Abstract
Two dogs from Quebec were diagnosed with granulocytic anaplasmosis. They both displayed fever, lethargy, and anorexia. Other clinical signs included vomiting, uveitis, polyarthritis, hepatomegaly, and splenomegaly. Thrombocytopenia, anemia, and lymphopenia were identified in both cases. Cytoplasmic inclusions were observed within neutrophils, and Anaplasma phagocytophilum infection was confirmed by polymerase chain reaction in both dogs.
Résumé
Anaplasmose granulocytaire chez deux chiens au Québec. Deux chiens originaires du Québec ont été diagnostiqués avec une anaplasmose granulocytaire. Les chiens ont manifesté de façon aiguë de la fièvre, un abattement et de l’anorexie. D’autres signes cliniques ont été observés incluant vomissement, uvéite, polyarthrite, hépatomégalie et splénomagalie. Une thrombocytopénie, une anémie et une lymphopénie ont été détectées chez les deux chiens. Des inclusions intracytoplasmques étaient également présentent dans les neutrophiles et l’infection à Anaplasma phagocytophilum a été confirmée par réaction d’amplification en chaîne par la polymérase chez les deux chiens.
(Traduit par les auteurs)
Canine granulocytic anaplasmosis (CGA) is a widely distributed zoonotic tick-borne disease (TBD). The causative agent, Anaplasma phagocytophilum, is an obligate intracellular Gram-negative bacterium that infects neutrophils. The bacterium is usually transmitted by Ixodes spp. ticks (1). The severity of the canine disease varies from mild subclinical to severe acute, and most frequent clinicopathological signs include fever, lethargy, anorexia, weight loss, musculoskeletal pain, thrombocytopenia, anemia, and lymphopenia (2–8).
Several methods are available to diagnose CGA including blood smear evaluation, serological testing, and DNA detection by polymerase chain reaction (PCR). However, each method has limitations and results depend on the stage of infection. Therefore, in some cases (1,9), multiple diagnostic modalities may be needed to maximize the likehood of reaching an accurate diagnosis and to confirm CGA. Diagnostic criteria for human granulocytic anaplasmosis (HGA) can be applied to dogs (1). Presumptive HGA is defined by suggestive clinical signs and laboratory findings together with detection of morulae within neutrophils or a single A. phagocytophilum antibody titer ≥ 640. Confirmation of HGA requires a 4-fold change in antibody titer or seroconversion, a positive PCR of blood, isolation of the bacterium from blood, or detection of antigen in tissue sample by immunohistochemistry (10).
In Canada, prevalence of A. phagocytophilum exposure in dogs ranges from 0.19% to 1.8% with highest rates recorded in Manitoba (0.75%), Saskatchewan (0.34%), and Ontario (1.8%) (11,12). Villeneuve et al (11) reported 0.09% of dogs seropositive in Quebec in contrast to 2 other studies that failed to detect seropositive dogs in this region (12,13). To date, only 5 cases of CGA have been confirmed by DNA detection in Vancouver Island (14,15) and Saskatoon (16). We describe here the first 2 autochtonous cases of CGA from Quebec.
Case descriptions
Case 1
A 10-year-old, 64.5-kg, male Great Pyrenees dog was presented for an acute onset of lethargy and anorexia. The presenting signs were observed 2 d before consultation. The owner also observed bilateral mucopurulent ocular discharge and polypnea. The dog lived outdoors and had access to the forest. He was regularly vaccinated and treated preventively against heartworm with ivermectin/pyrantel (Heartgard Plus; Mérial, Baie-d’Urfé, Québec). No travel outside of Quebec was reported. The dog was followed for hypothyroidism diagnosed 4 y earlier and treated with levothyroxin (Thyro-Tab; Lloyd, Peterborough, Ontario), 0.8 mg, PO, q24h.
Upon physical examination, the dog was alert but lethargic. Hyperthermia (40.2°C), tachycardia (120 beats/min), and polypnea (> 60 breaths/min) were recorded. No abnormalities were detected on thoracic auscultation and abdominal palpation. Pale mucous membranes and 7% dehydration were also noted. The dog had bilateral enophthalmos and mucopurulant ocular discharge. Ophthalmologic examination revealed conjunctival hyperemia and Tyndall effect in both eyes in addition to corneal edema, and blood and fibrin in the anterior chambers.
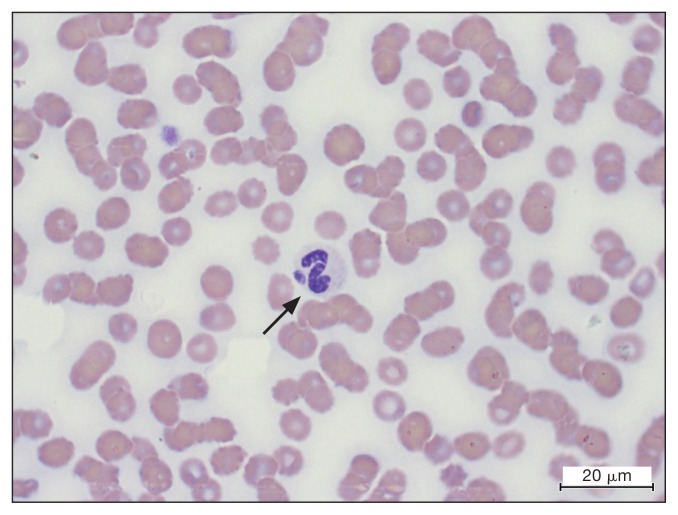
Blood tests including a biochemistry profile, a complete blood (cell) count (CBC), and coagulation tests and urinalysis were carried out. Biochemistry abnormalities included a mild decrease in total protein [56.4 g/L; reference interval (RI): 56.6 to 74.8 g/L] and albumin (23.50 g/L; RI: 29.1 to 39.70 g/L). Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were within reference ranges. Urinalysis did not reveal any relevant abnormalities. Hematologic changes included mild thrombocytopenia (102 × 109/L, RI: 143 to 400 × 109/L), mild lymphopenia (1.07 × 109/L, RI: 1.3 to 4.4 × 109/L), mild neutrophilia (8.4 × 109/L, RI: 3.9 to 8.0 × 109/L), and a moderate normocytic normochromic nonregenerative anemia [red blood (cell) count: 4.5 × 1012/L, RI: 5.7 to 8.8 × 1012/L; hemoglobin concentration: 100 g/L, RI: 129 to 184 g/L; hematocrit: 29%, RI: 37 to 57%]. Blood smear examination revealed a left shift of neutrophils with an increased band cell count (0.76 × 109/L, RI: 0.0 to 0.3 × 109/L) and metamyelocyte count (0.1 × 109/L, RI: 0.0 to 0.0 × 109/L). Toxic changes of neutrophils (presence of Dohle bodies) was also observed. Small oval basophilic intracytoplasmic inclusions measuring about 2 to 3 μm that were compatible with Anaplasma phagocytophilum or Ehrlichia ewingii morulae were present in a small number of neutrophils (Figure 1). A multiplex real-time PCR targeting the A. phagocytophilum msp2 gene was positive on blood, but the in-clinic qualitative SNAP 4DX was negative to Anaplasma spp. antibodies at first presentation.
Figure 1.
Inclusion (arrow) within a neutrophil on a blood smear from a dog with Anaplasma phagocytophilum infection. Modified Wright’s stain. Bar = 20 μm.
The dog was hospitalized for 3 d and received fluid therapy (Plasma-Lyte A; Baxter Healthcare, Alliston, Ontario), famotidine (Famotidine; Omega, Montreal, Quebec), 0.28 mg/kg body weight (BW), IV, q24h, and doxycycline (Apo-Doxy; Apotex, Toronto, Ontario), 10.9 mg/kg BW, PO, q24h. The uveitis was treated with atropine sulfate drops 1% (Atropine Alcon; Alcon, Mississauga, Ontario), q8h and dexamethasone/ neomycin/polymyxin B drops (Maxidrol; Alcon) q6h. The dog improved quicky with resolution of fever 8 h after the start of doxycycline and improvement of appetite after 24 h. He was discharged with at-home treatment consisting of doxycycline (Apo-Doxy; Apotex), 10.9 mg/kg BW, PO, q24h for a total of 4 wk, famotidine (Apo-Famotidine; Apotex), 0.6 mg/kg BW, PO, q24h for 1 wk, and treatment for uveitis (atropine q8h for 3 d followed by q24h for 1 mo as well as a progressively decreasing dose of dexamethasone). Preventive therapy against ectoparasites was advised each month or every 2 wk if ticks were still observed, especially between spring and autumn. A follow-up examination performed 4 wk after the first presentation did not reveal any clinical abnormalities. Signs of uveitis and CBC modifications had completely resolved. A second SNAP 4DX test was performed and was positive for Anaplasma spp., suggesting seroconversion. The owner was advised to stop the treatment for uveitis and to continue with the doxycyline therapy as prescribed.
Case 2
A 9-year-old neutered male Siberian husky dog was presented to his veterinarian for anorexia, apathy, and lameness of the left hind leg. The owner reported some episodes of diarrhea. The dog had never travelled outside of Quebec.
On physical examination, the dog had a fever (40.2°C) and pain on manipulation of the hips and left knee. No other significant physical abnormalities were recorded. A CBC was performed and revealed a marked thrombocytopenia (43 × 109/L, RI: 148 to 484 × 109/L), a mild decrease in hematocrit (35.7%, RI: 37.3% to 61.7%) with the red blood cell count (5.70 × 1012/L, RI: 5.65 to 8.87 × 1012/L) and hemoglobin concentration (135 g/L, RI: 131 to 205 g/L) at the low end of the reference interval. Mild leukopenia (4.6 × 109/L, RI: 5.1 to 16.8 × 109/L) secondary to lymphopenia (0.3 × 109/L, RI: 1.1 to 5.1 × 109/L) and eosinopenia (0 × 109/L, RI: 0.1 to 1.2 × 109/L) was also recorded. A complete biochemistry profile did not reveal any abnormality. An in-clinic SNAP 4 DX PLUS test (IDEXX Laboratories; Westbrook, Maine, USA) was negative. Clinical signs did not improve with Tramadol (Apo-Tramadol; Apotex, Toronto, Ontario) 2.3 mg/kg BW, PO, q24h. Two days after the first examination, the dog was presented to the Faculté de Medecine Vétérinaire, Université de Montréal for anorexia and persistent fever. Physical examination revealed bilateral swelling of the carpal and tarsal joints, with greater swelling on the tarsal joints. A tick was found attached on the right flank.
A CBC revealed a mild normocytic (68.8 fl, RI: 62 to 73 fl), normochromic (344.1 g/L, RI: 325 to 373 g/L) non-regenerative (reticulocyte count 4940 × 106/L, RI: 0 to 9100 × 106/L) anemia (hematocrit: 0.34 L/L, RI: 0.40 to 0.56 L/L, hemoglobin concentration: 117 g/L, RI: 139 to 198 g/L; erythrocyte count: 4.9 × 1012/L, RI: 5.4 to 8.6 × 1012/L) and a mild leukopenia (3.7 × 109/L, RI: 5.1 to 14.2 × 109/L) secondary to a moderate lymphopenia (0.15 × 109/L, RI: 0.7 to 3.87 × 109/L). On microscopic blood smear examination, 25% of neutrophils and a few platelets contained 1 to 4 round to oval granular basophilic intracytoplasmic inclusions measuring about 1 to 4 μm and resembling Anaplasma phagocytophilum or Ehrlichia ewingii morulae, and Anaplasma platys morulae, respectively (Figure 2). Urinalysis showed a midly decreased urine specific gravity (1.021), a mild proteinuria (protein to creatinine ratio: 0.60), and a physiologic mild bilirubinuria (dipstick 1+) associated with occasional bilirubin crystals.
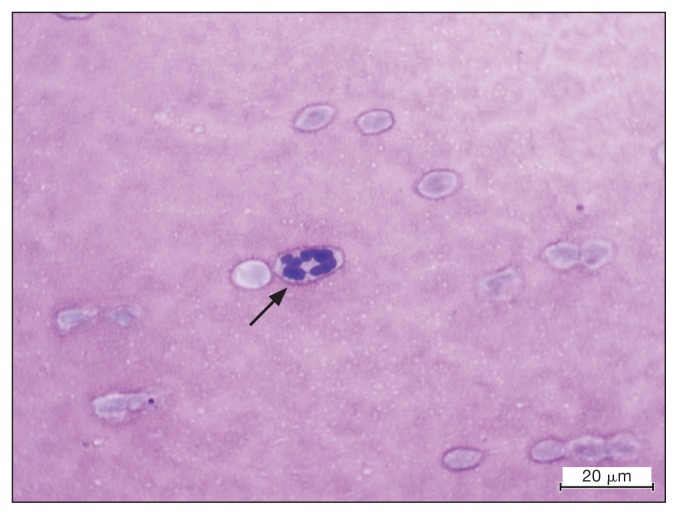
Figure 2.
Inclusion (arrow) within a neutrophil on a cytologic examination of synovial fluid of a dog with Anaplasma phagocytophilum infection. Modified Wright’s stain. Bar = 20 μm.
To investigate the patient’s lameness, cytologic examination of the synovial fluid from the right carpus and both right and left tarsi was performed and revealed a mild neutrophilic inflammation with a few neutrophils containing morulae (Figure 2). Polymerase chain reaction (PCR) and DNA sequencing on blood targeting the 16S gene (partial gene sequence: TAAGATAGTTAGTGGCAGACGGGTGAGTAATGCAT AGGAATCTACCTAGTAGTATGGGATAGCCACTAGAAA TGGTGGGTAATACTGTATAATCCCTGCGGGGGAAAG ATTTATCGCTATTAGATGAGCCTATGTTAGATTAGCT AGTTGGTAGGGTAAAGGCCTACCAAGGCGATGATC TATAG) to identify the genus Anaplasma and then targeting the p44 gene partial gene sequence: (AGCAAGATAAGAGA TTTTAGTATAAGGGAGAGTAACGGAGAGACTAAGGC AGTATTCCATACTTAAAGGATGGAAAGAGTGTAAAGC TAGAGTCACACAAGTTTGACTGGAACACACCTGATC CTCGGATTGGGA) to specifically identify the Anaplasma species was performed and confirmed the presence of A. phagocytophilum but not A. platys. Gene sequencing was conducted by the College of Veterinary Medicine, Vector-Borne Diagnostic Laboratory, North Carolina State University, Raleigh, North Carolina, USA.
The tick was identified as Ixodes scapularis and was positive for A. phagocytophilum DNA by PCR.
The dog was hospitalized for 2 d and received treatment with dexamethasone, 0.25 mg/kg BW, IV, q24h, doxycycline (Novo Doxylin), 300 mg, PO, q24h, dantroprazole, 1 mg/kg BW, IV, q12h, and tramadol, 150 mg, PO, q12h. The patient’s temperature normalized within 8 h after treatment initiation. The dog was discharged with at-home medication consisting of prednisone (Apo-prednisone), 25 mg, PO, q12h and doxycycline (Novo Doxylin), 300 mg, PO, q24h for a month. The dog was lost to follow-up.
Discussion
A suitable environment for Ixodes scapularis and I. pacificus, the main vectors for A. phagocytophilum in North America, seems to have expanded in Canada (11). Although only 1 study reported a low seroprevalence of A. phagocytophilum in dogs in Quebec (11), a previous survey detected the DNA of the bacterium in 15% of ticks collected from hunter-caught deer in this region in 2007 (17).
Uveitis has infrequently been reported in CGA (16,18,19). In canine monocytic ehrlichiosis, over-activity of B-lymphocytes gives rise to hypergammaglobulinemia and the formation of immune complexes of antigen, antibody, and complement, causing immune-mediated glomerulonephritis and uveitis (20). Studies on immune-mediated disease associated with A. phagocytophilum infection in dogs are scarce, although immune-mediated polyarthritis, thrombocytopenia, and anemia have been reported (4,8,18). Circulating immune complexes (CIC) have also been described in dogs with CGA. Dogs displaying higher CIC also had decreased platelet counts, lower albumin to globulin ratios, and higher gammaglobulin concentrations (21). Polyarthritis has been reported in tick-borne diseases including CGA caused by A. phagocytophilum (4,22) and neutrophilic inflammation of the joints associated with A. phagocytophilum-like inclusion has been described (22). A type II immune-mediated polyarthritis was reported in an A. phagocytophilum infected dog (23). The cause of the arthritis is not clear, but an immune-mediated process is suspected to be involved (4,23).
Both dogs herein showed mild to marked thrombocytopenia and mild anemia. Thrombocytopenia is considered the most relevant clinicopathologic abnormality in CGA after detection of morulae (6,24). The severity of thrombocytopenia varies from mild to severe and the platelet count has been reported to range from 5000 to 164 000 cells/μL (2–4,7). Anemia is an inconsistent hematological finding associated with CGA (2,4). Often, CGA-associated anemia is mild to moderate nonregenerative, normocytic, and normochromic (1). This type of anemia commonly occurs with infections by ehrlichial agents and generally occurs in the chronic phase of the disease due to suppressive effects on bone marrow (7). Both patients had mild lymphopenia but different neutrophil patterns. Lymphopenia is suggested to be a common white blood cell count abnormality in CGA (8,16). However, several other modifications have been described including lymphocytosis, eosinopenia, monocytosis, monocytopenia, neutropenia, neutrophilia, and left shift regeneration of neutrophils (2,4,7,16,18,19).
Both dogs had a small number of neutrophils containing A. phagocytophilum-like morulae and a positive PCR to A. phagocytophilum. In addition, the dog in case 1 showed seroconversion to Anaplasma spp. antibodies. Morulae are usually present transiently during the bacteremic phase (4 to 14 d after inoculation) and persist for 4 to 8 d (5). The proportion of neutrophils containing morulae varies from < 1% to 42% (2,3,5,9) and some case reports failed to identify these inclusions (18,19). In addition, the morulae of A. phagocytophilum cannot be distinguished from those of E. ewingii, which can lead to misdiagnosis in regions in which both pathogens are present. Therefore, serology and PCR are needed to confirm the diagnosis. Antibodies (immunoglobulin class G) can be detected using indirect immunofluorescence assay (IFA), enzyme-linked immunosorbent assay (ELISA), or Western immunoblotting; with IFA the most frequently used in diagnostic laboratories. A point-of-care ELISA-based test (SNAP 4DX Plus; IDEXX Laboratories) is also available for the detection of Anaplasma spp. antibodies (1,9,24). Positive antibody titers appear approximately 1 wk after initial exposure, 2 to 5 d after the appearance of morulae. As a result, during acute illness, antibodies may be undetectable and PCR may be more useful for diagnosis of acute infection when morulae are not present. Because antibodies may persist for months and reflect previous exposure, demonstration of a 4-fold rise or increase in titer between the acute and convalescent phases (3 to 4 wk) is required to serologicaly confirm A. phagocytophilum infection (1,4). When serial assays are needed to determine a changing titer, using the same laboratory is important because strain variability among isolates of A. phagocytophilum may cause variation among aliquots of the same sample sent to different laboratories (24). Another limitation of serologic testing is the possibility of cross-reaction with other Anaplasma species, regardless of the method used (9,24). Polymerase chain reaction techniques have been developed for the detection of A. phagocytophilum DNA from peripheral blood, buffy coat, bone marrow, tissue specimens, and from lymph node and spleen aspirates (1,24). Several genes can be targetted including the 16S rRNA, groEL, ankA, msp2, and msp4 (1,9,24). Polymerase chain reaction is a sensitive method for early diagnosis of CGA since infected dogs have positive PCR results 6 to 8 d before the appearance of morulae on blood smear (1,5). However, both false-negative and false-positive PCR results have been described. False-negative results can occur due to variations in levels of circulating bacteria or antibiotic treatment and should not be interpreted as evidence of absence of infection. Conversly, false-positve results were reported even when care was taken to avoid contamination (9,24). Indeed, depending on the type of assay used and the concentration of other organisms in the sample, DNA of other α–proteobacteria can be amplified (24), especially when using conserved genes such as the 16S rRNA (1,9). Some PCR methods (real-time PCR with fluorophore-containing DNA probes or reverse line blot hybridization) can avoid the amplification of non-specific DNA (24). Finally, the sequencing of a representative fragment of a non-conserved gene should be used for the final confirmation (1,24).
Our patients had a quick improvement after initiation of doxycycline therapy. This antibiotic is considered the treatment of choice for CGA (1). In vitro studies have shown susceptibility of A. phagocytophilum to doxycycline, rifampin, and some quinolones (levofloxacin, ciprofloxacin, moxifloxacin) (9,24–26). Both doxycycline and rifampin have bactericidal activity in vitro (26). For puppies under 1 y of age, chloramphenicol has been recommended to avoid yellowing of teeth, although doxycycline is unlikely to cause this effect (24). In addition, A. phagocytophilum only had limited in vitro susceptibility to chloramphenicol according to one survey (26). There have been no controlled studies to evaluate the optimal dose or duration of treatment in dogs or humans (6,7). However, a 2 to 4 wk course of doxycyline at 5 mg/kg BW, q12h or 10 mg/kg BW, q24h PO has been recommended in dogs (1,6,7). In human medicine, the recommended duration of treatment is 7 to 10 d (10). Most dogs in clinical studies showed clinical improvement within 1 to 6 d of antibiotic treatment (3,6,7). However, in 1 report a small number of dogs required 1 to 3 wk to show improvement (6). In severely affected patients, supportive therapies including blood transfusion, parenteral fluids, and/or corticosteroids should be administered (9). A lack of response to treatment with doxycycline may indicate that another etiology may be responsible for the illness or the presence of co-morbidities (9,24). In 1 study, 6 of 9 dogs that were initially responsive to doxycycline developed signs consistent with CGA during the subsequent year. These dogs were treated empirically with an appropriate dose of doxycycline, and signs improved within 48 h (8). Therefore, preventive measures are necessary to avoid re-infection with A. phagocytophilum or infection with other tick-borne pathogens. Infection may be prevented by keeping ticks from attaching to the host using regular ectoparasiticides or ectoparasite repellants, and prompt removal of ticks (1,27–29). Nevertheless, infections have been documented in dogs apparently receiving monthly tick preventatives (4).
These cases highlight the importance of considering CGA in the differential diagnoses of dogs in Quebec presenting with lethargy, anorexia, fever, lameness, splenomegaly particularly in the context of thrombocytopenia, although the prevalence of A. phagocytophilum in Quebec is low. The negative initial serologic test with positive PCR and observed morulae on blood smear emphasizes the need for multiple diagnostic modalities to confirm the diagnosis. Veterinarians, therefore, should be aware of the limitations of each method and the possible difficulty in achieving a reliable final diagnosis.
Acknowledgment
The authors thank the Vector-Borne Diagnostic Laboratory NCSU, College of Veterinary Medicine, Raleigh, North Carolina for performing the gene sequencing on Case 2. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Carrade DD, Foley JE, Borjesson DL, Sykes JE. Canine granulocytic anaplasmosis: A review. J Vet Intern Med. 2009;23:1129–1141. doi: 10.1111/j.1939-1676.2009.0384.x. [DOI] [PubMed] [Google Scholar]
- 2.Greig B, Asanovich KM, Armstrong PJ. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol. 1996;34:44–48. doi: 10.1128/jcm.34.1.44-48.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poitout FM, Shinozaki JK, Stockwell PJ, Holland CJ, Shukla SK. Genetic variants of Anaplasma phagocytophilum infecting dogs in western Washington State. J Clin Microbiol. 2005;43:796–801. doi: 10.1128/JCM.43.2.796-801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohn B, Galke D, Beelitz P, Pfister K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J Vet Intern Med. 2008;22:1289–1295. doi: 10.1111/j.1939-1676.2008.0180.x. [DOI] [PubMed] [Google Scholar]
- 5.Egenvall A, Bjöersdorff A, Lilliehöök I, et al. Early manifestations of granulocytic ehrlichiosis in dogs inoculated experimentally with a Swedish Ehrlichia species isolate. Vet Rec. 1998;143:412–417. doi: 10.1136/vr.143.15.412. [DOI] [PubMed] [Google Scholar]
- 6.Granick JL, Armstrong PJ, Bender JB. Anaplasma phagocytophilum infection in dogs: 34 cases (2000–2007) J Am Vet Med Assoc. 2009;234:1559–1565. doi: 10.2460/javma.234.12.1559. [DOI] [PubMed] [Google Scholar]
- 7.Eberts MD, Vissotto de Paiva Diniz PP, Beall MJ, Stillman BA, Chandrashekar R, Breitschwerdt EB. Typical and atypical manifestations of Anaplasma phagocytophilum infection in dogs. J Am Anim Hosp Assoc. 2011;47:86–94. doi: 10.5326/JAAHA-MS-5578. [DOI] [PubMed] [Google Scholar]
- 8.Mazepa AW, Kidd LB, Young KM, Trepanier L. Clinical presentation of 26 Anaplasma phagocytophilum-seropositive dogs residing in an endemic area. J Am Anim Hosp Assoc. 2010;46:405–412. doi: 10.5326/0460405. [DOI] [PubMed] [Google Scholar]
- 9.Allison RW, Little SE. Diagnosis of rickettsial diseases in dogs and cats. Vet Clin Pathol. 2013;42:127–144. doi: 10.1111/vcp.12040. [DOI] [PubMed] [Google Scholar]
- 10.Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin N Am. 2015;29:341–355. doi: 10.1016/j.idc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villeneuve A, Goring J, Marcotte L, Overvelde S. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, Ehrlichia canis, and Dirofilaria immitis among dogs in Canada. Can Vet J. 2011;52:527–530. [PMC free article] [PubMed] [Google Scholar]
- 12.Qurollo BA, Chandrashekar R, Hegarty BC, et al. A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species-specific peptides. Infect Ecol Epidemiol. 2014;4 doi: 10.3402/iee.v4.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gary AT, Webb JA, Hegarty BC, Breitschwerdt EB. The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can Vet J. 2006;47:1194–1200. [PMC free article] [PubMed] [Google Scholar]
- 14.Lester SJ, Breitschwerdt EB, Collis CD, Hegarty BC. Anaplasma phagocytophilum infection (granulocytic anaplasmosis) in a dog from Vancouver Island. Can Vet J. 2005;46:825–827. [PMC free article] [PubMed] [Google Scholar]
- 15.Kowalski J, Cruickshank D, Macartney M. Anaplasmosis in a dog on Vancouver Island. Can Vet J. 2015;56:761–762. [PMC free article] [PubMed] [Google Scholar]
- 16.Cockwill KR, Taylor SM, Snead EC, et al. Granulocytic anaplasmosis in three dogs from Saskatoon, Saskatchewan. Can Vet J. 2009;50:835–840. [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchard C, Leighton PA, Beauchamp G, et al. Harvested white-tailed deer as sentinel hosts for early establishing Ixodes scapularis populations and risk from vector-borne zoonoses in southeastern Canada. J Med Entomol. 2013;50:384–393. doi: 10.1603/me12093. [DOI] [PubMed] [Google Scholar]
- 18.Bexfield NH, Villiers EJ, Herrtage ME. Immune-mediated haemolytic anaemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J Small Anim Pract. 2005;46:543–548. doi: 10.1111/j.1748-5827.2005.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 19.Arsenault WG, Messick JB. Acute granulocytic ehrlichiosis in a rottweiler. J Am Anim Hosp Assoc. 2005;41:323–326. doi: 10.5326/0410323. [DOI] [PubMed] [Google Scholar]
- 20.Harrus S, Day MJ, Waner T, Bark H. Presence of immune-complexes, and absence of antinuclear antibodies, in sera of dogs naturally and experimentally infected with Ehrlichia canis. Vet Microbiol. 2001;83:343–349. doi: 10.1016/s0378-1135(01)00431-x. [DOI] [PubMed] [Google Scholar]
- 21.Ravnik U, Bajuk BP, Lusa L, Tozon N. Serum protein profiles, circulating immune complexes and proteinuria in dogs naturally infected with Anaplasma phagocytophilum. Vet Microbiol. 2014;173:160–165. doi: 10.1016/j.vetmic.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Foley J, Drazenovich N, Leutenegger CM, Chomel BB. Association between polyarthritis and thrombocytopenia and increased prevalence of vectorborne pathogens in Californian dogs. Vet Rec. 2007;160:159–162. doi: 10.1136/vr.160.5.159. [DOI] [PubMed] [Google Scholar]
- 23.Kjelgaard-Hansen M, Jensen AL, Houser GA, Jensen LR, Kristensen AT. Use of serum C-reactive protein as an early marker of inflammatory activity in canine type II immune-mediated polyarthritis: Case report. Acta Vet Scand. 2006;48:9. doi: 10.1186/1751-0147-48-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diniz PP, Breitschwerdt EB. Infectious Diseases of the Dog and Cat. 4th ed. St. Louis, Missouri: Elsevier Saunders; 2012. pp. 244–254. [Google Scholar]
- 25.Hunfeld KP, Bittner T, Rödel R, Brade V, Cinatl J. New real-time PCR-based method for in vitro susceptibility testing of Anaplasma phagocytophilum against antimicrobial agents. Int J Antimicrob Agents. 2004;23:563–571. doi: 10.1016/j.ijantimicag.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Woldehiwet Z. In-vitro studies on the susceptibility of ovine strains of Anaplasma phagocytophilum to antimicrobial agents and to immune serum. J Comp Path. 2010;143:94–100. doi: 10.1016/j.jcpa.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Blagburn BL, Spencer JA, Billeter SA, et al. Use of imidacloprid-permethrin to prevent transmission of Anaplasma phagocytophilum from naturally infected Ixodes scapularis ticks to dogs. Vet Ther. 2004;5:212–217. [PubMed] [Google Scholar]
- 28.McCall JW, Baker CF, Mather TN, et al. The ability of a topical novel combination of fipronil, amitraz and (S)-methoprene to protect dogs from Borrelia burgdorferi and Anaplasma phagocytophilum infections transmitted by Ixodes scapularis. Vet Parasitol. 2011;179:335–342. doi: 10.1016/j.vetpar.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 29.Honsberger NA, Six RH, Heinz TJ, Weber A, Mahabir SP, Berg TC. Efficacy of sarolaner in the prevention of Borrelia burgdorferi and Anaplasma phagocytophilum transmission from infected Ixodes scapularis to dogs. Vet Parasitol. 2016;222:67–72. doi: 10.1016/j.vetpar.2016.02.010. [DOI] [PubMed] [Google Scholar]