Abstract
Clinical isolates are important to antimicrobial resistance surveillance efforts because clinically ill animals are the direct targets of antimicrobial treatments. Thus, clinical data may provide a surveillance tool for identifying emerging resistance threats. The purpose of this study was to describe resistance trends in Escherichia coli and Salmonella spp. from clinically ill animals over time and evaluate the utility of these laboratory data as a passive surveillance tool. Susceptibility results of isolates from chickens, swine, and cattle recovered between 2007 and 2015 at a major veterinary diagnostic laboratory in Ontario, Canada were analyzed. Relative to other antimicrobials tested, visible trends highlighted high resistance to ampicillin and tetracycline in chicken E. coli, consistently high resistance to tetracycline, sulfisoxazole, and ampicillin among swine isolates, and an increase in cattle E. coli resistant to ampicillin and cephalothin over time. While the data show potential for use in surveillance, there are limitations of such a clinical dataset for predicting overall trends and guiding empirical treatment decisions.
Résumé
Surveillance passive de l’antibiorésistance dans les isolats de Salmonella et d’Escherichia coli chez le bétail de l’Ontario, 2007–2015. Les isolats cliniques sont importants pour la surveillance de l’antibiorésistance parce que les animaux cliniquement malades sont les cibles directes des traitements antimicrobiens. Par conséquent, les données cliniques peuvent fournir un outil de surveillance pour identifier les nouvelles menaces de résistance. Le but de cette étude consistait à décrire dans le temps les tendances de résistance d’Escherichia coli et de Salmonella spp. chez les animaux cliniquement malades et d’évaluer l’utilité de ces données de laboratoire en tant qu’outil de surveillance passive. On a analysé les résultats de susceptibilité des isolats récupérés entre 2007 et 2015 auprès de poulets, de porcs et de bovins dans un grand laboratoire de diagnostic vétérinaire en Ontario, au Canada. Pour les antimicrobiens testés, les tendances visibles ont souligné une résistance importante d’E. coli à l’ampicilline et à la tétracycline chez les poulets, une résistance importante constante à la tétracycline, au sulfisoxazole et à l’ampicilline parmi les isolats des porcs et, chez les bovins, une progression d’E. coli résistant à l’ampicilline et à la céphalothine dans le temps. Même si les données montrent un potentiel d’utilisation pour la surveillance, il y a des limitations pour un tel ensemble de données cliniques en vue de la prédiction des tendances générales et de l’orientation des décisions de traitement empiriques.
(Traduit par Isabelle Vallières)
Introduction
Antimicrobial resistance (AMR) is a major public health concern. The emergence of antimicrobial resistant bacteria in food animals has the potential to increase exposure of humans via foodborne transmission, and the transfer of resistance genes from bacteria of animal origin to those of pathogenic significance to humans (1,2). For example, Escherichia coli is a common commensal bacterium of both humans and animals, but pathogenic variants can cause urinary tract infections, neonatal meningitis, nosocomial bacteremia, childhood enteritis, and traveler’s diarrhea in humans (3). Similarly, nontyphoidal Salmonella is a major cause of foodborne illness and bloodstream infections in humans (4). Salmonella and pathogenic variants of E. coli can also have economic impacts and cause severe illness among food-producing animals, including chickens, swine, and cattle. For example, E. coli can cause diarrhea in calves and young pigs (5,6). In poultry, E. coli related respiratory and systemic infections were among the most commonly reported indications for antibiotic therapy by Ontario veterinarians (7). The potential inability to treat such infections as a result of antimicrobial resistance warrants further research and surveillance across the farm-to-fork continuum.
National surveillance programs such as the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) and the National Antimicrobial Resistance Monitoring System (NARMS) in the United States are used to monitor resistance trends among specific pathogens or indicator species through both active and passive means (8,9). Use of data from large veterinary diagnostic laboratories for passive surveillance of AMR trends has been attempted previously. Quebec established a passive surveillance program in 1993 through the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ), utilizing data from the Faculté de médecine vétérinaire diagnostic laboratory at the Université de Montréal (10). This program played a role in supporting the association made by CIPARS between ceftiofur use in ovo in the provincial poultry industry and cephalosporin resistance in chicken and human Salmonella spp. isolates between 2005 and 2008 (11). More recently, data from the MAPAQ passive surveillance program were used to investigate the determinants of resistance to antimicrobials used at the hatchery level in Quebec, which lead to the identification of a likely association between spectinomycin-lincomycin use and gentamicin resistance (12). A study examining susceptibility data from the Animal Health Laboratory (AHL) of the University of Guelph for the swine pathogens E. coli F4, Pasteurella multocida, and Streptococcus suis found that there were challenges with recording consistency, data management, and the number of isolates submitted for resistance testing. Nonetheless, it was concluded that some of the data could be used for temporal analyses in a prospective surveillance system for these swine pathogens (13).
Passive surveillance has limitations as the data may not be representative of the general animal or bacterial population, and potential biases exist with sample submission (14). However, clinical isolates are important to surveillance efforts because clinically ill animals are the direct targets of antimicrobial treatments, and therefore bacterial isolates from these animals potentially undergo the greatest selective pressure (15). Passive surveillance is also thought to be less costly compared to active surveillance, and can provide valuable information as an early warning system for emerging resistance threats (14). Having an indication of the emerging resistance trends within veterinary clinical isolates in Ontario could better inform policy decisions and prevention efforts.
The objectives of this study were to assess the availability and utility, in terms of passive surveillance, of antimicrobial susceptibility data for E. coli and Salmonella spp. in chickens, cattle, and swine in the AHL database, and to describe trends in resistance over time. Additionally, the results of the Ontario AHL Salmonella spp. data were compared to the results of the veterinary clinical Salmonella spp. data from the CIPARS program for the period 2007 to 2013 to help assess the utility of clinical isolate data from the AHL for surveillance purposes in Ontario.
Materials and methods
Antimicrobial susceptibility test results were obtained from the AHL, along with the following data fields: “date of submission,” “species,” “commodity code,” “age,” “age units,” “sample type,” “client sample ID,” “history,” “case type,” “client postal code,” and “owner postal code.” Data were obtained for all Salmonella spp. and E. coli isolates from chicken, swine, and cattle submissions between 2007 and 2015 (see Table 1 for sample sizes). All susceptibility testing was done using the Kirby-Bauer disk diffusion method, according to the most recent Clinical and Laboratory Standards Institute (CLSI) guidelines for antimicrobial zone diameter breakpoints (16).
Table 1.
Total number and range of yearly isolations of Salmonella spp. and E. coli isolates tested for susceptibility at the Animal Health Laboratory (May 2007 to December 2015).
| Salmonella spp. | E. coli | |||
|---|---|---|---|---|
|
|
|
|||
| Species | Total number | Range | Total number | Range |
| Chicken | 501 | 17 to 90 | 7465 | 216 to 1250 |
| Swine | 548 | 38 to 102 | 671 | 45 to 101 |
| Cattle | 540 | 34 to 81 | 2695 | 219 to 370 |
All isolates from samples submitted from outside of Ontario and all isolates identified as research cases were excluded from the analysis for all species groups. Duplicate isolates which contained the same sample submission ID were also excluded from the analysis to avoid sampling bias from including non-independent observations. Results reported as “intermediate” were grouped with the “susceptible” isolates in order to remain consistent with the methods used in other surveillance systems (8).
Data analysis
All data analyses were carried out in Excel 2010 (Microsoft Corporation, Redmond, Washington, USA) and STATA version 14 (STATA Corporation, College Station, Texas, USA). Descriptive statistics were used to assess which data fields could potentially be included in a prospective surveillance system. Prevalence of resistance was tabulated by year for all antimicrobials with ≥ 10 susceptibility test results in each species in each year between 2007 and 2015. These included ampicillin, sulfisoxazole, gentamicin, kanamycin, trimethoprim-sulfamethoxazole, tetracycline, and ceftiofur. Resistance patterns over time were described graphically for E. coli isolates using moving average plots modeled as 12-month weighted averages including 6 previous months and 5 following months, as previously described (17).
For all Salmonella spp. isolates, temporal changes in susceptibility were compared to corresponding clinical data from CIPARS using the 2-sample Z-test for proportions. Significance was set at P < 0.05. The CIPARS non-human clinical data were extracted from annual reports between 2007 and 2013. These data included a national distribution with all 10 provinces submitting isolates at some point over the study period, with a range of 105 to 342 isolates per commodity (including chickens, swine, and cattle) tested for susceptibility each year.
Results
Suitability of antimicrobial susceptibility data for surveillance
While the number of E. coli isolates with susceptibility test results was over 600 for each animal species, the number of Salmonella spp. isolates was much smaller (Table 1). Thus, it was decided that there were insufficient Salmonella spp. data to assess temporal trends. However, temporal trends were compared to the results of the clinical Salmonella spp. data from CIPARS to compare surveillance outcomes.
Some data fields were found to have too many missing values to be useful for analysis or surveillance (Table 2), while others were limited in their use due to inconsistency in data recording (e.g., free text fields). The “client postal code” field was consistently recorded, but only reflected the submitting veterinary clinic and therefore was limited in its use as each clinic could serve a substantial number of producers in a large geographic area. The “sample type” field was also consistently recorded, but commonly only specified “tissue” or “swab” and thus was also of limited use in analysis.
Table 2.
Percent of laboratory Salmonella spp. and E. coli submissions that had recorded values for selected data fields (May 2007 to December 2015).
| Percent of recorded values | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Salmonella spp. | E. coli | |||||
|
|
|
|||||
| Data field | Chickens | Swine | Cattle | Chickens | Swine | Cattle |
| Client postal code | 100 | 100 | 100 | 100 | 100 | 100 |
| Owner postal code | 8 | 28 | 37 | 12 | 31 | 50 |
| Age | 89 | 50 | 56 | 89 | 55 | 38 |
| Age units | 90 | 55 | 60 | 88 | 61 | 80 |
| Commodity | 100 | 77 | 84 | 100 | 79 | 82 |
| Sample type | 100 | 100 | 100 | 100 | 100 | 100 |
Escherichia coli temporal resistance trends
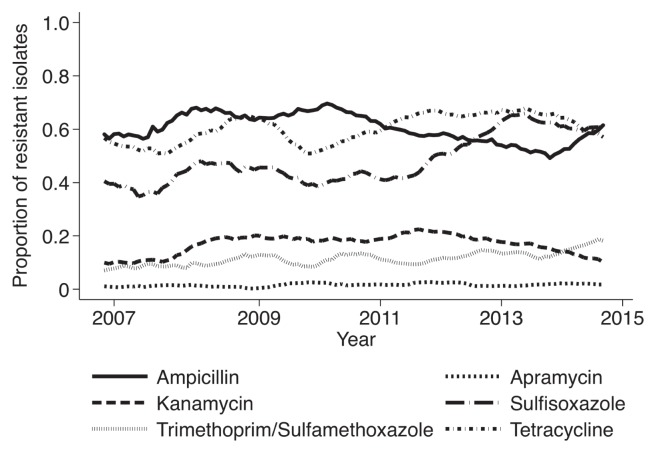
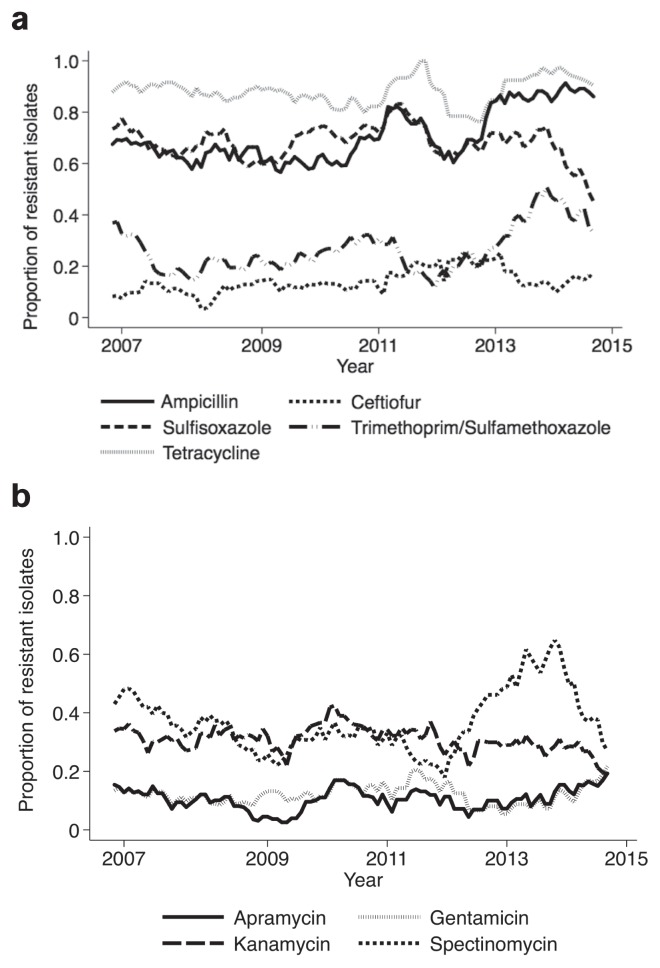
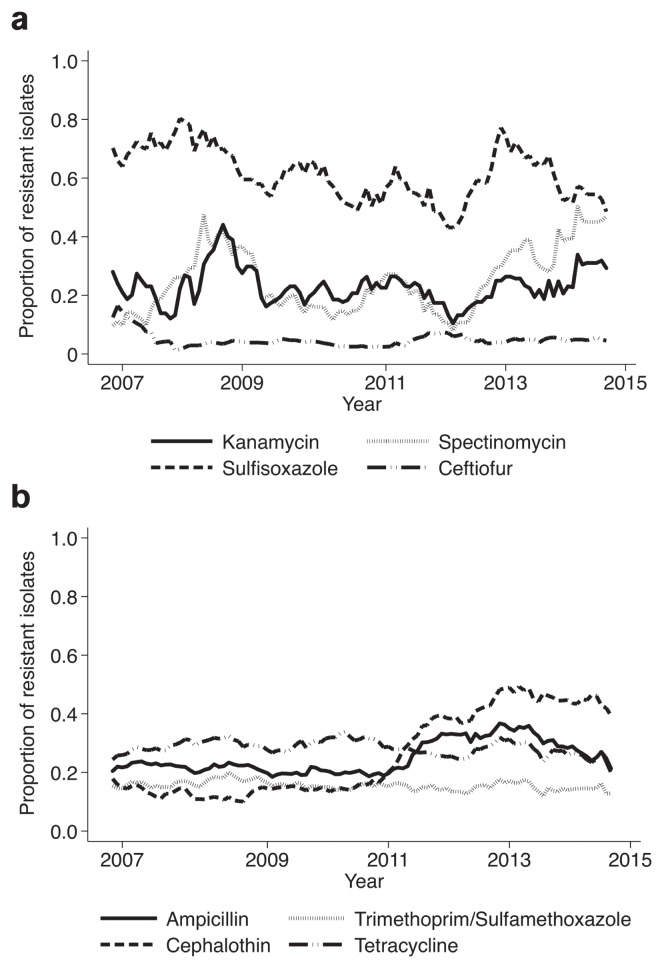
Temporal resistance trends for E. coli from chickens, swine, and cattle are shown in Figures 1, 2, and 3, respectively. Notable findings for E. coli chicken isolates include resistance to ampicillin and tetracycline that was consistently over 50%; and resistance to sulfisoxazole, which increased gradually from 39% in 2007 to 60% in 2015. The percentage of swine E. coli isolates resistant to ampicillin, sulfisoxazole, and tetracycline was consistently over 50% (Figure 2a). The percentage of cattle E. coli isolates resistant to sulfisoxazole fluctuated between 54% and 73% (Figure 3a). There was an increase in the prevalence of cattle E. coli resistance to ampicillin and cephalothin between 2011 and 2014, from 20% to 34% and from 15% to 45%, respectively (Figure 3b).
Figure 1.
Moving average plot of the proportion of chicken E. coli isolates resistant to select antimicrobials between 2007 and 2015.
Figures 2a, b.
Moving average plot of the proportion of swine E. coli isolates resistant to select antimicrobials between 2007 and 2015.
Figures 3a, b.
Moving average plot of the proportion of cattle E. coli isolates resistant to select antimicrobials between 2007 and 2015.
Comparison of resistance trends in Salmonella spp. with CIPARS data
Chicken and swine Salmonella spp. isolates did not show significant differences in resistance patterns in most years when compared with clinical data from CIPARS (Tables 3 and 4). However, there were significant differences in susceptibility test results for gentamicin and kanamycin among all 3 species groups (Tables 3–5). Cattle isolates had comparable results until 2011, at which point there was a greater number of significant differences showing fewer resistant isolates among the AHL data compared with the CIPARS data (P < 0.05) (Table 5).
Table 3.
Proportion of chicken Salmonella spp. isolates resistant to selected antimicrobials in the AHL and CIPARS (C) datasets (May 2007 to December 2013).
| Ampicillin | Sulfisoxazole | Gentamicina | Kanamycina | Trim/Sulfa | Tetracycline | Ceftiofur | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||
| Year | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C |
| 2007 | 0.1515 | 0.1620 | 0.0606 | 0.0290 | 1.0000b | 0.0290 | 1.0000b | 0.0290 | 0.0000 | 0.0000 | 0.1515 | 0.1330 | 0.1515 | 0.1330 |
| 2008 | 0.1556 | 0.2110 | 0.1034 | 0.0530 | 1.0000b | 0.0240 | 1.0000b | 0.0140 | 0.0000 | 0.0000 | 0.1222 | 0.1820 | 0.1000 | 0.1630 |
| 2009 | 0.1646 | 0.1071 | 0.1013b | 0.0429 | 1.0000b | 0.0179 | 1.0000b | 0.0000 | 0.0127 | 0.0000 | 0.1266 | 0.1500 | 0.1013 | 0.0857 |
| 2010 | 0.1910 | 0.2047 | 0.1098 | 0.0731 | 1.0000b | 0.0877 | 1.0000b | 0.0146 | 0.0112 | 0.0088 | 0.0667b | 0.2076 | 0.1011 | 0.1374 |
| 2011 | 0.2754b | 0.1197 | 0.0580 | 0.0247 | 1.0000b | 0.0704 | 1.0000b | 0.0704 | 0.0145 | 0.0070 | 0.2029 | 0.2183 | 0.1739 | 0.0915 |
| 2012 | 0.2456 | 0.1925 | 0.1053 | 0.0435 | 1.0000b | 0.0062 | 1.0000b | 0.0186 | 0.0351 | 0.0062 | 0.1754 | 0.1615 | 0.1754 | 0.1491 |
| 2013 | 0.2895 | 0.2253 | 0.1579 | 0.0769 | 1.0000b | 0.0604 | 1.0000b | 0.0330 | 0.0263b | 0.0000 | 0.2895 | 0.1868 | 0.2632 | 0.2143 |
CIPARS reports in vitro susceptibility results for aminoglycosides, while the AHL reports them as resistant based on clinical effectiveness.
Indicates a significant difference by the Z-test (P < 0.05).
Table 4.
Proportion of swine Salmonella spp. isolates resistant to selected antimicrobials in the AHL and CIPARS (C) datasets (May 2007 to December 2013).
| Ampicillin | Sulfisoxazole | Gentamicina | Kanamycina | Trim/Sulfa | Tetracycline | Ceftiofur | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||
| Year | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C |
| 2007 | 0.5682 | 0.5030 | 0.6591 | 0.6680 | 1.0000b | 0.0270 | 1.0000b | 0.2890 | 0.0909 | 0.1930 | 0.6364 | 0.7060 | 0.0000 | 0.0210 |
| 2008 | 0.5789 | 0.4490 | 0.7105 | 0.5890 | 1.0000b | 0.0190 | 1.0000b | 0.1770 | 0.0000b | 0.0950 | 0.6053 | 0.6580 | 0.0263 | 0.0130 |
| 2009 | 0.5283 | 0.4420 | 0.7358 | 0.6640 | 1.0000b | 0.0440 | 1.0000b | 0.2570 | 0.0943 | 0.1590 | 0.6792 | 0.7390 | 0.0189 | 0.0400 |
| 2010 | 0.5472 | 0.4468 | 0.5882 | 0.5532 | 1.0000b | 0.0383 | 1.0000b | 0.1830 | 0.0755 | 0.1362 | 0.6415 | 0.6596 | 0.0000 | 0.0596 |
| 2011 | 0.5965 | 0.4922 | 0.6842 | 0.6062 | 1.0000b | 0.0415 | 1.0000b | 0.1762 | 0.0351 | 0.0985 | 0.6316 | 0.6943 | 0.0000 | 0.0311 |
| 2012 | 0.6304 | 0.4980 | 0.6739 | 0.5800 | 1.0000b | 0.0310 | 1.0000b | 0.1370 | 0.0435 | 0.0940 | 0.7174 | 0.7250 | 0.0652 | 0.0240 |
| 2013 | 0.4833 | 0.4660 | 0.5932 | 0.6350 | 1.0000b | 0.0410 | 1.0000b | 0.1720 | 0.0500 | 0.1420 | 0.6667 | 0.7230 | 0.1167b | 0.0340 |
CIPARS reports in vitro susceptibility results for aminoglycosides, while the AHL reports them as resistant based on clinical effectiveness.
Indicates a significant difference by the Z-test (P < 0.05).
Table 5.
Proportion of cattle Salmonella spp. isolates resistant to selected antimicrobials in the AHL and CIPARS (C) datasets (May 2007 to December 2013).
| Ampicillin | Sulfisoxazole | Kanamycina | Trim/Sulfa | Tetracycline | Ceftiofur | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| Year | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C | AHL | C |
| 2007 | 0.1071 | 0.2140 | 0.1429 | 0.2070 | 1.0000b | 0.1290 | 0.0357 | 0.0210 | 0.1607 | 0.2430 | 0.0357 | 0.0210 |
| 2008 | 0.2593 | 0.3280 | 0.2933 | 0.3430 | 1.0000b | 0.2390 | 0.0617 | 0.0670 | 0.2716 | 0.3280 | 0.0617 | 0.0450 |
| 2009 | 0.4048 | 0.5650 | 0.4048 | 0.5500 | 1.0000b | 0.3820 | 0.0476 | 0.0760 | 0.3810 | 0.5110 | 0.0714 | 0.0760 |
| 2010 | 0.5294 | 0.5105 | 0.6000 | 0.5594 | 1.0000b | 0.4476 | 0.0294 | 0.0839 | 0.5000 | 0.5455 | 0.0588 | 0.1818 |
| 2011 | 0.4211b | 0.5847 | 0.3929b | 0.6356 | 1.0000b | 0.3644 | 0.0175 | 0.0678 | 0.3333b | 0.6102 | 0.0351 | 0.1186 |
| 2012 | 0.2222b | 0.5030 | 0.2639b | 0.5820 | 1.0000b | 0.2600 | 0.0833 | 0.0400 | 0.2778b | 0.5990 | 0.0972b | 0.2660 |
| 2013 | 0.1385b | 0.6130 | 0.1538b | 0.6610 | 1.0000b | 0.2220 | 0.0615 | 0.0560 | 0.1538b | 0.6450 | 0.0615b | 0.4270 |
CIPARS reports in vitro susceptibility results for aminoglycosides, while the AHL reports them as resistant based on clinical effectiveness.
Indicates a significant difference by the Z-test (P < 0.05).
Discussion
For E. coli isolates from poultry, similar susceptibility results to those presented here were found with the MAPAQ program: for ampicillin and tetracycline, an average of 52% and 61% of isolates, respectively, had resistance between 2011 to 2015 (10). In contrast, Huang et al (18) found that 79% of clinical chicken E. coli isolates from veterinary laboratories across the US were resistant to tetracycline, while only 40% of isolates were resistant to ampicillin. The MAPAQ program found that resistance of E. coli to trimethoprim-sulfamethoxazole was below 20%, which is also consistent with the results of this study (10).
Escherichia coli isolates from swine had a slight increase in resistance to most antimicrobials tested over time, but resistance was generally higher for ampicillin, sulfisoxazole, and tetracycline. While MAPAQ does not include sulfisoxazole on their testing panel, their results were comparable, with the highest resistance rates reported for tetracycline, followed by ampicillin (10).
A case-control study for post-weaning E. coli diarrhea, which looked at antimicrobial use and resistance among swine nursery farms selected from the AHL database, found that apramycin was the most commonly used water-soluble medication, while the most common injectable antimicrobial used was trimethoprim sulfadoxine on case farms (6). Varga et al (19) found that in-feed chlortetracycline use was significantly associated with ampicillin and tetracycline resistance among generic E. coli isolates from finishing swine farms in Alberta.
Resistance results for bovine E. coli appeared to be more variable than those for swine and chickens. The prevalence of resistance to sulfisoxazole in E. coli was also reported to be high by MAPAQ in 2015, with 64% of isolates showing resistance (10). Among E. coli isolated from diarrheal disease in calves in the US, isolates were found to be resistant to β-lactams, including the extended spectrum cephalosporins, aminoglycosides, sulphonamides, tetracycline, and fluoroquinolones (5).
The similarity in resistance patterns evident between the AHL and CIPARS data in chicken and swine Salmonella isolates was expected, as the isolates tested by the AHL were also sent to the CIPARS laboratory for susceptibility testing, and included in their national surveillance program. The difference in the proportion of isolates resistant to gentamicin and kanamycin is likely due to the difference in interpretation used by the AHL and CIPARS. According to the CLSI guidelines, Salmonella spp. isolates should always be reported as resistant to aminoglycosides because these antimicrobials are not clinically effective for treating Salmonella spp. (16). However, major surveillance programs such as CIPARS and NARMS report in vitro susceptibility results for aminoglycosides in order to monitor resistance trends (8,9).
The differences in resistance patterns in Salmonella spp. observed in the data from this study compared to results from CIPARS surveillance over the same time period may also be due to regional differences in commodity groups and disease patterns represented by the respective data sets. For example, while the Ontario cattle industry is weighted heavily toward dairy production, the national CIPARS program includes data from major beef-producing regions in western Canada (20).
While testing for antimicrobial resistance at the genetic level is now considered one of the most sensitive, specific, and rapid methods for detecting resistance, the Kirby-Bauer method used by the AHL as well as the microbroth dilution method used by CIPARS remain standard phenotypic testing modalities (21). However, these methods are not uniform across laboratories, and differences in methodology may lead to inconsistencies in the resulting data.
Comparison of the AHL data with active surveillance data from CIPARS’ Ontario region may be useful to further explore the utility of these diagnostic data. Previous comparisons of CIPARS’ active and passive surveillance data demonstrated the importance of passive surveillance in detecting rare and emerging resistance phenotypes of Salmonella compared with active surveillance (14).
There are limitations in the use of the laboratory data for passive surveillance. As the AHL is the largest diagnostic veterinary laboratory in Ontario, it is potentially the most representative of clinically ill livestock in Ontario compared with other laboratories in the province. However, extrapolation of these data to all Ontario livestock must be done with caution, as the samples submitted for diagnostic testing are inherently biased toward more severe/unusual infections or those that failed to respond to initial treatment. This may ultimately overestimate the true prevalence of resistance in clinical isolates in the general livestock population in Ontario.
The small number of isolates tested each year may also limit the external validity of the results. While it is challenging to quantify an adequate sample size for surveillance (22), the greater the number of isolates included, the greater the statistical power. Since some years included fewer than 30 Salmonella spp. isolates, this may not be a large enough sample size to make inferences about resistance trends over time. Nonetheless, MAPAQ’s passive surveillance program uses similar sample sizes for their Salmonella spp. isolates (10). The challenge of having few isolates tested per year could be overcome by combining a range of years together in order to compare cumulative data. However, this would limit the ability to detect yearly changes in the data.
The utility of data from a clinical diagnostic laboratory such as the AHL could be improved by offering incentives for veterinarians to provide complete submission data and enforcing the provision of these incentives. Recording consistency could then be further improved by training of laboratory staff and innovations to streamline standardized data entry without interfering with or creating undue burden on day-to-day operations. For example, use of a standardized checklist with defined sample sources, such as “liver,” “heart,” “yolk sac,” and “bone marrow” could improve the consistency of data recording. This could also be done by using a standardized electronic submission form, or by requiring that lab staff use a standardized dropdown list for data entry. Some items, such as “sample source” or “commodity group,” could be included as required fields on an electronic submission form. Caprioli et al (15) further recommend that details on animal husbandry as well as animal identification for cattle be included with submission of clinical isolates in order to track resistance with animal movement.
Results for the chicken data highlight high levels of E. coli resistance to ampicillin and tetracycline. Data for swine indicate consistently high resistance rates to tetracycline, sulfisoxazole, and ampicillin. While the data trends for cattle are less well-defined, the results indicate an increase in E. coli resistance to ampicillin and cephalothin since 2011. These trends may in part be associated with antimicrobial use patterns; however, there are many other factors to consider, including stage of production, type of operation, animal husbandry factors, in addition to infection control and biosecurity measures. Nonetheless, the purpose of this study was not to provide recommendations on antimicrobial use, but rather to inform surveillance efforts and describe resistance trends.
Use of pre-existing data from diagnostic laboratories is advantageous in that it does not require any additional time or effort on the part of producers or veterinarians. However, the utility of the data for surveillance purposes could be significantly improved by encouraging veterinarians to provide complete and accurate submission data, and working with laboratory staff to improve consistency of data recording. Further investigation of trends in the prevalence of resistance within different commodity groups is warranted to better characterize the changes and drivers of resistance. Further research is also needed to identify additional pathogens from the AHL database that could be included in a passive surveillance system. Methods for routine dissemination of results, while clearly acknowledging the limitations of such surveillance, must also be established.
Acknowledgments
The authors thank Dr. Durda Slavic (Animal Health Laboratory) and Dr. Jane Parmley (Public Health Agency of Canada) for their assistance while conducting this study, and for critically reviewing this manuscript. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Berglund B. Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infect Ecol Epidemiol. 2015;5:28564. doi: 10.3402/iee.v5.28564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manges AR, Johnson JR. Foodborne origins of Escherichia coli causing extraintestinal infections. Clin Infect Dis. 2012;55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- 3.Von Baum H, Marre R. Antimicrobial resistance of Escherichia coli and therapeutic implications. Int J Med Microbiol. 2005;295:503–511. doi: 10.1016/j.ijmm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Antimicrobial Resistance: Global Report on Surveillance: 2014 summary. [Last accessed April 10, 2018]. Available from: http://www.who.int/drugresistance/documents/surveillancereport/en/
- 5.Bradford PA, Petersen PJ, Fingerman IM, White DG. Characterization of expanded-spectrum cephalosporin resistance in E. coli isolates associated with bovine calf diarrhoeal disease. J Antimicrob Chemother. 1999;44:607–610. doi: 10.1093/jac/44.5.607. [DOI] [PubMed] [Google Scholar]
- 6.Amezcua R, Friendship RM, Dewey CE, Gyles C, Fairbrother JM. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can J Vet Res. 2002;66:73–78. [PMC free article] [PubMed] [Google Scholar]
- 7.Ontario veterinary stewardship of antibiotic use in food-producing animals. Ontario Veterinary Medical Association; c2015. [Last accessed April 10, 2018]. The College of Veterinarians of Ontario Growing Forward 2 Project. Available from: https://www.ovma.org/assets/1/6/CVO_AMR_Report.pdf. [Google Scholar]
- 8.Government of Canada. Public Health Agency of Canada [homepage on the internet] Guelph, Ontario: c2007. [Last accessed April 10, 2018]. Canadian Integrated Program for Antimicrobial Resistance Surveillance. [updated 2007 July 23]. Available from: http://www.phac-aspc.gc.ca/cipars-picra/about_ov-eng.php. [Google Scholar]
- 9.United States of America Government. National Antimicrobial Resistance Monitoring System US Food and Drug Administration [homepage on the internet] Silver Spring, Maryland: c1996–2016. [Last accessed April 10, 2018]. [updated 2016 November 18]. Available from: http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ [Google Scholar]
- 10.Le Ministère de l’Agriculture, des Pêcheries, et de l’Alimentation du Québec. Programme québécois d’antibiosurveillance vétérinaire : Rapport 2015 résultat de la surveillance passive de l’antibiorésistance. c2015. [Last accessed April 10, 2018]. Available from: http://www.mapaq.gouv.qc.ca/fr/Productions/santeanimale/maladies/antibio/antibioresistance/Pages/resultats_surveillance.aspx.
- 11.Dutil L, Irwin R, Finley R, et al. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis. 2010;16:48. doi: 10.3201/eid1601.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalmers G, Cormier AC, Nadeau M, Côté G, Reid-Smith RJ, Boerlin P. Determinants of virulence and of resistance to ceftiofur, gentamicin, and spectinomycin in clinical Escherichia coli from broiler chickens in Québec, Canada. Vet Microbiol. 2017;203:149–157. doi: 10.1016/j.vetmic.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Glass-Kaastra SK, Pearl DL, Reid-Smith RJ, et al. Antimicrobial susceptibility of Escherichia coli F4, Pasteurella multocida, and Streptococcus suis isolates from a diagnostic veterinary laboratory and recommendations for a surveillance system. Can Vet J. 2014;55:341–348. [PMC free article] [PubMed] [Google Scholar]
- 14.Mather AE, Reeve R, Mellor DJ, et al. Detection of rare antimicrobial resistance profiles by active and passive surveillance approaches. PLoS One. 2016;11:e0158515. doi: 10.1371/journal.pone.0158515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caprioli A, Busani L, Martel JL, Helmuth R. Monitoring of antibiotic resistance in bacteria of animal origin: Epidemiological and microbiological methodologies. Int J Antimicrob Agents. 2000;14:295–301. doi: 10.1016/s0924-8579(00)00140-0. [DOI] [PubMed] [Google Scholar]
- 16.Patel JB, Cockerill FR, Bradford PA. Performance Standards for Antimicrobial Susceptibility Testing. M100-S25. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 17.Glass-Kaastra SK, Pearl DL, Reid-Smith RJ, et al. Surveillance of antimicrobial resistance in clinical isolates of Pasteurella multocida and Streptococcus suis from Ontario swine. Can J Vet Res. 2014;78:241–249. [PMC free article] [PubMed] [Google Scholar]
- 18.Huang TM, Lin TL, Wu CC. Antimicrobial susceptibility and resistance of chicken Escherichia coli, Salmonella spp., and Pasteurella multocida isolates. Avian Dis. 2009;53:89–93. doi: 10.1637/8268-021608-Reg.1. [DOI] [PubMed] [Google Scholar]
- 19.Varga C, Rajić A, McFall ME, et al. Associations between reported on-farm antimicrobial use practices and observed antimicrobial resistance in generic fecal Escherichia coli isolated from Alberta finishing swine farms. Prev Vet Med. 2009;88:185–192. doi: 10.1016/j.prevetmed.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Statistics Canada. Snapshot of Canadian agriculture. c2016. [Last accessed April 10, 2018]. [updated 2016 January 25]. Available from: http://www.statcan.gc.ca/pub/95-640-x/2011001/p1/p1-03-eng.htm.
- 21.Guidelines 3.1: Laboratory methodologies for bacterial antimicrobial susceptibility testing. Manual of Diagnostic Testing and Vaccines for Terrestrial Animals 2012 World Organization for Animal Health Available from: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.1_ANTIMICROBIAL.pdfLast accessed April 10, 2018s
- 22.Lewis D. Antimicrobial resistance surveillance: Methods will depend on objectives. J Antimicrob Chemother. 2002;49:3–5. doi: 10.1093/jac/49.1.3. [DOI] [PubMed] [Google Scholar]