Abstract
Three-dimensional printing technology is getting more attention recently, especially in the craniofacial region. This is a review of literature enlightening the materials that have been used to date and the application of such technology within the scope of maxillofacial surgery.
Keywords: 3D printing, facial reconstruction, maxillofacial surgery, surgical education
Three-dimensional (3D) printing technology, also referred as additive manufacturing or rapid prototyping or solid-freeform technology, was first demonstrated in 1986,1 since then this innovative technique has attracted significant attention, especially within the head and neck surgical specialities; maxillofacial, otorhinolaryngology and plastic surgery, owning to its incredible ability to create complex constructs with high precision.2 A systematic review stated that publications produced using such technology in the craniofacial region has accounted for the second highest percentage compared to other fields.3 Reconstruction, rehabilitation and regeneration have been the main areas benefitting from research projects using this technology, as it potentially offers reproducible, precise and durable patient-specific models for different surgical application; moreover, this was extended also to include teaching and education.4
Application of 3D printing in facial reconstructive surgery
The surgical application of such technology can be mainly focussed on four different aspects:
Obtaining highly accurate anatomic prototype models to ease preoperative planning and improve postoperative facial contour symmetry, for example, reconstruction of the mandible, the maxilla and the orbits.4,5 This may help clinicians to inspect anatomy preoperatively, practice different techniques and consequently reduce the operating time and minimise errors.6
Virtually planning and printing pre-contoured grafts and plates to improve surgical outcomes and reduce operating time.7,8
Offering high-accuracy prostheses that can enhance the aesthetic and psychological status of patients suffering from significant scarring, deformation and asymmetry.9,10
Proposing cutting-edge simulation models to enhance surgical education at both undergraduate and postgraduate level.11
The concept of 3D printing
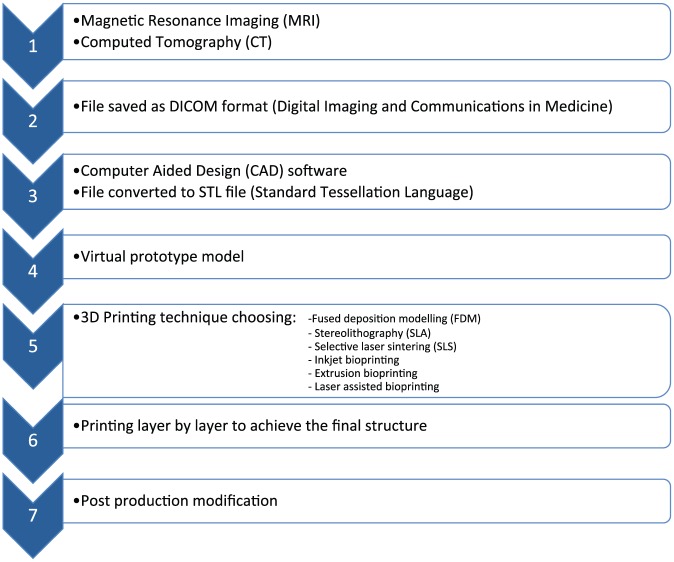
The concept behind 3D printing in the medical field is to capture anatomical scans using imaging techniques such as magnetic resonance imaging (MRI) and computed tomography (CT) scans. The image from these modalities will be saved in a standard format such as Digital Imaging and Communications in Medicine (DICOM) format and later with the help of computer-aided design (CAD) software will create a virtual 3D prototype with Standard Tessellation Language (STL) format to allow 3D printing and deposition of the material layer by layer to achieve the final structure. Depending on the application, appropriate printing technique is selected, for example, fused deposition modelling (FDM), stereolithography (SLA), selective laser sintering (SLS), inkjet bioprinting, extrusion bioprinting and laser-assisted bioprinting. Finally, the printed object will go through a post-printing modification to obtain the final printed object.12–14
Materials of choice for 3D printing
Although autogenous graft is still considered to be the gold standard for bone grafting materials as it benefits from having osteoinductive, osteoconductive and osteogenic properties,15 it has some disadvantages, such as donor-site morbidity, availability in limited quantities, chronic postoperative pain, resorption unpredictability, risk of wound infection, increased blood loss and prolonged anaesthesia time.16 This indeed can be added to its lack of delivering the ideal geometry. Consequently, tissue engineering has arisen as a potential tool combining material science, principles of engineering and biology to restore, replace or improve biological function (Figure 1).17
Figure 1.
The flowchart of 3D printing process.
Among the many degradable polymers that have been investigated for maxillofacial defect repair, poly(glycolic acid) (PGA), polylactic acid (PLA) and copolymer (PLGA) were used broadly in the clinical environment.18 Solid PLGA has osteoconductive properties in vivo, and the end product can be cleared by metabolic processes. Although, it has been noted, when large PLGA prosthetics undergo mechanical strain, a rapid decrease in molecular weight and loss of strength will lead to bulk degradation, releasing high levels of lactic acid and glycolic acid resulting in pH drop and tissue loss.19 Another polymer that has been widely investigated for craniofacial reconstruction is poly(ε-caprolactone) (PCL), which offers good biocompatibility and mechanical properties. A recent study investigated the combination of a 3D-printed polycaprolactone scaffold and dual spatiotemporal growth factor delivery for regeneration of the temporomandibular joint articular disc.20 Although the mechanical properties of the scaffold were in the range of the native tissue, introduction of a harder polymer matrix in a soft cartilaginous tissue carries the risk of adjacent articular surface damage due to stress shielding.
Mechanical properties of cement containing poly (propylene fumarate) (PPF) polymer was investigated in a study by Lalwani et al.21 for the purpose of mandibular reconstruction; cross-linked microparticles were found to significantly improve the compressive modulus and reduce the temperature increase on cross-linking. This was consistent with a previous clinical study by Trantolo et al.,22 which establishes a potential for future use of this bone cement in the repair of maxillofacial fractures (other biochemical properties of this composite are yet to be assessed).
In another study, the osteogenic potential of mesenchymal stem cells on a polyamide/hydroxyapatite scaffold was investigated. This composite showed excellent biocompatibility and cell attachment, but the mechanical stiffness was limited.23
Another case report has shown the translational potential of 3D-printed scaffolds for patient-specific applications in the craniofacial area.24 Electron beam melting was used to construct an anatomically precise mandible made of titanium. This was then implanted into a patient who had undergone severe osteotomy due to resection of a squamous cell carcinoma; 9-month follow-up demonstrated satisfactory aesthetic and implant stability outcomes with optimum osseointegration with the titanium 3D-printed implant. In a proof of concept study, the potential use of 3D-printed bioceramic implants have been investigated for craniofacial reconstruction. In this research, the customised scaffolds were 3D printed from a bioceramic powder bed leading to formation of a brushite/monetite resorbable implant. This was further examined by placing the scaffold in a 3D-printed human skull model where the fixation of the bioceramic implant was achieved by using titanium screws and plates.25 Although this is a promising proof of concept, the biomechanical viability of the implant was not assessed; hence, the application of bioceramics in a load-bearing area still requires further testing (Table 1).
Table 1.
Different 3D-printed materials with their main advantages and disadvantages.
| Materials of choice for 3D printing | |||
|---|---|---|---|
| Degradable | Non-degradable | ||
| Natural (collagen, alginate, chitosan) | Ad: high biocompatibility, similar morphology to the extracellular matrix and hydrophilicity | Metals (titanium) | Ad: optimum mechanical properties, biocompatibility, corrosion resistance and satisfactory osseointegration |
| Dis: lack of mechanical strength | Dis: release of trace of material over time, immunological response, mechanical irritation from underlying fixation devices and infection | ||
| Synthetic (PLGA) | Ad: osteoconductive properties | Polymers (poly (methyl methacrylate)) | Ad: protective, defect-filling replacement that lacks postoperative inflammation |
| Dis: creating a strong acidic environment upon degradation | Dis: highly exothermic polymerisation, prone to infection and lacks osseointegration | ||
| Synthetic (PCL) | Ad: optimum mechanical properties and biocompatibility | Ceramics (calcium phosphates) | Ad: high osteoconductivity |
| Dis: Slow degradation | Dis: Brittleness | ||
3D: three-dimensional; PLGA: copolymer; PCL: poly(ε-caprolactone); Ad: advantage; Dis: disadvantage.
Current limitations
Despite the potential cost limitation, the price of 3D technology is continuing to be driven down in terms of the price of devices, materials and software.3 It would be more objective, however, to evaluate this using some cost-efficiency methods. For example, from an educational perspective, more 3D-printed educational models are becoming more available. The actual cost of producing them should be compared to the cost of obtaining and storing their human tissue substitutes. Once the 3D printer and its software is installed, the cost of these models might vary depending on the material used. The accuracy of these models, however, is still a challenge to completely alternate human tissue and this yet to be an ongoing concern.9
In terms of its surgical application, there is a significant need to design randomised clinical trials to prove the superiority of adopting 3D planning over the classical surgical approaches. The articles published to date are more focussed on clinical case reports with only a few small sample trials performed.26 Another possible limitation is the time to produce a 3D-printed model.1 This includes the time required to capture anatomical scans, create a virtual 3D prototype, 3D print of the material layer by layer and finally modify the final structure. This can also vary between 1 and 24 h, depending on the size of the object and the resolution of the printing.
Although the aforementioned limitations are yet to be overcome, many of them can be addressed via formulating suitable materials with desirable mechanical and biological properties that can be printed directly without the need of obtaining a mould first. This indeed will reduce both the printing time and cost.
Future application
In summary, the utilisation of additive manufacturing in the oral and maxillofacial area has significant promise and can extend way beyond the production of custom-fit implants, as it can be used for surgical planning and training. There is a view that there is a need to work towards increasingly higher resolution printing and this should obviously be achieved without sacrificing the strength, handleability and shape of the final construct, but this should be assessed in conjunction with surgeons about whether there is an actual need for very-high-resolution printing, particularly for custom-fit implants. The production of implants at high resolution significantly increases the print time. It may be that the surfaces in contact with bone need to fit closely to ensure new bone growth, but for other surfaces, in contact with soft tissue, then a lower resolution may suffice. Perhaps one of the most pressing needs is the development of materials that are suitable for printing and that have appropriate mechanical and biological properties. While there is significant work being undertaken to print materials such as hydrogels, these are not appropriate for hard-tissue augmentation due to the lack of mechanical properties.
Therefore, there is a need for a novel, biocompatible and mechanically strong bioink that can easily be adapted to fit specific needs such as flexibility, stiffness and surface energy by small variations in the manufacturing process. A 3D scaffold can be developed subsequently by using direct 3D printing technology, which will allow fabrication of complex bone grafts with precise control over the internal channel networks of the scaffold, necessary for cell proliferation and eventually bone ingrowth. This will address the drawbacks of current techniques by maintaining the shape and location of the graft material during the consolidation phase. It will not require an additional surgery to remove the material and finally can be shaped into different configurations to follow the unique contour of craniofacial defects.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Alaa Aldaadaa  https://orcid.org/0000-0002-9841-3406
https://orcid.org/0000-0002-9841-3406
References
- 1. Zhong N, Zhao X. 3D printing for clinical application in otorhinolaryngology. Eur Arch Otorhinolaryngol 2017; 274(12): 4079–4089. [DOI] [PubMed] [Google Scholar]
- 2. Choi JW, Kim N. Erratum: clinical application of three-dimensional printing technology in craniofacial plastic surgery. Arch Plast Surg 2015; 42(4): 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tack P, Victor J, Gemmel P, et al. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016; 15(1): 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marconi S, Pugliese L, Botti M, et al. Value of 3D printing for the comprehension of surgical anatomy. Surg Endosc 2017; 31(10): 4102–4110. [DOI] [PubMed] [Google Scholar]
- 5. Azuma M, Yanagawa T, Ishibashi-Kanno N, et al. Mandibular reconstruction using plates prebent to fit rapid prototyping 3-dimensional printing models ameliorates contour deformity. Head Face Med 2014; 10: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel A, Levine J, Brecht L, et al. Digital technologies in mandibular pathology and reconstruction. Atlas Oral Maxillofac Surg Clin North Am 2012; 20(1): 95–106. [DOI] [PubMed] [Google Scholar]
- 7. Sieira Gil R, Roig AM, Obispo CA, et al. Surgical planning and microvascular reconstruction of the mandible with a fibular flap using computer-aided design, rapid prototype modelling, and precontoured titanium reconstruction plates: a prospective study. Br J Oral Maxillofac Surg 2015; 53(1): 49–53. [DOI] [PubMed] [Google Scholar]
- 8. Zimmerer RM, Ellis E, 3rd, Aniceto GS, et al. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J Craniomaxillofac Surg 2016; 44(9): 1485–1497. [DOI] [PubMed] [Google Scholar]
- 9. Crafts TD, Ellsperman SE, Wannemuehler TJ, et al. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg 2017; 156(6): 999–1010. [DOI] [PubMed] [Google Scholar]
- 10. Ledgerwood LG, Chao J, Tollefson TT. Prosthetic reconstruction of complicated auricular defects: use of a hybrid prosthetic fabrication technique. JAMA Facial Plast Surg 2014; 16(2): 153–154. [DOI] [PubMed] [Google Scholar]
- 11. Lichtenstein JT, Zeller AN, Lemound J, et al. 3D-printed simulation device for orbital surgery. J Surg Educ 2017; 74(1): 2–8. [DOI] [PubMed] [Google Scholar]
- 12. Marro A, Bandukwala T, Mak W. Three-dimensional printing and medical imaging: a review of the methods and applications. Curr Probl Diagn Radiol 2016; 45(1): 2–9. [DOI] [PubMed] [Google Scholar]
- 13. Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010; 5(4): 335–341. [DOI] [PubMed] [Google Scholar]
- 14. Frame M, Huntley JS. Rapid prototyping in orthopaedic surgery: a user’s guide. Sci World J 2012; 2012: 838575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farre-Guasch E, Wolff J, Helder MN, et al. Application of additive manufacturing in oral and maxillofacial surgery. J Oral Maxillofac Surg 2015; 73(12): 2408–2418. [DOI] [PubMed] [Google Scholar]
- 16. Sharif F, Ur Rehman I, Muhammad N, et al. Dental materials for cleft palate repair. Mater Sci Eng C Mater Biol Appl 2016; 61: 1018–1028. [DOI] [PubMed] [Google Scholar]
- 17. Lanza RP, Langer RS, Vacanti J. Principles of tissue engineering. 2011. [Google Scholar]
- 18. Whitcombe MJ, Kirsch N, Nicholls IA. Molecular imprinting science and technology: a survey of the literature for the years 2004-2011. J Mol Recognit 2014; 27(6): 297–401. [DOI] [PubMed] [Google Scholar]
- 19. Xie XH, Wang XL, Zhang G, et al. Biofabrication of a PLGA-TCP-based porous bioactive bone substitute with sustained release of icaritin. J Tissue Eng Regen Med 2015; 9(8): 961–972. [DOI] [PubMed] [Google Scholar]
- 20. Legemate K, Tarafder S, Jun Y, et al. Engineering human TMJ discs with protein-releasing 3D-printed scaffolds. J Dent Res 2016; 95(7): 800–807. [DOI] [PubMed] [Google Scholar]
- 21. Lalwani G, Henslee AM, Farshid B, et al. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules 2013; 14(3): 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trantolo DJ, Sonis ST, Thompson BM, et al. Evaluation of a porous, biodegradable biopolymer scaffold for mandibular reconstruction. Int J Oral Maxillofac Implants 2003; 18(2): 182–188. [PubMed] [Google Scholar]
- 23. Wang H, Li Y, Zuo Y, et al. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007; 28(22): 3338–3348. [DOI] [PubMed] [Google Scholar]
- 24. Suska F, Kjeller G, Tarnow P, et al. Electron beam melting manufacturing technology for individually manufactured jaw prosthesis: a case report. J Oral Maxillofac Surg 2016; 74(8): 1706.e1–1706.e15. [DOI] [PubMed] [Google Scholar]
- 25. Klammert U, Gbureck U, Vorndran E, et al. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Craniomaxillofac Surg 2010; 38(8): 565–570. [DOI] [PubMed] [Google Scholar]
- 26. Pedersen TH, Gysin J, Wegmann A, et al. A randomised, controlled trial evaluating a low cost, 3D-printed bronchoscopy simulator. Anaesthesia 2017; 72(8): 1005–1009. [DOI] [PubMed] [Google Scholar]