Abstract
Tinnitus masking and residual inhibition (RI) are two well-known psychoacoustic measures of tinnitus. While it has long been suggested that they may provide diagnostic and prognostic information, these measures are still rarely performed in clinics, as they are too time consuming. Given this issue, the main goal of the present study was to validate a new method for assessing these measures. An acoustic sequence made of pulsed stimuli, which included a fixed stimulus duration and interstimulus interval, was applied to 68 tinnitus patients at two testing sites. First, the minimum masking level (MML) was measured by raising the stimulus intensity until the tinnitus was unheard during the stimulus presentation. Second, the level of the stimulus was further increased until the tinnitus was suppressed during the silence interval between the acoustic pulses. This level was called the minimum residual inhibition level (MRIL). The sequential measurement of MML and MRIL from the same stimulus condition offers several advantages such as time efficiency and the ability to compare results between the MRIL and MML. Our study confirms that, from this new approach, MML and MRIL can be easily and quickly obtained from a wide variety of patients displaying either normal hearing or different hearing loss configurations. Indeed, MML was obtained in all patients except one (98.5%), and some level of MRIL was found on 59 patients (86.7%). More so, this approach allows the categorization of tinnitus patients into different subgroups based on the properties of their MRIL.
Keywords: tinnitus, residual inhibition, minimum masking level, masking, psychoacoustics
Introduction
Tinnitus is defined as an auditory perception that is not induced by an external acoustic stimulation. Objective tinnitus (5% of tinnitus cases) results from an acoustic source originating from within the body (spontaneous otoacoustic emissions, vascular issues, contraction of the middle ear muscles, etc.), while subjective tinnitus (95% of tinnitus cases) is not produced by any acoustic source. Tinnitus is very prevalent in the general population and can severely impair an individual’s quality of life (McCormack, Edmondson-Jones, Fortnum et al., 2014; McCormack, Edmondson-Jones, Somerset, & Hall, 2016).
Tinnitus is considered as a symptom that can arise from many different causes. The research on tinnitus mechanisms has been very active over the past several years (Eggermont & Roberts, 2015; Schaette, 2013; Shore, Zhou, & Koehler, 2007). Many proposed mechanisms can be subdivided into two broad categories, namely peripheral and central tinnitus (see Noreña, 2015 for a review). Cochlear tinnitus has been defined as a tinnitus subtype resulting from aberrant activity generated at the periphery of the auditory system, thus as early as the cochlear nerve (Puel & Guitton, 2007). On the other hand, central tinnitus is believed to be the result of central changes triggered by hearing loss for instance (Noreña, 2011, 2015; Noreña & Farley, 2012). It is possible for several forms of tinnitus to coexist in a single individual.
One current limitation in the field of tinnitus is the inability to objectively characterize the tinnitus-related signal in a single individual. At best, neural biomarkers of tinnitus have been reported in groups of tinnitus patients, but these findings are still debated and may reflect the presence of hearing loss rather than tinnitus (Adjamian, Sereda, Zobay, Hall, & Palmer, 2012; Ortmann, Müller, Schlee, & Weisz, 2011; Sedley et al., 2015; Weisz, Moratti, Meinzer, Dohrmann, & Elbert, 2005). Moreover, researchers and clinicians have currently no means of identifying the specific causes of tinnitus in a given patient or determining whether the tinnitus is of peripheral or central origin. The difficulty to identify and select a given subtype of tinnitus is a significant barrier for developing and testing therapeutic approaches.
Although the internal tinnitus-related signal is still difficult to detect by available tools in neuroscience (electrophysiology, functional magnetic resonance imaging, etc.), at least at an individual level, the psychoacoustic properties of tinnitus may provide, on the other hand, some insights into the underlying mechanisms of tinnitus and the classification of its different subtypes. The tinnitus percept can be described according to several dimensions: the tinnitus percept itself (the timbre, the pitch, and the loudness) and the modulation of the tinnitus percept induced by acoustic stimulation (Henry & Meikle, 2000). Tinnitus can resemble a pure tone, a narrow or a broadband noise, or a combination of each. The dominant perceived pitch of tinnitus corresponds most often to the frequency range of the hearing loss (Basile, Fournier, Hutchins, & Hébert, 2013; Fournier & Hébert, 2013; Norena, Micheyl, Chéry-Croze, & Collet, 2002; Roberts, Moffat, Baumann, Ward, & Bosnyak, 2008). The perceived tinnitus loudness is typically relatively low, often only a few decibels above hearing threshold (HT) (Basile et al., 2013; Henry & Meikle, 2000; Moffat et al., 2009; Norena et al., 2002; Roberts et al., 2008). Tinnitus can be masked by pure tones and narrow or broadband stimuli, and the prolonged exposure to such an acoustic stimulation can temporarily reduce or even suppress tinnitus following the cessation of the stimulation (Feldmann, 1971; Hazell & Wood, 1981; Terry, Jones, Davis, & Slater, 1983; Vernon, 1977). The former is called the minimum masking level (MML), which is defined as the lowest intensity level required to just cover or mask tinnitus. The latter phenomenon is called the residual inhibition (RI) of tinnitus, which is defined as a temporary decrease of tinnitus after a prolonged acoustic stimulation.
The modulation of tinnitus by acoustic stimulation may provide prognostic information on the long-term effects of clinical treatments using auditory stimulation. Intuitively, it seems that patients with tinnitus that can be easily masked have a higher chance of benefiting from acoustic stimulation treatments (i.e., hearing aids, maskers, customized acoustic stimulation) than patients with tinnitus that is difficult to mask (Vernon, 1977). Indeed, the relief associated with tinnitus masking would be maximal if the discomfort produced by the acoustic stimulation is minimal, thus if the MML is low. Similarly, it seems plausible that patients with long-lasting RI (>30 s) would benefit more from a clinical approach based on acoustic stimulation than patients with no RI or poor lasting RI (<10 s). Coincidentally, RI and masking seem maximal when using noise centred at or close to the tinnitus frequency (Roberts et al., 2008; Roberts, Moffat, & Bosnyak, 2006). More so, acoustic stimulation using hearing aids for tinnitus relief is also more effective when amplification is specifically targeted to those frequencies (Schaette, König, Hornig, Gross, & Kempter, 2010).
While masking and RI of tinnitus may have prognostic value for clinical applications of acoustic stimulation, these measures are not performed consistently in clinics (Langguth et al., 2007). This is especially true for RI because the protocol used to assess it may be too time consuming and detailed for regular clinical practice. The classic method used to assess RI is aimed at estimating the depth and duration of RI after an acoustic stimulus, presented for 30 or 60 s at an intensity of MML + 10 dB, is stopped (Henry & Meikle, 2000; Roberts et al., 2008; Terry et al., 1983; Vernon & Meikle, 2003). Patients have to first indicate the depth of RI immediately after the cessation of the noise and second report when the tinnitus reappears and when its loudness returns to the initial level (i.e., before the stimulus was presented). This method revealed some general properties of RI as a function of the stimulus characteristics, namely that RI depth and duration tend to be larger for high-level and long-duration stimuli (Roberts et al., 2008; Terry et al., 1983).
We believe that the assessment of tinnitus masking and RI would be performed more routinely if the method was improved and accounted for the typical clinical time constraints. As such, we have devised a new method to assess the masking and RI of tinnitus. The main goal of the present study was to validate this new method.
Methods
Participants
In total, the data files of 68 tinnitus patients aged between 14 and 79 years old were included in this retrospective study (mean age: 54.2, SD: 14). Half of them were tested at the IMERTA clinic in Marseille (Marseille site; mean age: 50.6, SD: 12.9) and the other half at an audiology clinic in Lyon (Lyon site; mean age: 58, SD: 14.3). The data files were chosen for patients who reported tinnitus and performed the psychoacoustic tasks during standard clinical assessment. Some patients were able to report a possible etiology of their tinnitus that included acoustic trauma, stress, work noise exposure, head trauma, sudden hearing loss, acoustic neuroma, and whiplash. However, most patients reported an unknown etiology. The description of the tinnitus also varied between patients with most of them reporting a ringing or a high-pitch sound. Some patients also reported humming, low-pitch, and noise-type sounds. The patients’ samples varied slightly between the Marseille and Lyon sites, with mostly bilateral tinnitus patients in Marseille (bilateral, n = 27; unilateral left, n = 5; unilateral right, n = 2) and only unilateral tinnitus patients in Lyon (left, n = 20; right, n = 8; missing data, n = 6). All patients gave verbal consent for the use of their medical data. The Ramsay Général de Santé institution review board approved this study: Ethics approval number is COS-RGDS-2017-09-001.
Audiological Assessment Measures
HTs were measured monaurally in both ears for all patients from 0.25 to 8 kHz using the conventional clinical procedures using TDH 39 P earphones with a GSI-61 audiometer (Gradson-Sadler Inc., Eden Prairie, Minnesota, USA) for the Marseille site and TDH 39 earphones with an Aurical audiometer (Otometrics Inc., Taastrup, Denmark) for the yon site. For the Marseille site, the Sennheiser HDA 280 earphones were used to assess HTs for frequencies above 8 kHz. Most patients displayed a high-frequency sloping configuration of hearing loss (n = 40), but other configurations such as flat hearing loss (n = 10), low-frequency hearing loss (n = 2), notched hearing loss (n = 1), and normal hearing (n = 14) were also found. Normal hearing was defined as thresholds of ≤25 dB HL from 0.25 to 8 kHz; however, one patient included in the normal-hearing group had a single threshold at 30 dB HL. For patients tested at the Marseille site, HTs for standard frequencies (0.25 to 8 kHz) were more elevated for the left ears compared with the right (right ears, mean threshold = 36.9 dB HL; left ears, mean threshold =43 dB HL, F(1,32) = 5.1, p = .03). As for patients at the Lyon site, the HTs were significantly higher in the tinnitus ears (mostly left ears) compared with the contralateral ears (tinnitus ears, mean threshold = 44.8 dB HL; contralateral ears, mean threshold = 24.4 dB HL, F(1,32) = 21.9, p < .001). In addition, loudness discomfort levels (LDLs) using an ascending psychoacoustic approach were measured in each ear for all frequencies from 0.25 to 12.5 kHz for all patients at the Lyon site only. The intensity of a pure tone was increased by 2 dB steps, and patients had to verbally indicate the level which the presented sound was judged uncomfortable. The LDLs in dB HL did not differ significantly between the tinnitus ears (M = 87.8 ± 15.7 dB HL) and the contralateral ears (M = 85.3 ± 17.8 dB HL; F = 1) but differed significantly when expressed in dB SL with means of 46.1 ± 20 dB SL for the tinnitus ears compared with 61 dB SL ± 17.5, F(1,22) = 12.4, p = .002 for the control ears. These results suggest a more restrictive dynamic range in the ears with the tinnitus compared with the control ears of unilateral tinnitus patients, which is in accordance with the enhanced auditory sensitivity in tinnitus ears and the central gain hypothesis (Hébert, Fournier, & Noreña, 2013). The measurements of LDLs and HTs were repeated using pulsed narrowband noises instead of pure tones in the tinnitus ears (Lyon site, see later) and yielded similar results.
Psychoacoustic Measures
Equipment
Psychoacoustic measures were performed on different sets of equipment at the Marseille and Lyon sites. For Marseille, all the psychoacoustic measurements were performed using Sennheiser HD-600 supra-auricular headphones and a Sound Blaster X-fi HD model SB1240 sound card. A MATLAB program, created in-house, allowed the clinician or the research assistant to control manually all the stimulus parameters such as the duration, the intensity level, the center frequency, and the bandwidth of any sound presented. For Lyon, all the measurements were performed under TDH 39 earphones using an Aurical audiometer (Otometrics Inc.) that allowed the clinician to manually control most parameters. All the psychoacoustic measurements were performed binaurally at the Marseille site and monaurally at the Lyon site.
Predominant tinnitus pitch and loudness
For tinnitus pitch matching, a 1-kHz pure tone target was first presented. The patient was asked to judge the likeness of the target sound to the tinnitus in terms of pitch. If the tinnitus was judged as higher pitched, then the frequency of the target was increased by half-octave steps until the tinnitus pitch, and the target, were judged as qualitatively similar. The target was decreased by the same number of steps in the reverse case (i.e., tinnitus judged as lower compared with the target tone). The same procedure was repeated until the participant was satisfied with the pitch similarity between the target and the tinnitus. Other modifications such as smaller frequency steps or decreased/increased bandwidth could be made to establish a better correspondence between the target sound and the pitch and timbre of the tinnitus. For tinnitus loudness matching, a target sound at the tinnitus frequency was presented at a low intensity level (around threshold) and was increased by 3 dB steps until the level of the sound and the tinnitus were judged similar by the patient.
MMLs and minimum residual inhibition levels
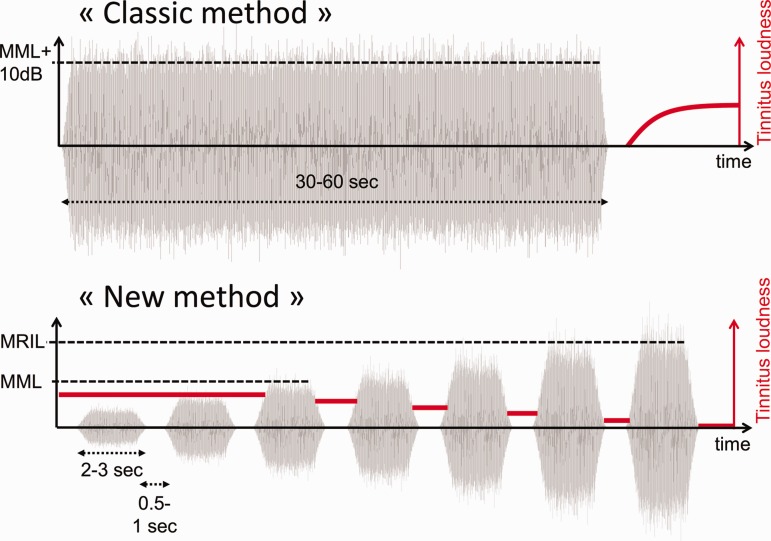
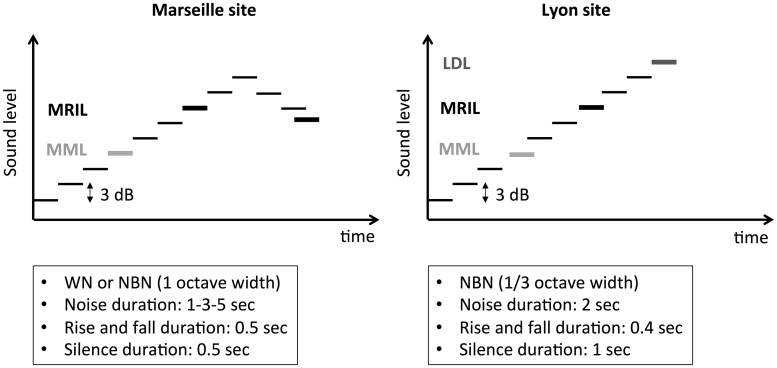
The MML and minimum residual inhibition level (MRIL) were assessed using the same stimulus sequence made of pulsed broadband or narrowband noises (1 or 1/3 octave width, in Marseille and Lyon, respectively). The idea behind this new approach was to replace a measurement of RI duration after a prolonged presentation of a noise by the measurement of an intensity level producing RI for a fixed period of time (Figures 1 and 2). Indeed, the classic method consisted of measuring the time needed for the tinnitus to reappear after the cessation of a 30 or 60 s noise presentation (broadband or a narrowband) at an intensity of 10 dB above the MML (Figure 1). In the new method, the acoustic sequence made of pulsed acoustic stimulation of fixed duration and fixed interstimulus intervals were first used to measure MML (Figure 1). First, the level of the stimulus was presented at low but audible levels and was then raised by 3 dB steps until the tinnitus was masked during the stimulus presentation (Figure 1). Second, the level of the stimulus was further raised by 2 or 3 dB steps until the tinnitus was suppressed during the silent interval, between the acoustic pulses (Figure 2). For the Lyon site, the duration of the stimulation was kept constant at 2 s and the silent intervals at 1 s (rise/fall time 0.4 s). For the Marseille site, the effects of several parameters on the MML and MRIL, such as the duration of the stimulation (1, 3, or 5 s), were investigated. However, the silent intervals were always kept constant at 0.5 s (rise/fall time was 0.5 s; Figure 2).
Figure 1.
Schematic of the classic (continuous noise) versus the new method of RI (pulsed noise). In the classic method, a noise either broad or narrowband is presented for 1 min at 10 dB above the minimal masking level. The time for the tinnitus to reappear (T1) and return to its initial stage (T2) is measured after the cessation of the noise. In addition, the patient can be asked to rate on a visual analog scale the depth of the inhibition. In the new method, the silent intervals are fixed at 1 s. The MML is obtained by raising the intensity of the pulse noise until the tinnitus is masked during the stimulation. The MRIL is obtained by further increasing the stimulation until the tinnitus is suppressed during the 1-s interval of silence (the dark gray line indicates tinnitus loudness decreasing as the stimulation intensity increases).
RI = residual inhibition; MML = minimum masking level; MRIL = minimum residual inhibition level.
Figure 2.
Schematic of the MML and MRIL experimental procedure at the two testing sites (Marseille and Lyon). Marseille (left panel): The sound level is first raised until the MML is reached (i.e., when the tinnitus is not perceived during the noise stimulation). Then, the level is raised again until the tinnitus is not perceived during the 0.5-s silence intervals (MRIL ascending). After the RI is reached, an additional 6 dB is added to the MRIL ascending value, and the level is decreased until the tinnitus barely reappears in the 0.5-s silence intervals (MRIL descending). Lyon (right panel): The sound level is first raised until the MML is reached. Then, the level is raised again until the tinnitus is not perceived during the 1-s silence intervals. Finally, the level is raised further until the patients report that the stimulus sequence produces discomfort (loudness discomfort level).
MML = minimum masking level; MRIL = minimum residual inhibition level; RI = residual inhibition.
Procedure
The tinnitus assessment took place in a sound booth at the Lyon site and in a quiet medical office at the Marseille site. The typical assessment session usually started with HT evaluation for both ears from 0.25 to 16 kHz for the Marseille site and 0.25 to 12.5 kHz for Lyon site before the tinnitus psychoacoustic measures were assessed.
Marseille site
Patients were asked, before and after the assessment, to rate the loudness of their tinnitus on a visual analogue scale (VAS) ranging from 0 (Inaudible) to 10 (maximum loudness). The session started with psychoacoustic measurements of tinnitus pitch and loudness followed by the measurements of MML and MRIL (obtained from ascending and descending procedures) using broadband noise at three different durations of 1, 3, or 5 s. The ascending MRIL is simply the MRIL obtained from an ascending procedure. For the descending MRIL, the level is raised by +6 dB from the ascending MRIL and then decreased by 3 dB steps until the tinnitus reappears during the silent periods (Figure 2). The descending MRIL indicates the last intensity value for which the tinnitus is suppressed during the silent periods. The descending MRIL was assessed to investigate a putative hysteresis of tinnitus suppression when the level was increased. Then, the MML and MRIL, with a 3-s stimulation only (3 dB steps), were also measured with a narrowband noise centred at three different frequencies: the tinnitus frequency, a frequency within the slope of the hearing loss, and a frequency outside of the hearing loss region. The frequency of the slope of the hearing loss was chosen by visual inspection for each individual patient audiogram. A hearing loss slope was present mostly for patients with presbycusis. For most cases (n = 46), the chosen frequency was between the cutoff frequency and the first frequency that reached a difference threshold of ≥15 dB and equalled the threshold of the cutoff frequency. The frequency 4 kHz was chosen when no frequency slope could be clearly determined visually. The frequency outside of the hearing loss region was generally 1 kHz or lower when the threshold was in the hearing loss range at 1 kHz. Finally, the MML and MRIL were tested with 1 and 5 s of stimulation at the center frequency with the best inhibition (i.e., the lowest MRIL level in dB SL) using the 3-s stimulation. The maximum intensity value set for the MML or the MRIL was 95 dB sound pressure level (SPL) for all the conditions obtained in Marseille. More so, for MRIL ascending, the level was raised until the tinnitus completely disappeared in the silent intervals or until the maximum stimulation value was reached. In the latter case, the patient was asked to rate the loudness of the tinnitus on the same VAS as the one used at the beginning of the experiment. In the case where the RI persisted after a condition was performed, a short break of a few minutes was given to the patient for the tinnitus to come back to normal levels (i.e., prestimulus intensity level). A VAS was used to validate the return of the tinnitus intensity to a normal level. If the tinnitus did not reappear after the short break, these measurements were stopped. The complete assessment of all measures usually lasted around 45 min. Still, some patients did not complete all the conditions because of time constraints, lack of motivation, or fear of worsening their tinnitus. Finally, at the end of the session, the patients were presented again with the four different centred noise pulsed stimulations (broadband, tinnitus frequency, frequency of the HL slope, and outside the HL region) at an audible level, and they were asked the question: If you had to hear one sound for several hours a day for several weeks in the context of a treatment, which one would you prefer between those four sounds?
Lyon site
For the Lyon site, the pulsed acoustic stimulation of fixed duration (2-s stimulation and 1-s silent intervals) was used to measure pulse noise thresholds, MMLs, MRILs, and the LDLs in a sequential order (Figure 2). Indeed, for each center frequency tested, from 0.25 to 12.5 kHz, the pulsed narrowband noise was first used to measure the HT. Then, the intensity of the noise was further increased in 2 dB steps to measure, in a sequential order, the MML, the MRIL, and finally the LDL (Figure 2). Patients were asked to signal the clinician when they hear a noise for the HT measurement, when the tinnitus is just masked by the noise during the noise presentation for the MML, when you don’t hear the tinnitus during the silence intervals for the MRIL, and finally, when the loudness of the sound is uncomfortable for the LDLs. The MML or MRIL measurements were stopped if they were not obtained at levels below the LDLs. Contrary to what has been done at the Marseille site, the MRIL measurement was considered for the analysis only if total inhibition was obtained, as partial inhibition was not considered. More so, the broadband noise stimulation was not tested at the Lyon site. The pitch and loudness tinnitus matching psychoacoustic assessment were performed thereafter. Finally, the classic RI technique (described earlier, Figure 1) was performed at one or two frequencies depending on time with the goal of comparing the sensitivity of the classic method to the new method and to assess any associations between the two measures. A chronometer was started immediately after the cessation of a noise presented continuously for 60 s. Patients were then asked to report (a) when the tinnitus reappeared (T1) and (b) when the tinnitus had come back to its initial level (T2). The clinician noted those two time values. The time required to complete the whole session was around 1 hr. More specifically, a complete sequence of HT, MML, MRIL, and LDL could take 2 to 5 min per frequency. The complete testing of this particular sequence for all frequencies thus depends on the number of frequencies tested, but in most cases, the complete sequence for all frequencies was performed in about 20 min.
Statistical Analysis
HTs and LDLs were averaged for each frequency for each ear and were then compared using a repeated measures analysis with the within-subject measures of frequency and ear.
Raw MML and MRIL data, expressed in either dB HL or dB SPL, were transformed into dB SL by subtracting the HT of the corresponding center frequency of the noise to the raw values of MML and MRIL. For the broadband noise, the threshold at 1 kHz was used for transforming into dB SL or a lower frequency if the threshold at 1 kHz indicated the presence of hearing loss. All the analysis on MML and MRIL were performed in dB SL values. To assess the similarity of the results obtained from the two different sites, the MML and MRIL results at the tinnitus frequency, the frequency of the HL slope, and the frequency outside the HL region were extracted from the large set of MML and MRIL (0.25 to 12.5 kHz) results for each patient tested in Lyon using the same approach as the one described for the Marseille site. The relationships between the MML/MRIL, MRIL ascending/descending, and MRIL/classic method of RI (T1, T2) were investigated by using the coefficient of determination (r2), the Pearson correlation coefficient (r), or paired-sample t tests. Repeated measure analysis of variance (ANOVA) or mixed ANOVA were used for the analysis of multiple noise durations, the analysis of frequency specificity of the MML and MRIL, as well as the frequency specificity of the old classic inhibition paradigm. Post hoc Bonferroni pairwise tests were performed to follow-up on main significant effects when applicable. Other post hoc statistical tests included paired-sample t tests.
Results
Characteristics of MML and MRIL
Distribution
The MML was obtained for all 34 patients tested on the new method at the Marseille site and for all patients, except one (33 out of 34), at the Lyon site. For the MRIL, complete inhibition was obtained in at least one condition for 28 out of the 34 patients at the Marseille site and 31 out of 34 at the Lyon site. Overall, when merging the data of the two sites, MML and MRIL were obtained in 98.5% and 86.7% of all patients, respectively.
Individual examples
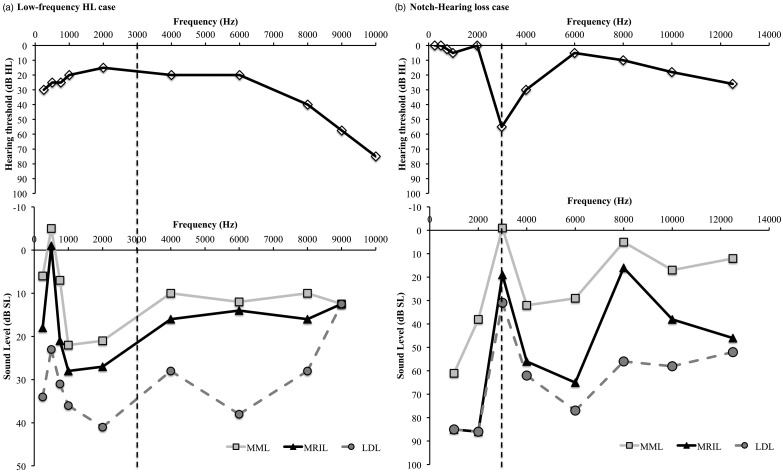
Figure 3 shows two interesting cases: one with a notched hearing loss at 3 kHz and one with a low-frequency hearing loss. For the notched hearing loss case, the MML was the most effective (in dB SL) when the noise was centered at the frequency of the notch. Interestingly, the MML and MRIL were also most effective when the noise was centered at 8 kHz, suggesting that tinnitus may have a second (minor) component at this frequency. For the low-frequency hearing loss case, the LDLs were very low across the tested frequencies (around 60 dB HL). Still, effective MML and MRIL were obtained below those levels across frequencies. Interestingly, the most effective MML and MRIL were produced when the center noise frequency was at 500 Hz (in the low-frequency hearing loss region). It is possible that tinnitus in this patient also had two frequency components, one near 500 Hz and another one near 3000 Hz. To note, the MML and MRIL of tinnitus were also successfully obtained in an ear with profound hearing loss by stimulating the contralateral ear (n = 2, Marseille site).
Figure 3.
Results obtained at the Lyon site. Two special cases are presented: (a) a low-frequency mild hearing loss with low LDLs case and (b) a notched hearing loss case. The dotted line is the tinnitus frequency for each case.
LDL = loudness discomfort level.
Defining subgroups and their characteristics
The types of possible RI outcomes were further refined at the Marseille site only. Indeed, the patients reported different types of outcomes that were classified into five types of RI: (a) complete inhibition, (b) partial inhibition, (c) persistent inhibition, (d) increased tinnitus loudness, and (e) change in tinnitus pitch (description in Table 1). Because the number of patients is distributed unequally between the five different groups, no statistical tests were run between the five groups. Still, the patients reporting positive RI outcomes (complete, partial, and persistent) were generally older and had a higher tinnitus pitch (see Table 1) and higher HTs (see Supplementary Table 1) than the ones reporting negative outcomes (increased tinnitus loudness) or other outcomes (change in pitch). More so, when comparing the positive outcome groups, the patients with partial inhibition were the oldest and displayed the highest HTs. The two groups with the loudest tinnitus were those with partial inhibition and increased tinnitus loudness with 13 dB SL measured for both.
Table 1.
Description and Characteristics of the Different Residual Inhibition Subgroups for Marseille Site Only.
| Types of RI (Outcome) | Description | Number of patients | Age in years (SD) | Duration of tinnitus in years (SD) | Sex (Male/ Female) | Tinnitus pitch in Hz (SD) | Tinnitus loudness in dB SL (SD) |
|---|---|---|---|---|---|---|---|
| Positive | |||||||
| Complete | Tinnitus completely disappeared during the 1-s silence gap and reappeared when the stimulation procedure stopped | 16 | 51.5 (13.4) | 5 (4.3) | 6/10 | 5265 (3485) | 8 (6) |
| Partial | Tinnitus loudness was reduced by at least 2 points on a visual analogue scale, but the tinnitus could not be completely suppressed | 8 | 55 (10.4) | 11 (6.4) | 6/2 | 5963 (2325) | 13 (7) |
| Persistent | Tinnitus completely disappeared during the 1-s gap and persisted after the cessation of the stimulation from minutes to hours | 4 | 51.3 (6.9) | 5 (4.4) | 2/2 | 4211 (3467) | 11 (3) |
| Negative | |||||||
| Increased tinnitus loudness | The tinnitus loudness increased during the stimulation procedure (but back to initial level right after the cessation of the stimulation) | 4 | 39 (19.9) | 7 (10.8) | 3/1 | 2527 (3648) | 13 (9) |
| Other | |||||||
| Change in tinnitus pitch | The tinnitus pitch changed during the stimulation procedure (but back to initial level right after the cessation of the stimulation) | 2 | 47.5 (2) | <1 | 2/0 | 1038 (531) | 7 (8) |
| Total | 34 | 50.56 (12.9) | 5.9 (5.9) | 19/15 | 4734 (3608) | 9.8 (7) | |
Note. RI = residual inhibition.
These groups can also be compared based on the measured levels required for MML and MRIL of frequency-centered noise stimulus. For the broadband noise and the noise with a center frequency outside the hearing loss region, the MML was similar across all groups (see Supplementary Table 1). More so, the positive RI outcome groups had much lower levels (in dB SL) for the MML when the center frequency was the tinnitus pitch or the hearing loss frequency slope. This was not the case for the negative RI outcome groups. Finally, for all the positive RI outcome groups, the MRIL was obtained at lower levels for noises with central frequency at the tinnitus frequency or at the hearing loss slope frequency compared with a broadband noise or a narrowband noise with a center frequency outside the hearing loss region (see Supplementary Table 1).
The VAS of tinnitus loudness, taken before and after the experiment, also revealed differences between the five groups (see Supplementary Table 1). Indeed, the positive RI outcome groups reported a decreased in tinnitus loudness at the end of the experiment of −2, −3, and −4 points for the complete, partial, and persistent group, respectively. The increased tinnitus loudness and change in tinnitus pitch groups reported no change (0) and a minimal but positive change (−1.25), respectively. To note, the change of tinnitus pitch toward a lower frequency was reported as pleasant by the two affected patients. The new sound and the modification were both noted as pleasant by the patients.
MML and MRIL applied to different hearing loss configurations
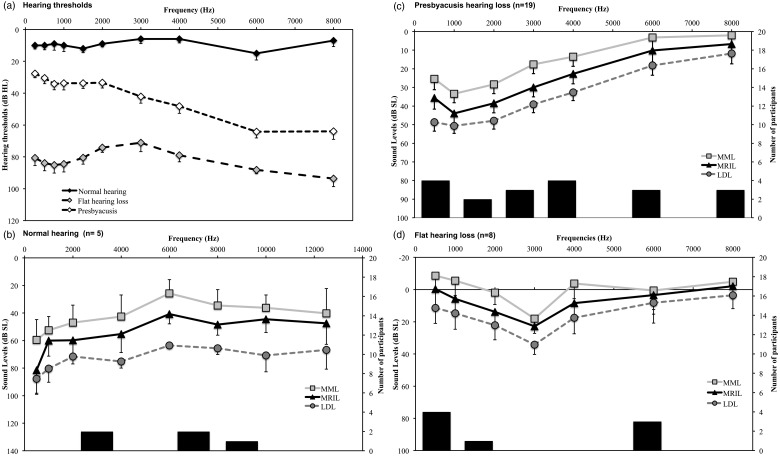
The applicability of the MML and MRIL techniques for different types of hearing loss configurations could be investigated for the data from the Lyon site only. The patients were regrouped into three types of hearing loss configurations: normal hearing, presbycusis, and flat hearing loss groups (Figure 4). It was possible to obtain the MML and MRIL in all three groups at levels under the LDL for all the frequencies tested. For the normal-hearing group, the MML and MRIL seemed to require lower intensity levels for frequencies close to the tinnitus pitch (i.e., around 6000 Hz) compared with frequencies well above or below. For the presbycusis group, the levels required for obtaining MML and MRIL were stable across frequencies when considering the levels in dB HL but were most effective in the hearing loss region when considering the levels in dB SL. Finally, the MML and MRIL were also obtained in the flat hearing loss group despite significant severe hearing loss (around 80 dB HL thresholds for means across frequencies). For this group, the MML and MRIL values were more variable between frequencies than the other two groups.
Figure 4.
Results obtained at the Lyon site. Data were divided as a function of the audiogram shape. (a) Audiometric thresholds of the three groups. The MML, MRIL, and LDL expressed in dB SL are presented for each group separately, (b) normal audiograms group, (c) presbycusis-type audiograms, and (d) flat hearing loss. The secondary axis bar chart represents the distribution of predominant tinnitus frequency for each group. The error bars represent the standard error of the mean.
MML = minimum masking level; MRIL = minimum residual inhibition level; LDL = loudness discomfort level.
Frequency specificity on the MML and MRIL
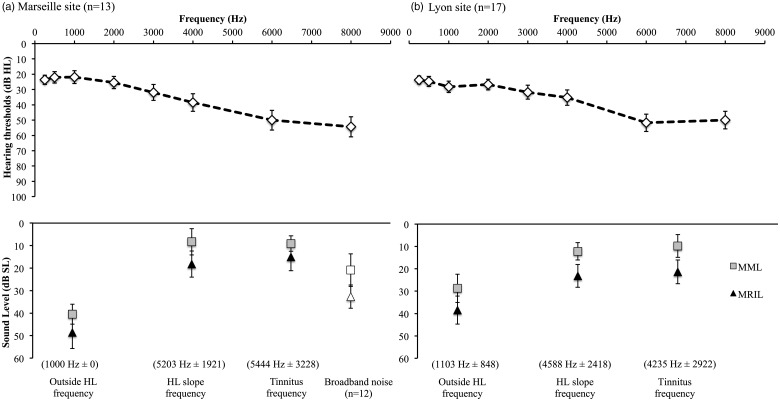
The impact of threshold shifts on the MML and MRIL and the effect of the center frequency of noise was investigated by comparing the levels, in dB SL, obtained when the noise was centered at the tinnitus frequency, at the frequency of the slope of the hearing loss, and at a frequency outside the region of the hearing loss for MML and MRIL. This is one of the only measurements that are common to both the Marseille and Lyon sites. A 2 × (2 × 3) mixed ANOVA that included the sites as the between-subject factors (Marseille, Lyon), and type of measure (i.e., MML, MRIL) and frequency of the noise (i.e., tinnitus frequency, HL slope frequency, outside HL frequency) as the within-subject factors was conducted. The analysis revealed a significant interaction between frequency and the sites, F(2, 80) = 9, p = .004 (higher values at tinnitus frequency and HL slope frequency for the Lyon site compared with Marseille site, Figure 5). As expected, there was a significant main effect of type of measure with MRIL requiring higher levels (mean difference: 9 dB) than MML, F(1,40) = 72.7, p < .001 (Figure 6(b)). Finally, there was a significant main effect of the frequency of the noise, F(2, 80) =28.2, p < .001. Post hoc tests revealed that a noise centred at the tinnitus frequency or the frequency of the slope showed significantly (p < .001) lower MML and MRIL intensity (in dB SL) compared with noise centred outside the HL frequency with mean differences of 24 and 21 dB SL, respectively (Figure 5). There was no significant difference between the tinnitus frequency and the frequency of the slope. More so, the MRIL and MML obtained with the broadband stimulation were significantly higher than the stimulation obtained for the tinnitus frequency and the HL slope frequency but significantly lower than the frequency outside the HL region (see Supplementary Table 2).
Figure 5.
Effects of central noise frequency on MML and MRIL. Comparison between the data collected in Marseille (a) for with those obtained in Lyon (b) for the three principal frequencies, that is, the tinnitus frequency, a frequency outside the hearing loss region (outside HL frequency), and a frequency in the slope of the hearing loss (HL slope frequency). Only participants with normal hearing or presbycusis hearing loss were included for both sites. The results obtained for the broadband noise (Marseille site only) was also added. The error bars represent the standard error of the mean.
MML = minimum masking level; MRIL = minimum residual inhibition level.
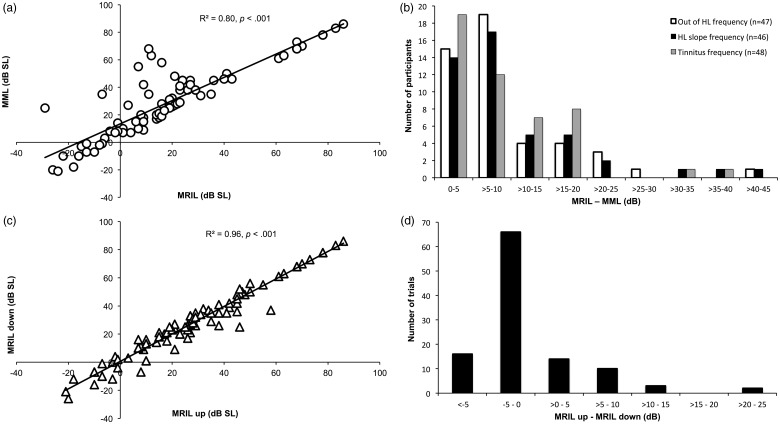
Figure 6.
(a) MRIL as a function of MML, Marseille only. (b) Distribution of the difference between MRIL and MML (data obtained from the two sites were pooled). (c) MRIL ascending as a function of MRIL descending, Marseille only. (d) Distribution of the difference between MRIL up and down, Marseille only.
MRIL = minimum residual inhibition level; MML = minimum masking level.
Relations between MML and MRIL
The relationship between MML and MRIL was investigated. Figure 6(a) displays the significant correlation between the MRIL and the MML for all the data points obtained from all the patients for all the conditions at the Marseille site (p < .001). The R2 value of .87 indicates that 87% of the variance of the MRIL is explained by the variance of the MML. Most of the data points are close to the sectional line suggesting that, for the majority of patients, the added level required to obtain the MRIL from the MML is stable (around 10 dB). Still, in a minority of cases, the added level required to obtain the MRIL was much higher than 10 dB above the MML. Similar high correlations between MML and MRIL levels were found for different stimulation frequencies (500 to 8000 Hz) at the Lyon site (see Supplementary Table 3). The mean differences between MRIL and MML for each frequency (500 to 8000 Hz) were all significant and varied from 6 to 13 dB. When merging the data from the two sites, the correlations between MML and MRIL for the three shared frequencies of stimulation (i.e., tinnitus frequency, HL slope frequency, and outside HL frequency) were also very high. The mean differences for the three frequencies of stimulation were significant and varied between 8.5 to 10.3 dB. Although most patients displayed mean differences around 10 dB, for those three frequencies, there were patients who displayed much higher levels as shown by the distribution of the mean differences (Figure 6(b)).
Relations between MRIL ascending and MRIL descending
The relationship between MRIL ascending and MRIL descending was investigated for all the data for all the conditions at the Marseille site. A paired-sample t test revealed no significant differences between the minimal level of MRIL ascending and descending, t(110) = 0.16, p = .87 (mean difference = 0.08, SD: 5.2). More so, the two measurements were highly correlated with an R2 value of .96 (Figure 6(c) and (d)).
Effects of Stimulation Duration on the MML and MRIL
Effect of stimulation duration
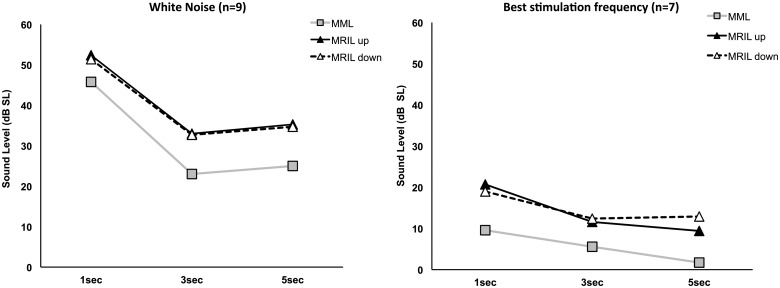
The effect of the duration of the noise on the MML and MRIL was investigated by comparing the levels obtained between 1, 3, and 5 s of stimulation. These measurements were only performed at the Marseille site. A (3 × 3) repeated measures ANOVA including type of measure (i.e., MML, MRIL up, MRIL down) and stimulation duration (i.e., 1, 3, and 5 s) as the within-subject factors was conducted for the broadband noise (n = 9) and best frequency (n = 7) groups separately. To note, the best frequency was the tinnitus frequency for five patients and the frequency of the slope of the HL for the remaining two. Only patients who performed all the conditions within each group (broadband noise or best frequency) were kept for the statistical analysis. The analysis revealed that the MML was obtained at lower levels than the MRIL (up and down) for all three durations (1, 3, and 5 s) for both the broadband noise (Figure 7(a)) and the best stimulation frequency (Figure 7(b)). The mean difference between MML and MRIL (up and down, all duration) was 8.5 dB for the broadband noise (min: −3, max: 27) and 8.7 dB for the best frequency (min: −6, max: 42; all ps ≤ .01).
Figure 7.
Effects of noise duration on MML and MRIL. Data obtained from the Marseille site, for broadband (a) and narrowband noises (1 octave width) centered at the frequency at which MRIL was minimum (best stimulation frequency) (b). The error bars represent the standard error of the mean.
MML = minimum masking level; MRIL = minimum residual inhibition level.
Interestingly, a significant main effect of stimulus duration was also found for the broadband noise, F(2,16) = 9.14, p = .002, but not for the best frequency, F(2,12) = 3.3, p = .07. Following the stimulus duration main effect for the broadband noise, the post hoc tests revealed that the 1-s stimulus required significantly higher intensity levels than the 3 s but only marginally for 5 s (mean difference = 20.3 dB, p = .03; mean difference = 18.1 dB, p = .076, respectively). More so, some patients reported that 1 s was too short to be able to perform the MML task (i.e., to determine at what level the tinnitus was masked by the noise). Indeed, out of the 34 patients in total tested in Marseille, 16 patients reached the intensity level limit, set at 95 dB SPL, for the 1-s duration broadband noise stimulation compared with 1 and 2 patients only for the 3 and 5 s durations, respectively.
Clinical Management
The new RI paradigm versus the classic approach
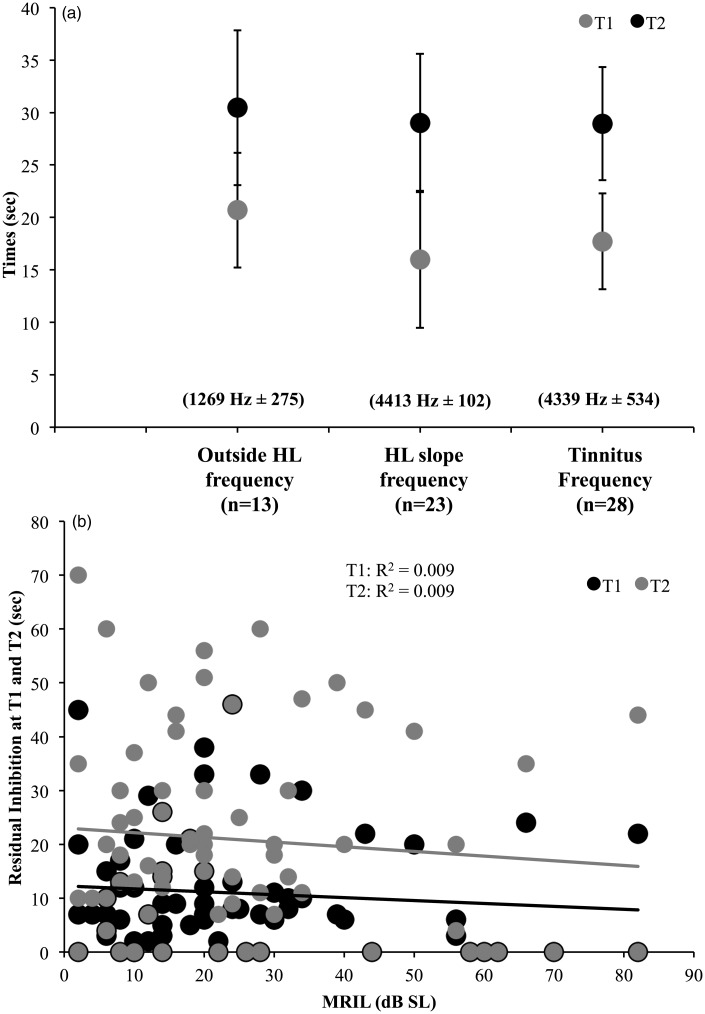
The classic RI technique that consists of presenting a high-intensity broadband noise (10 dB over the MML) for 1 min and assessing the time when the tinnitus reappears (T1) and returns to normal levels (T2) after the cessation of the noise was measured only at the Lyon site. As the classic RI technique requires a significant amount of testing time, it was tested only at one or two frequencies per patient. Still, it was possible to compare the results obtained for three different center noise frequencies (i.e., tinnitus frequency, HL slope frequency, and outside HL frequency) using a between-group analysis. Importantly, there were no significant differences between the three center noise frequencies for T1 and T2 (Fs < 1; Figure 8(a)). When merging all the data for all the three frequencies, the time at T1 and T2 differed significantly by around 10.7 s in mean, t(80) = 6.6, p < .001. More so, T1 and T2 were highly correlated (r = .86, p < .001). The relationship between the classic RI technique and the MRIL was investigated: There were no significant correlations between the old technique (T1 and T2) and the MRIL (Figure 8(b)).
Figure 8.
(a) Duration of the RI when using the classic RI paradigm for three different centered noises (i.e., noises centered at the tinnitus frequency, at the frequency of the slope, or a frequency outside of the hearing loss). T1 is the time for the tinnitus to reappear, and T2 is the time for the tinnitus to come back to its normal or original level. (b) T1 and T2 of the classic RI paradigm as a function of MRIL. The error bars represent the standard error of the mean.
RI = residual inhibition; MRIL = minimum residual inhibition level.
Patient sound preferences
Twenty-five patients were asked about their preferred sound at the Marseille site only; the preferences were chosen as follows: 10 preferred the frequency of the slope of the HL (40%), 8 the broadband noise (32%), 3 the tinnitus frequency (12%), 3 had no real preferences (12%), and 1 preferred equally the broadband noise and the frequency of the slope (4%). The frequency outside of the hearing loss region was not reported to be the preferred frequency by any of the patients.
Discussion
The main objective of the current study was to validate the feasibility and the clinical applicability of a new method using a pulsed noise of fixed interstimulus intervals to measure tinnitus masking and RI. These measures were easily and reliably obtained at two different sites, for several types of hearing loss configurations, under monaural and binaural conditions. The assessment at the Marseille site had, as a first objective, to explore the effects of stimulus duration and spectrum of the new method, while the main objective pursued at the Lyon site was to validate the clinical applicability of the method.
Overall, tinnitus masking was obtained in at least one condition for all of the 64 tinnitus patients tested except one (98.5%), and some level of RI was obtained for 59 patients (86.7%). For most patients, tinnitus masking and RI were achieved on multiple occasions and for different conditions indicative of the robustness and reliability of the new technique. The prevalence of tinnitus masking and RI reported here was similar to what has been shown in previous studies with prevalence values around 94% (Mitchell, 1983; Roberts et al., 2008) for the former, and values between 70% and 88% for the latter (Henry & Meikle, 2000; Roberts et al., 2008; Vernon & Meikle, 2003). Moreover, many of the properties of RI uncovered by earlier research with the classic method were observed with the new method. For example, these include (a) RI optimal (MRIL in dB SL lowest) when the center frequency of the masker is at, or near, the dominant tinnitus frequency, which is also the hearing loss region when threshold shifts are present (Mitchell, 1983; Roberts et al., 2008); (b) this principle holds in notched cases, where threshold shifts and tinnitus frequencies coincide (Eggermont & Roberts, 2015); (c) RI is observed when MRIL is <20 dB higher than the MML (Roberts et al., 2008; Terry et al., 1983); (d) RI duration increases with masker duration (Terry et al., 1983); (e) tinnitus increases were seen after masking in a small minority of patients (Roberts et al., 2006; Sedley et al., 2012). These results suggest that this new technique is, at least, as effective as the classic method to achieve tinnitus masking and RI while requiring less effort from the patient and shorter amounts of time. It is noteworthy that RI assessed by our procedure could be obtained using short bursts of noise (≤5 s), while a unique presentation of noise for 10 s has been reported to produce RI in only one tinnitus participant over six (Terry et al., 1983). This result suggests that the repeated stimulus sequence (pulsed noise) may have a fast (within minutes) cumulative action on the auditory system, which may lower MRIL and extend RI duration during the course of the experiment.
In terms of stimulation duration, the 3- and 5-s stimulation provided both optimal masking and inhibition, compared with the 1-s stimulation. This result indicates that 3 s is close to the optimal stimulus duration for clinical use: It is long enough to produce RI and short enough allowing several stimulus conditions to be tested. More so, patients reported that the MML task was more difficult to perform with the 1-s stimulation because the duration of the noise was reported as too short to evaluate with confidence if the tinnitus was masked or not. As this was not reported for the 3- and 5-s stimulation, the 3-s stimulation could thus be considered the best stimulation duration option for maximizing time without compromising effectiveness. Moreover, the best stimulus to maximize masking and RI was investigated. In agreement with previous literature, the best center noise frequencies requiring the lowest levels of stimulation to produce MML and MRIL were the ones at the tinnitus frequency and at the frequency of the hearing loss slope. Those results will be discussed in turn.
RI Subgroups
The measurement of MRIL at the Marseille site allowed a better refinement of RI outcomes into different subgroups. Indeed, not all tinnitus patients had the same RI outcome, most classified as positive and others as negative. The positive outcome groups included patients for which total, partial, or persistent tinnitus suppression was reached. For the total and partial inhibition subgroups, tinnitus came back to normal level only a few seconds after the cessation of the stimulation. For persistent inhibition, however, the suppression by those patients lasted from minutes to hours, which is not an uncommon phenomenon (Feldmann, 1971; Roberts et al., 2008; Vernon & Meikle, 2003). The negative outcome groups, on the other hand, included patients for whom the tinnitus loudness increased during the stimulation. These cases, although rare, were also reported in previous studies (Henry & Meikle, 2000; Roberts et al., 2008; Vernon & Meikle, 2003). Moreover, our results show that the difference between MML and MRIL is around 10 dB in most patients but not all. Indeed, in a substantial number of patients, the MRIL is well above the MML, by 15 dB or more in some conditions. Indeed, around 40.5% of all patients who were tested with the three centered noise conditions had at least one value (MRIL minus MML) of more than 15 dB. We propose that the difference between MML and MRIL in each patient may also be used to group/categorize patients.
Clear distinct clinical profiles of each individual subgroup could not be achieved by the comparative analysis of the sociodemographic and psychoacoustic characteristics. The same experience with a larger number of patients might lead to the establishment of distinct clinical features for each subgroup. Still, there was a significant trend for the HTs. Indeed, the partial inhibition group had the highest HTs between all subgroups. It is thus plausible that the elevated HTs in this group have prevented sufficient stimulation to affect the auditory system to achieve total suppression of tinnitus. One can presume that a higher level of stimulation, exceeding the limit of the equipment used in the Marseille site (95 dB SPL), might lead to total suppression of tinnitus in this subgroup. This argument is consistent with the fact that total RI could be achieved in tinnitus patients with moderate to severe flat hearing losses at the Lyon site. Alternatively, the lack of RI might also be explained by the presence of inner hair cell dead regions that are most prevalent in the presence of steeply sloping high-frequency hearing loss (Moore, 2004; see later for possible mechanisms of RI). Previous studies have observed the presence of inner hair cells dead regions in tinnitus cohorts with and without the presence of HTs elevations (Etchelecou, Coulet, Derkenne, Tomasi, & Noreña, 2011; Kiani, Yoganantha, Tan, Meddis, & Schaette, 2013; Weisz, Hartmann, Dohrmann, Schlee, & Norena, 2006). Another interesting feature of the partial inhibition subgroup was the highest tinnitus duration in years compared with other groups. Finally, increased tinnitus loudness in the presence of moderate- to high-level background noises is a well-known complaint of some tinnitus sufferers. The new RI method could thus be used to objectify this complaint.
Possible RI Mechanisms
The underlying mechanisms of tinnitus masking and RI are still unknown. Still one can presume that any auditory stimulus is associated to a given pattern of neural activity in the auditory pathways during the stimulation and also produces some durable neural changes after the stimulation ceases (Alves-Pinto, Baudoux, Palmer, & Sumner, 2010; Bleeck, Sayles, Ingham, & Winter, 2006; Harris & Dallos, 1979; Ingham, Itatani, Bleeck, & Winter, 2016; Javel, 1996; Nelson, Smith, & Young, 2009; Relkin & Turner, 1988; Smith, 1977; Young & Sachs, 1973). Tinnitus masking might thus provide some insight into the overlap or fusion between the stimulus-related activity and the tinnitus-related activity (TRA), while RI, on the other hand, might reflect whether an acoustic stimulation can durably change the TRA in a way that the tinnitus percept is reduced or even suppressed, at least, for a certain amount of time. One possible mechanism of RI might be neural adaptation at peripheral or central levels after acoustic stimulation (Galazyuk, Voytenko, & Longenecker, 2016). In brief, adaptation is usually defined as a decrease of neuronal discharges, at a single or population level, during stimulation (Pérez-González & Malmierca, 2014). The typical pattern of stimulus-induced activity is a fast increase in discharge rates at stimulus onset, followed by a rapid decrease (rapid adaptation) within a few milliseconds after stimulus onset and another smaller and slower reduction in discharge rate several seconds later (long-term adaptation; Javel, 1996). Studies have shown that adaptation can last for a while after prolonged acoustic stimulation, thereby affecting both stimulus-evoked and spontaneous neuronal discharge rates (Galazyuk et al., 2016; Javel, 1996; Young & Sachs, 1973). The pulsed noise stimulation of the current method could thus provoke rapid and long-term adaptation leading to a decrease in evoked and spontaneous activity. The reduction of spontaneous activity would be perceived as a reduction or suppression of tinnitus during the silent periods. The depth of inhibition would thus reflect the depth of neural adaptation: Partial to complete inhibition would result in partial to complete tinnitus suppression. Interestingly, smaller reductions in the discharge rates that accumulate over a period of minutes have also been shown (Javel, 1996; Young & Sachs, 1973) and could possibly be a correlate of persistent tinnitus inhibition. Indeed, Young and Sachs (1973) have also reported that occasionally, some fibers displayed a cumulative decrease of discharge rates over several presentations of their paradigm. The level of discharge was reported to be lower than the preexposure one. This effect occurred despite a quiet period of 60 to 80 s between the different presentations of the paradigm, suggesting that the effect can last for more than a minute. The cumulative effect of the adaptation process might thus reduce the TRA at a level where the signal becomes unnoticeable, that is, hidden within the background activity. More so, although less prevalent, enhancement of discharge rates instead of adaptation or reduction in discharge a few milliseconds to seconds after stimulus onset has also been reported (Galazyuk et al., 2016). This phenomenon could possibly explain the enhancement of tinnitus loudness in some rare patients during the pulsed noise presentation. Overall, the different types of neurophysiological adaptation (or absence of adaptation) could reflect different types of RI outcomes obtained in tinnitus patients.
Alternatively, one can also imagine that a prolonged acoustic stimulation may alter the functional properties of the tinnitus-related central network durably, by modifying the synaptic weights or inhibitory neurotransmission, for instance. As a consequence of these modifications, the tinnitus-related network may shift from a pathological state, associated to an abnormal neural activity (i.e., the TRA) to a tinnitus-free state. The network may stay in this configuration for a certain amount of time until the tinnitus network retrieves its initial condition (Tass & Popovych, 2012).
Intriguingly, tinnitus can sometimes disappear for hours after a prolonged acoustic stimulation (Vernon & Meikle, 2003). The TRA is very small, just above the background activity, but is still detected and associated to an auditory percept possibly because the central nervous system is trained to detect this small signal: Tinnitus can be interpreted by patients as an alert signal, and this focuses attention on it. Selective attention has been shown to modify pure tone detection (Greenberg & Larkin, 1968; Scharf, Quigley, Aoki, Peachey, & Reeves, 1987). Indeed, the percentage of correct detected pure tones was lower when the frequency of the tone was unexpected (i.e., different from the target). A pure tone is thus better detected, at lower levels, when participants expect this specific target. This result is suggestive of a modulatory influence of focused attention on the sensitivity of the auditory system. Thus, the transient reduction of the TRA may be enough to hinder the ability of the high-level cortical areas to track down the TRA and focus attention on it. We propose that this process may contribute to explaining why tinnitus can disappear for hours after stimulus presentation.
The results obtained for the different center noise frequencies, at both the Marseille and Lyon sites, revealed that tinnitus masking and RI are the most effective when the noise is centered at, or close to, the tinnitus frequency. This effect was previously reported for tinnitus masking (Feldmann, 1971; Mitchell, 1983) as well as for RI using the classic method (Roberts et al., 2006, 2008). As previously reported (Eggermont & Roberts, 2015), this principle also holds in terms of notched hearing loss where the threshold shifts and the tinnitus frequencies coincide. From the current data, it seems that, for the normal-hearing patients, the TRA resembles a relatively wide bell-shaped curve, with the top of the curve corresponding to the dominant pitch of tinnitus. This result argues in favor of stimulating at/or close to the tinnitus frequency even in the absence of audiometric hearing loss to have a stronger effect on the TRA. If tinnitus is related to abnormal activity within a specific frequency region of the auditory system, the pulsed stimulation is thus more efficient to interact with this activity when centered close to the tinnitus frequency. Conversely, the results obtained using the classic method in Lyon did not reveal any frequency specificity effects for the classic method when measuring the duration of RI for different frequencies. Indeed, at both T1 and T2, the duration was similar between noises centered at the tinnitus frequency, at a frequency within the hearing loss slope, and at a frequency outside the hearing loss region. These results were not consistent with those of Roberts et al. (2008). The variability of RI duration combined with the small sample and the between-group comparisons might explain the absence of frequency specificity in the present report. Indeed, the study by Roberts et al. (2008) tested 59 participants and reported a significant frequency effect for both, RI depth and duration, with deeper and longer lasting inhibition for characteristic frequencies in the ranges 3 to 10 kHz. Still, they reported a large variability between participants for both measures.
Importantly, measuring the MRIL in decibels from our approach rather than the RI depth and duration from the classic method might thus be a more sensitive method to detect small effect. In addition, the duration of RI using the classic method and the MRIL does not correlate, suggesting that they may measure different attributes of the same phenomenon. The MRIL results confirmed the widely used clinical standard of adding 10 dB above the MML to generate RI. Still, there was some patients displaying differences of more than 15 dB between the MRIL and the MML in one or more of the three different noises centred frequency condition (approximately 21%, 22%, and 19% of all patients for noise centred at the tinnitus frequency, the HL slope frequency, and outside the HL region, respectively). More so, only two patients displayed a difference more or equal to 15 dB in all three conditions; all the other patients with a 15-dB difference in one condition showed a difference of less than 15 in at least one of the two other conditions. Finally, although MML and MRIL are closely related, MML cannot invariably predict MRIL, as some patients with reliable MML could not achieve RI.
Clinical Management
The new technique offers several advantages over the classic RI technique for clinical use. First, the measurements of HT, MML, MRIL, and LDL can be all achieved within the same sequence of stimulation for each frequency. Indeed, at the Lyon site, the pulsed noise was first presented at an inaudible level and was then slightly increased to measure, in a sequential order, these four measures by only changing the instructions given to the patient. This allows the clinician to maximize the collection of clinical information within a reduced testing time period. Interestingly, the MML and MRIL measures could be achieved on many different hearing loss configurations using either binaural or monaural stimulation. Of high clinical interest is the measurement of MML and MRIL in moderately severe to severe hearing loss cases. Indeed, the classic testing of RI is usually not performed in those patients to avoid (a) discomfort and (b) possible hearing damage caused by the high-intensity stimulation presented for a relative long period of time (e.g., 30 s to 1 min of stimulation at MML + 10 dB intensity level). The new technique developed here thus offers the possibility to test RI quickly in those patients avoiding discomfort and hearing damage. More so, MML and MRIL measurements could be measured at intensity levels under LDLs even when they were very low (see special cases, Figure 3 for an example). The MML and MRIL were also achievable by stimulating the contralateral ear of two patients with unilateral severe hearing loss and bilateral tinnitus. One reported persistent complete RI bilaterally and the other one, partial inhibition. Tinnitus suppression by contralateral masker presentation has been reported in a few rare cases (Feldmann, 1971; Vernon, 1977), but this procedure has also been shown to be ineffective (Terry et al., 1983; Vernon, 1977). Different results from homolateral versus contralateral masker presentation may provide information on the origin (peripheral vs. central) of tinnitus generation. Indeed, homolateral RI in a unilateral tinnitus case might suggest a peripheral origin of the tinnitus in a given patient, while contralateral RI might argue in favor of a central origin. This hypothesis deserves further investigation. Finally, the expression of MRIL in decibels compared with RI depth or duration used in the classic method offers several clinical advantages. The value of RI can now be compared or correlated to other psychoacoustic and physiological measures widely used in clinics such as HT, LDL, and tinnitus loudness for the former and stapedial reflex thresholds or auditory evoked potentials for the latter. This may, in turn, reveal unknown associations between RI and those measurements, which can provide a better understanding of the mechanisms underlying RI.
The new RI method might also provide several therapeutic implications. First, this new technique might guide the clinician and the patient in their choice for the best treatment. Indeed, a patient with a total or persistent RI during the clinical examination could be given a very good prognostic for any acoustic treatments such as, for instance, hearing aids or acoustic maskers. Conversely, a patient with increased tinnitus loudness during the RI would be given a poor prognostic for acoustic treatments and could be thus suggested to try another type of treatment.
In addition, the pulsed noise signal, by itself, could be used as a therapeutic signal. Indeed, similar to programming a hearing aid by balancing speech comprehension and listening comfort, the pulsed noise signal could be customized for each individual patient to balance tinnitus inhibition and listening comfort. If the RI is effective and well adapted in a given patient, the signal could be, at least, used during a tinnitus crisis or before going to bed for the patient to have some control over their symptoms, in a similar fashion to taking a pill before or during headaches. At best, there is also the possibility that long-term exposition to the pulsed noise for several hours every day for several weeks might help reduce tinnitus loudness, extend RI duration, or lower MML and MRIL, or even suppress tinnitus permanently. Indeed, we can speculate that the signal may impact the TRA in several ways. First, the stimulus sequence may have a lasting impact on the central auditory nervous system, by restoring part of the sensory inputs lost with cochlear damages or shifting the tinnitus-related network to a tinnitus-free state (Noreña, 2011; Noreña & Chery-Croze, 2007; Noreña & Eggermont, 2005; Noreña & Farley, 2012; Tass & Popovych, 2012; Schaette & Kempter, 2006). In addition, by suppressing tinnitus during periods of stimulation and silence, the signal may contribute in reducing the salience of the tinnitus-related neural networks and in fine allows the brain to release its attention from the tinnitus-related signal. Interestingly, tinnitus sound-based treatments have been shown to reduce MML and extend RI duration (Davis, Wilde, Steed, & Hanley, 2008; Hanley, Davis, Paki, Quinn, & Bellekom, 2008; McKinney, Hazell, & Graham, 1999; Vernon & Meikle, 2003). The putative long-term effects of the stimulus sequence would need further investigation.
Conclusion
The new RI method allows the quick assessment of absolute thresholds, MML, MRIL, and LDL. We have shown, in broad agreement with previous studies, that RI is stronger (lower MRIL from our approach) within the frequency region close to the tinnitus frequency. While the mechanisms of MRIL are not well understood, we believe that this should not prevent clinicians from measuring it. On the contrary, we suggest that more studies should be dedicated to elucidate RI underlying mechanisms and provide more insights into the clinical utility of this measure. Indeed, we believe that the MRIL may provide crucial prognostic information on clinical approaches based on acoustic stimulation. Moreover, the procedure in itself leading to transient tinnitus reduction can be very helpful for reassuring tinnitus patients and preventing an escalation of negative thoughts and reactions if used properly during the counseling process. Finally, the stimulus sequence used for assessing the MRIL (or derived from it) may also be used as therapeutic acoustic stimulation. We hope that the simplicity and rapidity of this new approach may contribute in launching new research projects on RI.
Supplemental Material
Supplemental material, Supplementary Tables for A New Method for Assessing Masking and Residual Inhibition of Tinnitus by Philippe Fournier, Anne-Flore Cuvillier, Stéphane Gallego, Fabien Paolino, Michel Paolino, Anne Quemar, Alain Londero and Arnaud Norena in Trends in Hearing
Acknowledgments
The authors thank Victoria Milloy for a thorough revision of the article.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been conducted with the financial assistance of CNRS, Aix-Marseille Université, B2V, and the supplementary pension institution of Klesia. Philippe Fournier was supported by the Fonds de Recherche du Québec – Santé and by the Canadian Institute of Health Research.'
Supplemental Material
Supplementary material for this article is available online.
References
- Adjamian P., Sereda M., Zobay O., Hall D. A., Palmer A. R. (2012) Neuromagnetic indicators of tinnitus and tinnitus masking in patients with and without hearing loss. Journal of the Association for Research in Otolaryngology: JARO 13(5): 715–731. doi:10.1007/s10162-012-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Pinto A., Baudoux S., Palmer A. R., Sumner C. J. (2010) Forward masking estimated by signal detection theory analysis of neuronal responses in primary auditory cortex. Journal of the Association for Research in Otolaryngology: JARO 11: 477–494. doi:10.1007/s10162-010-0215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile C. É., Fournier P., Hutchins S., Hébert S. (2013) Psychoacoustic assessment to improve tinnitus diagnosis. PLoS One 8(12): e82995 doi:10.1371/journal.pone.0082995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeck S., Sayles M., Ingham N. J., Winter I. M. (2006) The time course of recovery from suppression and facilitation from single units in the mammalian cochlear nucleus. Hearing Research 212: 176–184. doi:10.1016/j.heares.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Davis P. B., Wilde R. A., Steed L. G., Hanley P. J. (2008) Treatment of tinnitus with a customized, dynamic acoustic neural stimulus: Underlying principles and clinical efficacy. Trends in Amplification 12(3): 210–222. doi:10.1177/1084713808319942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont J. J., Roberts L. E. (2015) Tinnitus: Animal models and findings in humans. Cell Tissue Research 361: 311–336. doi:10.1007/s00441-014-1992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchelecou M. C., Coulet O., Derkenne R., Tomasi M., Noreña A. J. (2011) Temporary off-frequency listening after noise trauma. Hearing Research 282(1): 81–91. doi:10.1016/j.heares.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Feldmann H. (1971) Homolateral and contralateral masking of tinnitus by noise-bands and by pure tones. Audiology 10: 138–144. [DOI] [PubMed] [Google Scholar]
- Fournier P., Hébert S. (2013) Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: Does tinnitus fill in the gap? Hearing Research 295: 16–23. doi:10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Galazyuk A. V., Voytenko S. V., Longenecker R. J. (2016) Long-lasting forward suppression of spontaneous firing in auditory neurons: Implication to the residual inhibition of tinnitus. Journal of the Association for Research in Otolaryngology: JARO 18(2): 343–353. doi:10.1007/s10162-016-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg G. Z., Larkin W. D. (1968) Frequency-response characteristic of auditory observers detecting signals of a single frequency in noise: The probe-signal method. Journal of the Acoustical Society of America 44: 1513–1523. doi:10.1121/1.1911290. [DOI] [PubMed] [Google Scholar]
- Hanley P. J., Davis P. B., Paki B., Quinn S. A., Bellekom S. R. (2008) Treatment of tinnitus with a customized, dynamic acoustic neural stimulus: Clinical outcomes in general private practice. Annals of Otology, Rhinology & Laryngology 117(11): 791–799. [DOI] [PubMed] [Google Scholar]
- Harris D. M., Dallos P. (1979) Forward masking of auditory nerve fiber responses. Journal of Neurophysiology 42(4): 1083–1107. doi:10.1152/jn.1979.42.4.1083. [DOI] [PubMed] [Google Scholar]
- Hazell J. W., Wood S. (1981) Tinnitus masking-a significant contribution to tinnitus management. British Journal of Audiology 15: 223–230. doi:10.3109/03005368109081442. [DOI] [PubMed] [Google Scholar]
- Hébert S., Fournier P., Noreña A. (2013) The auditory sensitivity is increased in tinnitus ears. Journal of Neuroscience 33: 2356–2364. doi:10.1523/JNEUROSCI. 3461-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Meikle M. B. (2000) Psychoacoustic measures of tinnitus. Journal of the American Academy of Audiology 11: 138–155. [PubMed] [Google Scholar]
- Ingham N. J., Itatani N., Bleeck S., Winter I. M. (2016) Enhancement of forward suppression begins in the ventral cochlear nucleus. Brain Research 1639: 13–27. doi:10.1016/j.brainres.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javel E. (1996) Long-term adaptation in cat auditory-nerve fiber responses. The Journal of the Acoustical Society of America 99: 1040–1052. doi:10.1121/1.414633. [DOI] [PubMed] [Google Scholar]
- Kiani F., Yoganantha U., Tan C. M., Meddis R., Schaette R. (2013) Off-frequency listening in subjects with chronic tinnitus. Hearing Research 306: 1–10. doi:10.1016/j.heares.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Langguth B., Goodey R., Azevedo A., Bjorne A., Cacace A., Crocetti A., Vergara R. (2007) Consensus for tinnitus patient assessment and treatment outcome measurement: Tinnitus research initiative meeting, Regensburg, July 2006. Progress in Brain Research 166: 525–536. doi:10.1016/S0079-6123(07)66050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Fortnum H., Dawes P., Middleton H., Munro K. J., Moore D. R. (2014) The prevalence of tinnitus and the relationship with neuroticism in a middle-aged UK population. Journal of Psychosomatic Research 76: 56–60. doi:10.1016/j.jpsychores.2013.08.018. [DOI] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Somerset S., Hall D. (2016) A systematic review of the reporting of tinnitus prevalence and severity. Hearing Research 337: 70–79. doi:10.1016/j.heares.2016.05.009. [DOI] [PubMed] [Google Scholar]
- McKinney, C., Hazell, J., & Graham, R. (1999). An evaluation of the TRT method. In Jonathan Hazell (Ed.), Proceedings of the Sixth International Tinnitus Seminar, Jonathan Hazell, Cambridge, UK (pp. 99–105). London: Tinnitus and Hyperacusis Centre.
- Mitchell C. (1983) The masking of tinnitus with pure tones. Audiology 22: 73–87. [DOI] [PubMed] [Google Scholar]
- Moffat G., Adjout K., Gallego S., Thai-Van H., Collet L., Noreña A. J. (2009) Effects of hearing aid fitting on the perceptual characteristics of tinnitus. Hearing Research 254: 82–91. doi:10.1016/j.heares.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Moore B. C. (2004) Dead regions in the cochlea: Conceptual foundations, diagnosis, and clinical applications. Ear and Hearing 25(2): 98–116. doi:10.1097/01.aud.0000120359.49711.d7. [DOI] [PubMed] [Google Scholar]
- Nelson P. C., Smith Z. M., Young E. D. (2009) Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. Journal of Neuroscience 29: 2553–2562. doi:10.1523/JNEUROSCI.5359-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena A., Micheyl C., Chéry-Croze S., Collet L. (2002) Psychoacoustic characterization of the tinnitus spectrum: Implications for the underlying mechanisms of tinnitus. Audiology and Neurootology 7: 358–369. doi:10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- Noreña A. J. (2011) An integrative model of tinnitus based on a central gain controlling neural sensitivity. Neuroscience & Biobehavioral Reviews 35: 1089–1109. doi:10.1016/j.neubiorev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Noreña A. J. (2015) Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. Audiology and Neurootology 20(Suppl 1): 53–59. doi:10.1159/000380749. [DOI] [PubMed] [Google Scholar]
- Noreña A. J., Chery-Croze S. (2007) Enriched acoustic environment rescales auditory sensitivity. Neuroreport 18: 1251–1255. doi:10.1097/WNR.0b013e3282202c35. [DOI] [PubMed] [Google Scholar]
- Noreña A. J., Eggermont J. J. (2005) Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. Journal of Neuroscience 25: 699–705. doi:10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña A. J., Farley B. J. (2012) Tinnitus-related neural activity: Theories of generation, propagation, and centralization. Hearing Research 295: 161–171. doi:10.1016/j.heares.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Ortmann M., Müller N., Schlee W., Weisz N. (2011) Rapid increases of gamma power in the auditory cortex following noise trauma in humans. European Journal of Neuroscience 33: 568–575. doi:10.1111/j.1460-9568.2010.07542.x. [DOI] [PubMed] [Google Scholar]
- Pérez-González D., Malmierca M. S. (2014) Adaptation in the auditory system: An overview. Frontiers in Integrative Neuroscience 8: 19 doi:10.3389/fnint.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel J.-L., Guitton M. J. (2007) Salicylate-induced tinnitus: Molecular mechanisms and modulation by anxiety. Progress in Brain Research 166: 141–146. doi:10.1016/S0079-6123(07)66012-9. [DOI] [PubMed] [Google Scholar]
- Relkin E. M., Turner C. W. (1988) A reexamination of forward masking in the auditory nerve. The Journal of the Acoustical Society of America 84: 584–591. doi:10.1121/1.396836. [DOI] [PubMed] [Google Scholar]
- Roberts L. E., Moffat G., Baumann M., Ward L. M., Bosnyak D. J. (2008) Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. Journal of the Association for Research in Otolaryngology 9: 417–435. doi:10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. E., Moffat G., Bosnyak D. J. (2006) Residual inhibition functions in relation to tinnitus spectra and auditory threshold shift. Acta Oto-Laryngologica 126(Suppl. 556): 27–33. doi:10.1007/s10162-008-0136-9. [DOI] [PubMed] [Google Scholar]
- Schaette R. (2013) Tinnitus in men, mice (as well as other rodents), and machines. Hearing Research 311: 63–71. doi:10.1016/j.heares.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Schaette R., Kempter R. (2006) Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: A computational model. European Journal of Neuroscience 23: 3124–3138. doi:10.1111/j.1460-9568.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- Schaette R., König O., Hornig D., Gross M., Kempter R. (2010) Acoustic stimulation treatments against tinnitus could be most effective when tinnitus pitch is within the stimulated frequency range. Hearing Research 269(1): 95–101. doi:10.1016/j.heares.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Scharf B., Quigley S., Aoki C., Peachey N., Reeves A. (1987) Focused auditory attention and frequency selectivity. Attention, Perception, & Psychophysics 42: 215–223. doi:10.3758/BF03203073. [DOI] [PubMed] [Google Scholar]
- Sedley W., Gander P. E., Kumar S., Oya H., Kovach C. K., Nourski K. V., Griffiths T. D. (2015) Intracranial mapping of a cortical tinnitus system using residual inhibition. Current Biology 25: 1208–1214. doi:10.1016/j.cub.2015.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedley W., Teki S., Kumar S., Barnes G. R., Bamiou D. E., Griffiths T. D. (2012) Single-subject oscillatory gamma responses in tinnitus. Brain 135(10): 3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S., Zhou J., Koehler S. (2007) Neural mechanisms underlying somatic tinnitus. Progress in Brain Research 166: 107–123. doi:10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L. (1977) Short-term adaptation in single auditory nerve fibers: Some poststimulatory effects. Journal of Neurophysiology 40: 1098–1111. doi:10.1121/1.2002474. [DOI] [PubMed] [Google Scholar]
- Tass P. A., Popovych O. V. (2012) Unlearning tinnitus-related cerebral synchrony with acoustic coordinated reset stimulation: Theoretical concept and modelling. Biological Cybernetics 106: 27–36. doi:10.1007/s00422-012-0479-5. [DOI] [PubMed] [Google Scholar]
- Terry A. M., Jones D. M., Davis B. R., Slater R. (1983) Parametric studies of tinnitus masking and residual inhibition. British Journal of Audiology 17: 245–256. doi:10.3109/03005368309081485. [DOI] [PubMed] [Google Scholar]
- Vernon J. (1977) Attempts to relieve tinnitus. Ear and Hearing 2(4): 124–131. [PubMed] [Google Scholar]
- Vernon J. A., Meikle M. B. (2003) Tinnitus: Clinical measurement. Otolaryngologic Clinics of North America 36: 293–305. doi:10.1016/S0030-6665(02)00162-7. [DOI] [PubMed] [Google Scholar]
- Weisz N., Hartmann T., Dohrmann K., Schlee W., Norena A. (2006) High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hearing Research 222(1): 108–114. doi:10.1016/j.heares.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Weisz N., Moratti S., Meinzer M., Dohrmann K., Elbert T. (2005) Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Medicine 2: e153 doi:10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Sachs M. B. (1973) Recovery of detection probability following sound exposure: Comparison of physiology and psychophysics. Journal of the Acoustical Society of America 54: 1544–1553. doi:10.1121/1.1914452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary Tables for A New Method for Assessing Masking and Residual Inhibition of Tinnitus by Philippe Fournier, Anne-Flore Cuvillier, Stéphane Gallego, Fabien Paolino, Michel Paolino, Anne Quemar, Alain Londero and Arnaud Norena in Trends in Hearing