, placebo-controlled phase III MAGRIT trial [ClinicalTrials.gov identifier: NCT00480025].27 MAGE-A3-positive patients with completely resected stage IB, II or IIIA NSCLC, and adjuvant chemotherapy as clinically indicated, were randomly assigned in a 2:1 fashion to the MAGE-A3 vaccine or to placebo. Between 18 October 2007 and 17 July 2012, a total of 13,849 surgical patients in 443 centers in 34 countries were screened for MAGE-A3 expression: 4210 had MAGE-A3 expression and 2272 were ultimately treated (active vaccine 1515; placebo 757). In the MAGE-A3 group, 784 patients also received adjuvant chemotherapy, as did 392 in the placebo group. At the time of the report, median follow up was 38.1 months in the MAGE-A3 group and 39.5 months in the placebo group. In the overall population, median DFS was 60.5 months (95% CI 57.2–not reached) in the MAGE-A3 vaccine group and 57.9 months (55.7–not reached) in the placebo group (HR 1.02, 95% CI 0.89–1.18; p = 0.74). The frequency of grade 3 or worse adverse events was similar: 246/1515 (16%) in the MAGE-A3 group and 122/757 (16%) in the placebo group. The immunological responses to the MAGE-A3 vaccine documented in previous experience were thus not translated into clinical benefits. One of the hypotheses is that the generated T cells with cancer killing properties did not travel nor enter easily in tumors, and even if they did, they encountered checkpoints that blocked their potential benefit. Further development of the MAGE-A3 vaccine for NSCLC was stopped.
In conclusion, at this moment, there is no indication for antigen-specific immunotherapy in early stage NSCLC.
Immune checkpoint inhibitor immunotherapy
This type of immunotherapy has revolutionized the approach to metastatic NSCLC, for example, in the Keynote 024 trial in patients with advanced highly PD-L1 (programmed death receptor 1 ligand) expressing (⩾50%) NSCLC. In that study, there was a significantly superior progression-free survival (PFS) with first-line pembrolizumab immunotherapy compared with standard doublet chemotherapy.15
These successes in advanced NSCLC obviously lead to the very important question of whether the outcome of early stage NSCLC can be improved by checkpoint inhibitor immunotherapy.
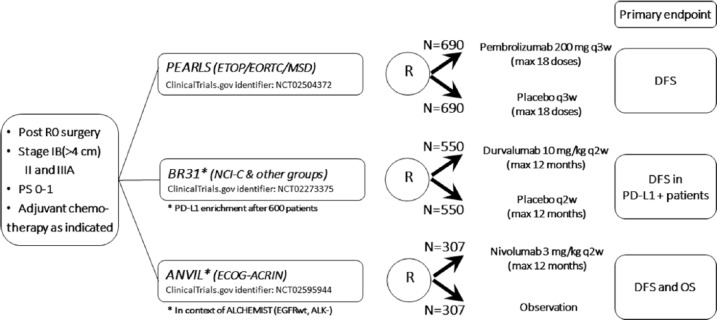
Several phase III trials with adjuvant checkpoint inhibitor immunotherapy in early stage NSCLC are ongoing (Figure 2). They all have a similar set-up. Inclusion criteria are complete resection, stage IB (>4 cm)/stage II/stage IIIA NSCLC, performance status of 0 or 1, and absence of contra-indications such as autoimmune disease. Adjuvant chemotherapy is administrated if this is part of the standard of care. The common primary end point is DFS. In the PEARLS trial, pembrolizumab is compared with placebo, in the BR31 trial durvalumab is compared with placebo, and in the ANVIL trial nivolumab is administrated without a placebo in the control arm. The therapy is given for a maximum of one year. As for all adjuvant studies, quite some years will be needed for recruitment and sufficiently long follow-up to draw sound conclusions.
Figure 2.
Ongoing phase III randomized controlled trials of adjuvant immune checkpoint inhibitor immunotherapy in patients with completely resected NSCLC.
ACRIN, American College of Radiology Imaging Network; ALK, anaplastic lymphoma kinase; DFS, disease-free survival; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; EORTC, European Organization for Research and Treatment of Cancer; ETOP, European Thoracic Oncology Platform; MSD, Merck Sharp & Dohme; NCI-C, National Cancer Institute Canada; NSCLC, non-small cell lung cancer; OS, overall survival; PD-L1, programmed death receptor 1 ligand; PS, performance status; q2w, every 2 weeks; q3w, every 3 weeks; RO, complete resection.
Another area of recent interest is immunotherapy in the neoadjuvant setting. Preclinical data based on a mouse model for triple negative breast cancer demonstrated an improved efficacy of neoadjuvant versus adjuvant immunotherapy in preventing metastatic disease using four different types of immunotherapy: T-regulatory cell depletion, anti-C25 and anti-programmed death receptor 1 (PD-1) alone or in combination with anti CD137.28 The higher antitumor response of neoadjuvant immunotherapy may be due to an increase and maintenance of tumor-specific CD8+ T cells in the blood early after immunotherapy. High levels of CD8+ T cells predicted long-term survival in this mouse model.
The first clinical data in NSCLC were presented at the 2016 meeting of the European Society for Medical Oncology (ESMO). In a feasibility study with 18 patients with untreated, resectable, stage I–IIIA NSCLC, two doses of nivolumab 3 mg/kg were given 4 and 2 weeks prior to surgery.29 This proved to be feasible and did not jeopardize the chance for surgical resection. Exploratory endpoints were most interesting: in 6 of 15 resected tumors, there was a major pathological response (i.e. <10% residual viable tumor cells, a surrogate predictor of OS), and the resection specimens showed new infiltration of CD8+ T-cell clones. Although the effects of nivolumab on tumor shrinkage are promising, the value of this approach now needs to be proven in randomized controlled trials. Several larger trials with preoperative anti-PD-1 or anti-PD-L1 checkpoint inhibitors are now being planned or launched. The results of major pathological response will be available after a short delay, but the results of the long-term efficacy will only be available after several years of follow up.
In conclusion, both adjuvant and neoadjuvant immunotherapy are intensively studied in different phases of preclinical and clinical trials in NSCLC. The preclinical results of a better efficacy of neoadjuvant over adjuvant immunotherapy need to be confirmed. At present, there are no studies with both strategies in NSCLC. In one phase II trial in patients with stage III or oligometastatic stage IV melanoma (n = 40), patients receive neoadjuvant anti-PD-1 with or without anti-CTLA-4 immunotherapy and adjuvant anti PD-1 after surgery [ClinicalTrials.gov identifier: NCT02519322]. Primary outcome parameters are pathological response of neoadjuvant nivolumab versus combination nivolumab and ipilimumab. Recruitment is finished but no results are published yet. Also the hypothesis that immunotherapy may be less effective after lymph node dissection intuitively seems to favor neoadjuvant checkpoint inhibition.
A possible concern with neoadjuvant immunotherapy may be that the immune-related toxicity in the first few cycles potentially hampers timely surgery, although this was not the case in the data presented in NSCLC.29
Unresectable stage III NSCLC
Approximately one third of patients with NSCLC present with unresectable stage III disease. The treatment of these patients remains one of the major challenges of respiratory oncology, despite gradual progress over the past decades. In the 1980s, patients with unresectable stage III NSCLC were treated with radiotherapy as a single modality, resulting in a median OS of about 10 months. In a meta-analysis of clinical trials from the 1990s, it became clear that adding cisplatin-based chemotherapy to radiotherapy improved median OS to 14 months.30 Subsequently, another meta-analysis established that the concurrent delivery of both modalities, compared with sequential delivery, further improved median OS by approximately 4 months to a total of 18 months, which corresponded to an absolute 4.5% gain in 5-year OS, leading to an expected 5-year survival of about 15%.31 Concurrent therapy achieves better local control at the price of more esophageal toxicity, but without increased pulmonary toxicity. Based on these results, the concurrent delivery of 60 Gy of chest radiation and two to four cycles of a cisplatin-based doublet chemotherapy is our current standard of care for fit patients with unresectable stage III NSCLC.17
Strategies to further increase survival focused on improvements in both local and systemic therapy, and on consolidation therapy.
Radiotherapy
For quite some time, the general belief was that the delivery of higher doses of radiotherapy, in a setting with good quality control, would improve OS without significant impact on toxicity. This was effectively studied in the phase III intergroup RTOG 0617 trial that compared standard (60 Gy) with high-dose (74 Gy) radiotherapy. Quite unexpectedly, higher doses proved to be inferior, both in terms of locoregional control and OS (28.7 versus 20.3 months).32
In conclusion, in unresectable locally advanced NSCLC, high-dose thoracic radiotherapy with 74 Gy resulted in inferior locoregional control and OS, and a dose of 60–66 Gy remains the standard of care.
Chemotherapy and targeted agents
Cisplatin-etoposide has been the doublet of choice for a long time, mainly because it can be delivered in full dose concurrently with radiotherapy. Indeed, more modern doublets with, for example, vinorelbine, docetaxel or gemcitabine need a significant dose reduction of the non-platinum agent in concurrent treatment schedules. Pemetrexed doublets, however, are one of the most effective regimens in advanced nonsquamous NSCLC,2 and were therefore studied in the setting of concurrent chemoradiotherapy as well. The PROCLAIM trial investigated whether pemetrexed doublets would be a step forward for stage III unresectable nonsquamous NSCLC.33 Unfortunately, cisplatin–pemetrexed did not significantly improve OS over cisplatin–etoposide: median OS 26.8 versus 25.0 months, respectively. However, there was a better safety profile and drug delivery, so that cisplatin–pemetrexed did become a more convenient standard chemotherapy regimen for unresectable stage III nonsquamous NSCLC.34
The combination of targeted agents and chemoradiotherapy is also a very attractive concept. However, hardly any of the agents that are successful in advanced NSCLC, such as gefinitb, erlotinib and bevacizumab, have made it to phase III trials in the setting of unresectable stage III NSCLC. The addition of the EGFR antibody cetuximab to concurrent chemoradiotherapy was studied in the above-described phase III RTOG 0617 trial. There was no significant benefit in OS (25 versus 24 months in the placebo arm), while there was a significant increase in grade 3 or 4 drug-related adverse events with cetuximab.32
In conclusion, the pemetrexed doublet showed no difference in OS, but had a better safety profile and ease of administration than the etoposide doublet. Therefore, it is currently preferred by many teams in the concurrent chemoradiotherapy for patients with locally advanced unresectable adenocarcinoma. The addition of a targeted therapy has until now not proven to be beneficial in this patient group.
Consolidation therapy with antigen-specific immunotherapy
For several years there were no major advances in the OS outcomes of patients with stage III unresectable NSCLC. As systemic maintenance therapy has been shown to improve OS in advanced NSCLC,3 several attempts to similarly consolidate the results of the concurrent treatment in stage III NSCLC have been made. Consolidation with, for instance, docetaxel chemotherapy or gefitinib targeted therapy has been assessed. However, these strategies did not improve OS rates and even tended to result in increased toxicity.35,36 Hence, there was great hope that novel immunotherapeutic approaches might address this unmet need.
As for antigen-specific immunotherapy or therapeutic cancer vaccination, the phase III placebo-controlled, randomized START trial assessed the tumor vaccine tecemotide – with the MUC1 protein as antigen target – following concurrent or sequential chemoradiotherapy for stage III NSCLC.37 No significant difference in median OS was observed between patients who received tecemotide and those who received placebo (25.6 versus 22.3 months). The authors did identify a favorable effect of the tecemotide vaccine in the predefined large (n = 806) subgroup of patients initially treated with concurrent chemoradiotherapy, with a remarkable 10.2-month improvement in median OS (30.8 versus 20.8 months in the placebo group). In contrast, patients who had previously been treated with sequential chemoradiotherapy did not obtain clinical benefit. Importantly, tecemotide was very well tolerated. However, a follow-up study (EMR 63325-009 phase I/II study of tecemotide versus placebo in Japanese patients with stage III NSCLC) could not support the initial promising results and hence the sponsor decided to stop the development of the drug.
In conclusion, consolidation therapy with antigen-specific immunotherapy initially showed a marked improvement in median OS in the concurrently treated subgroup of the START trial, but the results were not confirmed in later trials. At the moment there is no antigen-specific immunotherapy indicated in patients with locally advanced unresectable NSCLC.
Consolidation therapy with immune checkpoint inhibitor immunotherapy
The main hope then shifted towards the success of immune checkpoint blockade in advanced NSCLC, and its possible translation into stage III NSCLC. Preclinical and early clinical data indeed suggest a synergistic effect of PD-1/PD-L1 blockade and radiotherapy. Radiotherapy has been shown to activate key elements of the immune system that may help to turn the immunosuppressive environment of NSCLC into an inflamed and immunoreactive environment.38–40 Radiotherapy helps antigen presentation by upregulating major histocompatibility complex class I molecules and tumor-associated antigens. Furthermore, radiotherapy provokes immunogenic cell death, activates dendritic cells, promotes chemokines, and lowers the activity of immunosuppressive regulatory T cells. Thereby, the ‘immune effects’ of radiotherapy not only improve local tumor control, but also promote a systemic antitumor immune action, the so-called ‘abscopal’ effect on distant metastases.41 Moreover, PD-1 and PD-L1 are upregulated by radiotherapy, which makes the combination with checkpoint inhibitors even more attractive.
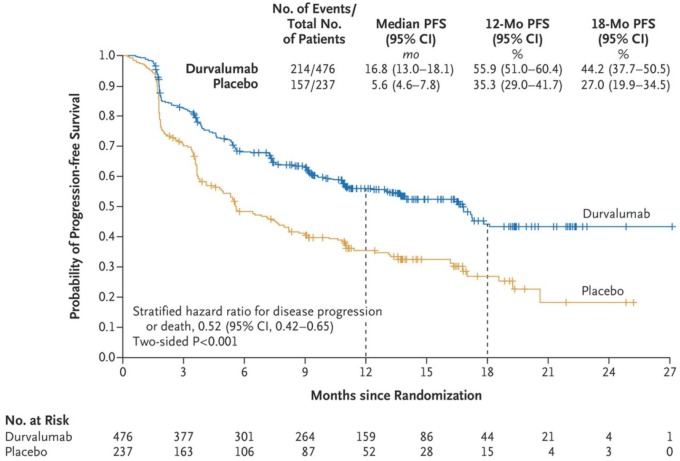
Hence, many clinical trials assessing this new concept in NSCLC are ongoing. At the 2017 meeting of the ESMO, interim results of the phase III randomized, double-blind, placebo-controlled PACIFIC trial were reported and simultaneously publihed.42 PACIFIC evaluated consolidation therapy with durvalumab in patients with disease control after chemoradiotherapy for stage III unresectable NSCLC. Durvalumab is a monoclonal antibody that blocks PD-L1 binding to PD-1 and CD80, thereby enhancing effector T-cell function and tumor cell killing. In the study, fit patients without progression after at least two cycles of platinum-based chemotherapy concurrent with radiotherapy (54–66 Gy) were randomized in a 2:1 fashion to durvalumab (10 mg/kg, n = 476) versus placebo (n = 237) every 2 weeks for up to 12 months. Coprimary endpoints were PFS (assessed by blinded independent central review) and OS. The first preplanned interim PFS analysis, after a median follow up of 14.5 months, showed a highly significant difference in median PFS in favor of consolidation therapy with durvalumab, with a clinically relevant improvement in PFS from 16.8 versus 5.6 months from randomization (HR 0.52) (Figure 3). Interestingly, benefit with durvalumab was observed in all prespecified subgroups, including in never smokers, in squamous and nonsquamous NSCLC, and irrespective of PD-L1 status. Time to death or distant metastasis was also significantly longer in the durvalumab arm: 23.2 versus 14.6 months from randomization (HR 0.52).
Figure 3.
Progression-free survival (PFS) in the intention-to-treat population of the two arms in the PACIFIC study (reprinted with permission from Antonia and colleagues42). CI, confidence interval
At the start of the trial there had been much concern that the concurrence of radiation pneumonitis and immunotherapy-induced lung damage might result in unacceptable pulmonary toxicity. But the safety profile turned out to be quite manageable. Treatment-related adverse events occurred in 67.8% of cases in the durvalumab arm and in 53.4% of cases in the placebo arm, respectively, resulting in treatment discontinuation in 15.4% versus 9.8% of cases. As expected, immune-mediated adverse advents occurred more frequently in the durvalumab arm (24.2% versus 8.1%), however, with only a minor difference in grade 3 or 4 events between both treatment arms (3.4% versus 2.6%). Above all, symptoms such as cough or pneumonitis were common adverse events, but this was true for both the durvalumab and the placebo arm. Any grade pneumonitis was reported in 33.9% of cases in the durvalumab arm and 24.8% of cases in the placebo arm. However, the incidence of grade 3 or 4 pneumonitis did not significantly differ between both treatment arms (3.4% versus 2.6%). Hence, overall, there was a slight increase in toxic effects in the durvalumab group, but the rates of severe immune-related adverse events, and of pneumonitis in particular, were not significantly different. Mature OS data are now eagerly awaited. However, the clinically meaningful difference in PFS and the manageable safety profile now already support a role for durvalumab as part of standard therapy for patients with stage III unresectable NSCLC. In addition, the positive results of the PACIFIC trial bring, for the first time in nearly two decades, hope for a new treatment option and improved survival for unresectable stage III NSCLC.
The timing and duration of immune checkpoint blockade require further investigation. At least five other phase II and III trials with nivolumab, pembrolizumab and atezolizumab are ongoing in this setting (Table 1). One of these trials, the phase II NICOLAS trial of the European Thoracic Oncology Platform (ETOP), incorporates immunotherapy (nivolumab) from the start of radiotherapy (i.e. concurrently), with a flat dose of 360 mg every 3 weeks for the first four doses and then followed by a flat dose of 480 mg every 4 weeks up to 1 year from the start of immunotherapy. The primary endpoint of this feasibility trial is grade 3 or higher pneumonitis observed any time during 6 months from the end of radiotherapy, while secondary endpoints include PFS and OS. This trial will bring the first data on concurrent use of radiotherapy and immunotherapy in stage III NSCLC.
Table 1.
Reported (PACIFIC) or ongoing trials with immune checkpoint inhibitor immunotherapy in patients with unresectable stage III non-small cell lung cancer (NSCLC).
| Agent | Phase | Number (n) | Primary endpoints | Immunotherapy timing | Register | Sponsor | Dosage |
|---|---|---|---|---|---|---|---|
| Durvalumab | III | 713 | OS/PFS | 1–42 days after CRT | PACIFIC [ClinicalTrials.gov identifier: NCT 02125461] |
AstraZeneca | 10 mg/kg IV every 2 weeks for 12 months |
| Nivolumab | III | 660* | OS/PFS | 4–12 weeks after CRT | RTOG 3505 [ClinicalTrials.gov identifier: NCT 02768558] |
RTOG | 240 mg IV every 2 weeks for 12 months |
| Pembrolizumab | II | 93 | OS/PFS | 4–7 weeks after CRT | [ClinicalTrials.gov identifier: NCT 02343952] | Hoosier Group | 200 mg IV every 3 weeks for 12 months |
| Pembrolizumab | I | 30$ | Safety | G1: 2–6 weeks after CRT G2: 2 weeks before the end of CRT G3: at start of CRT |
[ClinicalTrials.gov identifier: NCT 02621398] | Rutgers | 200 mg IV every 3 weeks for 54 weeks |
| Atezolizumab | II | 40$ | Safety/timing | 4 weeks after CRT (one group receives one dose of atezolizumab in this interval) |
[ClinicalTrials.gov identifier: NCT 02525757] | MD Anderson | 1200 mg IV every 3 weeks, twice concurrent with two additional cycles of chemotherapy, then atezolizumab alone up to 12 months |
| Nivolumab | II | 78$ | Safety | Concurrent from start of RT | NICOLAS [EudraCT 2014-005097-11] |
ETOP | 360 mg IV every 3 weeks for four cycles then 480 mg IV every 4 weeks, total 12 months |
Trial currently on hold with 13 patients included.
Trial still recruiting patients.
CRT, concurrent chemoradiotherapy; ETOP, European Thoracic Oncology platform; G1, group/cohort 1; G2, group/cohort 2; G3, group/cohort 3; IV, intravenous; OS, overall survival; PFS, progression-free survival; RT, radiotherapy; RTOG, Radiation Therapy Oncology Group.
Conclusion
Many of the steps forward in the treatment of patients with stage IV NCSLC led to disappointing results in randomized controlled trials in the curative setting of nonmetastatic NSCLC. The recent breakthroughs with immune checkpoint inhibitor immunotherapy in metastatic NSCLC, however, seem to be the way forward, to start with durvalumab after concurrent chemoradiotherapy in patients with unresectable stage III NSCLC. Until now, in this patient group, only 15–20% are alive 5 years after chemoradiotherapy. This percentage has not been increased by use of radiotherapy dose escalation or consolidation with chemotherapy, targeted agents or vaccination. Importantly, some of these approaches appeared to even worsen outcome and should therefore not be applied in clinical practice, at least not outside of the context of a clinical trial. We have only recently observed initial exciting progress with durvalumab consolidation therapy after concurrent chemoradiotherapy with a clinically meaningful increase in PFS of almost 1 year. On the basis of the interim analysis of the PACIFIC trial, the US Food and Drug Administration approved durvalumab (Imfinzi, AstraZeneca UK Limited, 1 Francis Crick Ave, Cambridge, England CB2 0AA) on 16 February 2018 as consolidation therapy for patients without progression after concurrent chemoradiotherapy and irrespective of PD-L1 status. Durvalumab is administered intravenously every 2 weeks in a dose of 10 mg/kg until disease progression or for a maximum of 12 months. The approval process with the European Medicines Agency is ongoing. In addition to the final survival data of the PACIFIC trial, more data are needed on the ideal timing of immunotherapy in relation to chemo- and radiotherapy delivery and, importantly, also on patient selection. Indeed, given the potential curability of concurrent chemoradiotherapy alone and given the cost of checkpoint inhibitor immunotherapy, there is a high need for biomarker discovery to predict who will need and who will benefit from consolidative immunotherapy. Finally, ongoing trials are further defining the role of immune checkpoint inhibitor immunotherapy in earlier stages of NSCLC as well. Especially in the neoadjuvant setting some exciting data have been observed and may have the potential to further change clinical practice in the setting of nonmetastatic NSCLC.
Acknowledgments
GD and DG contributed equally to the manuscript.
Footnotes
Funding: This is an academic manuscript written without any external support or writing assistance.
Conflict of interest statement: JV: advisory functions with Apotex, AstraZeneca, Boehringer Ingelheim, MSD and Novartis.
Contributor Information
Griet Deslypere, Respiratory Oncology Unit, Department of Respiratory Medicine, University Hospitals KU Leuven, Leuven, Belgium.
Dorothée Gullentops, Respiratory Oncology Unit, Department of Respiratory Medicine, University Hospitals KU Leuven, Leuven, Belgium.
Els Wauters, Respiratory Oncology Unit, Department of Respiratory Medicine, University Hospitals KU Leuven, Leuven, Belgium.
Johan Vansteenkiste, Respiratory Oncology Unit, Department of Respiratory Medicine, University Hospitals KU Leuven, Herestraat 49, B-3000 Leuven, Belgium.
References
- 1. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 2. Scagliotti G, Parikh P, Von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced stage non-small cell lung cancer. J Clin Oncol 2008; 26: 3543–3551. [DOI] [PubMed] [Google Scholar]
- 3. Paz-Ares L, De Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with cisplatin plus pemetrexed for advanced non-squamous non-small cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012; 13: 247–255. [DOI] [PubMed] [Google Scholar]
- 4. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med 2006; 355: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 5. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957. [DOI] [PubMed] [Google Scholar]
- 6. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–246. [DOI] [PubMed] [Google Scholar]
- 7. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–3334. [DOI] [PubMed] [Google Scholar]
- 8. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 9. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371: 2167–2177. [DOI] [PubMed] [Google Scholar]
- 10. Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017; 389: 917–929. [DOI] [PubMed] [Google Scholar]
- 11. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in untreated ALK-positive non-small cell lung cancer. N Engl J Med 2017; 377: 829–838. [DOI] [PubMed] [Google Scholar]
- 12. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 14. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 16. The International Adjuvant Lung Cancer Trial Collaborative Group, Arriagada R, Bergman B, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small cell lung cancer. N Engl J Med 2004; 350: 351–360. [DOI] [PubMed] [Google Scholar]
- 17. Vansteenkiste J, De Ruysscher D, Eberhardt W, et al. Early and locally advanced non-small cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl. 6): vi89–vi98. [DOI] [PubMed] [Google Scholar]
- 18. Kreuter M, Vansteenkiste JF, Fischer JR, et al. Randomized phase 2 trial on refinement of early stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: The TREAT study. Ann Oncol 2013; 24: 986–992. [DOI] [PubMed] [Google Scholar]
- 19. Kreuter M, Vansteenkiste J, Fischer JR, et al. Three-year follow-up of a randomized phase II trial on refinement of early stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine (the TREAT study). J Thorac Oncol 2016; 11: 85–93. [DOI] [PubMed] [Google Scholar]
- 20. Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2017; 18: 1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goss GD, O‘Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013; 31: 3320–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol 2015; 33: 4007–4014. [DOI] [PubMed] [Google Scholar]
- 23. Wu YL, Zhong W, Wang Q, et al. Gefitinib (G) versus vinorelbine+cisplatin (VP) as adjuvant treatment in stage II-IIIA (N1-N2) non-small cell lung cancer (NSCLC) with EGFR-activating mutation (ADJUVANT): a randomized, Phase III trial (CTONG 1104). J Clin Oncol 2017; 35(Suppl.): Abstract 8500. [Google Scholar]
- 24. Kruit WH, Suciu S, Dreno B, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer melanoma group in metastatic melanoma. J Clin Oncol 2013; 31: 2413–2420. [DOI] [PubMed] [Google Scholar]
- 25. Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small cell lung cancer: phase II randomized study results. J Clin Oncol 2013; 31: 2396–403. [DOI] [PubMed] [Google Scholar]
- 26. Ulloa-Montoya F, Louahed J, Dizier B, et al. Predictive gene signature in MAGEA3 antigen-specific cancer immunotherapy. J Clin Oncol 2013; 31: 2388–2395. [DOI] [PubMed] [Google Scholar]
- 27. Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016; 17: 822–35. [DOI] [PubMed] [Google Scholar]
- 28. Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016; 6: 1382–1399. [DOI] [PubMed] [Google Scholar]
- 29. Forde PM, Smith KN, Chaft JE, et al. Neoadjuvant anti-PD1, nivolumab, in early stage resectable non-small cell lung cancer. Ann Oncol 2017; 27(Suppl. 6): LBA 41. [Google Scholar]
- 30. Auperin A, Le Pechoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006; 17: 473–483. [DOI] [PubMed] [Google Scholar]
- 31. Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small cell lung cancer. J Clin Oncol 2010; 28: 2181–2190. [DOI] [PubMed] [Google Scholar]
- 32. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16: 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Senan S, Brade A, Wang L, et al. PROCLAIM: a randomized, phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced non-squamous non-small cell lung cancer. J Clin Oncol 2016; 34: 953–962. [DOI] [PubMed] [Google Scholar]
- 34. Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28(Suppl. 4): iv1–iv21. [DOI] [PubMed] [Google Scholar]
- 35. Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008; 26: 5755–5760. [DOI] [PubMed] [Google Scholar]
- 36. Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small cell lung cancer: SWOG S0023. J Clin Oncol 2008; 26: 2450–2456. [DOI] [PubMed] [Google Scholar]
- 37. Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 59–68. [DOI] [PubMed] [Google Scholar]
- 38. Weichselbaum RR, Liang H, Deng L, et al. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol 2017; 14: 365–379. [DOI] [PubMed] [Google Scholar]
- 39. Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16: e498–e509. [DOI] [PubMed] [Google Scholar]
- 40. De Ruysscher D, Reynders K, Van Limbergen E, et al. Radiotherapy in combination with immune checkpoint inhibitors. Curr Opin Oncol 2017; 29: 105–111. [DOI] [PubMed] [Google Scholar]
- 41. Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015; 41: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. N Engl J Med 2017; 377: 1919–1929. [DOI] [PubMed] [Google Scholar]