Abstract
Hypogylcemia in dogs is defined as a blood glucose concentration of less than 3.3 mmol/L (60 mg/dL) and is a relatively common problem encountered in veterinary practice. This metabolic disorder can have an array of clinical signs, ranging from subtle abnormalities to a life-threatening emergency. Hypoglycemia can be due to several physiological processes or etiologies. It is imperative that the clinician is astute in diagnosing hypoglycemia, proficient in providing rapid symptomatic treatment (if indicated) and has a clear diagnostic plan to elucidate the underlying cause. This article reviews the pathophysiology, most common etiologies, and emergency management of hypoglycemia, and presents a diagnostic approach for this problem.
Résumé
Hypoglycémie chez les chiens : causes, gestion et diagnostic. L’hypoglycémie chez les chiens se définit comme une concentration de glucose dans le sang de moins de 3,3 mmol/L (60 mg/dL) et elle représente un problème assez fréquemment observé en pratique vétérinaire. Ce trouble métabolique peut comporter un éventail de signes cliniques, allant d’anomalies subtiles à une urgence potentiellement mortelle. L’hypoglycémie peut être causée par plusieurs processus ou étiologies physiologiques. Il est impératif que le clinicien soit perspicace afin de diagnostiquer l’hypoglycémie, compétent afin de fournir un traitement rapide des symptômes (si cela est indiqué) et qu’il ait un plan diagnostique clair afin d’élucider la cause sous-jacente. Cet article examine la pathophysiologie, les étiologies les plus courantes et la gestion des urgences de l’hypoglycémie et présente une approche diagnostique pour ce problème.
(Traduit par Isabelle Vallières)
Introduction
Why can the presence of hypoglycemia be a diagnostic dilemma?
Being confronted with a dog with hypoglycemia is not an infrequent occurrence in veterinary practice and may be a disconcerting problem for the clinician. This may be due in part to the numerous physiological and pathological causes for hypoglycemia, which are listed in Table 1. However, even this table is not exhaustive. Hypoglycemia may also be the product of artifactual causes which may lead the clinician astray. In order to better understand the potential causes of hypoglycemia it is necessary to start with background information on normal glucose homeostasis.
Table 1.
Causes of hypoglycemia in the dog broadly divided by the presence or absence of an underlying disease resulting in hypoglycemia (1,6).
| Physiological causes of hypoglycemia | Chief mechanism or mechanisms |
|---|---|
| Extreme exercise (e.g., hunting dog hypoglycemia) | Excess glucose utilization and inadequate glycogen stores |
| Neonatal/juvenile or toy breed juvenile hypoglycemia | Inadequate glycogen stores, limited fat and muscle mass |
| Malnutrition/starvation | Inadequate intake and depletion of glycogen stores |
| Drug and toxin associated causes such as iatrogenic insulin overdose, xylitol toxicity, oral hypoglycemic agents (usually sulfonylureas), beta blockers | Excess glucose utilization due to hypersecretion of insulin and increased tissue sensitivity to insulin. Beta blockers via suspected interference of counter- regulatory mechanisms |
|
| |
| Pathological causes of hypoglycemia | |
|
| |
| Severe hepatic disease such as hepatitis, cirrhosis, neoplasia, amyloidosis, hepatotoxins | Decreased hepatic gluconeogenesis |
| Congenital portosystemic shunt | Decreased hepatic gluconeogenesis |
| Hypoadrenocorticism | Decreased glucose production from lack of a counter-regulatory hormone (i.e., cortisol) |
| Hypopituitarism | Decreased glucose production from lack of a counter-regulatory hormone (i.e., growth hormone or adrenocorticotropic hormone) |
| Insulinoma | Excess glucose utilization due to hypersecretion of insulin |
| Islet cell hyperplasiaa | Excess glucose utilization due to hypersecretion of insulin |
| Extra-pancreatic tumors (e.g., hepatocellular carcinoma, hepatoma, leiomyosarcoma, leiomyoma) | Increased glucose utilization by the tumor but also due to secretion of insulin analogues |
| Chronic renal failure | Decreased hepatic gluconeogenesis |
| Pancreatitis | Unknown |
| Infection (e.g., sepsis, severe canine babesiosis) | Decreased hepatic glycogenesis and increased glucose utilization |
| Glycogen storage disease | Deficiency of enzymes required for glycogen conversion |
|
| |
| Artifactual/spurious | Laboratory error from improper sample handling or submission, use of a human glucometer, leukemia/polycythemia vera |
Reported as a rare cause of hypoglycemia in humans.
This pancreatic pathology has been documented in dogs but without hyperinsulinemic hypoglycemia syndrome.
Glucose homeostasis
Glucose in the body is derived from 3 sources: i) intestinal absorption from the digestion of carbohydrates, ii) dissolution of glycogen (the storage form of glucose) via glycogenolysis predominantly in the liver but also in the muscle, and iii) synthesis of glucose (gluconeogenesis), mostly by the liver, from non-carbohydrate sources e.g., lactate, pyruvate, amino acids, and glycerol, but also a significant amount by the kidneys (1).
In the clinically normal animal, the body maintains euglycemia primarily via equilibrium between the glucose-lowering hormone insulin and the glucose-elevating hormones glucagon, cortisol, epinephrine, norepinephrine, and growth hormone (diabetogenic hormones or counter-regulatory hormones) (2). However, hepatic autoregulation, independent of counter regulatory hormones is also vitally important in modulating blood glucose levels (3). After a meal, glucose, amino acids, and gastrointestinal hormones (gastrin, secretin, cholecystokinin, and gastric inhibitory peptide) rise in the plasma. The release of insulin from pancreatic beta cells is signaled when glucose is > 6 mmol/L (110 mg/dL) (4). Insulin serves to temporarily halt gluconeogenesis and glycogenolysis, stimulate glucose uptake and utilization by insulin-sensitive cells, promote production and storage of glycogen, and inhibit glucagon secretion (1,2); the net effect being prevention of sustained hyperglycemia. Insulin also promotes production of triglyceride in adipose tissue and of protein and glycogen in skeletal muscle (1). After the initial post-prandial insulin peak, insulin concentrations begin to decline due to inhibitory feedback from decreasing plasma glucose concentration (< 3.3 mmol/L or 60 mg/dL) and the excretion of insulin by the kidney (1,5). Decreasing blood glucose concentration simultaneously stimulates the release of the counter-regulatory hormones. Glucagon, from pancreatic alpha cells, and epinephrine from the adrenal medulla, are the initial hormones that are released within minutes in response to hypoglycemia but have a short duration of action. The actions of glucagon and epinephrine are physiologically counteractive to those of insulin and therefore these hormones promote glycogenolysis, gluconeogenesis, inhibit insulin secretion, and limit peripheral glucose uptake by tissues (1). Gluconeogenesis is primarily achieved by the conversion of specific amino acids and glycerol to glucose and urea (2,6). Cortisol and growth hormone are released after 1 to 2 hours of hypoglycemia being detected but have a more protracted effect in raising blood glucose, through gluconeogenesis, reducing peripheral glucose utilization and promoting lipolysis so adipose tissue can be used an alternative energy source (1). Thus, in the fasting (post-absorptive) state, euglycemia is maintained via endogenous sources and in the postprandial state, glucose is directly acquired through exogenous sources (7).
Pathophysiology, definition, and mechanisms of hypoglycemia
In the clinically normal dog, glucose concentration is maintained within a narrow range (3.3 mmol/L to 6.2 mmol/L or 60 mg/dL to 111 mg/dL) (2). Hypoglycemia in dogs is defined by a blood glucose level of ≤ 3.3 mmol/L (≤ 60 mg/dL) (1,4,6–8). Hypoglycemia occurs when there is perturbation of glucose homeostasis in which glucose utilization exceeds glucose production and/or entry into circulation (1). There are 4 mechanisms by which hypoglycemia may arise: i) poor dietary intake of glucose and other substrates used in hepatic gluconeogenesis; ii) increased glucose uptake and utilization by normal or neoplastic cells due to an increase in demand or secondary to hyperinsulinism; iii) dysfunctional hepatic glycogenolytic or gluconeogenic pathways; and iv) endocrine abnormalities resulting in a deficiency of counter-regulatory hormones such as cortisol (1,8). In many disease processes causing hypoglycemia, the origin of the hypoglycemia is multifactorial.
Clinical signs of hypoglycemia
Although clinicopathologic hypoglycemia is diagnosed by a blood glucose level of < 3.3 mmol/L (< 60 mg/dL), clinical signs do not typically manifest until the blood glucose concentration is < 2.2 mmol/L to 2.8 mmol/L (< 40 mg/dL to 50 mg/dL) (1,8).
The clinical signs may vary, are often non-specific, and can wax and wane. Clinical signs may include altered mentation and behavior, seizures, syncope, muscle twitching/fasciculations, somnolence, exercise intolerance, muscle tremors, collapse, ataxia, weakness, and impaired vision. These clinical signs are attributable to neuroglycopenia (cerebral hypoglycemia) (8). The brain’s poor tolerance for hypoglycemia is due to its high and continuous requirement for glucose, the brain’s predominant energy substrate; the brain cannot generate glucose, nor adequately store glucose in the form of glycogen (1). Diminished neuronal glucose concentration leads to inadequate ATP production within the neurons, leading to vascular permeability, vasospasm, vascular dilation, and edema. This leads to neuronal death from anoxia (4). Interestingly, in humans it has been documented that acute hypoglycemia can lead to changes within the brain on magnetic resonance imaging (MRI), tantamount to an ischemic stroke (9); highlighting the profound pathology that hypoglycemia can impose on the brain. Hypoglycemic dogs may concurrently have as their prevailing clinical signs, those referable to activation of the adrenergic nervous system such as unsettledness/nervousness, tachypnea, trembling, tachycardia, and gastrointestinal signs such as vomiting, diarrhea, polyphagia, and ptyalism (1,8,10). This is due to adrenergic activation attempting to counteract the declining blood glucose concentration (1,8). Seldom, dogs with hypoglycemia may be presented with bradycardia, and clinical signs of circulatory collapse, for which the pathophysiology is unclear (11).
Dogs which have experienced chronic or repeated episodes of hypoglycemia may appear clinically normal. Extrapolated from human medicine, this is defined as hypoglycemia unawareness (12,13). The absence of clinical signs in hypoglycemia is postulated to be in part due to upregulation of cerebral glucose uptake and lack of premonitory sympathetic nervous system clinical signs (1,12,13).
In summary, the clinical presentation of a hypoglycemic dog is varied and is dependent on the underlying cause, the degree of hypoglycemia, rate of decline of glucose, duration of hypoglycemia, and competence of the counter-regulatory hormone mechanisms (1,4).
Spurious or artifactual causes of hypoglycemia
Artifactual hypoglycemia is a frequent cause of apparent hypoglycemia in dogs. Artifactual hypoglycemia may be caused by using human-specific portable blood glucose meters (PBGM) in dogs. Several studies have established that use of a human PBGM has a propensity to underestimate a dog’s blood glucose concentration when compared to reference laboratory methods (14–18). In 2 studies, canine blood glucose concentrations in the normoglycemic range, as determined by the reference laboratory, were underestimated by a human PBGM by mean differences of 1.1 and 1.2 mmol/L (19 and 22 mg/dL) (17,18). In another study, when 1 PBGM underestimated the blood glucose, it was typically 15% less than the determined reference method value (16). These studies suggest that the underestimation of blood glucose by some human PBGMs is of a modest magnitude but can be clinically significant. This underestimation may be more marked if the dog has a hematocrit of > 55%, which is not uncommon in some sighthounds and dehydrated dogs (19). Thus, it is prudent to ensure that hypoglycemia as determined by a human PBGM is always verified by an external reference laboratory or a point-of-care chemistry analyzer.
The other main contributor to artifactual hypoglycemia is the generation of pseudohypoglycemia from improper handling of samples. Blood should be submitted to an external reference laboratory in a sodium fluoride tube, which prevents continual glucose consumption via glycolysis by erythrocytes and leukocytes. Post-sampling glucose utilization can be particularly marked if the dog has polycythemia or leukocytosis (8).
If collected whole blood is not submitted in a sodium fluoride tube, prolonged storage of blood before separation into plasma or serum should be avoided, as this will cause the glucose concentration to decrease at a rate of approximately 0.4 mmol/L per hour (7 mg/dL per hour) (8).
Physiological and iatrogenic causes of hypoglycemia
Fasting
While prolonged fasting or starvation can theoretically lead to hypoglycemia, this is seldom a cause for significant hypoglycemia in the adult dog without concomitant disease affecting glucose homeostasis (1,2,4). This contrasts with adult humans, in whom fasting hypoglycemia is reported to be a frequent event (20). The reason behind this interspecies difference is unclear.
Exertional hypoglycemia (hunting dog hypoglycemia)
Intense exercise or prolonged physical activity can significantly increase glucose utilization and rapidly deplete glycogen stores, especially in lean dogs, with the sequela of hypoglycemia (2). This is referred to as exertional hypoglycemia and colloquially as “hunting or working dog hypoglycemia” (1), as it is considered more common in this type of dog due to their natural behavior. The prevalence of hunting dog hypoglycemia is unknown. Other than an abstract of a case report on 3 dogs with suspected exertional hypoglycemia and an experimental study of the physiological effects of exercise-induced hypoglycemia in dogs, there is a lack of published information on this phenomenon (21,22). The paucity of reported information on this condition is likely due to the fact that the clinical signs are often self-limiting, effective counter-regulatory mechanisms come into play, and by the time the dog is presented for examination at the veterinary hospital, the dog is asymptomatic and blood glucose concentration has normalized.
In humans, continuous exercise for 2 to 3 h at 65% maximal oxygen uptake results in the development of hypoglycemia (20). Therefore, it is more than conceivable that hypoglycemia would readily occur in dogs undertaking extreme exercise coupled with the presence of poor body condition. The word extreme is emphasized as one should not anticipate that well-conditioned working dogs participating in routine field and search and rescue activities, should readily develop hypoglycemia. Two studies did not document hypoglycemia in working dogs subjected to field training (23,24).
Thus, while hunting dog hypoglycemia should be considered in dogs with an appropriate signalment and history, it is nevertheless a diagnosis of exclusion and other differentials for hypoglycemia should always be considered. This point is reinforced by a case report in which a hunting dog had recurring seizures associated with exercise but diagnostic investigation revealed that the dog’s hypoglycemia was in fact secondary to hypoadrenocorticism (25).
Neonatal/juvenile and toy breed hypoglycemia
Neonatal dogs have a propensity for developing hypoglycemia due to several factors: they have limited glycogen reserves, decreased ability for hepatic gluconeogenesis, a low body mass index leading to lack of lipolysis for an alternative fuel source, immature counter-regulatory hormonal systems, and the heart, in addition to the brain, relies heavily on glucose for energy (26). These factors cause neonates to poorly cope with stressors such as inadequate or poor-quality food intake, fasting, dehydration, infection, and hypothermia (1,26), all of which rapidly deplete their blood glucose, in a setting of diminished ability to conserve and replenish glucose. In the absence of normal compensatory mechanisms, hypoglycemia may ensue in a neonate within 2 to 3 h of decreased food intake (26). Similar to neonates, small stature juveniles, especially toy and miniature breeds, are at an increased risk of developing hypoglycemia due to their low body mass index (BMI). In addition to this, a suspected alanine deficiency, which contributes to dysregulation of gluconeogenesis during the fasted state, may also be a contributing factor (6). Diagnosis of neonatal or toy breed hypoglycemia is based on signalment; however, concurrent diseases such as sepsis and portosystemic shunt should be explored, especially if episodes of hypoglycemia persist into adulthood (6).
Hyperinsulinemia: Iatrogenic insulin overdose and xylitol toxicity
Iatrogenic insulin overdose should be easily identified based on the history of a diabetic patient receiving insulin. Possible causes include an absolute overdose, e.g., an owner accidentally administering an increased dose of insulin versus a relative overdose, i.e., normal insulin dose administered with increased glucose utilization due to aberrant physical activity or concurrent illness or inadequate food intake.
Xylitol is a sugar alcohol which is used commercially as an artificial sweetener and has anti-microbial properties. It is commonly found in an array of products such as candy, sugar-free chewing gums, toothpaste, and baked goods (27,28). Xylitol can cause hypoglycemia in dogs through a dose-dependent release of insulin. This insulin surge can lead to hypoglycemia because the amount of insulin released is 2.5 to 7 times greater than if an equal amount of glucose was administered. Hypoglycemia ensues within 30 to 60 min of ingestion (29,30). A xylitol dose as low as 0.03 g/kg body weight (BW) may cause clinical hypoglycemia (31). In addition to hypoglycemia, xylitol may lead to liver disease, characterized by elevation of hepatocellular enzymes, chiefly alanine aminotransferase (ALT) and/or hyperbilirubinemia (27,28,31,32). Less commonly, fulminant hepatic failure may occur in which the most consistent finding is elevation of clotting times (27,28,33). Consequently, the clinical picture of xylitol ingestion may be misdiagnosed as hepatic failure. The clinician presented with a patient with hypoglycemia and concurrent liver disease must therefore ensure that an adequate toxicology history is obtained. Interestingly, erythritol, another common sugar substitute related to xylitol, does not cause toxicity in dogs (34).
The most common pathological causes of hypoglycemia in the dog
Although the list of causes of hypoglycemia is vast, there are 5 common causes of pathological hypoglycemia reported in the literature: sepsis, extrapancreatic neoplasia, insulinoma, hypoadrenocorticism, and liver dysfunction (6,8). There have been no published studies assessing the proportional prevalence of these diseases in causing hypoglycemia in the dog. However, at the authors’ veterinary institution, between 2002 and 2016, the most common pathological causes of hypoglycemia in 55 dogs were: insulinoma (69%, based on the results of an insulin assay and documentation of a pancreatic mass on imaging or exploratory celiotomy), extrapancreatic tumor (14%), sepsis (7%), hypoadrenocorticism (6%) and hepatic failure (4%). It is acknowledged that a selection bias applies since these cases were derived from a referral institution.
Other less common causes of hypoglycemia include: pituitary dwarfism; renal disease (tubular acidosis, Fanconi-like syndrome); acetylcholinesterase inhibitors such as edrophonium, neostigmine, organophosphates, physostigmine; ethanol; disopyramide, propranolol; salicylate; sulfonylurea compounds and toxins from herb fenugreek (Trigonella foenum-graecum), bitter melon gourd (Momordica charantia), climbing ivy gourd (Coccinia indica), mamijava (Enicostemma littorale), Asian ginseng (Panax ginseng), American ginseng (Panax quinquefolius), Siberian ginseng (Eleutherococcus senticosus), ackee tree (Blighia sapida), prickly pear (Opuntia robusta), oleander plant (Nerium oleander), and yellow bells [Tecoma stans (family Bignoniaceae)] (6,35).
Insulinoma
Insulinomas are functional beta cell tumors of the pancreas, which cause hypoglycemia via secretion of insulin independent of the normal suppressive effects of normoglycemia or hypoglycemia (36). The diagnosis of an insulinoma can be a challenge for a few reasons.
Firstly, the blood glucose may fluctuate in and out of the normal range, because of counter-regulatory mechanisms and the effects of feeding (4,6). Therefore, patients with a suspected insulinoma may require that multiple blood glucose assessments are performed during a 12-hour fasting period to avoid missing a hypoglycemic episode (10). However, if provocative testing is employed, then diligent monitoring of blood glucose every hour should be undertaken to minimize the risk of an unobserved hypoglycemic crisis (37). In some cases of insulinoma, despite serial monitoring of fasting blood glucose, the blood glucose can be consistently within the reference range. Measurement of fructosamine may prove helpful in these difficult cases (38).
Secondly, in cases of insulinomas, seizures may be a more common clinical sign than with other pathological causes for hypoglycemia (6). It may be difficult to determine whether the hypoglycemia is the cause of the seizure, or whether the hypoglycemia is due to increased skeletal muscle utilization of glucose secondary to seizure activity. To help clarify this conundrum, the fulfilment of Whipple’s triad can be helpful. Whipple’s triad consists of clinical symptoms supportive of hypoglycemia, documentation of a low blood glucose, and resolution or improvement of clinical signs with correction of the hypoglycemia (39). Historically in human medicine, fulfilment of Whipple’s triad was tantamount to the diagnosis of an insulinoma (37). However, the criteria are not definitive and patients with other causes of hypoglycemia will readily fulfil these requirements.
A third diagnostic problem with insulinomas is that specific clinicopathologic testing for an insulinoma, namely the serum insulin concentration, is not 100% sensitive or specific for an insulinoma. To maximize the diagnostic yield of this test, blood for the insulin concentration should be measured at the same time as documented severe hypoglycemia. An insulin concentration above the upper limit of normal, or a normal insulin level, in the upper half of the reference range, in the face of significant hypoglycemia is suggestive of an insulinoma (6). However, in some cases, it is ambiguous as to whether the insulin value is inappropriate for the degree of hypoglycemia; e.g., hypoglycemia, with a low normal insulin value: this may be consistent with an insulinoma but also other causes of hypoglycemia (6). In this circumstance, a repeat insulin assay may be performed. Some laboratories will perform an insulin:glucose ratio or an amended insulin:glucose ratio in equivocal insulinoma cases. However, use of any insulin:glucose ratio is not advocated but particularly the amended glucose ratio because the formula used to generate this value is extrapolated from blood glucose concentrations in clinically normal humans and the test lacks specificity (4,6).
When the history, clinical signs, and results of an insulin concentration in context of hypoglycemia are suggestive of an insulinoma, imaging such as ultrasound or computed tomography (CT) should be undertaken. The sensitivity of abdominal ultrasound in detecting an insulinoma varies from 28% to 75% (37); thus ultrasound has only a modest diagnostic yield for insulinomas. Computed tomography may fare better in the detection of insulinomas, with 1 study reporting a sensitivity of 71% compared to 35% with abdominal ultrasound (40). Preoperative diagnosis can be arduous and therefore intra-operative lesion localization with histopathology confirmation, is considered the gold standard (6). Before embarking on an exploratory celiotomy for a suspected insulinoma, staging with diagnostic imaging should be performed. In 1 study, metastatic disease was detected grossly in 30% to 50% of cases at the time of surgery (4).
Extrapancreatic neoplasia
Virtually any non-pancreatic neoplasm has the potential to cause hypoglycemia. The mechanism is often multifactorial and includes: a paraneoplastic effect through the liberation of insulin or insulin analogs, and direct tumor effects such as excessive glucose utilization by the tumor and impaired hepatic glucose homeostasis due to a primary liver tumor or metastasis to the liver (1,4).
The most common tumors associated with hypoglycemia are hepatocellular carcinoma, hepatoma, leiomyoma, and leiomyosarcoma (1,2,4,5). In 1 study of canine leiomyosarcomas, 6/11 dogs had documented hypoglycemia; however, sepsis from peritonitis was suspected to be responsible for the hypoglycemia in 4/6 dogs (41). Diagnosis of extra-pancreatic neoplasms can usually be made based on physical examination findings, clinicopathologic data, and imaging tests, but some tumors may be occult and therefore difficult to identify prior to surgery.
Sepsis
Hypoglycemia secondary to sepsis is postulated to be from a culmination of processes, including decreased caloric intake, hepatic dysfunction, and increased insulin-independent glucose consumption by bacteria, neutrophils, and peripheral tissues, which is attributable to inflammatory mediators and insulin analogs (1,6). Septic patients are usually moribund and diagnosis in dogs is via documenting at least 2 of the 4 criteria for Systemic Inflammatory Response Syndrome (SIRS) and identifying a nidus of infection (42).
Any severe bacterial infection can cause hypoglycemia from sepsis and some viral infections are commonly associated with hypoglycemia. In 1 study evaluating biochemical changes, all 14 puppies with parvovirus infection and 6 of 8 puppies with coronavirus infection were hypoglycemic (43). However, as the dogs in the aforementioned study were puppies, juvenile hypoglycemia may have been a factor. Furthermore, because septic patients may have leucocytosis, hypoglycemia may have been exacerbated due to artifactual reasons.
Babesiosis
Canine babesiosis is considered an emerging disease in Canada. Increased incidence of acquired Babesia infections may be the result of increased global movement of pets, establishment or identification of appropriate vectors in Canada and administration of canine blood products from various locations in North America (44). Hypoglycemia is thought to ensue via mechanisms similar to those for bacterial sepsis (1) and there is a relatively high prevalence of hypoglycemia in dogs infected with Babesia spp. (45).
Hepatic disease
Because glucose homeostasis largely relies on hepatic glycogen storage, hepatic gluconeogenesis and glycogenolysis, severe perturbation of hepatic function can foreseeably lead to hypoglycemia. The hypoglycemia is usually relatively mild and an incidental finding (1). Seventy percent of hepatic mass needs to be lost before hypoglycemia ensues and most dogs will have abnormalities in other functional hepatic indices such as prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT) (1,2,46). Causes of hepatic dysfunction include vascular anomalies such as a portosystemic shunt, chronic hepatitis, primary or metastatic hepatic neoplasia, hepatic lipidosis, and hepatic fibrosis/cirrhosis. Diagnosis is based on clinicopathologic data, dynamic liver functional tests, imaging studies and hepatic biopsies.
Hypoadrenocorticism
Dogs with primary hypoadrenocorticism (including atypical) and secondary hypoadrenocorticism are all deficient in cortisol, which diminishes hepatic gluconeogenesis and increases peripheral insulin sensitivity (47). It is estimated that up to 22% of dogs with primary hypoadrenocorticism and up to 43% of dogs with secondary hypoadrenocorticism will have hypoglycemia (47), but this information is based on a definition of hypoglycemia of < 3.9 mmol/L (70 mg/dL). Nevertheless, hypoglycemia that is severe enough to cause neurologic manifestations is an uncommon presentation in hypoadrenocorticism, and similar to the hypoglycemia that may be encountered in hepatic dysfunction, is usually not the prevailing problem (8,47). Profound hypoglycemia in cases of hypoadrenocorticism may be due to concurrent sepsis from gastrointestinal ulceration and bacterial translocation (47). Definitive diagnosis of hypoadrenocorticism is by an ACTH stimulation test.
Emergency management of hypoglycemia
While treatment or management directed at the specific underlying cause of the hypoglycemia is essential for long-term resolution or prevention in a dog with significant clinical signs secondary to hypoglycemia, prompt emergency management is imperative. Protracted neuroglycopenia can lead to irreversible brain damage and neurological deficits that are refractory to treatment (1).
The symptomatic treatment for hypoglycemia is glucose. If the patient is at home, owners can be instructed to rub corn syrup, honey, glucose syrup, or 50% dextrose on the tissues of the mouth, lining the cheek, followed by giving the same solution by mouth once the patient can swallow; then seek immediate veterinary medical attention. In hospitalized hypoglycemic patients, glucose is generally administered in the form of intravenous dextrose. This practice, at least in part, is derived from the incretin effect, in which oral glucose administration increases insulin secretion to a greater extent than does peripheral glucose infusion, through the induction of gut hormones (incretin) (48). Dextrose is routinely administered at a dose rate of 0.5 to 1 mL/kg BW of 50% dextrose, diluted with saline in at least a 1:2 dilution (to minimize phlebitis and hemolysis), and given via a peripheral vein (1,2,49). In dogs with clinical hypoglycemia, parenteral dextrose usually alleviates the signs within approximately 5 min and the dose can be repeated if hypoglycemia persists (1,8,49). If, after the initial bolus of dextrose, the patient has appropriate mentation, feeding of frequent small meals, containing a complex carbohydrate is recommended (1,8). If this action is unsuccessful, then maintenance of euglycemia can be achieved via administration of 2.5% to 5% dextrose (49) diluted into a balanced crystalloid solution and administered as a constant rate infusion (CRI) at maintenance rate, but the rate modulated dependent on the blood glucose concentration. Blood glucose concentration should be monitored hourly with a goal of maintaining blood glucose concentrations between 3.3 and 8.3 mmol/L (60 and 150 mg/dL) (49).
An important caveat regarding the emergency treatment of hypoglycemia with dextrose is that excessive dextrose solution can lead to an osmotic diuresis and dehydration (26). Furthermore, in dogs with insulinomas or extrapancreatic tumors secreting insulin analogs, infusion of a rapid, large volume of dextrose can be counterproductive, as elevated glucose in the circulation exacerbates hyperinsulinemia through normal homeostatic mechanisms. This situation creates a vicious cycle of hyperglycemia and rebound hypoglycemia (1,6). Therefore, dextrose should always be slowly infused over 5 to 10 min (1,26).
If dextrose fails to alleviate the hypoglycemia, then a glucagon constant rate infusion can be considered as an alternative therapy. Glucagon infusion may be particularly helpful in cases in which the hypoglycemia is due to iatrogenic insulin overdose, insulinoma, or extrapancreatic secreting tumor (4). In a case series of 9 dogs with hypoglycemia refractory to dextrose administration, a glucagon CRI successfully raised blood glucose concentrations in all dogs (50). In the afforementioned study, the median bolus of glucagon was 50 ng/kg BW followed by a median maximum dose of a glucagon CRI of 15 ng/kg BW per minute (50).
A diagnostic approach to hypoglycemia
Once hypoglycemia is documented, is a repeatable finding, spurious causes for hypoglycemia have been excluded, and there is no overt iatrogenic or physiological cause based on signalment and history, the clinician must then compile a list of the most likely differential diagnoses for pathological causes of hypoglycemia. In some cases, based on signalment, history, physical examination findings, and results of preliminary diagnostic tests, there will be “red flags” to help hone in on the most likely differential diagnoses. For example, a puppy, especially if unvaccinated, that has gastrointestinal signs should have parvovirus as a top differential for hypoglycemia; or an adult dog, with chronic or waxing and waning gastrointestinal signs, should always have hypoadrenocorticism considered as a cause for the hypoglycemia.
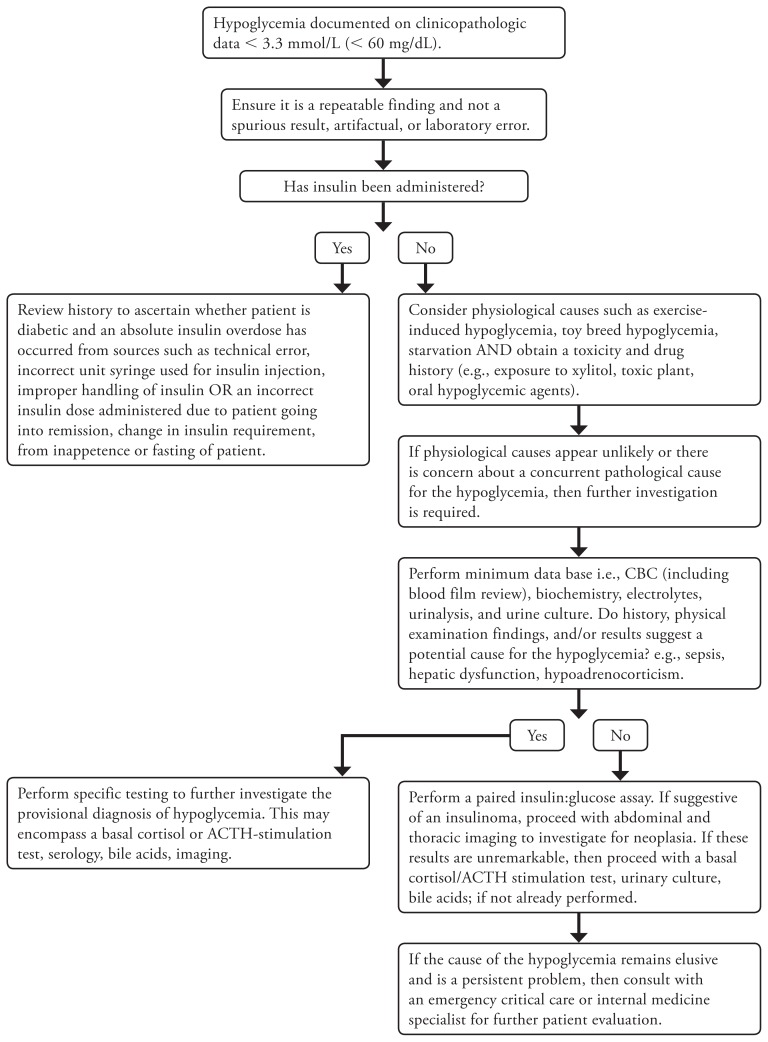
When the clinician is not presented with “red flags,” then a systematic approach rather than strictly pattern recognition will be required (Figure 1).
Figure 1.
A diagnostic tree for the investigation of hypoglycemia in dogs.
If not already done, a complete blood (cell) count (including blood film review), biochemistry, electrolytes, and urinalysis should be performed. These results may suggest hypoadrenocorticism, hepatic disease, or sepsis, which will help guide the clinician to the next phase of appropriate testing. If the blood analysis is unremarkable, other than hypoglycemia, or the changes in the profile are non-specific, then the clinician may proceed with a paired insulin and glucose assay. If this test is suggestive of an insulinoma, then diagnostic imaging to evaluate for a pancreatic mass and metastasis should be conducted. If the insulin and glucose assay result is not consistent with an insulinoma, it is still appropriate to proceed with diagnostic imaging to evaluate the thorax and abdomen for an extra-pancreatic mass, occult nidus of infection such as stump pyometra, pyelonephritis, and to assess the liver for vascular and parenchymal abnormalities. If at this stage, there are still no further clues as to the cause of the patient’s hypoglycemia, fasting and postprandial bile acids, a urinary culture, a basal cortisol, and an ACTH-stimulation test should be conducted. However, a basal cortisol of > 55.2 nmol/L (> 2 μg/dL) sufficiently excludes hypoadrenocorticism and thus an ACTH-stimulation test is unnecessary (47).
If after exploring the more common causes of hypoglycemia, there is still no diagnosis, this is the time when the clinician should consult a veterinary internal medicine or emergency and critical care specialist, for further evaluation of the patient.
In conclusion, veterinarians will almost certainly be faced with or have already been confronted with a dog that has hypoglycemia. While this can be a disconcerting problem, being confident in how to symptomatically treat this patient is an important initial step and with a systematic approach, the cause of the hypoglycemia can usually be identified. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Koenig A. Hypoglycemia. In: Hopper KH, Silverstein DC, editors. Small Animal Critical Care Medicine. 1st ed. St Louis, Missouri: Saunders Elsevier; 2009. pp. 295–298. [Google Scholar]
- 2.Smith SA. The hypoglycemic crisis. Proc International Veterinary Emergency and Critical Care Symposium; San Diego, California. 2004. pp. 1–5. [Google Scholar]
- 3.Frizzell RT, Hendrick GK, Biggers DW, et al. Role of gluconeogenesis in sustaining glucose production during hypoglycemia caused by continuous insulin infusion in conscious dogs. Diabetes. 1988;37:749–756. doi: 10.2337/diab.37.6.749. [DOI] [PubMed] [Google Scholar]
- 4.Scott-Moncrieff JC. Logical approach to diagnosis and management of hypoglycemia. Proc CVC; Kansas City, Missouri. 2011. pp. 1–4. [Google Scholar]
- 5.Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27:351–357. doi: 10.1007/BF00304849. [DOI] [PubMed] [Google Scholar]
- 6.Nelson RW. The liver, the pancreas and hypoglycemia. Proc ECVIM-CA/ESVIM Congress; Munich, Germany. September 19–21, 2002; pp. 1–2. [Google Scholar]
- 7.Nelson RW, editor. Canine and Feline Endocrinology. 4th ed. St Louis, Missouri: Saunders Elsevier; 2015. pp. 348–375. [Google Scholar]
- 8.Nelson RW, editor. Small Animal Internal Medicine. 5th ed. St Louis, Missouri: Mosby Elsevier; 2014. pp. 777–823. [Google Scholar]
- 9.Yong AW, Morris Z, Schuler K, Smith C, Wardlaw J. Acute symptomatic hypoglycemia mimicking ischaemic stroke on imaging: A systematic review. BMC Neurol. 2012;12:1–12. doi: 10.1186/1471-2377-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knottenbelt C. Hypoglycemia. In: Villers E, Blackwood L, editors. BSAVA Manual of Canine and Feline Pathology. 2nd ed. Gloucester, England: British Small Animal Veterinary Association; 2005. pp. 248–259. [Google Scholar]
- 11.Little CJ. Hypoglycaemic bradycardia and circulatory collapse in a dog and a cat. J Small Anim Pract. 2005;46:445–458. doi: 10.1111/j.1748-5827.2005.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 12.Cryer PE, Axelrod L, Grossman AB, et al. Evaluation and management of adult hypoglycemia: A clinical practical guideline. J Clin Endocrinol Metab. 2009;94:709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Timon I, Del Cañizo-Gómez FJ. Mechanisms of hypogylcemia unawareness and implications in diabetic patients. World J Diabetes. 2015;6:912–926. doi: 10.4239/wjd.v6.i7.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domori A, Sunahara A, Tateno M, Miyama TS, Setoguchi A, Endo Y. The clinical utility of two human portable blood glucose meters in canine and feline practice. Vet Clin Pathol. 2014;1:55–62. doi: 10.1111/vcp.12115. [DOI] [PubMed] [Google Scholar]
- 15.Cohen TA, Nelson RW, Kass PH, Christopher MM, Feldman EC. Evaluation of six portable blood glucose meters for measuring blood glucose concentrations in dogs. J Am Vet Med Assoc. 2009;253:276–280. doi: 10.2460/javma.235.3.276. [DOI] [PubMed] [Google Scholar]
- 16.Cohn LA, McCaw DL, Tate DJ, Johnson JC. Assessment of five portable blood glucose meters, a point-of-care analyzer, and color test strips for measuring blood glucose concentration in dogs. J Am Vet Med Assoc. 2000;216:198–202. doi: 10.2460/javma.2000.216.198. [DOI] [PubMed] [Google Scholar]
- 17.Kang MH, Kim DH, Jeong IS, Choi GG, Park HM. Evaluation of four portable blood glucose meters in diabetic and non-diabetic dogs and cats. Vet Q. 2016;36:2–9. doi: 10.1080/01652176.2015.1092617. [DOI] [PubMed] [Google Scholar]
- 18.Wess G, Reusch G. Evaluation of five portable blood glucose meters for use in dogs. J Am Vet Med Assoc. 2000;216:203–209. doi: 10.2460/javma.2000.216.203. [DOI] [PubMed] [Google Scholar]
- 19.Paul AE, Shiel RE, Juvet F, Mooney CT, Mansfield CS. Effect of haematocrit on accuracy of two point of care glucometers for use in dogs. Am J Vet Res. 2011;72:1204–1208. doi: 10.2460/ajvr.72.9.1204. [DOI] [PubMed] [Google Scholar]
- 20.Romijn JA. Hypoglycemia. Proc ECVIM-CA/ESVIM Congress; Munich, Germany. September 19–21, 2002; pp. 1–2. [Google Scholar]
- 21.Lord P, Olsson SE, Audell L. Acute pulmonary edema and seizures in hunting dogs. Nord Vet Med. 1975;27:112–116. [PubMed] [Google Scholar]
- 22.Koyama O, Galassetti P, Cocker RH, et al. Prior exercise and the response to insulin-induced hypoglycemia in the dog. Am J Physiol Endocrinol Metab. 2002;282:1128–1138. doi: 10.1152/ajpendo.00370.2001. [DOI] [PubMed] [Google Scholar]
- 23.Rovira S, Munoz A, Benito M. Effect of exercise on physiological, blood and endocrine parameters in search and rescue-trained dogs. Veterinarni Medicina. 2008;6:333–346. [Google Scholar]
- 24.Steiss J, Ahmad HA, Cooper P, Ledford C. Physiologic responses in healthy Labrador Retrievers during field trial training and competition. J Vet Intern Med. 2004;18:147–151. doi: 10.1892/0891-6640(2004)18<147:prihlr>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Syme HM, Scott-Moncrieff JC. Chronic hypoglycemia in a hunting dog due to secondary hypoadrenocorticism. J Small Anim Pract. 1998;39:348–351. doi: 10.1111/j.1748-5827.1998.tb03726.x. [DOI] [PubMed] [Google Scholar]
- 26.Harmon M. Critical care of the feline neonate. Proc International Veterinary Emergency and Critical Care Symposium; Grapevine, Texas. September 7–11, 2016; pp. 1–4. [Google Scholar]
- 27.Zhaofei X, Liyuan C, Yuying H, Wan J, Yu J. Xylitol poisoning of dogs is associated with increased glycogenolysis, coagulopathy and oxidative stress. Toxicol Environ Chem. 2013;95:337–343. [Google Scholar]
- 28.Xia Z, He Y, Yu J. Experimental acute toxicity of xylitol in dogs. J Vet Pharmacol Therap. 2009;32:465–469. doi: 10.1111/j.1365-2885.2009.01065.x. [DOI] [PubMed] [Google Scholar]
- 29.Kuzuya T, Kanazawa Y, Kosaka K. Stimulation of insulin secretion by xylitol in dogs. Endocrinol. 1969;84:200–207. doi: 10.1210/endo-84-2-200. [DOI] [PubMed] [Google Scholar]
- 30.Kuzuya T, Kanazawa Y, Kosaka K. Plasma insulin response to intravenously administered xylitol. Metabolism. 1966;15:1149–1152. doi: 10.1016/0026-0495(66)90105-3. [DOI] [PubMed] [Google Scholar]
- 31.Duhadway MR, Sharp CR, Meyers KE, Koenigshof AM. Retrospective evaluation of xylitol ingestion in dogs: 192 cases (2007–2012) J Vet Emerg Crit Care (San Antonio) 2015;25:646–654. doi: 10.1111/vec.12350. [DOI] [PubMed] [Google Scholar]
- 32.Dunayer EK, Gwaltney-Brant SM. Acute hepatic failure and coagulopathy associated with xylitol ingestion in eight dogs. J Am Vet Med Assoc. 2006;229:1113–1117. doi: 10.2460/javma.229.7.1113. [DOI] [PubMed] [Google Scholar]
- 33.Todd JM, Powell LL. Xylitol intoxication associated with fulminant hepatic failure in a dog. J Vet Emerg Crit Care (San Antonio) 2007;17:286–289. [Google Scholar]
- 34.Eapen AK, de Cock P, Crincoli CM, Means C, Wismer T, Pappas C. Acute and sub-chronic oral toxicity studies of erythritol in Beagle dogs. Food Chem Toxicol. 2017;105:448–455. doi: 10.1016/j.fct.2017.04.049. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs R. Hypoglycemia. In: Allen DG, Kruth SA, Garvey MS, editors. Small Animal Medicine. Philadelphia, Pennsylvania: JB Lippincott; 1991. p. 997. [Google Scholar]
- 36.Steiner JM, Bruyete DS. Canine insulinoma. Compend Cont Vet Educ Pract Vet. 1996;18:13–23. [Google Scholar]
- 37.Goutal CM, Brugmann BL, Ryan KR. Insulinoma in dogs: A review. J Am Anim Hosp Assoc. 2012;48:151–163. doi: 10.5326/JAAHA-MS-5745. [DOI] [PubMed] [Google Scholar]
- 38.Mellanby RJ, Herrtage ME. Insulinoma in a normoglycemic dog with low serum fructosamine. J Small Anim Pract. 2002;43:506–508. doi: 10.1111/j.1748-5827.2002.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 39.Whipple AO, Frants VK. Adenoma of islet cells with hyperinsulinism: A review. Ann Surg. 1935;101:1299–1335. doi: 10.1097/00000658-193506000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robben JH, Pollak YW, Kirpensteijn J, et al. Comparison of ultrasonography, computed tomography and single-photon emission computed tomography for the detection and localization of canine insulinoma. J Vet Intern Med. 2005;19:15–22. doi: 10.1892/0891-6640(2005)19<15:coucta>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Cohen M, Post GS, Wright JC. Gastrointestinal leiomyosarcoma in 14 dogs. J Vet Intern Med. 2003;17:107–110. doi: 10.1892/0891-6640(2003)017<0107:glid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Brady CA, Otto CM. Systemic inflammatory response syndrome, sepsis and multiple organ dysfunction. Vet Clin North Am. 2001;31:1115–1389. doi: 10.1016/s0195-5616(01)50097-2. [DOI] [PubMed] [Google Scholar]
- 43.Castro TX, Cubel Garcia RN, Goncalves LP, et al. Clinical, haematological, and biochemical findings in puppies with coronavirus and parvovirus enteritis. Can Vet J. 2013;54:885–888. [PMC free article] [PubMed] [Google Scholar]
- 44.Vince AR. Fatal babesiosis in a dog imported into Canada. Animal Health Laboratory (AHL) newsletter [homepage on the Internet] 2016. [Last accessed April 9, 2018]. Available from: http://www.wormsandgermsblog.com/files/2016/02/ANwsl20-1-Mar2016-pub2010-p12.pdf.
- 45.Keller N, Jacobson LS, Nel M, deClerg M, Thompson PN, Schoeman JP. Prevalence and risk factors for hypoglycemia in virulent canine babesiosis. J Vet Intern Med. 2004;18:265–270. doi: 10.1892/0891-6640(2004)18<265:parfoh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Lester C, Cooper J, Peters RM, Webster CR. Retrospective evaluation of acute liver failure in dogs (1995–2012) J Vet Emerg Crit Care (San Antonio) 2016;26:559–567. doi: 10.1111/vec.12482. [DOI] [PubMed] [Google Scholar]
- 47.Klein SC, Peterson ME. Canine hypoadrenocorticism: Part 1. Can Vet J. 2010;51:63–69. [PMC free article] [PubMed] [Google Scholar]
- 48.Kohler H, Schroter-Printzen I, Nustede R, Barthel M, Ebert R, Schafmayer A. Endocrine response to intragasrtic and intravenous glucose challenge in the denervated dog pancreas. Int J Pancreatol. 1992;11:117–124. doi: 10.1007/BF02925983. [DOI] [PubMed] [Google Scholar]
- 49.Panciera DL. Fluid therapy in endocrine and metabolic disorders. In: Dibartola SP, editor. Fluid, Electrolyte, and Acid-Base disorders in Small Animal Practice. 4th ed. Philadelphia, Pennsylvania: Elsevier; 2011. pp. 500–513. [Google Scholar]
- 50.Datte K, Guillaumin J. Retrospective evaluation of the use of glucagon infusion as adjunctive therapy for hypoglycemia in dogs: 9 cases (2005–2014) J Vet Emerg Crit Care (San Antonio) 2016;26:775–781. doi: 10.1111/vec.12513. [DOI] [PubMed] [Google Scholar]