Abstract
Background:
Recent evidence suggests that patients with herpes simplex virus (HSV) encephalitis may relapse because of autoimmunity against the N-methyl-D-aspartate receptor (NMDAR). We present a case series of post-HSV relapsing encephalopathy associated with antibodies to central nervous system (CNS) synaptic antigens.
Patient/Methods:
Sera and cerebrospinal fluid (CSF) from five patients with HSV encephalitis who relapsed after antiviral therapy were tested for anti-NMDAR, gamma-aminobutyric acid b receptor (GABAbR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), Leucine-rich, glioma inactivated 1 (LGI1), anti -contactin-associated protein-like 2 (CASPR2) and dipeptidyl-peptidase-like protein-6 (DDPX) antibodies using cell-based assays.
Results:
Five patients (two infants, one child and two adults) developed post-HSV autoimmune encephalitis. The infants, aged 9 months and 10 months, after prompt and seemingly successful anti-HSV therapy, were readmitted with typical signs of NMDAR-encephalitis evolving within days, with NMDAR antibodies detected in both serum and CSF. Although they were promptly treated with intravenous immunoglobulin (IVIg) and with IVIg followed by rituximab, respectively, they were both left with psychomotor deficits. A 14-year-old girl with seizures due to HSV encephalitis improved with anti-HSV therapy. Later, she manifested intractable seizures and she was found positive for anti-NMDAR antibodies which persist. The two adults were women, aged 58 and 33 years. The first recovered after anti-HSV therapy and remained asymptomatic for 6 months, until she developed generalized seizures with persisting CSF anti-NMDAR antibodies; the second, who continued to be encephalopathic after 2 weeks of anti-HSV therapy, tested positive for anti-NMDAR antibodies in the serum and anti-GABAbR antibodies in the serum and CSF. She recovered fully following IVIg therapy but her serum anti-GABAbR antibodies persist 34 months later.
Discussion:
Infection of the CNS with HSV can trigger CNS autoimmunity associated not only with anti-NMDAR but also with anti-GABAbR antibodies. These antibodies can persist in the serum, even without associated symptoms, but their presence in the CSF is firmly associated with disease development. In contrast to children and adults who responded well to therapies, the infants had an incomplete recovery with severe psychomotor deficits probably due to the interference of anti-NMDAR antibodies with neuro-developmental processes.
Keywords: autoimmunity, autoantibodies, CSF, encephalitis, herpes simplex virus
Introduction
Herpes simplex virus (HSV) encephalitis may have a relapsing course in up to 12% of adults and 14–35% of children, despite antiviral treatment. In the relapsing patients, polymerase chain reaction (PCR) for HSV-1 or HSV-2 is often negative. It is not clear whether relapses are due to latent virus reactivation or to a postinfectious autoimmune process against central nervous system (CNS) antigens released following the HSV-induced brain damage.1
It has only been recently described that a foremost cause of relapse is associated with the occurrence of anti-N-methyl-D-aspartate receptor (NMDAR) antibodies.2,3 Anti-NMDAR encephalitis is a well-studied form of autoimmune encephalitis and the antibodies are considered pathogenic4,5 as they bind to the NR1 subunit of the NMDA receptor and likely cause an effect by promoting the internalization of the receptor. The presence of antibodies in the cerebrospinal fluid (CSF) is of diagnostic significance, compared with the serum, and these patients respond favourably to immunotherapy.6 Treatment strategies are mostly empirical and include corticosteroids, intravenous immunoglobulin (IVIg), cyclophosphamide, plasmapheresis or rituximab. There is no difference in the overall therapeutic algorithm between adults and infants.
We present a case series of post-HSV encephalopathy in adults and infants associated with antineuronal antibodies and report on their response to therapy and long-term course.
Patients and methods
We report on five incidental cases that had a clinical relapse following HSV encephalitis in spite of prompt antiviral therapy. The diagnosis of HSV encephalitis was based on clinical, laboratory (HSV testing by CSF PCR and anti-HSV antibody testing in both serum and CSF) and radiological evidence. Sera and CSF were examined for anti-NMDAR, gamma-aminobutyric acid b receptor (GABAbR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), leucine-rich, glioma inactivated 1 (LGI1), anti -contactin-associated protein-like 2 (CASPR2) and dipeptidyl-peptidase-like protein-6 (DDPX) antibodies using commercially available cell-based assays (Euroimmun, Lübeck, Germany). Antiviral antibodies in CSF were also confirmed retrospectively using a commercially available ELISA (Virion/Serion, Würzburg, Germany). CSF and serum samples were tested serially, as dictated by the patients’ response to therapies. The University of Athens Medical School ethics committee provided approval (approval no 5107). Subjects or their next of kin provided informed consent for inclusion in the case series.
Case series
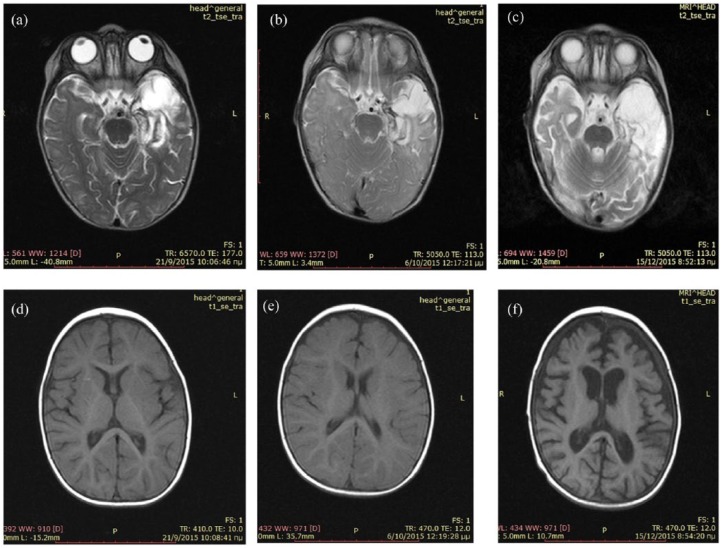
Case 1: A 9-month-old infant presented with malaise and mild fever. On day 4, she manifested an episode of staring. CSF showed 332 cells per mm3 and HSV-1 DNA positivity by PCR. She was started on 60 mg/kg/d acyclovir. The magnetic resonance imaging (MRI) on day 15 showed a haemorrhagic lesion in the left temporal lobe (Figure 1a, 1d). She was discharged after a 21-day course of acyclovir in excellent neurological condition; at discharge, her CSF had become negative for the virus by PCR. However, 2 days later, she was readmitted with fever, agitation, choreoathetotic and dystonic movements and seizures. At this time, she tested positive for anti-NMDAR antibodies in both serum and CSF; retrospective testing of her previous serum and CSF samples drawn 3 weeks earlier were NMDAR-negative. MRI did not show significant changes (Figure 1b, 1e); repeated PCR for HSV remained negative. Interestingly, both of her CSF samples (the first, NMDAR negative, and the second, NMDAR positive) were positive for intrathecal synthesis of immunoglobulin G (IgG) anti-HSV 1 antibodies, prompting another course of antiviral therapy with acyclovir. She was, however, concurrently started on immunotherapy with IVIg 2 g/kg and steroids (5-day pulse IV methylprednisolone/30 mg/kg/d followed by prednisolone 2 mg/kg/d with slow tapering over 1 year).
Figure 1.
Magnetic resonance imaging of case 1.
(a) T2-MRI on day 15 after disease onset shows oedema in the left temporal lobe and the surrounding tissue; (b) T2-MRI at the time point of NMDAR-positive testing shows no further changes; (c) T2-MRI at discharge shows atrophy and gliosis in the left temporal lobe; (d) T1-MRI on day 15 shows normal ventricular size and mild dilatation of the left Sylvian fissure; (e) T1-MRI at NMDAR encephalitis diagnosis shows no further changes in comparison with the previous one; (f) T1-MRI at discharge shows a significant dilatation of the lateral and third ventricles and subarachnoid space.
T1, ; T2,; MRI, magnetic resonance imaging; NMDAR, N-methyl-D-aspartate receptor.
In spite of the immediate immunotherapy initiation, she clinically worsened with continuous violent jerking abnormal movements, suppressed level of consciousness, apnoeas and bradycardia. She was admitted to the intensive care unit and received additional symptomatic treatment with clonidine, lorazepam, phenobarbital, phenytoin, midazolam, ketamine, fentanyl and chloral hydrate 4% pro re nata. On day 30, rituximab (375 mg/m2 every week for 4 weeks) was also added for her anti-NMDAR encephalitis. Gradual clinical improvement in the level of consciousness, autonomic dysfunction and abnormal movements was noted 2 weeks after the onset of rituximab. She was discharged after 98 days of hospitalization with clonidine, levetiracetam, phenobarbital, prednisolone, acyclovir, cotrimoxazole, fluconazole and ranitidine. Brain MRI at discharge showed significant dilatation of subarachnoid spaces with enlargement of the third and lateral ventricles (Figure 1f); the prior haemorrhagic lesion in the left temporal lobe related to HSV-1 infection remains now as a gliotic area (Figure 1c). On discharge, she was not able to sit, had feeding difficulties, very poor visual contact and impaired social interactions.
At 1 year later, anti-NMDAR antibodies, tested again in both serum and CSF, were negative. No increased IgG intrathecal synthesis for HSV-1 antibodies was detected. The infant has been left with a significant neurodevelopmental delay, mainly in speech, cognitive and social skills. Her motor development is better, without abnormal muscle tone, and a steady but slow improvement in her gross motor skills. All drugs except antiepileptics were discontinued.
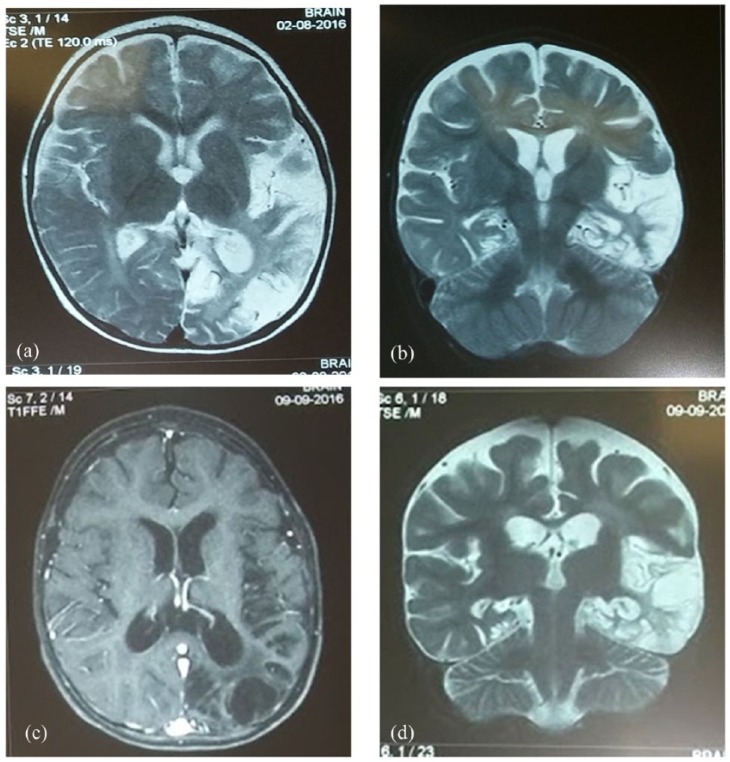
Case 2: A 10-month-old infant was admitted because of intense agitation lasting 20 days. A 3-day febrile infection that spontaneously resolved preceded the irritability. Although the infant was afebrile, a lumbar puncture was performed because of persisting irritability, which revealed 40–45 leukocytes/mm3 and 83 mg/dl of protein. Intravenous ceftriaxone and acyclovir were initiated. CSF culture and PCR for HSV-1 and -2 were negative. However, antibodies for HSV-1 at high titres were found in the serum [both immunoglobulin M (IgM) and IgG] and increased intrathecal synthesis of anti-HSV-1 IgG was detected in the CSF. Brain MRI revealed the presence of increased T2-MRI signal diffusely at the left temporal and occipital lobes (Figure 2a, 2b), consistent with the diagnosis of HSV encephalitis. Although agitation was slightly improved in the ensuing days, the child started to show signs of psychomotor regression. A second lumbar puncture performed at day 23 of admission revealed CSF pleocytosis with 185 leukocytes/mm3 and increased protein of 75 mg/dl; glucose was 50 mg/dl and cultures were negative. A second PCR for HSV-1 and -2 was also negative but the increased intrathecal synthesis of anti-HSV-1 IgG persisted. The CSF now tested positive for anti-NMDAR antibodies.
Figure 2.
Magnetic resonance imaging of case 2.
(a), (b) T2-weighted-images show high-signal intensity and oedema in the left temporal and occipital lobes; (c), (d) Follow-up MRIs show areas of necrotic lesions in the same regions, seen on T1-weighted images (c) and T2 (d).
T1, ; T2,; MRI, magnetic resonance imaging.
In the following days, the child developed choreoathetotic movements, generalized hypotonia, automatisms and impaired feeding. Immunotherapy with IVIg 2 g/kg/day divided in 5 days was initiated followed by methylprednisolone (30 mg/kg/day for 5 days) and gradual oral tapering for 6 weeks. Levetiracetam was added. A new CSF analysis 2 weeks later revealed a higher titre of anti-NMDAR antibodies. Follow-up brain MRI, performed 1 month after immunotherapy, revealed areas of necrotic lesions mainly at the temporal and occipital lobes (Figure 2c, 2d). The child was treated for almost 1 month with intravenous acyclovir 45 mg/kg/day, followed by oral acyclovir prophylaxis for 2 months. The patient, despite the abnormal MRI findings, shows gradual clinical and psychomotor improvement in a 3-month follow up.
Case 3: A 14-year-old girl with no previous medical history presented with a febrile viral infection lasting for 3 days, followed by intense headache, somnolence, confusion and generalized epileptic seizures 8 days later. She was diagnosed with herpetic encephalitis based on positive PCR for HSV-1 in the CSF. CSF analysis revealed 165 cells per mm3, glucose 57 mg/dl and protein 39 mg/dl. Oligoclonal bands and IgG intrathecal synthesis were negative. Brain MRI revealed symmetrical focal lesions in bilateral temporal lobes and basal ganglia without contrast enhancement. She tested negative for antineuronal antibodies in both serum and CSF. The patient was treated with a 3-week course of IV acyclovir plus antiepileptics (sodium valproate) and she was recovering steadily and uneventfully. At 2 months after the initial viral encephalitis, however, she presented with complex partial seizures (manifested with a sense of weakness, staring, dysarthria and difficulty speaking, followed by drowsiness); levetiracetam was added. She continued, however, to experience sporadic epileptic episodes despite adjustments to the antiepileptic therapy.
At 1 year later, the patient was readmitted to the hospital after a more severe epileptic episode and altered level of consciousness. She manifested staring, reduced responsiveness to verbal stimuli, flaccid muscle tone and irregular jerks of arms and legs lasting approximately 1 minute, followed by a short period of weakness and dizziness. For the 3 weeks prior to readmission, she experienced daily long-lasting episodes of headaches, accompanied by photophobia and phonophobia and the last 3 days became somnolent. Repeated diagnostic work up at the time of admission revealed negative CSF PCR for HSV-1 and -2, negative intrathecal synthesis for anti-HSV IgG and negative CSF anti-NMDAR antibodies. Her serum, however, tested positive for anti-NMDAR antibodies that persisted when retested 3 months later. There were no abnormal findings in the electroencephalogram (EEG). Immunotherapy for autoimmune anti-NMDAR encephalitis was not initiated because she had clinically improved and the CSF was negative.
Case 4: A 58-year-old woman was transferred to our hospital with hyperpyrexia (39.0°C) and headache. She was drowsy but easily awoken. CSF analysis showed 140 cells per mm3 and tested positive for HSV-1 in the CSF by PCR. MRI revealed increased T2 signal intensity in the right temporal lobe. She was started on intravenous acyclovir for 21 days and then continued on oral valacyclovir. She gradually improved and normalized. After treatment and before discharge, the HSV-1 PCR in the CSF was negative but the intrathecal synthesis of anti-HSV-1 antibodies persisted; her serum tested positive for anti-NMDAR antibodies, even though she had no residual abnormal neurological findings. During the next 2 months she started to develop dysosmias, sleep disturbance and anxiety. Serum testing for anti-NMDAR autoantibodies continued to be positive. At 6 months later, she developed prosopagnosia and personality changes, followed by an episode of loss of consciousness and generalized epileptic seizures for which she was readmitted to the hospital. Brain MRI revealed gliosis and atrophy of the right temporal lobe, as previously observed. Anti-NMDAR autoantibodies remained positive in the serum, but this time were also detected in the CSF; the intrathecal synthesis of anti-IgG HSV-1 antibodies persisted. She was treated only symptomatically with levetiracetam and improved. She denied treatment with rituximab. Follow-up antibody testing showed persistence of anti-NMDAR antibodies in the serum 3, 12 and 24 months later, even though her clinical condition remains stable.
Case 5: A 33-year-old woman presented with seizures, but with normal orientation, MRI and EEG, afebrile at initial presentation. She was started on oral levetiracetam. At 5 days afterward, she had two more epileptic episodes. She was stable until 10 days later, when she had four new episodes of generalized tonicoclonic spasms occurring within a 24-hour period that led to hospitalization. Neurological examination revealed horizontal nystagmus, confusion and disorientation. She was treated with a single dose of 1000 mg of IV levetiracetam. EEG showed a slow wave pattern, while MRI showed a lesion, probably inflammatory, in the left hippocampus. The CSF revealed 15 cells per mm3 and was negative for HSV-1 and -2 by PCR. Her serum tested positive for IgM HSV-1 and HSV-2 antibodies and she was diagnosed as having HSV encephalitis for which she was treated with IV acyclovir. Orientation improved after 21 days of therapy but then she worsened, becoming confused. A new CSF study 1 month later was negative for HSV-1 IgG antibodies and for HSV-1 and -2 by PCR. However, the patient tested positive for NMDAR and GABAbR antibodies in the serum and for anti-GABAbR antibodies in the CSF. The patient was started on IV corticosteroids followed by IVIg (5 days, 120 g total). After 1 month, she was discharged without any residual symptoms or the need for long-term immunotherapy. Testing after 1, 4 and 22 months revealed persistent seropositivity for both anti-NMDAR and anti-GABAbR antibodies, even though she was clinically normal. Repeated testing after 34 months showed persistence of anti-GABAbR but not anti-NMDAR antibodies.
The case series are explained below in Table 1.
Table 1.
A summary of clinical and laboratory characteristics of all five patients during their infectious phase and during their autoimmune phase.
| Patient/sex/age | HSV |
Autoimmune relapse |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Imaging | Laboratory | Treatment | Follow up | Symptoms | Imaging | Laboratory | Treatment and outcome | |
| #1, female, 9 months (12 months follow up) |
Fever, staring | Haemorrhagic lesion temporal lobe | 332 WBC, HSV-1 PCR (+) in CSF | Acyclovir 21 days | HSV-1 PCR (−) in CSF | Fever, agitation, choreoathetotic movements, seizures | Gliotic lesions in temporal and occipital lobes; cortical atrophy | 30 WBC serum NMDAR (+); CSF NMDAR (+); HSV-1 PCR (−) |
Steroids, IVIg: 2 g/kg divided in five daily dosages; symptomatic: rituximab 375 g/m2 per week for 4 weeks; poor outcome |
| #2, female, 10 months (3 months follow up) |
Fever, agitation | Haemorrhagic lesion temporal lobe and occipital lobe | 45 WBC, IgM HSV-1 and 2 serum antibodies (+), PCR (−) in CSF | Acyclovir 21 days, ceftriaxone | 185 WBC in CSF, HSV-1 PCR (-) in CSF | Agitation, hypotonia, choreoathetotic movements |
Necrotic /gliotic lesions | Serum NMDAR (+); CSF NMDAR (+) | IVIg: 400 mg/kg/day for 5 days; steroids; gradual improvement |
| #3, female, 14 years (16 months follow up) |
Fever, headache, confusion, seizures | Symmetrical focal lesions; temporal lobes and basal ganglia | 165 WBC, HSV-1 PCR (+) in CSF; NMDAR (−) in CSF and serum | Acyclovir 21 days, sodium valproate | HSV-1 PCR (−) in CSF | Headache, somnolence, staring, altered state of consciousness | Normal MRI | Serum NMDAR (+), CSF NMDAR (−) | Symptomatic therapy, mild symptomatology |
| #4, female, 58 years (24 months follow up) |
Fever, headache, drowsiness | T2 increased signal intensity in right temporal lobe | 140 WBC, HSV-1 PCR (+) in CSF | Acyclovir 21 days | Serum NMDAR (+) | Dysosmias, sleep disturbance, anxiety, generalized seizures | Gliosis and atrophy in right temporal lobe | Serum NMDAR (+); CSF NMDAR (+); HSV-1 PCR (−) | Levetiracetam; resolution of symptoms |
| #5, female, 33 years (34 months follow up) |
Seizures, confusion, horizontal nystagmus | MRI lesion left hippocampus | IgM HSV-1 and -2 serum antibodies (+), PCR (−) in CSF | Levetiracetam; acyclovir for 21 days |
− | Confusion | MRI compatible with encephalitis | Serum NMDAR and GABAbR (+); CSF GABAbR (+), NMDAR (−) | Steroids, IVIg: 20 g total in 5 days; complete resolution of symptoms |
CSF, cerebrospinal fluid; GABAbR, gamma-aminobutyric acid b receptor; HSV, herpes simplex virus; NMDAR, N-methyl-D-aspartate receptor; IgM, immunoglobulin M; IVIg, intravenous immunoglobulin; T2,; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; WBC, white blood cell.
Discussion
It has only recently been established that following HSV-1 encephalitis, a number of patients relapse due to development of autoimmune encephalitis triggered by the virus, either as a result of molecular mimicry or due to release of synaptic antigens following the virus-induced neuronal damage. At least 40 cases of post-HSV autoimmune encephalitis have now been reported.3,7 Our study corroborates these findings by describing five new cases and presenting additional immunological findings. Our data also reiterate the view that it is the antibodies in the CSF rather than the serum (in four out of five patients), which are strongly associated with disease flares. Our series also highlights some clinical differences among the infantile, paediatric and adult cases. It seems that infants develop more typical NMDAR disease with a distinct motor component and severe symptomatology;8 in contrast, in the child and the adult patients, the postinfectious autoimmunity was manifested as an epileptic disorder with an overall milder symptomatology to the point that two of them did not require immunotherapy. The variable course and the need for heightened suspicion or early application of immunotherapy are illustrated in case 3, where the initial worsening manifested as partial complex seizures, and was not suspected as possibly related to an evolving autoimmune encephalitis. Consequently, the patient was not tested for anti-NMDAR antibodies to initiate early immunotherapy until her epilepsy became therapy resistant.
Our first paediatric case also revealed an interesting phenomenon; antibody titres were negative early in the infection but were first detected at the time of relapse when psychomotor symptoms became prominent, suggesting that the autoimmune disease is clinically manifested when sufficient antibody levels are produced several days after the viral trigger. Of significant clinical importance is also the observation that infants had a different outcome compared with adults. In spite of early initiation of immunotherapy, including the anti-B cell drug rituximab in the first case, in both infants, the symptoms did not fully resolve, suggesting that the antibodies might have exerted a deleterious effect in synapse development and plasticity or in arresting maturation. Apart from the synaptic deregulation, the widespread autoimmune insult may have led to structural abnormalities, as shown by the diffuse cortical atrophy, and to overt developmental delay.
Case 5 has the novelty that apart from anti-NMDAR antibodies, anti-GABAb antibodies may be also induced following acute CNS HSV infection (as evidenced by the presence of IgM anti-HSV antibodies). This complements another recent report in which post-HSV encephalitis was associated with voltage-gated calcium channel antibodies.9 It is therefore likely that the post-HSV antisynaptic antibodies may not develop as a result of molecular mimicry but rather due to self immunization following the release of antigens from the damaged brain tissue. In most cases of postviral encephalitis, the most commonly observed reactivity is against anti-NMDAR, probably because it is more abundant, compared with other receptors or channels.
Cases 4 and 5 illustrate that antibodies (anti-NMDAR and anti-GABAbR) may persist for long periods (24 and 34 months, respectively), particularly in the serum, even without causing any additional clinical symptoms. This is probably the first demonstration of such long persistence for anti-GABAbR antibodies in fully recovered and seemingly asymptomatic patients. Long-term antibody persistence has been previously shown for idiopathic NMDAR encephalitis10,11 and suggests that certain B-cell clones, probably long-lived plasmablasts, persist in the circulation; whether these represent negative prognostic factors for future relapses, if gaining access to the CNS remains to be determined. The similarity in antibody persistence between autoimmune (nonparaneoplastic) and postviral encephalitis may suggest a similar autoimmunization process or perpetuation of autoimmunity.
Finally, the cases emphasize the merit of prompt therapy. Apart from the infants, the adult and the child case responded favourably to therapy (either symptomatic or immunotherapy) suggesting that post-HSV autoimmune encephalopathy occurring in the mature brain is a treatable condition, particularly when symptoms are mild. The observed benefit with IVIg in these cases may be related to supply of idiotypic antibodies or complement inhibition.12 Other types of immunotherapy could also be considered in the postinfectious cases, such as immunoadsorption, which has previously been employed in autoimmune encephalitides.13 Why the infants did not respond as favourably, despite prompt diagnosis and early initiation of aggressive immunotherapy, requires further study; it is likely that the antibodies in immature brain may interfere with normal development, resulting in cortical atrophy and developmental delay.
Footnotes
Funding: This study was funded by the Institute of Autoimmune Systemic and Neurological Disorders.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Harry Alexopoulos, Department of Pathophysiology, National and Kapodistrian University of Athens, Athens, Greece.
Sofia Akrivou, Department of Pathophysiology, National and Kapodistrian University of Athens, Athens, Greece.
Sotiria Mastroyanni, Department of Neurology, ‘P & A Kyriakou’ Children’s Hospital, Athens, Greece.
Maria Antonopoulou, Department of Neurology, 251 Hellenic Air Force Military Hospital, Athens, Greece.
Argirios Dinopoulos, Third Department of Pediatrics, National and Kapodistrian University of Athens, Athens, Greece.
Melpo Giorgi, Third Department of Pediatrics, National and Kapodistrian University of Athens, Athens, Greece.
Kostas Konstantinou, Department of Pathophysiology, National and Kapodistrian University of Athens, Athens, Greece.
Evangelos Kouremenos, Department of Neurology, 251 Hellenic Air Force Military Hospital, Athens, Greece.
Maria Lariou, Department of Neurology, ‘P & A Kyriakou’ Children’s Hospital, Athens, Greece.
Dimitrios Naoumis, Department of Neurology, 251 Hellenic Air Force Military Hospital, Athens, Greece.
Efterpi Pavlidou, Second Department of Pediatrics, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Evaggelos Pavlou, Second Department of Pediatrics, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Konstantinos Voudris, Department of Neurology, ‘P & A Kyriakou’ Children’s Hospital, Athens, Greece.
Panayotis Vlachoyiannopoulos, Department of Pathophysiology, National and Kapodistrian University of Athens, Athens, Greece.
Marinos C. Dalakas, Neuroimmunology Unit, Department of Pathophysiology, Faculty of Medicine, National and Kapodistrian University of Athens, Greece.
References
- 1. Bradshaw MJ, Venkatesan A. Herpes simplex virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics 2016; 13: 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pruss H, Finke C, Holtje M, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012; 72: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armangue T, Moris G, Cantarin-Extremera V, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 2015; 85: 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Planaguma J, Leypoldt F, Mannara F, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain 2015; 138: 94–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wright S, Hashemi K, Stasiak L, et al. Epileptogenic effects of NMDAR antibodies in a passive transfer mouse model. Brain 2015; 138: 3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sutcu M, Akturk H, Somer A, et al. Role of autoantibodies to N-Methyl-d-Aspartate (NMDA) receptor in relapsing herpes simplex encephalitis: a retrospective, one-center experience. J Child Neurol 2016; 31: 345–350. [DOI] [PubMed] [Google Scholar]
- 8. Pruss H. Postviral autoimmune encephalitis: manifestations in children and adults. Curr Opin Neurol 2017; 30: 327–333. [DOI] [PubMed] [Google Scholar]
- 9. Bradshaw MJ, Pawate S, Lennon VA, et al. Herpes simplex virus 1 encephalitis associated with voltage-gated calcium channel autoimmunity. Neurology 2015; 85: 2176–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alexopoulos H, Kosmidis ML, Dalmau J, et al. Paraneoplastic anti-NMDAR encephalitis: long term follow-up reveals persistent serum antibodies. J Neurol 2011; 258: 1568–1570. [DOI] [PubMed] [Google Scholar]
- 11. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014; 13: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther 2004; 102: 177–193. [DOI] [PubMed] [Google Scholar]
- 13. Dogan Onugoren M, Golombeck KS, Bien M, et al. Immunoadsorption therapy in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e206. [DOI] [PMC free article] [PubMed] [Google Scholar]