Abstract
This study aimed to investigate the clinical response and short-term survival and further explore the comprehensive factors for predicting clinical outcomes in patients with liver cancer treated by drug-eluting beads transarterial chemoembolization . Forty-nine patients with liver cancer who received drug-eluting beads transarterial chemoembolization treatment were consecutively enrolled in this cohort study. Demographic features, medical histories, clinicopathological properties, biochemical indexes, previous treatments, and chemoembolization reagents were recorded. Ten (20.4%) patients achieved complete response and 31 (63.3%) patients achieved partial response after drug-eluting beads transarterial chemoembolization treatment, with overall response rate of 83.7%. Logistic analysis revealed that high aspartate aminotransferase (P = .041), high carbohydrate antigen 199 (P = .030), and low hemoglobin (P = .020) could independently predict less possibility for complete response achievement. As to survival analysis, high alkaline phosphatase (P = .040), low albumin (P = .033) low hemoglobin (P = .018), portal vein invasion (P = .025), higher Eastern Cooperative Oncology Group performance status (P = .011), and higher Child-pugh stage (P = .001) were independent predictors for worse overall survival. In conclusion, the present study validated that drug-eluting beads transarterial chemoembolization was effective and well tolerated for patients with liver cancer, and high aspartate aminotransferase, high alkaline phosphatase, low albumin, low hemoglobin, portal vein invasion, higher Child-pugh stage, higher Barcelona Clinic Liver Cancer stage, higher Eastern Cooperative Oncology Group performance status were correlated with worse outcomes.
Keywords: drug-eluting beads transarterial chemoembolization (DEB-TACE), clinical response, overall survival, predictive factors, liver cancer
Introduction
Liver cancer, as one of the malignant tumors and the second leading cause of cancer deaths in men of less developed countries, is a crucial threat imperiling human health worldwide.1,2 The 2015 Global cancer statistics report discloses that roughly 0.78 million people were diagnosed with liver cancer contributing to 6% of new patients with cancer over the world in 2012, and approximately 0.75 million patients died from liver cancer accounting for 9% deaths in all cancers, among which half of the new liver cancers and deaths derives from China.2 Despite the great improvements in early diagnosis, targeted therapies, immune therapies, personalized treatments as well as integrated patients’ care, the prognosis of both hepatocellular carcinoma (HCC, 70%-90% of all liver cancers) and intrahepatic cholangiocarcinoma (ICC), as 2 main categories of liver cancers, is still far more from satisfaction.3-7
Transarterial chemoembolization (TACE), as a first-line treatment for HCC in intermediate stage that was recommended by Barcelona Clinic Liver Cancer (BCLC) tumor staging and management, is recently widely utilized as a common modality in patients with HCC, especially for patients unsuitable to receive surgery and/or ablation.8-10 Due to severe cytotoxic effect combined with ischemia, lack of calibrated operative techniques and heterogeneities according to chemotherapeutic agents, treatment devices, and schedule, conventional TACE (cTACE) is gradually replaced by drug-eluting beads (DEBs)-TACE gradually in clinical practice, which better standardizes the procedure and improves the delivering capacity of higher dose of chemotherapy agents.11-13 Multiple previous studies have explored the predictive factors for clinical response or long-term survival in patients treated with cTACE.14-17 However, few studies evaluating prognostic biomarkers for DEB-TACE treatment have been carried out. Therefore, this study aimed to investigate the clinical response and short-term survival and further explore the comprehensive factors in predicting clinical outcomes in patients with liver cancer treated by DEB-TACE.
Materials and Methods
Patients
Forty-nine patients with hepatic tumor in Sir Run Run Shaw Hospital from January 2016 to November 2016 were enrolled in this cohort study. The inclusion criteria were as follows: (1) patients diagnosed with HCC, intrahepatic cholangiocarcinoma (ICC), or secondary hepatic tumor via pathologic assessment or noninvasive diagnostic criteria according to American Association for the Study of the Liver Diseases (AASLD) guidelines,18 (2) age older than 18 years, and (3) patients were about to receive DEB-TACE treatment by clinical demand. Meanwhile, the exclusion criteria were as follows: (1) patients who received previous liver transplantation, (2) patients who lacked histological grade information, (3) patients with incomplete laboratory values, (4) patients with contraindication for artery puncture, (5) patients with severe liver failure or kidney failure, (6) patients with cognitive impairment or unable to understand the study consents,and (7) women who were in gestation or lactation period.
This study was approved by the Ethical Committee of Sir Run Run Shaw Hospital, and all written informed consents from patients were obtained. Moreover, this study was performed according to the Declaration of Helsinki.
Drug-Eluting Beads Transarterial ChemoembolizationProcedure
Drug-eluting beads transarterial chemoembolization was performed in all patients on demand, the indication of which was determined by the assessment of multidisciplinary teamwork.
CalliSpheres beads (CBs; Jiangsu Hengrui Medicine Co, Ltd, Jiangsu, China) with the diameter ranging from 100 to 300 μm were used as carriers. Bead loading was conducted using adriamycin drug (adriamycin, pirarubicin, or epirubicin) 50 to 80 mg, and the mean dose was 60 mg for patients with primary liver cancer. In terms of patients with secondary liver cancer, the bead loading was performed using irinotecan 100 mg. Chemotherapy reagent was made to 20 mg/mL solution extracted by a 10 mL injector for further application. The CBs were shaken up, subsequently the bead suspension was extracted into a 20 mL injector and placed for 5 minutes, and the liquid supernatant was pushed out of the injector. The chemotherapy reagent solution and CBs were mixed continuously in a 20-mL injector and shaken up every 5 minutes and placed for 30 minutes in room temperature at 23°C to 28°C. Finally, nonionic contrast agent was administered at the ratio of 1:1, and the mixture was placed for another 5 minutes in room temperature at 23°C to 28°C for use. For tumors that did not reach the embolization end point after a bottle of CBs was emptied, ordinary embolization agents were utilized.
The DEB-TACE was performed under local anesthesia. Hepatic angiography was performed to detect the tumor-supplying vessels, and microcatheter (MC-PE27131, Terumo, Japan) was used for the embolization of tumor-supplying vessel. The CB mixture with contrast agent was pulsed injected to vessel at the rate of 1 mL/min, and the injection was stopped when the flow of contrast agent slowed down. After 5 minutes, the angiography was conducted for the second time, and the embolization was continued if tumor blush still existed until all blushed tumors vanished. If there were still blushed, tumors existed when a bottle of CBs was emptied, the embolization was continued using Embosphere beads until there was no more blushed tumors. Subsequently, the microcatheter was pulled out, and the hemostasis by compression was performed, and the punctured wound was binded up. All patients postembolization were told to lie on one side and extend the punctured leg for 6 to 12 hours.
Post-DEB-TACE Treatment
Patients with postoperative nausea and vomiting were treated with an intravenous injection of tropisetron and ondansetron. Pethidine, dexamethasone, and lidocaine were given as analgesic treatment for pain. In addition, patients with infection were treated with sulperazone 2 mg/ every 12 hours and levofloxacin.
Clinical and Pathological Features
Comprehensive baseline properties of patients were collected to analyze their predictive values for clinical outcomes, which included (1) demographic features: age and gender; (2) medical history: hepatic B virus hepatitis, drink, and cirrhosis; (3) clinicopathological features: histology, tumor distribution, largest nodule size, tumor location, portal vein invasion, hepatic vein invasion, Eastern Cooperative Oncology Group (ECOG) performance status, child-pugh stage, and Barcelona Clinic Liver Cancer (BCLC) Stage; (4) biochemical indexes: while blood cell, red blood cell, absolute neutrophil count, hemoglobin, platelet, albumin (ALB), total protein, total bilirubin, total bile acid, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, blood creatinine, blood urea nitrogen (BUN), alpha-fetoprotein (AFP), carcino-embryonic antigen (CEA), and carbohydrate antigen199 (CA199); (5) previous treatments: cTACE, surgery, systematic chemotherapy, radiofrequency ablation, and targeted therapy; (6) chemoembolization reagents: adriamycin drug and irinotecan; and (7) combination of ordinary embolization agent.
Definitions and Follow-Ups
The imaging response including computerized tomography and magnetic resonance imaging as well as blood test results were recorded. Imaging response was assessed according to modified Response Evaluation Criteria in Solid Tumors (mRECIST) 19: (1) complete response (CR)—no existence of arterial enhancement of targeted tumors; (2) partial response (PR)— decrease in diameter of targeted tumor (with arterial enhancement) <30%; (3) stable disease (SD)—decrease in diameter of targeted tumor (with arterial enhancement) did not achieve PR or less than PD; and (4) progressive disease (PD)—increase in diameter of targeted tumor (with arterial enhancement) ≥20% or new tumor existed. Overall response rate (ORR) was defined as the portion of patients who achieved CR and PR. In addition, the clinical response was evaluated at 1 to 3 months after DEB-TACE. Overall survival (OS) was calculated from the time of DEB-TACE operation to the date of death or last follow-up. Safety was assessed according to the count and percentage of adverse events after DEB-TACE. The median follow-up duration was 120 (range from 30 to 236) days, and the last follow-up date was December 12, 2016.
Statistics
Statistical analysis was performed using SPSS 22.0 software (IBM, USA). Data were presented as count (%), mean (standard deviation), or median (25th-75th). Logistic regression and Cox proportional hazards regression were performed to evaluate the predicting factors for CR, ORR, and OS of patients. Kaplan-Meier (K-M) curves were conducted to analyze OS in patients with different clinicopathological features or laboratory values. P < .05 was considered significant.
Results
Characteristics
Forty-nine patients treated using DEB-TACE with mean age 59.95 ± 11.38 years were included in this study, of which 38 (77.6%) cases were male and 11 (22.4%) cases were female. Thirty-eight (77.5%) cases were patients with primary HCC, 2 (4.1%) cases were patients with primary ICC, while 9 (18.4%) cases were patients with secondary hepatic tumor. Thirty-two (65.3%) patients were multifocal and 17 (34.7%) patients were unifocal, with median largest nodule size of 5.70 (3.00-8.55) cm. Portal vein invasion and hepatic vein invasion were observed in 18 (36.7%) and 10 (20.4%) patients, respectively. Besides, 35 (71.4%), 9 (18.3%), 2(4.1%), and 3(6.2%) patients were at ECOG performance status 0, 1, 2, and 3, respectively. With regard to the stages for primary liver cancer, 31 (77.5%) patients were categorized into Child-pugh stage A, 9 (22.5%) patients were stage B, and 24 (60%), 7 (17.5%), and 9 (22.5%) patients were at BCLC stages A, B, and C, respectively. Other detailed information about clinicopathological features, biochemical indexes, previous treatments, and combination of chemoembolization reagents and ordinary embolization agent is given in Table 1.
Table 1.
Baseline Characteristics of Patients With Liver Cancers.a
| Parameters | Patients (n = 49) |
|---|---|
| Age, years | 59.95 ± 11.38 |
| Gender, female/male | 11/38 |
| HBV, n (%) | 35 (71.4%) |
| Drink, n (%) | 17 (34.6%) |
| Cirrhosis, n (%) | 21 (26.5%) |
| Histology | |
| Primary HCC, n (%) | 38 (77.5%) |
| Primary ICC, n (%) | 2 (4.1%) |
| Secondary hepatic tumor, n/% | 9 (18.4%) |
| Tumor distribution | |
| Multifocal, n (%) | 32 (65.3%) |
| Unifocal, n (%) | 17 (34.7%) |
| Largest nodule size, cm | 5.70 (3.00-8.55) |
| Tumor location | |
| Left liver, n (%) | 10 (20.4%) |
| Right liver, n (%) | 23 (46.9%) |
| Bilobar, n (%) | 16 (32.7%) |
| Portal vein invasion, n (%) | 18 (36.7%) |
| Hepatic vein invasion, n (%) | 10 (20.4%) |
| ECOG performance status | |
| 0, n (%) | 35 (71.4%) |
| 1, n (%) | 9 (18.3%) |
| 2, n (%) | 2 (4.1%) |
| 3, n (%) | 3 (6.2%) |
| Child-pugh stage (n = 40) | |
| A, n (%) | 31 (77.5%) |
| B, n (%) | 9 (22.5%) |
| BCLC stage (n = 40) | |
| A, n (%) | 24 (60%) |
| B, n (%) | 7 (17.5%) |
| C, n (%) | 9 (22.5%) |
| Biochemical indexes | |
| WBC, × 109 cell/L | 4.80 (3.75-6.60) |
| RBC, × 1012 cell/L | 4.01 (3.43-4.45) |
| ANC (%) | 64.10 (54.70-73.60) |
| HB, g/L | 12.70 (11.65-14.10) |
| PLT, × 109 cell/L | 97.00 (54.50-137.00) |
| ALB, g/L | 37.20 (33.60-42.05) |
| TP, g/L | 66.70 (58.60-70.50) |
| TBIL, μmol/L | 17.10 (11.85-31.70) |
| TBA, I/L | 14.35 (6.70-28.38) |
| ALT, μ/L | 31.00 (23.50-51.00) |
| AST, μ/L | 40.00 (31.50-51.50) |
| ALP, μ/L | 147.00 (100.00-213.00) |
| BCr, μmol/L | 66.00 (55.00-72.50) |
| BUN, mmol/L | 3.92 (2.93-5.33) |
| AFP, μg/L | 18.46 (3.15-1065.56) |
| CEA, μg/L | 2.74 (2.13-5.04) |
| CA199, kU/L | 24.80 (15.60-70.68) |
| Previous treatments | |
| cTACE, n (%) | 15 (30.6%) |
| Surgery, n (%) | 32 (65.3%) |
| Systematic chemotherapy, n (%) | 11 (22.4%) |
| Radiofrequency ablation, n (%) | 6 (12.2%) |
| Targeted therapy, n (%) | 2 (4.1%) |
| Chemoembolization reagents | |
| Adriamycin drug, n (%) | 40 (81.6%) |
| Irinotecan, n (%) | 9 (18.4%) |
| Combination of ordinary embolization agent | 23 (46.9%) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ANC, absolute neutrophil count; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALB, albumin; BUN, blood urea nitrogen; BCr, blood creatinine; BCLC, Barcelona Clinic Liver Cancer; CEA, carcino-embryonic antigen; cTACE, conventional transarterial chemo-embolization; CA199, carbohydrate antigen 199; ECOG, Eastern Cooperative Oncology Group; HBV, hepatic b virus; HCC, hepatocellular carcinoma; HB, hemoglobin; ICC, intrahepatic cholangiocarcinoma; PLT, platelet; RBC, red blood cell; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; WBC, while blood cell.
aData were presented as mean (standard deviation), median (25th-75th), or count (%).
Clinical Response of DEB-TACE Treatment
As presented Table 2, 10 (20.4%) patients achieved CR and 31 (63.3%) patients achieved PR after DEB-TACE treatment, with ORR of 83.7%. In addition, 7 (14.3%) patients were SD, while 1 (2.0)% patient with disease progressed (PD).
Table 2.
Clinical Response of DEB-TACE Treatment in All Patients.a
| Parameters | n (%) |
|---|---|
| Total patients | 49 (100.0%) |
| CR | 10 (20.4%) |
| PR | 31 (63.3%) |
| ORR | 41 (83.7%) |
| SD | 7 (14.3%) |
| PD | 1 (2.0%) |
Abbreviations: CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease; PD, progress disease.
aData were presented as count (%).
Comprehensive Analysis of Factors Predicting CR and ORR
To investigate the comprehensive predictive factors affecting clinical response, univariate and multivariate logistic regression analysis were performed.
As to CR achievement, univariate analysis illuminated that high AST (P = .041, odds ratio [OR]: 0.174, 95% confidence interval [CI]: 0.033-0.929) and high CA199 (P = .030, OR: 0.156, 0.029-0.837) could predict less possibility for CR achievement, while high BUN (P = .020, OR: 12.937, 95% CI: 1.489-112.437) was associated with greater possibility for CR achievement (Table 3). Factors with P value < .1 were further analyzed by multivariate model, and no factors had independently predicted value for CR achievement, while high BUN disclosed a potential value in predicting CR (P = .055, OR: 11.659, 95% CI: 0.945-143.874) but without statistical significance. However, due to the lack of CR events or non-CR events, “secondary versus primary hepatic tumor,” “multifocal versus unifocal,” “hepatic vein invasion,” “higher ECOG,” “higher child-pugh stage,” “previous systematic chemotherapy,” “previous radiofrequency ablation,” “adriamycin drug (chemoembolization reagents),” “irinotecan (chemoembolization reagents)” and “combination of ordinary agent” were not available for univariate logistic model.
Table 3.
Predicting Factors for CR of DEB-TACE Treatment.a
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| P Value | OR | 95% CI | P Value | OR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| Age ≥60 years | .526 | 0.633 | 0.154 | 2.600 | - | - | - | - |
| Gender, Female | .835 | 0.833 | 0.149 | 4.650 | - | - | - | - |
| HBV | .911 | 0.917 | 0.200 | 4.198 | - | - | - | - |
| Drink | .727 | 0.765 | 0.170 | 3.438 | - | - | - | - |
| Cirrhosis | .063 | 4.167 | 0.927 | 18.719 | .153 | 4.629 | 0.566 | 37.849 |
| Secondary vs Primary hepatic tumorb | - | - | - | - | - | - | - | - |
| Multifocal vs Unifocalb | - | - | - | - | - | - | - | - |
| Largest nodule size ≥ 5.7 cm | .113 | 0.298 | 0.067 | 1.330 | - | - | - | - |
| Bilobar vs Unilobar | .841 | 0.857 | 0.190 | 3.870 | - | - | - | - |
| Portal vein invasion | .079 | 0.144 | 0.017 | 1.248 | .398 | 0.319 | 0.023 | 4.500 |
| Hepatic vein invasionb | - | - | - | - | - | - | - | - |
| Higher ECOGb | - | - | - | - | - | - | - | - |
| Higher Child-pugh stageb | - | - | - | - | - | - | - | - |
| Higher BCLC stage | .329 | 0.614 | 0.230 | 1.637 | - | - | - | - |
| WBC >4.80, × 109 cell/L) | .942 | 1.053 | 0.262 | 4.224 | - | - | - | - |
| RBC >4.01, × 1012 cell/L) | .438 | 1.750 | 0.426 | 1.190 | - | - | - | - |
| ANC% >64.10 | .147 | 0.331 | 0.074 | 1.474 | - | - | - | - |
| HB >12.70, g/L | .188 | 2.722 | 0.612 | 12.101 | - | - | - | - |
| PLT >97.00, × 109 cell/L | .227 | 2.400 | 0.580 | 9.930 | - | - | - | - |
| ALB >37.20, g/L | .113 | 3.354 | 0.752 | 14.964 | - | - | - | - |
| TP >66.70, g/L | .358 | 1.941 | 0.472 | 7.988 | - | - | - | - |
| TBIL >17.10, μmol/L | .438 | 0.571 | 0.139 | 2.438 | - | - | - | - |
| TBA >14.35 (I/L) | .438 | 0.571 | 0.139 | 2.438 | - | - | - | - |
| ALT >31.00 (μ/L) | .438 | 0.571 | 0.139 | 2.438 | - | - | - | - |
| AST >40.00, μ/L | .041 | 0.174 | 0.033 | 0.929 | .438 | 0.441 | 0.056 | 3.490 |
| ALP >147.00, μ/L | .358 | 1.941 | 0.472 | 7.988 | - | - | - | - |
| BCr >66.00, μmol/L | .113 | 3.354 | 0.752 | 14.964 | - | - | - | - |
| BUN >3.92, mmol/L | .020 | 12.937 | 1.489 | 112.437 | .055 | 11.659 | 0.945 | 143.874 |
| AFP >18.46, μg/L | .526 | 1.579 | 0.385 | 6.438 | - | - | - | - |
| CEA >2.74, μg/L | .828 | 0.857 | 0.213 | 3.442 | - | - | - | - |
| CA199 >24.80, kU/L | .030 | 0.156 | 0.029 | 0.837 | .081 | 0.133 | 0.014 | 1.281 |
| Previous cTACE | .473 | 1.697 | 0.400 | 7.196 | - | - | - | - |
| Previous surgery | .727 | 1.307 | 0.291 | 5.870 | - | - | - | - |
| Previous systematic chemotherapyb | - | - | - | - | - | - | - | - |
| Previous radiofrequency ablationb | - | - | - | - | - | - | - | - |
| Previous targeted therapy | .325 | 4.222 | 0.240 | 74.130 | - | - | - | - |
| Adriamycin drug (chemoembolization reagents) | .454 | 2.323 | 0.255 | 21.116 | - | - | - | - |
| Irinotecan (chemoembolization reagents) | .454 | 0.431 | 0.047 | 3.914 | - | - | - | - |
| Combination of ordinary embolization agent | .238 | 0.407 | 0.092 | 1.809 | ||||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; AFP, alpha fetoprotein; ALB, albumin; ANC, absolute neutrophil count; BUN, blood urea nitrogen; BCr, blood creatinine; BCLC, Barcelona Clinic Liver Cancer; CA199, carbohydrate antigen199; CEA, carcino-embryonic antigen; cTACE, conventional transarterial chemo-embolization; ECOG, Eastern Cooperative Oncology Group; HB, hemoglobin; HBV, hepatic b virus; PLT, platelet; TP, total protein; RBC, red blood cell; TBIL, total bilirubin; TBA, total bile acid; WBC, while blood cell.
aData were presented as P value, odds ratio (OR), and 95% CI. Significance was determined by univariate and multivariate logistic regression analysis. All factors with P value < .1 in univariate model were further analyzed using multivariate model. P value < .05 was considered significant.
bDue to the lack of CR events or non-CR events, “Secondary vs Primary hepatic tumor,” “Multifocal vs Unifocal,” “Hepatic vein invasion,” “Higher ECOG,” “Higher Child-pugh stage,” “Previous systematic chemotherapy,” and “Previous radiofrequency ablation” were not available for univariate logistic model. The boldface values stand for values with statistical significance.
As to ORR, portal vein invasion (P = .009, OR: 0.052, 95% CI: 0.006-0.476), higher child-pugh stage (P = .014, OR: 0.086, 95% CI: 0.012-0.603), and higher BCLC stage (P = .038, OR: 0.321, 95% CI: 0.109-0.939) were predictors for not achieving ORR by univariate analysis (Table 4). Additionally, multivariate model was not available because of relative small sample (49 cases) according to too many variables (8 factors P < .1 in univariate model were included). Due to the lack of ORR events or non-ORR events, “cirrhosis,” “high BUN,” “previous targeted therapy,” and “adriamycin drug (chemoembolization reagents)” and “combination of ordinary agent” were not available for univariate logistic model.
Table 4.
Predicting Factors for ORR of DEB-TACE Treatment.a
| Univariate Logistic Regression | Multivariate Logistic Regressionb | |||||||
|---|---|---|---|---|---|---|---|---|
| P Value | OR | 95% CI | P Value | OR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| Age ≥60 years | .051 | 8.944 | 1.007 | 79.457 | - | - | - | - |
| Gender (Female) | .471 | 2.258 | 0.247 | 20.650 | - | - | - | - |
| HBV | .544 | 1.636 | 0.334 | 8.019 | - | - | - | - |
| Drink | .855 | 0.864 | 0.180 | 4.155 | - | - | - | - |
| Cirrhosisc | - | - | - | - | - | - | - | - |
| Secondary vs primary hepatic tumor | .599 | 0.618 | 0.103 | 3.719 | - | - | - | - |
| Multifocal vs Unifocal | .179 | 0.233 | 0.025 | 1.989 | - | - | - | - |
| Largest nodule size ≥5.7 cm | .850 | 1.158 | 0.254 | 5.272 | - | - | - | - |
| Bilobar vs Unilobar | .062 | 2.220 | 0.045 | 1.078 | - | - | - | - |
| Portal vein invasion | .009 | 0.052 | 0.006 | 0.476 | - | - | - | - |
| Hepatic vein invasion | .550 | 1.969 | 0.213 | 18.163 | - | - | - | - |
| Higher ECOG | .519 | 0.767 | 0.342 | 1.719 | - | - | - | - |
| Higher Child-pugh stage | .014 | 0.086 | 0.012 | 0.603 | - | - | - | - |
| Higher BCLC stage | .038 | 0.321 | 0.109 | 0.939 | - | - | - | - |
| WBC >4.80, ×109 cell/L | .950 | 0.952 | 0.209 | 4.334 | - | - | - | - |
| RBC >4.01, ×1012 cell/L | .154 | 3.474 | 0.626 | 19.283 | - | - | - | - |
| ANC% >64.10 | .950 | 1.050 | 0.231 | 4.778 | - | - | - | - |
| HB >12.70, g/L | .408 | 1.930 | 0.407 | 9.160 | - | - | - | - |
| PLT >97.00, ×109 cell/L | .656 | 0.708 | 0.155 | 3.325 | - | - | - | - |
| ALB >37.20, g/L | .561 | 1.587 | 0.335 | 7.530 | - | - | - | - |
| TP >66.70, g/L | .561 | 1.587 | 0.335 | 7.530 | - | - | - | - |
| TBIL >17.10, µmol/L | .154 | 0.288 | 0.052 | 1.598 | - | - | - | - |
| TBA >14.35, I/L | .408 | 1.930 | 0.407 | 9.160 | - | - | - | - |
| ALT >31.00, µ/L | .950 | 1.050 | 0.231 | 4.778 | - | - | - | - |
| AST >40.00, µ/L | .154 | 0.288 | 0.052 | 1.598 | - | - | - | - |
| ALP >147.00, µ/L | .099 | 0.236 | 0.042 | 1.314 | - | - | - | - |
| BCr >66.00, µmol/L | .189 | 3.150 | 0.568 | 17.477 | - | - | - | - |
| BUN >3.92, mmol/Lc | - | - | - | - | - | - | - | - |
| AFP >18.46, μg/L | .950 | 1.050 | 0.231 | 4.778 | - | - | - | - |
| CEA >2.74, μg/L | .342 | 2.130 | 0.448 | 10.120 | - | - | - | - |
| CA199 >24.80, kU/L | .561 | 0.630 | 0.133 | 2.989 | - | - | - | - |
| Previous cTACE | .707 | 1.393 | 0.247 | 7.858 | - | - | - | - |
| Previous surgery | .179 | 0.223 | 0.025 | 1.989 | - | - | - | - |
| Previous systematic chemotherapy | .054 | 0.206 | 0.041 | 1.027 | - | - | - | - |
| Previous radiofrequency ablation | .981 | 0.972 | 0.098 | 9.645 | - | - | - | - |
| Previous targeted therapyc | - | - | - | - | - | - | - | - |
| Adriamycin drug(chemoembolization reagents) | .599 | 1.619 | 0.269 | 9.748 | - | - | - | - |
| Irinotecan (chemoembolization reagents) | .599 | 1.618 | 0.103 | 3.719 | - | - | - | - |
| Combination of ordinary embolization agent | .099 | 0.236 | 0.042 | 1.314 | ||||
Abbreviations: ANC, absolute neutrophil count; AFP, alpha fetoprotein; ALT, alanine aminotransferase; ALB, albumin; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCLC, Barcelona Clinic Liver Cancer; BUN, blood urea nitrogen; BCr, blood creatinine; CEA, carcino-embryonic antigen; CA199, carbohydrate antigen 199; cTACE, conventional transarterial chemo-embolization; ECOG, Eastern Cooperative Oncology Group; HB, hemoglobin; HBV, hepatic b virus; RBC, red blood cell; PLT, platelet; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; WBC, while blood cell.
aData were presented as P value, OR (odds ratio), and 95% CI. Significance was determined by univariate and multivariate logistic regression analysis. All factors with P value < .1 in univariate model were further analyzed by multivariate model. P value < .05 was considered significant.
bMultivariate model was not available due to relative small sample (49 cases) according to too many variables (8 were included).
cDue to the lack of ORR events or non-ORR events, “cirrhosis,” “high BUN,” and “previous targeted therapy” were not available for univariate logistic model. The boldface values stand for values with statistical significance.
Overall Survival Analysis
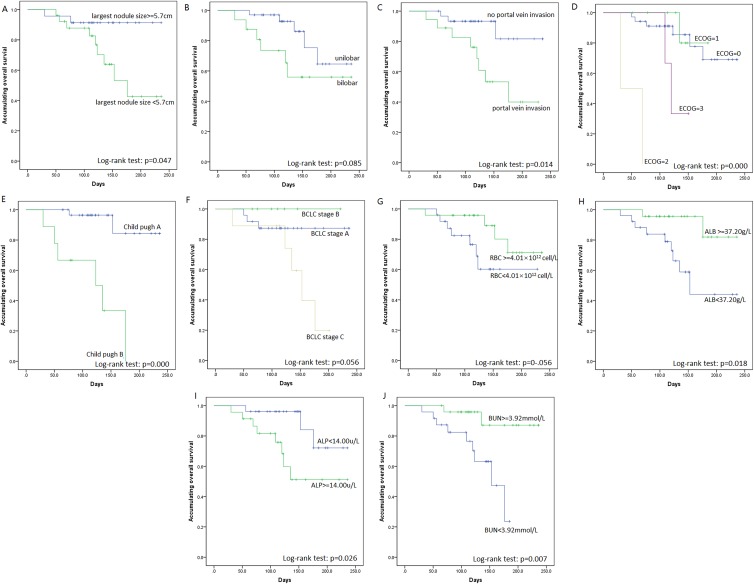
Overall survival was calculated from the date of DEB-TACE to death of patients or lost follow-up. One hundred eighty days of OS was 62.3% ± 10.5% for all patients with liver cancer. Patients were then divided into subgroups by patients’ features, and subgroup OS analysis by K-M curve and log-rank test were performed. All features associated with OS with P value < .1 by log-rank test is shown in Figure 1, which illustrated that largest nodule size ≥5.7 cm (P = .047, Figure 1A), portal vein invasion (P = .014, Figure 1C), higher ECOG performance status (P < .001, Figure 1D), higher Child-pugh stage (P < .001, Figure 1E), and high ALP (P = .026, Figure 1I) were associated with worse OS; while high ALB (P = .018, Figure 1H) and high BUN (P = .007, Figure 1J) were correlated with prolonged OS.
Figure 1.
OS analysis for subgroups by K-M curves. In DEB-TACE-treated patients with liver cancer, largest nodule size ≥5.7 cm (A), portal vein invasion (C), higher ECOG performance status (D), higher Child-pugh stage (E), and high ALP (I) were associated with shorter OS; while high ALB (H) and high BUN (J) were correlated with favorable OS. No correlation was observed in unilobar versus bilobar (B), BCLC stage (F), and RBC level (G). ALP indicates alpha-fetoprotein; ALB, albumin; BUN, blood urea nitrogen; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; RBC, red blood cells; OS, overall survival.
Comprehensive Analysis of Factors Predicting OS
To investigate the comprehensive predictive factors affecting OS, univariate and multivariate Cox hazard ratio regression analysis were conducted.
In univariate analysis, portal vein invasion (P = .025, hazard ratio [HR]: 4.571, 95% CI: 1.210-17.266), higher ECOG performance status (P = .011, HR: 1.984, 95% CI: 1.165-3.256), higher Child-pugh stage (P = .001, HR: 14.266, 95% CI: 2.830-71.508), and high ALP (P = .040, HR: 4.055, 95% CI: 1.068-15.404) were factors for predicting shorter OS, while high ALB (P = .033, HR: 0.186, 95% CI: 0.040-0.874) and high BUN (P = .018, HR: 0.153, 95% CI: 0.032-0.723) could predict favorable OS (Table 5). Factors with P value < .1 were further analyzed by multivariate model, and no factors had independently predictive value for OS.
Table 5.
Predicting Factors for OS in DEB-TACE Treatment.
| Parameters | Univariate Cox Regression | Multivariate Cox Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| P Value | HR | 95% CI | P value | HR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| Age ≥60 years | .132 | 0.305 | 0.065 | 1.482 | - | - | - | - |
| Gender, Female | .278 | 0.320 | 0.041 | 2.506 | - | - | - | - |
| HBV | .238 | 0.473 | 0.137 | 1.641 | - | - | - | - |
| Drink | .235 | 2.072 | 0.623 | 6.888 | - | - | - | - |
| Cirrhosis | .244 | 0.454 | 0.120 | 1,716 | - | - | - | - |
| Secondary vs Primary hepatic tumor | .592 | 1.439 | 0.390 | 5.444 | - | - | - | - |
| Multifocal vs Unifocal | .646 | 1.444 | 0.301 | 6.930 | - | - | - | - |
| Largest nodule size ≥5.7 cm | .067 | 4.186 | 0.903 | 19.401 | .830 | 0.413 | 0.000 | 1343.998 |
| Bilobar vs unilobar | .098 | 2.724 | 0.830 | 8.944 | .769 | 0.098 | 0.000 | 526330.905 |
| Portal vein invasion | .025 | 4.571 | 1.210 | 17.266 | .273 | 108.732 | 0.025 | 474160.746 |
| Hepatic vein invasion | .969 | 1.017 | 0.270 | 3.903 | - | - | - | - |
| Higher ECOG | .011 | 1.984 | 1.165 | 3.256 | .890 | 1.318 | 0.026 | 66.350 |
| Higher child-pugh stage | .001 | 14.266 | 2.830 | 71.508 | .261 | 36.391 | 0.069 | 19153.738 |
| Higher BCLC stage | .077 | 2.035 | 0.926 | 4.472 | .388 | 0.436 | 0.066 | 2.871 |
| WBC >4.80, ×109 cell/L) | .259 | 2.036 | 0.593 | 6.994 | - | - | - | - |
| RBC >4.01, ×1012 cell/L | .082 | 0.314 | 0.085 | 1.157 | .636 | 3.943 | 0.013 | 1164.723 |
| ANC% >64.10 | .271 | 1.998 | 0.580 | 6.876 | - | - | - | - |
| HB >12.70, g/L | .453 | 0.632 | 0.191 | 2.093 | - | - | - | - |
| PLT >97.00, ×109 cell/L | .467 | 1.544 | 0.473 | 5.100 | - | - | - | - |
| ALB >37.20, g/L | .033 | 0.186 | 0.040 | 0.874 | .213 | 0.000 | 0.000 | 191.478 |
| TP >66.70, g/L | .417 | 0.600 | 0.175 | 2.057 | - | - | - | - |
| TBIL >17.10, μmol/L | .161 | 2.596 | 0.684 | 9.851 | - | - | - | - |
| TBA >14.35, I/L | .305 | 0.525 | 0.153 | 1.795 | - | - | - | - |
| ALT >31.00, μ/L | .124 | 0.373 | 0.106 | 1.311 | - | - | - | - |
| AST >40.00, μ/L | .264 | 2.027 | 0.587 | 6.997 | - | - | - | - |
| ALP >147.00, μ/L | .040 | 4.055 | 1.068 | 15.404 | .631 | 9.415 | 0.001 | 88231.155 |
| BCr >66.00, μmol/L | .362 | 0.536 | 0.140 | 2.048 | - | - | - | - |
| BUN >3.92, mmol/L | .018 | 0.153 | 0.032 | 0.723 | .213 | 0.001 | 0.000 | 61.031 |
| AFP >18.46, μg/L | .225 | 0.466 | 0.136 | 1.598 | - | - | - | - |
| CEA >2.74, μg/L | .879 | 1.097 | 0.333 | 3.612 | - | - | - | - |
| CA199 >24.80, kU/L | .106 | 2.996 | 0.791 | 11.352 | - | - | - | - |
| Previous cTACE | .790 | 0.845 | 0.244 | 2.929 | - | - | - | - |
| Previous surgery | .242 | 2.231 | 0.582 | 8.559 | - | - | - | - |
| Previous systematic chemotherapy | .353 | 1.793 | 0.523 | 6.146 | - | - | - | - |
| Previous radiofrequency ablation | .569 | 0.550 | 0.070 | 4.310 | - | - | - | - |
| Previous targeted therapy | .675 | 0.047 | 0.000 | 7x104 | - | - | - | - |
| Adriamycin drug (chemoembolization reagents) | .647 | 1.442 | 0.302 | 6.880 | - | - | - | - |
| Irinotecan (chemoembolization reagents) | .647 | 0.694 | 0.145 | 3.331 | - | - | - | - |
| Combination of ordinary embolization agent | .154 | 2.633 | 0.697 | 9.947 | ||||
Abbreviations: ANC, absolute neutrophil count; AFP, alpha fetoprotein; ALT, alanine aminotransferase; ALB, albumin; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BCr, blood creatinine; BUN, blood urea nitrogen; BCLC, Barcelona Clinic Liver Cancer; CEA, carcino-embryonic antigen; CI, confidence interval; CA199, carbohydrate antigen199; cTACE, conventional transarterial chemo-embolization; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; HBV, hepatic b virus; HB, hemoglobin; PLT, platelet; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; RBC, red blood cell; WBC, while blood cell.
aData were presented as P value, HR, and 95% CI. Significance was determined by univariate and multivariate Cox regression analysis. Factors with P < .1 were further analyzed by multivariate model. P < .05 was considered significant.
Safety Profiles
Safety profiles during DEB-TACE operation and post-DEB TACE operation are presented in Table 6. During DEB-TACE operation, pain occurred in 22 (44.9%) patients, fever in 1 (2.0%) patient, and others in 1 (2.0%) patient. After DEB-TACE operation, 31 (63.3%) patients had pain, 27 (55.1%) patients had liver dysfunction, 31 (34.7%) patients had fever, 9 (18.4%) patients had nausea, and 14 patients (28.6) had vomiting. Each symptom was treated by clinical practice accordingly, and no SAE occurred.
Table 6.
Safety Profiles of DEB-TACE Treatment in All Patients.
| Parameters | n (%) |
|---|---|
| During DEB-TACE operation | 24 (49.0%) |
| Pain | 22 (44.9%) |
| Fever | 1 (2.0%) |
| Nausea | 0 (0.0%) |
| Vomiting | 0 (0.0%) |
| Others | 1 (2.0%) |
| After DEB-TACE operation | 46 (93.9%) |
| Pain | 31 (63.3%) |
| Fever | 17 (34.7%) |
| Nausea | 9 (18.4%) |
| Vomiting | 14 (28.6%) |
| Liver dysfunction | 27 (55.1%) |
| Alopecia | 0 (0.0%) |
| Chromatosis | 2 (4.1%) |
| Bone marrow toxicity | 6 (12.2%) |
| Others | 3 (6.1%) |
Abbreviation: DEB-TACE, drug-eluting beads transarterial chemoembolization.
aData were presented as count (%).
Subgroup Analysis of Patients With HCC
There were a total of 38 (77.6%) patients with HCC of 49 (100.0%) patients with liver cancer enrolled in our study and are presented in Table 7. Ten (26.3%) patients achieved CR and 23 (60.5%) patients achieved PR, the ORR was 86.8%, and the number of patients with SD and PD were 4 (10.5%) and 1 (2.6%), respectively. For the purpose of analyzing the predicting factors for CR in patients with HCC, univariate and multivariate logistic regression was performed (Table 8), which showed that AST > 40.00 μ/L (P = .025, OR: 0.139, 95% CI: 0.025-0.785), BUN >3.92 mmol/L (P = .037, OR: 20.385, 95% CI: 1.156-93.293), and CA199 >24.80 kU/L (P = .038, OR: 0.162, 95% CI: 0.029-0.908) correlated with worse CR in patients with HCC. In addition, CA 199 >24.80 kU/L (P = .041, OR: 0.066, 95% CI: 0.005-0.891) was an independent predictive factor for CR in patients with HCC.
Table 7.
Clinical Response of DEB-TACE Treatment in Patients With HCC.
| Parameters | n (%) |
|---|---|
| Total patients | 38 (100.0%) |
| CR | 10 (26.3%) |
| PR | 23 (60.5%) |
| ORR | 33 (86.8%) |
| SD | 4 (10.5%) |
| PD | 1 (2.6%) |
Abbreviations: CR, complete response; ORR, overall response rate; PD, progress disease; PR, partial response; SD, stable disease.
aData were presented as count (%).
Table 8.
Predicting Factors for CR of DEB-TACE Treatment in Patients With HCC.a
| Parameters | Univariate Logistic Regression | Multivariate Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| P Value | OR | 95% CI | P Value | OR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| Age ≥ 60 years | .464 | 0.578 | 0.133 | 2.505 | - | - | - | - |
| Gender, Female | .924 | 0.917 | 0.153 | 5.508 | - | - | - | - |
| HBVb | .088 | 0.179 | 0.025 | 1.293 | - | - | - | - |
| Drink | .603 | 0.662 | 0.140 | 3.123 | - | - | - | - |
| Cirrhosis | .282 | 2.333 | 0.499 | 10.907 | - | - | - | - |
| Multifocal vs unifocal | .968 | 0.971 | 0.222 | 4.243 | - | - | - | - |
| Largest nodule size ≥5.7 cm | .208 | 0.371 | 0.079 | 1.738 | - | - | - | - |
| Bilobar vs unilobar | .586 | 1.571 | 0.309 | 7.989 | - | - | - | - |
| Portal vein invasion | .117 | 0.172 | 0.019 | 1.551 | - | - | - | - |
| Hepatic vein invasionc | - | - | - | - | - | - | - | - |
| Higher ECOG c | - | - | - | - | - | - | - | - |
| Higher Child-pugh stagec | - | - | - | - | - | - | - | - |
| Higher BCLC stage | .267 | 0.570 | 0.211 | 1.537 | - | - | - | - |
| WBC >4.80, ×109 cell/L | .846 | 1.154 | 0.272 | 4.895 | - | - | - | - |
| RBC >4.01, ×1012 cell/L | .726 | 1.300 | 0.300 | 5.637 | - | - | - | - |
| ANC%>64.10 | .208 | 0.371 | 0.079 | 1.738 | - | - | - | - |
| HB >12.70, g/L | .478 | 1.750 | 0.373 | 8.201 | - | - | - | - |
| PLT >97.00, ×109 cell/L | .130 | 3.167 | 0.711 | 14.096 | - | - | - | - |
| ALB >37.20, g/L | .150 | 3.111 | 0.663 | 14.596 | - | - | - | - |
| TP >66.70, g/L | .464 | 1.731 | 0.399 | 7.505 | - | - | - | - |
| TBIL >17.10, μmol/L | .264 | 0.431 | 0.099 | 1.886 | - | - | - | - |
| TBA >14.35, I/L | .355 | 0.500 | 0.115 | 2.175 | - | - | - | - |
| ALT >31.00, μ/L | .464 | 0.578 | 0.133 | 2.505 | - | - | - | - |
| AST >40.00, μ/L | .025 | 0.139 | 0.025 | 0.785 | .073 | 0.089 | 0.006 | 1.251 |
| ALP >147.00, μ/L | .264 | 2.318 | 0.530 | 10.133 | - | - | - | - |
| BCr >66.00, μmol/L | .208 | 2.692 | 0.575 | 12.596 | - | - | - | - |
| BUN >3.92, mmol/La | .037 | 10.385 | 1.156 | 93.293 | - | - | - | - |
| AFP >18.46, μg/L | .968 | 0.971 | 0.222 | 4.243 | - | - | - | - |
| CEA >2.74, μg/Lb | - | - | - | - | - | - | - | - |
| CA199 >24.80, kU/L | .038 | 0.162 | 0.029 | 0.908 | .041 | 0.066 | 0.005 | 0.891 |
| Previous cTACE | .654 | 1.407 | 0.316 | 6.265 | - | - | - | - |
| Previous Surgeryc | - | - | - | - | - | - | - | - |
| Previous systematic chemotherapy c | - | - | - | - | - | - | - | - |
| Previous radiofrequency ablation c | - | - | - | - | - | - | - | - |
| Previous targeted therapyc | - | - | - | - | - | - | - | - |
| Adriamycin drug (chemoembolization reagents)c | - | - | - | - | - | - | - | - |
| Irinotecan (Chemoembolization reagents) | .775 | 1.444 | 0.117 | 17.904 | - | - | - | - |
| Combination of ordinary embolization agent | .208 | 0.371 | 0.079 | 1.738 | - | - | - | - |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALB, albumin; ANC, absolute neutrophil count; BUN, blood urea nitrogen; BCLC, Barcelona Clinic Liver Cancer; BCr, blood creatinine; CEA, carcino-embryonic antigen; CA199, carbohydrate antigen199; cTACE, conventional transarterial chemo-embolization; ECOG, Eastern Cooperative Oncology Group; HB, hemoglobin; HCC, hepatocellular carcinoma; HBV, hepatic b virus; PLT, platelet; RBC, red blood cell; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; WBC, while blood cell.
aData were presented as P value, OR (odds ratio), and 95% CI. Significance was determined by univariate and multivariate logistic regression analysis. All factors with P value < .1 in univariate model were further analyzed by multivariate model. P value < .05 was considered significant.
bDue to the high relevance among “HBV,” “AST,” “BUN,” and “CA199,” and lack of CR events or non-CR events, “HBV” and “BUN” were not available for multivariate logistic regression.
cDue to the lack of CR events or non-CR events, “hepatic vein invasion,” “higher ECOG,” “higher child-pugh stage,” “CEA >2.74 (μg/L),” “previous surgery,” “previous systematic chemotherapy,” “previous radiofrequency ablation,” “previous targeted therapy,” and “adriamycin drug(chemoembolization reagents)” were not available for univariate logistic model. The boldface values stand for values with statistical significance.
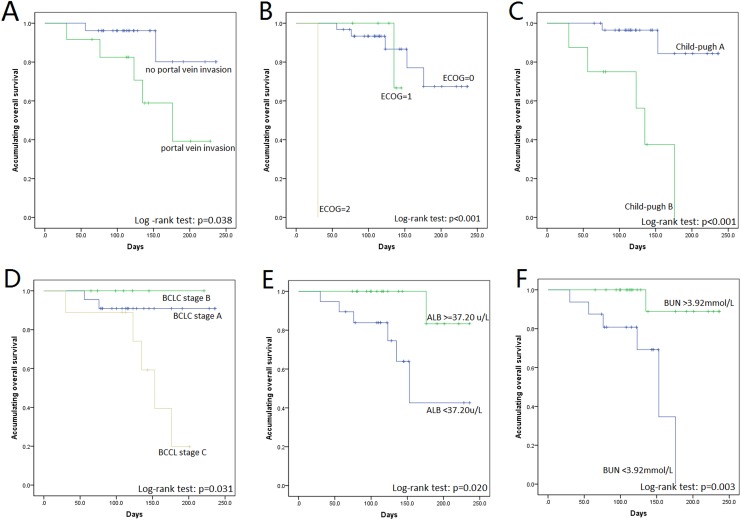
Subgroup analysis for OS in patients with HCC by K-M curves showed that portal vein invasion (P = .038), higher ECOG (P < .001), Child-pugh B (P < .001), and higher BCLC stage (P = .031) could predict worse OS, while ALB > 37.20 μ/L (P = .020) and BUN>3.92 mmol/L (P = .003) associated with better OS (Figure 2). In addition, the Cox regression was conducted for the predictive factor analysis of OS, which showed that higher ECOG (P = .046, HR: 4.752, 95% CI: 1.026-22.018), higher Child-pugh stage (P = .003, HR: 12.219, 95% CI: 2.334-63.984), and higher BCLC stage (P = .045, HR: 2.518, 95% CI: 1.020-6.215) negatively associated with OS in patients with HCC, while ALB >37.20 g/L (P = .049, HR: 0.117, 95% CI: 0.014-0.994) and BUN >3.92 mmol/L (P = .017, HR: 0.070, 95% CI: 0.008-0.617) were positively associated with OS (Table 9). In addition, multivariate Cox regression revealed that ALB >37.20 g/L (P = .042, HR: 0.004, 95% CI: 0.000-0.822) and BUN >3.92 mmol/L (P = .047, HR: 0.015, 95% CI: 0.000-0.945) were independent factors for predicting worse OS in patients with HCC.
Figure 2.
OS analysis for subgroups by K-M curves. In patients with HCC treated by DEB-TACE, portal vein invasion (A), higher ECOG performance status (B), higher child-pugh stage (C), and higher BCLC stage (D) were associated with worse OS. High ALB (E) and high BUN (F) were correlated with prolonged OS. ALB indicates albumin; BUN, blood urea nitrogen; BCLC, Barcelona Clinic Liver Cancer; DEB-TACE, drug-eluting beads transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; OS, overall survival.
Table 9.
Predicting Factors for OS of DEB-TACE Treatment in Patients With HCC.a
| Univariate Cox Regression | Multivariate Cox Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| P Value | HR | Lower | Higher | P Value | HR | Lower | Higher | |
| Age ≥60 years | .199 | 0.246 | 0.029 | 2.090 | - | - | - | - |
| Gender (Female) | .815 | 0.776 | 0.092 | 6.506 | - | - | - | - |
| HBV | .251 | 0.244 | 0.022 | 2.711 | - | - | - | - |
| Drink | .844 | 0.847 | 0.163 | 4.417 | - | - | - | - |
| Cirrhosis | .381 | 0.511 | 0.114 | 2.295 | - | - | - | - |
| Multifocal vs unifocal | .451 | 2.304 | 0.264 | 20.145 | - | - | - | - |
| Largest nodule size ≥5.7 cm | .306 | 2.364 | 0.455 | 12.273 | - | - | - | - |
| Bilobar vs unilobar | .301 | 2.226 | 0.489 | 10.141 | - | - | - | - |
| Portal vein invasion | .060 | 4.837 | 0.933 | 25.066 | .146 | 10.606 | 0.440 | 255.690 |
| Hepatic vein invasion | .601 | 0.566 | 0.067 | 4.777 | - | - | - | - |
| Higher ECOG | .046 | 4.752 | 1.026 | 22.018 | .327 | 2.730 | 0.367 | 20.314 |
| Higher child-pugh stage | .003 | 12.219 | 2.334 | 63.984 | .159 | 13.138 | 0.364 | 474.736 |
| Higher BCLC stage | .045 | 2.518 | 1.020 | 6.215 | .678 | 0.735 | 0.172 | 3.136 |
| WBC >4.80, ×109 cell/L | .259 | 2.584 | 0.496 | 13.449 | - | - | - | - |
| RBC >4.01, (×1012 cell/L | .474 | 0.560 | 0.115 | 2.736 | - | - | - | - |
| ANC% >64.10 | .139 | 3.463 | 0.667 | 17.988 | - | - | - | - |
| HB >12.70, g/L | .529 | 0.615 | 0.135 | 2.796 | - | - | - | - |
| PLT >97.00, ×109 cell/L | .821 | 1.189 | 0.265 | 5.331 | - | - | - | - |
| ALB >37.20, g/L | .049 | 0.117 | 0.014 | 0.994 | .042 | 0.004 | 0.000 | 0.822 |
| TP >66.70, g/L | .511 | 0.602 | 0.133 | 2.727 | - | - | - | - |
| TBIL >17.10, μmol/L | .202 | 55.574 | 0.117 | - | - | - | - | - |
| TBA >14.35, I/L | .146 | 0.295 | 0.057 | 1.527 | - | - | - | - |
| ALT >31.00, μ/L | .416 | 0.531 | 0.115 | 2.443 | - | - | - | - |
| AST >40.00, μ/L | .165 | 3.283 | 0.614 | 17.565 | - | - | - | - |
| ALP >147.00, μ/L | .370 | 1.987 | 0.443 | 8.920 | - | - | - | - |
| BCr >66.00, μmol/L | .476 | 0.542 | 0.101 | 2.913 | - | - | - | - |
| BUN >3.92, mmol/L | .017 | 0.070 | 0.008 | 0.617 | .047 | 0.015 | 0.000 | 0.945 |
| AFP >18.46, μg/L | .579 | 0.652 | 0.144 | 2.954 | - | - | - | - |
| CEA >2.74, μg/L | .803 | 0.826 | 0.183 | 3.174 | - | - | - | - |
| CA199 >24.80, kU/L | .180 | 3.083 | 0.595 | 15.986 | - | - | - | - |
| Previous cTACE | .683 | 0.725 | 0.154 | 3.407 | - | - | - | - |
| Previous surgery | .492 | 1.790 | 0.340 | 9.424 | - | - | - | - |
| Previous systematic chemotherapy | .207 | 4.099 | 0.458 | 36.729 | - | - | - | - |
| Previous radiofrequency ablation | .824 | 1.271 | 0.153 | 10.570 | - | - | - | - |
| Previous targeted therapy | .846 | 0.048 | - | - | - | - | - | - |
| Adriamycin drug (chemoembolization reagents) | .416 | 32.493 | - | - | - | - | - | - |
| Irinotecan (chemoembolization reagents) | .416 | 0.031 | - | - | - | - | - | - |
| Combination of ordinary embolization agent | .279 | 2.479 | 0.480 | 12.806 | - | - | - | - |
Abbreviations: ALT, alanine aminotransferase; ALB, albumin; ALP, alkaline phosphatase; ANC, absolute neutrophil count; AFP, alpha fetoprotein; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; BUN, blood urea nitrogen; CA199, carbohydrate antigen199; cTACE, conventional transarterial chemo-embolization; CEA, carcino-embryonic antigen; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HBV, hepatic b virus; HB, hemoglobin; PLT, platelet; RBC, red blood cell; TP, total protein; TBIL, total bilirubin; TBA, total bile acid; WBC, while blood cell.
aData were presented as P value, HR (hazard ratio), and 95% CI. Significance was determined by univariate and multivariate Cox regression analysis. All factors with P value < .1 in univariate model were further analyzed by multivariate model. P value < .05 was considered significant.
The safety profile of patients with HCC is listed in Table 10, which displayed that during DEB-TACE operation, 15 (39.5%) patients presented with pain, 1 (2.6%) patient had fever, and other adverse event was observed in 1 (2.6%) patient, while no nausea or vomiting event was discovered. After DEB-TACE operation, pain was observed in 23 (60.5%) patients, fever in 13 (34.2%) patients, nausea in 8 (21.1%) patients, vomiting in 11 (28.9%) patients, and liver dysfunction in 21 (55.3%) patients. The number of patients who were observed with epichrosis, bone marrow toxicity, and other adverse events were 2 (5.3%), 4 (10.5%), and 2 (5.3%), respectively.
Table 10.
Safety Profiles of DEB-TACE Treatment in Patients With HCC.
| Parameters | n (%) |
|---|---|
| During DEB-TACE operation | 17 (44.7) |
| Pain | 15 (39.5) |
| Fever | 1 (2.6) |
| Nausea | 0 (0.0) |
| Vomiting | 0 (0.0) |
| Others | 1 (2.6) |
| After DEB-TACE operation | 36 (94.7) |
| Pain | 23 (60.5) |
| Fever | 13 (34.2) |
| Nausea | 8 (21.1) |
| Vomiting | 11 (28.9) |
| Liver dysfunction | 21 (55.3) |
| Alopecia | 0 (0.0) |
| Chromatosis | 2 (5.3) |
| Bone marrow toxicity | 4 (10.5) |
| Others | 2 (5.3) |
Abbreviation: DEB-TACE, drug-eluting beads transarterial chemoembolization.
aData were presented as count (%).
Discussion
In the present study, we found (1) 83.7% patients with liver cancer achieved ORR by DEB-TACE treatment with tolerable side effects. (2) Comprehensive analysis revealed that patients with baseline high AST, high CA199, portal vein invasion, higher Child-pugh stage, and higher BCLC stage were seemed to less likely achieve clinical response, while high BUN could predict a increased possibility for achieving clinical response. (3) Portal vein invasion, higher ECOG performance status, higher Child-pugh stage, and high ALP were predictors for shorter OS, while high ALB and high BUN could predict favorable OS.
Liver cancer, with poor prognosis on account of late diagnosis and heterogeneity, is one of the most severe solid tumors worldwide, which mainly consists of HCC.9,20 In order to better improve the outcomes of liver cancers, BCLC staging and management divided the HCC into several stages by risk evaluation, in which TACE treatment is recommended as first-line treatment for intermediate HCC. Transarterial chemoembolization is carried out in many patients with early-stage HCC who are unsuitable for curative treatment due to physical condition, surgical contraindication, and so on, which account for nearly half of total cases with TACE.21 In line with the previous clinical experience, our study mainly included patients with BCLC stages A and B treated by DEB-TACE in the real-world setting.
Transarterial chemoembolization is categorized into cTACE and DEB-TACE. Recently, cTACE was gradually replaced by DEB-TACE due to the inconsistency in the technique and treatment schedule, and a large proportion of chemoembolization drugs may flow into the circulatory system subsequently inducing systemic toxicity in the duration between chemotherapy injection and embolic agent placement.11,13,22 DEB-TACE, first proposed in 2006 as commercial application, better standardizes the procedure, decreases treatment sessions, stabilizes the efficacy, builds up the drug-delivering capacity as well as reduces systemic toxicity technically.11-13,23,24 However, the objective clinical benefit of DEB-TACE compared to cTACE are still controversial, and a great amount of studies illuminated that DEB-TACE does not improve the clinical response or survival compared to cTACE but achieves fewer procedures, less liver toxicity benefit, better tolerance, and shorter hospital stay.18,21,25-29 While another 2 studies in Asia inversely illuminate that DEB-TACE elevates treatment response, postpones the progression, and prolongs OS when compared to cTACE.30,31 The main cause of this controversy might result from the gap of technical ability between non-Asian physicians and Asian physicians in performing cTACE. Prior large-scale comparative studies on cTACE performed by experienced institutions mainly from Europe and/or America predominantly improved the outcomes of cTACE. As to our institution, DEB-TACE becomes a standardized procedure for patients with liver cancer not appropriate to receive curative therapies, and in the present study, CBs loaded with adriamycin or irinotecan were used for TACE treatment and achieved ORR as high as 83.7%, which is consistent with previous studies in which ORR of DEB-TACE ranged from 50% to 90%; 20.4% patients achieved CR, which is partially in line with previous studies with a CR of 17% to 68.7%.18,21,25-31 In addition, for patients with tumor size larger than 5 cm, 1 bottle of CBs was not sufficient to reach embolization end point; thus, ordinary embolization agent was used in our study due to economic conditions of the patients. Earlier studies have reported relatively good efficacy for DEB-TACE as well. In 2011, a prospective, randomized, and single-center study using DEB-TACE to treat patients with HCC reveals a CR rate of 51.5% and a PR rate of 48.5%.32 In another case–control study, the CR and PR rates of DEB-TACE are 35% and 50%, respectively.33 A pilot study elucidates that patients who were refractory to cTACE achieved a CR rate of 40% and a PR rate of 60%.34 Due to different patient eligibilities, sample sizes, or the criteria that is used to evaluate the clinical response, the CR and PR rates vary among studies. The light difference in the clinical outcomes among studies are mainly due to the diversified inclusion criteria of patients enrolled, for example, in the present study, we not only enrolled the patients with primary liver cancer having BCLC stages A, B, and C but also included patients with secondary liver cancer which would reduce the response rate. We also found that 1 patient was PD after treatment, and the possible reason might be that the patient was BCLC stage C, multifocal, bilobar, relapsed HCC case, and with portal vein invasion, which correlates with worse clinical response.
Additionally, CBs were used in the DEB-TACE procedure in our study, and a previous animal experiment reveals that CBs loaded with doxorubicin (CBDOX) could achieve a relatively high drug concentration in rabbits compared to lipidol emusion; meanwhile, CBDOX could also deliver the drug to a distance of 200 μm and lasted for at least 1 month, indicating a good efficacy of drug release of CBDOX.35 The efficacy of CBs has also been proved by other experiments in vitro, which displayed that CBs could provide controlled release of doxorubicin with the half-life period more than 2 months.36,37 In vivo, it is reported that the drug can be detected after 2 weeks in rabbits post the DEB-TACE by CBs.38
Due to the diversified physical conditions, clinical properties, and biological features, the prognosis of patients with liver cancer receiving DEB-TACE treatment varies from each other.12,13,39,40 Thus, in order to better optimize the efficacy of DEB-TACE treatment and improve the prognosis of patients with liver cancer, it is essential to explore novel and convincing biomarkers for both clinical response and survival in patients by DEB-TACE treatment. A prospective historical cohort (mixed cohort design) study reveals that tumor size <5 cm and location in segments 1 or 4 correlates with higher possibility of CR in DEB-TACE-treated patients with HCC.39 In addition, another retrospective cohort study of DEB-TACE disclosed that tumor heterogeneity and tumor enhancement >50% predicts better CR but with a limited sample size (only 32 patients).40 Besides, a phase II trial illustrates that DEB-TACE achieves better objective response compared to cTACE in patients with HCC having Child-Pugh stage B, ECOG performance status 1, bilobar disease, or recurrent disease.18 In our study, we observed baseline high AST, high CA199, portal vein invasion, higher Child-pugh stage, and higher BCLC stage were associated with less possibility of clinical response by DEB-TACE treatment, while high BUN predicted a better clinical response achievement. Although the exact reason why these factors could predict clinical response was unclear, the possible explanation of the predictive value of these factors might be (1) AST was released to peripheral blood when hepatic cells are destroyed; thus in clinical practice, a high AST associates with a more severe liver function damage, meanwhile higher Child-pugh stage associates with worse liver function. CA199 has been used as a biomarker for diagnosing pancreatic cancer, rectal cancer, or liver cancer, and a higher CA199 might correlate with an advanced stage of cancer. Therefore, patients with baseline high AST and CA199 were less likely to achieve clinical response and might be explained by their worse liver function and more severe liver cancer.41,42 (2) Portal vein invasion and higher BCLC stage correlate with advanced liver cancer, which also suggest that those patients might not respond to DEB-TACE as good as patients with early-stage liver cancers. (3) Low BUN (divided as 3.92 mmol/L) correlated with more severe liver dysfunction, which reduced the response.
As for survival, a large sample size-based cohort study with 674 patients with HCC treated by DEB-TACE or cTACE presents that higher Child-pugh stage and portal vein invasion were independent predictors for worse OS.25 An randomized controlled trial study comparing DEB-TACE and cTACE found that higher ECOG stage and multiple tumors correlate with shorter OS independently in all patients with HCC.21 Another real-world setting study comparing DEB-TACE and cTACE disclosed that Bilobar and max diameter above 3.5 cm predicts lower OS.26 As to predictors for survival of DEB-TACE treatment alone, only a retrospective cohort study with limited patients (32 patients) reveals that tumor size above 6 cm is associated with worse OS.40 And a previous study in 2013 illuminates that high AFP, radiographically advanced HCC, high ECOG, high Child-pugh class, ascites, and high BUN are independently associated with worse survival, and the results are partly in accordance with ours.43 In this study, we disclosed that portal vein invasion, higher ECOG performance status, higher Child-pugh stage, and high ALP predict worse OS in DEB-TACE-treated patients, while high ALB and high BUN correlates with prolonged OS, which might result from the severe liver function, physical conditions, and lack of sensitivity to DEB-TACE associated with these factors influenced the OS. Additionally, 1 patient received surgery after DEB-TACE procedure, which might influence the prognosis of the patient. The patient who received surgery after DEB-TACE was a 63-aged female patient with HCC who was in BCLC stage A and Child-pugh stage A and with cirrhosis as well as largest nodule size >5.7 cm. The OS of the patient was 100 days, and the patient achieved CR after DEB-TACE procedure, indicating that DEB-TACE might be used as bridge therapy for patients with liver cancer about to receive surgery.
As to one of the most common adverse events of TACE, the occurrence rate of pain ranges from approximately 18.0% to 42.6% in patients treated with DEB-TACE, while in cTACE, the rate ranges roughly from 50.0% to 71.6%, suggesting DEB-TACE might be less painful compared to cTACE.21,44-46
This is the first study that analyzed the comprehensive factors affecting clinical response and survival in patients with liver cancer using DEB-TACE treatment, including demographic features, medical histories, clinicopathological properties, biochemical indexes, previous treatments, chemoembolization reagents. However, there were some limitations in this study. First, the relative small size sample with 49 patients limited the analysis of some key factors in univariate regression analysis due to the lack of effective events as well as multivariate were not available for ORR prediction due to too many variables compared to low size sample population enrolled. Second, the follow-up period was short, and the predictive value of the factors for long-term survival was not analyzed. Thus, further studies with larger sample size and longer follow-up duration are needed in the future.
In conclusion, this study observed that DEB-TACE was effective and well tolerated for patients with liver cancer, and high AST, high ALP, low ALB, low BUN, portal vein invasion, higher Child-pugh stage, higher BCLC stage, higher ECOG performance status were correlated with worse outcomes.
Abbreviations
- AFP
alpha-fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- BUN
blood urea nitrogen
- cTACE
conventional TACE
- CEA
carcino-embryonic antigen
- CA199
carbohydrate antigen199
- CR
complete response
- CBDOX
CBs loaded with doxorubicin
- DEB-TACE
drug-eluting beads transarterial chemoembolization
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- ICC
intrahepatic cholangiocarcinoma
- K-M
Kaplan-Meier
- ORR
overall response rate
- PR
partial response
- PD
progressed disease
- SD
stable disease.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No.81401493) and Zhejiang Provincial Natural Science Foundation of China under Grant No.LY18H180002.
References
- 1. Adult Primary Liver Cancer Treatment. Health Professional Version In: PDQ Adult Treatment Editorial Board, eds. PDQ Cancer Information Summaries. Bethesda, MD: National Cancer Institute; 2002. [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 3. Aravalli RN, Steer CJ. Immune-mediated therapies for liver cancer. Genes (Basel). 2017;8(2):E76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong GC, Liu Y, Chen N, et al. Reproductive factors, menopausal hormone therapies and primary liver cancer risk: a systematic review and dose-response meta-analysis of observational studies. Hum Reprod Update. 2016;23(1):126–138. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: approaching a personalized care. J Hepatol. 2015;62(1 suppl):S144–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12(8):436. [DOI] [PubMed] [Google Scholar]
- 7. Buettner S, van Vugt JL, IJzermans JN, Groot Koerkamp B. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther. 2017;10(10):1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63(5):844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med. 2015;5(9):a021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang K, Zhang XM, Yang L, Xu H, Peng J. Advanced imaging techniques in the therapeutic response of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2016;22(20):4835–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(3):212–220. [DOI] [PubMed] [Google Scholar]
- 12. Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;48(6):571–577. [DOI] [PubMed] [Google Scholar]
- 13. Woo HY, Heo J. Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: now and future. Clin Mol Hepatol. 2015;21(4):344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwan SW, Fidelman N, Ma E, Kerlan RK, Jr, Yao FY. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18(6):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bryant MK, Dorn DP, Zarzour J, et al. Computed tomography predictors of hepatocellular carcinoma tumour necrosis after chemoembolization. HPB (Oxford). 2014;16(4):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu HT, Kim JH, Lee LS, et al. Chemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J Vasc Interv Radiol. 2011;22(7):917–923. [DOI] [PubMed] [Google Scholar]
- 17. Lee MY, Chuang VP, Wei CJ, Cheng TY, Cherng MT. Histopathologic correlation of hepatocellular carcinoma after transcatheter arterial chemoembolization with polyvinyl alcohol particle of various sizes. Eur J Radiol. 2012;81(9):1976–1979. [DOI] [PubMed] [Google Scholar]
- 18. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. [DOI] [PubMed] [Google Scholar]
- 20. Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745–761. [DOI] [PubMed] [Google Scholar]
- 21. Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyayama S, Matsui O. Superselective conventional transarterial chemoembolization for hepatocellular carcinoma: rationale, technique, and outcome. J Vasc Interv Radiol. 2016;27(9):1269–1278. [DOI] [PubMed] [Google Scholar]
- 23. Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35(5):980–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Massani M, Stecca T, Ruffolo C, Bassi N. Should we routinely use DEBTACE for unresectable HCC? cTACE versus DEBTACE: a single-center survival analysis. Updates Surg. 2017;69(1):67–73. [DOI] [PubMed] [Google Scholar]
- 25. Kloeckner R, Weinmann A, Prinz F, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015;15(1):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Facciorusso A, Mariani L, Sposito C, et al. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(3):645–653. [DOI] [PubMed] [Google Scholar]
- 27. Arabi M, BenMousa A, Bzeizi K, Garad F, Ahmed I, Al-Otaibi M. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol. 2015;21(3):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duan F, Wang EQ, Lam MG, et al. Superselective chemoembolization of HCC: comparison of short-term safety and efficacy between drug-eluting LC beads, QuadraSpheres, and conventional ethiodized oil emulsion. Radiology. 2016;278(2):612–621. [DOI] [PubMed] [Google Scholar]
- 29. Megias Vericat JE, Garcia Marcos R, Lopez Briz E, et al. Trans-arterial chemoembolization with doxorubicin-eluting particles versus conventional trans-arterial chemoembolization in unresectable hepatocellular carcinoma: a study of effectiveness, safety and costs [in English and Spanish]. Radiologia (Roma). 2015;57(6):496–504. [DOI] [PubMed] [Google Scholar]
- 30. Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57(6):1244–1250. [DOI] [PubMed] [Google Scholar]
- 31. Rahman FA, Naidu J, Ngiu CS, et al. Conventional versus doxorubicin-eluting beads transarterial chemoembolization for unresectable hepatocellular carcinoma: a tertiary medical centre experience in Malaysia. Asian Pac J Cancer Prev. 2016;17(8):4037–4041. [PubMed] [Google Scholar]
- 32. Sacco R, Bargellini I, Bertini M, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22(11):1545–1552. [DOI] [PubMed] [Google Scholar]
- 33. Song MJ, Park CH, Kim JD, et al. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011;23(6):521–527. [DOI] [PubMed] [Google Scholar]
- 34. Song DS, Choi JY, Yoo SH, et al. DC bead transarterial chemoembolization is effective in hepatocellular carcinoma refractory to conventional transarteral chemoembolization: a pilot study. Gut Liver. 2013;7(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Deliv. 2017;24(1):1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lewis AL, Gonzalez MV, Lloyd AW, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17(2 pt 1):335–342. [DOI] [PubMed] [Google Scholar]
- 37. Gonzalez MV, Tang Y, Phillips GJ, et al. Doxorubicin eluting beads-2:methods for evaluating drug elution and in-vitro: in-vivo correlation. J Mater Sci Mater Med. 2008;19(2):767–775. [DOI] [PubMed] [Google Scholar]
- 38. Hong K, Kobeiter H, Georgiades CS, Torbenson MS, Geschwind JF. Effects of the type of embolization particles on carboplatin concentration in liver tumors after transcatheter arterial chemoembolization in a rabbit model of liver cancer. J Vasc Interv Radiol. 2005;16(12):1711–1717. [DOI] [PubMed] [Google Scholar]
- 39. Vesselle G, Quirier-Leleu C, Velasco S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016;26(6):1640–1648. [DOI] [PubMed] [Google Scholar]
- 40. Reis SP, Sutphin PD, Singal AG, et al. Tumor enhancement and heterogeneity are associated with treatment response to drug-eluting bead chemoembolization for hepatocellular carcinoma. J Comput Assist Tomogr. 2017;41(2):289–293. [DOI] [PubMed] [Google Scholar]
- 41. Ravel V, Streja E, Molnar MZ, et al. Association of aspartate aminotransferase with mortality in hemodialysis patients. Nephrol Dial Transplant. 2016;31(5):814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wada N, Kurokawa Y, Miyazaki Y, et al. The characteristics of the serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer cases. Surg Today. 2017;47(2):227–232. [DOI] [PubMed] [Google Scholar]
- 43. Sellers MT, Huggins S, Kegley K, et al. Multivariate analysis of prognostic factors for survival following doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2013;24(5):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24(4):437–443. [DOI] [PubMed] [Google Scholar]
- 45. Kalva SP, Iqbal SI, Yeddula K, et al. Transarterial chemoembolization with Doxorubicin-eluting microspheres for inoperable hepatocellular carcinoma. Gastrointest Cancer Res. 2011;4(1):2–8. [PMC free article] [PubMed] [Google Scholar]
- 46. Grosso M, Vignali C, Quaretti P, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol. 2008;31(6):1141–1149. [DOI] [PubMed] [Google Scholar]