Abstract
The objective of this study was to investigate the association between sow- and litter-level factors with mortality in a swine nursery barn experiencing a severe Streptococcus suis disease outbreak. All-cause mortality data from a 300-sow farrow-to-finish herd was analyzed using a Cox’s regression model. The data were recorded over 6 months and included 24 cohorts, 297 sows, 295 litters, and 2779 piglets with an average of 14.4% post-weaning mortality. If the sows had 2 litters within the study period and pigs from their first litter experienced mortality, then pigs from their subsequent litter had a decreased risk of mortality [hazard ratio (HR) = 0.34, P < 0.05]. Pigs were more likely to experience mortality if at least 1 additional littermate experienced mortality (HR = 9.22, P = 0.001). Under conditions of this study, the results suggest mechanisms related to sow immunity and within-litter spread that could have contributed to the risk of mortality during the S. suis outbreak.
Résumé
Facteurs contribuant à la mortalité durant une éclosion de Streptoccocus suis chez des porcelets en pouponnière. Cette étude avait pour objectif de faire enquête sur l’association entre les facteurs au niveau de la truie et de la portée en lien avec la mortalité dans une pouponnière de porcelets aux prises avec une grave éclosion de maladie causée par Streptococcus suis. Les données sur toutes les causes de mortalité provenant d’un troupeau de 300 truies de naissage-finition ont été analysées en utilisant un modèle de régression de Cox. Les données ont été enregistrées pendant 6 mois et incluaient 24 cohortes, 297 truies, 295 portées et 2779 porcelets avec une moyenne de mortalité après le sevrage de 14,4 %. Si les truies avaient 2 portées durant la période de l’étude et qu’il y avait de la mortalité chez les porcs de leur première portée, alors les porcs de leur portée subséquente présentaient un risque réduit de mortalité (taux de risque [TR] = 0,34, P < 0,05). Il était plus probable qu’il y ait de la mortalité chez les porcs si au moins 1 autre compagnon de portée était mort (TR = 9,22, P = 0,001). En vertu des conditions de cette étude, les résultats suggèrent des mécanismes associés à l’immunité des truies et à l’écart au sein de la portée qui ont pu contribuer au risque de mortalité durant l’éclosion de S. suis.
(Traduit par Isabelle Vallières)
Introduction
The early nursery phase is stressful for pigs due to various novel environmental factors in combination with an immature immune system that leaves pigs susceptible to infection with multiple pathogens, possibly leading to clinical disease. Streptococcus suis is one of the most important pathogens affecting nursery pigs (1). There are currently 35 serotypes of S. suis identified based on capsular polysaccharides (cps), in addition to untypable strains that are un-encapsulated, or do not contain a cps moiety (2). The bacteria may occur as commensals or as an opportunistic pathogen in the nasal cavity and tonsils of the majority of pigs (2). In most cases, the bacteria remain colonized in healthy pigs, but transition into a systemic infection causing clinical disease can occur (3,4). It is unknown what causes this transition in some pigs and not others; however, nursery pigs seem to be at high risk, in part due to the stress associated with this transitional period of development (5).
Infections caused by S. suis in nursery pigs are usually characterized by a low incidence (0% to 5%) of clinical cases that show a variety of signs related to septicemia, arthritis, pericarditis, and meningitis. The onset of clinical signs is typically rapid and sudden death can also be a common finding (2). Treatment with penicillin or broad-spectrum β-lactams such as ampicillin and amoxicillin can be effective; however, even with early treatment, prognosis is often poor (6). Outbreaks involving a large proportion of at risk-animals (> 20%) can occur, demonstrating the severe impact this disease can have on swine populations. The pathogenesis of S. suis is not fully understood, adding an additional layer of complexity when attempting to determine what triggers an outbreak of clinical disease (7).
Control of S. suis disease using individual animal or farm level treatments is challenging and vaccination trials provide inconsistent results, making prevention strategies within the nursery an important area of research (8). There is great interest in what factors cause the transition from healthy pigs carrying commensal bacteria to pigs with clinical disease. These risk factors may include the influence of a sow’s passive immunity, co-infection with other pathogens, environmental or genetic factors that leave some pigs more susceptible to S. suis disease than others.
The objectives of the current study were to identify sow- and litter-level risk factors associated with nursery pig mortality during a prolonged outbreak of clinical disease caused by S. suis, and to use this information to help guide potential prevention and control strategies for future outbreaks.
Materials and methods
Retrospective data set
The data used were the production records from a 300-sow farrow-to-finish farm experiencing an outbreak of S. suis within the nursery over the 6-month period, October 2011 to March 2012. Mortality data for this period included overall mortality due to any reason; however, most deaths were assumed to be due to S. suis based on clinical signs of acute meningitis. A subset of sick pigs had diagnostic postmortem examinations conducted to confirm S. suis as the cause of the disease.
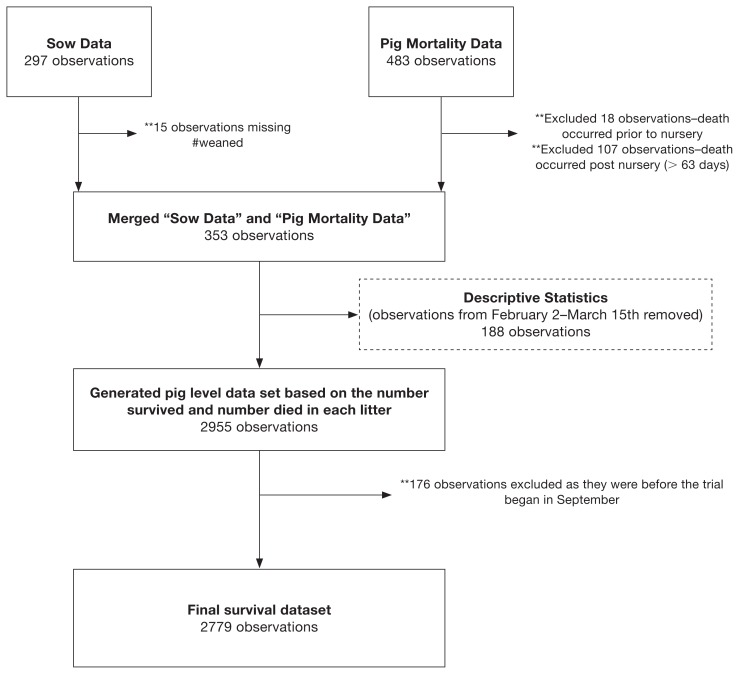
Only the all-cause (overall) mortality for the entire 6-month period was the outcome of interest. The dataset used for analysis was a compilation of data from 2 sources: i) pig-mortality data indicative of all-cause mortality recorded by the farm staff during the 6-month study period, and ii) individual sow production records for all sows that farrowed during the study period. For the pig-level mortality dataset, records were excluded if mortality occurred before or after time at risk during nursery phase of production (Figure 1). The time at risk was defined to be 63 d, or the approximate time a pig spent in this farm’s nursery and the period immediately following the nursery phase. Thus, deaths that occurred before or after the period of risk were excluded from the pig-level records (Figure 1). After removal of all missing observations the datasets were merged based on sow identification (ID) and processed so that it represented pig-level data from specific litters that entered the nursery between October 2011 and March 2012.
Figure 1.
Flowchart showing the step-by-step data manipulation starting with an online database and mortality records from a single farm over a 6-month period up until the point of survival analysis.
Three new variables were generated from the expanded dataset including pre-weaning mortality, previous-litter mortality, and within-litter mortality. Previous litter mortality was generated as a nominal variable with 3 levels based on nursery mortality levels in each litter in the following way: i) reference category — the first litter of sows with repeated farrowing’s that had 0% nursery mortality, ii) the risk factor of interest — the first litter of sows with repeated farrowings that had > 0% nursery mortality, and iii) sows with only 1 litter in the dataset (their records were classified into a third category as they could not contribute to evaluation of this risk factor of interest).
Parity was grouped into 6 categories; category 6 contained parities 6, 7, and 8 as there were only 6 sows in the dataset with a parity > 6. Pre-weaning mortality was organized into 6 groups based on percentile mortality within litters. The first category represents cross-fostered pigs, in which a litter received pigs from another sow and therefore yielded a percentage pre-weaning mortality of 0% if no mortality was experienced by those pigs. The rest of the observations were grouped into 10% mortality categories up to 50%. Then pre-weaning mortality between 50% to 100% was grouped together as few litters experienced pre-weaning mortality levels in this range.
Cox’s proportional hazard model building
Descriptive statistics were performed on each predictor variable of interest including sow parity, the month of weaning, age of weaning, the number of piglets weaned, pre-weaning mortality, previous litter mortality, and mortality within litters. Observations collected on pigs between February 2, 2012 and March 15, 2012 when a vaccination trial occurred were excluded from descriptive statistics to avoid potentially altering mortality patterns experienced at that time (Figure 1).
For the inferential analysis, the full dataset of 2779 observations coming from the merged sow- and pig-level datasets was used. The categorical variables were assessed using Kaplan-Meier survival curves, followed by a log-rank test for statistical significance (P < 0.05). Univariable analysis was conducted using Cox’s proportional hazard model on each of the categorical and continuous predictor variables of interest with a liberal P-value cut-off of P < 0.20 for inclusion in the main effects model. All continuous variables were also assessed for a significant quadratic term (P < 0.05) using Cox’s regression. The time to event variable for the Cox’s proportional hazard model was the time it takes for mortality to occur once a pig entered the nursery.
The interaction terms were tested based on the causal model. The main relationships of interest involved the age, number weaned, and pre-weaning mortality based on their potential to represent overall litter health and subsequent impact on mortality within the nursery (8). Once the final model was identified, the assumption of proportional hazards was evaluated for each variable using Schoenfeld residuals and deviance residuals and the overall fit of the model was evaluated using Cox-Snell residuals. Due to the potential of clustering within litters the model was adjusted using robust standard errors.
Results
Descriptive statistics of categorical and continuous variables provided at the sow- and pig-levels are presented in Table 1. Over the duration of the 6-month period, 12 pigs showing signs for acute meningitis were submitted for full postmortem analysis and all 12 were confirmed cases of S. suis disease. They were confirmed in the laboratory based on growth from meningeal swabs plated on Columbia agar, and confirmation of the isolate as S. suis via matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) technology.
Table 1.
Descriptive analysis of each categorical and continuous variable of interest based on the number of observations, percentage of the total observations at the pig-level and sow-level, and the mean and range of observations, respectively.
| Covariate | Category | Pig-level (N observations, % total) | Sow-level (N observations, % total) |
|---|---|---|---|
| Month of weaning | November 2011 | 524, 19 | 52, 17 |
| December 2011 | 378, 14 | 39, 13 | |
| January 2011 | 484, 17 | 54, 18 | |
| February 2011 | 377, 14 | 43, 14 | |
| March 2011 | 447, 16 | 46, 15 | |
| November 2011 | 569, 20 | 51, 15 | |
| Parity | Parity 1 | 672, 24 | 74, 30 |
| Parity 2 | 809, 29 | 82, 28 | |
| Parity 3 | 459, 17 | 54, 18 | |
| Parity 4 | 445, 16 | 51, 17 | |
| Parity 5 | 224, 8 | 24, 8 | |
| Parity 6,7,8 | 170, 6 | 12, 4 | |
| Pre-weaning mortality | Cross Fostered | 475, 23 | 52, 26 |
| ≥ 0% to 10% mortality | 302, 14 | 29, 15 | |
| > 10% to 20% mortality | 506, 24 | 52, 26 | |
| > 20% to 30% mortality | 351, 17 | 34, 18 | |
| > 30% to 40% mortality | 311, 15 | 33, 17 | |
| > 40% to 50% mortality | 121, 6 | 14, 7 | |
| > 50% to 100% mortality | 45, 2 | 8, 4 | |
| Missing | 668, 24 | 107, 35 | |
| Nursery mortality within the same litter | 0 to 1 pig dead | 971, 35 | 96, 35 |
| > 1 or 1 additional pig died within the same litter | 1808, 65 | 188, 65 | |
| Sow with previous litter having 0% mortality | 285, 10 | 25, 8 | |
| Sow with previous litter having > 0% mortality | 177, 7 | 17, 6 | |
| Sows with only 1 litter in data set | 2317, 83 | 252, 83 | |
| Final number of piglets weaned | [0,18] | 10.27 ± 2.17 | |
| Age of weaning in days | [8,57] | 27.63 ± 3.16 |
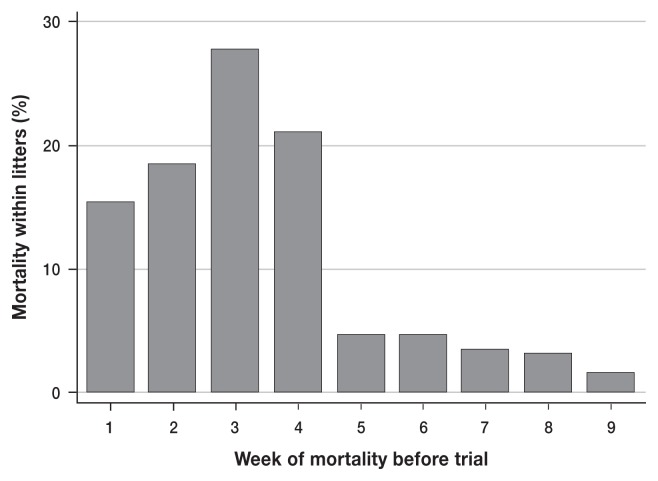
Approximately 40% of litters did not experience any mortality. In the litters in which mortality occurred, the most common level was between 0% and 30%. The deaths occurred most frequently during the 2nd to 4th wk after entry into the nursery, followed by a rapid decline in reported mortality after the 4th wk (Figure 2). In addition, levels of mortality were seen during the colder months of January and December. The full data set containing 2779 pigs, evaluated over the 6-month study had 358 pigs (13%) that died within the nursery and average within-litter mortality was 14.4%.
Figure 2.
Mortality within litters organized by the week of death within the nursery from October 2011 to January 2012, before the vaccination trial. The time within the nursery or time for potential mortality to occur is 63 days or 9 weeks where weeks 2, 3, and 4 are showing increased mortality rates.
The complete univariable analysis results can be found in Table 2. Univariable analysis based on Cox’s proportional hazard model revealed parity to be non-statistically significant (P > 0.05), in addition log-rank test indicated there was no significant difference between the categories (P > 0.05), resulting in the removal of parity from the model.
Table 2.
Univariable analysis representing each factor of interest LRT P-value, partial LRT P-value, and hazard ratios after each variable was run individually through a Cox’s proportional hazard regression model. The referent categories are listed below the table.
| Predictor LRT P-value | Covariate category | Hazard ratio | Partial LRT P-value | 95% Confidence interval |
|---|---|---|---|---|
|
aMonth of weaning 0.001 |
January | — | — | |
| February | 0.727 | 0.074 | 0.51, 1.03 | |
| March | 0.387 | 0.001 | 0.26, 0.57 | |
| October | 0.225 | 0.001 | 0.14, 0.36 | |
| November | 0.735 | 0.098 | 0.51, 1.06 | |
| December | 1.15 | 0.392 | 0.84, 1.56 | |
|
bParity 0.635 |
Parity 1 | — | — | — |
| Parity 2 | 0.962 | 0.803 | 0.71, 1.30 | |
| Parity 3 | 1.240 | 0.202 | 0.89, 1.72 | |
| Parity 4 | 1.052 | 0.773 | 0.74, 1.48 | |
| Parity 5 | 0.964 | 0.876 | 0.61, 1.51 | |
| Parity 6 | 1.247 | 0.344 | 0.78, 1.97 | |
|
cPre-weaning mortality (%) 0.034 |
Cross Fostered | — | — | — |
| Pre-weaning mortality 0 to 10 | 0.584 | 0.020 | 0.37, 0.91 | |
| Pre-weaning mortality 10 to 20 | 1.166 | 0.348 | 0.84, 1.61 | |
| Pre-weaning mortality 20 to 30 | 0.837 | 0.367 | 0.56, 1.23 | |
| Pre-weaning mortality 30 to 40 | 0.782 | 0.238 | 0.51, 1.18 | |
| Pre-weaning mortality 40 to 50 | 0.674 | 0.208 | 0.36, 1.25 | |
| Pre-weaning mortality 50 to 100 | 0.940 | 0.885 | 0.41, 2.17 | |
|
dNursery mortality within the same litter 0.001 |
0 or 1 death within litter | — | — | — |
| > 1 or 1 additional pig died within the same litter | 10.342 | 0.001 | 7.79, 13.72 | |
|
ePrevious litter mortality 0.001* |
Sow’s previous litter < 1% mortality | — | — | — |
| Sow’s previous litter having > 1% mortality | 0.553 | 0.016 | 0.36, 0.86 | |
| Sows with only 1 litter in data set | 0.126 | 0.009* | 0.04, 0.39 | |
| Age of weaning 0.002 |
Age at weaning | 0.943 | 0.002 | 0.91, 0.98 |
| Number of piglets weaned 0.001 |
Final number pigs weaned | 0.897 | 0.001 | 0.85, 0.94 |
Month weaned = January.
Parity = parity 1.
Pre-weaning mortality = < 0% or cross fostered.
Nursery mortality in the same litter = 0 or 1 death.
Previous litter mortality = previous mortality 0%.
Non-relevant P-value.
The final Cox’s regression model can be found in Table 3. Age of weaning had time varying effects based on Schoenfeld residuals and was left in the model due to its potential biological impact on mortality and statistically significant impact on the Cox’s regression model (P < 0.05). The model that was adjusted for the effect of clustering within litters using robust standard errors was adopted as the final model.
Table 3.
Final Cox’s regression model illustrating the hazard ratio for each factor of interest before and after adjustment for the effect of clustering within litters.
| Cox’s regression model using | Cox’s regression model robust standard error | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Covariate | Category | Hazard ratio | P-value | 95% CI | Hazard ratio | P-value | 95% CI |
| Number of weaned piglets | 0.913 | 0.001 | 0.86, 0.96 | 0.913 | 0.001 | 0.87, 0.95 | |
| Age at weaning | 1.077 | 0.002 | 1.02, 1.12 | 1.076 | 0.001 | 1.04, 1.10 | |
| Nursery mortality within the same litter *0–1 pig dead within a litter |
> 1 or 1 additional pig in litter died | 9.216 | 0.001 | 6.87, 12.36 | 9.216 | 0.001 | 7.21, 11.77 |
| Previous litter mortality *Sow with previous litter having 0% mortality |
Sow with previous litter having > 0% mortality | 0.337 | 0.082 | 0.10, 1.14 | 0.337 | 0.024 | 0.13, 0.87 |
| Sows with only 1 litter in data set | 1.158 | 0.623 | 0.64, 2.08 | 1.158 | 0.536 | 0.72, 1.84 | |
| Month of weaning *January |
October | 0.349 | 0.001 | 0.21, 0.57 | 0.349 | 0.001 | 0.22, 0.55 |
| November | 0.822 | 0.295 | 0.57, 1.19 | 0.822 | 0.318 | 0.55, 1.21 | |
| December | 0.768 | 0.103 | 0.56, 1.05 | 0.768 | 0.089 | 0.56, 1.04 | |
| February | 0.767 | 0.143 | 0.53, 1.09 | 0.767 | 0.152 | 0.53, 1.10 | |
| March | 0.925 | 0.771 | 0.55, 1.56 | 0.925 | 0.750 | 0.57, 1.49 | |
| Age at weaning *TVC |
0.996 | 0.001 | 0.99, 1.00 | 0.996 | 0.001 | 0.99, 0.99 | |
Referent category.
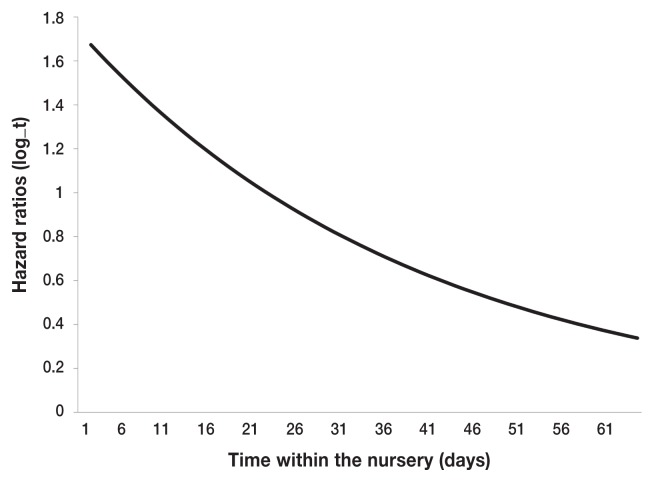
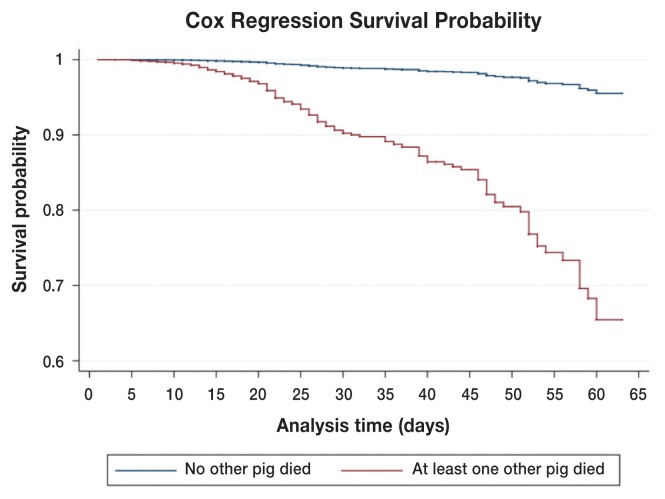
The factors that remained in the final model were the number of piglets weaned, age of weaning as well as its time varying component, within litter mortality, previous litter mortality, and month of weaning. No significant interaction terms were identified. Number of pigs weaned per litter had a protective effect on nursery mortality, specifically; the hazard of mortality was decreased if the number of pigs weaned increased (HR = 0.9, P < 0.05). Increasing the age of weaning increased the hazard of mortality within the nursery (HR = 1.1, P < 0.05, Table 3). However, as the time in nursery progressed, a higher weaning age started to demonstrate protective effect as suggested by the time-varying effect of the weaning age (HR = 0.9, P < 0.05). This concept is illustrated in Figure 3, which estimates that increasing weaning age increases the hazard of dying early in the nursery phase, and this hazard decreases in the later nursery phase. Litter-mort is illustrated in Figure 4. If an additional pig within a litter experienced mortality there was a significant increased hazard for another pig within that same litter to experience mortality (HR = 9.2, P < 0.05). Previous-mort had a significantly protective effect, indicating if a sow’s first litter experienced greater than 0% nursery mortality, a pig from a second litter would have a decreased risk of experiencing mortality in the nursery (HR = 0.3, P < 0.05). Finally, month-wean was found to have confounding effects on previous litter mortality and age of weaning, and a significant difference between mortality rates was observed between the months of October and January (HR = 0.3, P < 0.05).
Figure 3.
Expected development of hazard ratio (HR) over time for a hypothetical increase in weaning age of 7 days. Protective effect of increased weaning age (i.e., HR < 1) is expected to occur at approximately 21 days for this scenario, based on the final Cox’s regression model.
Figure 4.
Predicted survival curves for a pig from a litter in which no other pig died during the nursery period (blue line) and from a litter in which at least one other pig died (red line). Predictions are generated from the final Cox’s regression model and are based on pigs with baseline values for other variables in the model.
Discussion
This S. suis outbreak investigation provided important information on common mortality patterns and identified potential risk factors that may have influenced nursery mortality levels. Descriptive statistics identified a common pattern of mortality in the nursery, in which pigs generally experienced mortality due to S. suis in the 2nd, 3rd, or 4th week after entry. Other studies have also reported that mortality related to S. suis commonly occurs at a specific age within the nursery, although the age of mortality may vary among farms (3,5,9). One explanation for this pattern of mortality may be the transition from passive to active immunity that pigs experience, generally corresponding to the time pigs have been relocated from the farrowing room into the nursery (7). Pigs can be colonized at birth from the vaginal canals of sows and rely on the passive immunity received from the sow’s colostrum to protect them from clinical disease causing bacteria (10). After the initial colostrum intake, the maternal antibodies in the piglet gradually decline, while the pig mounts active immunity. The disease exposure at weaning may increase during this time of stress due to transition from the farrowing room to the nursery, leaving pigs susceptible to systemic infection with S. suis within these first few weeks post-weaning (7).
According to this dataset, if pigs survived past the first 2 to 4 wk within the nursery, they were most likely able to survive the entire duration of the nursery period. However, more investigation into this pattern should be conducted as some pigs remain healthy carriers of the bacteria throughout this transitional period, while others that appear to experience the same set of risk factors develop clinical disease due to S. suis infection (9). In addition, this dataset focuses on all-cause mortality and although a subset of piglets underwent postmortem analysis and the cases were confirmed to be caused by S. suis, the mortality patterns observed during this outbreak situation likely contain a small proportion of deaths due to other causes.
Another important factor to consider due to this stressful transition into the nursery is the optimal age for weaning to ensure a healthy transition. According to this outbreak investigation, increasing the weaning age by 7 d increases a pig’s hazard of dying on day 1 within the nursery and then the longer the pig survives within the nursery the hazard of mortality gradually reduces relative to the pig that was weaned earlier. This suggests older weaned pigs are expected to have a higher risk of mortality in the early phase of the nursery, but lower risk in the later phase. These conclusions, however, are not necessarily directly a result of the age of weaning, and may be indirectly related to the high mortality levels seen in the early phase of the nursery. One potential limitation to this finding is the use of all-cause mortality as an outcome, which may have resulted in an inflated mortality rate on the early days piglets enter the nursery. Additionally, there may be confounding factors that accompany field studies that could explain this pattern of mortality we observed. This outcome, however, does provide a good launching pad for future research investigating patterns and timing of mortality associated with weaning age and S. suis outbreaks in the nursery. Although there is currently no defined age at which to wean pigs that can help prevent S. suis outbreak situations, previous literature suggests weaning between 21 to 28 d of age to be an appropriate target for strong healthy pigs that are well-prepared to handle the stress of weaning (5).
The number of pigs weaned per sow also appears to have an impact on survival in the nursery. In this study, increasing the number of pigs weaned resulted in a significantly decreased risk of mortality within the nursery. This was hypothesized to be a result of the overall health status of the litter. More specifically, if there is an increase in the number of pigs born alive and surviving until weaning, it is likely this is representative of a healthier litter and subsequently these pigs should have greater success within the nursery. However, due to the potential influence of cross-fostering and factors that could not be controlled, this finding was treated as a potential confounder to the data and further investigation into this risk factor is necessary.
Once a pig has entered the nursery there are a few factors that can lead to significantly increased mortality during an S. suis outbreak. Specific to this study, there was a significantly increased hazard of mortality for pigs within a litter if at least 1 additional pig from the same litter died. It is possible that this increased hazard may be attributed to within pen transmission of S. suis. Studies on transmission of S. suis have demonstrated both direct and indirect spread of S. suis within litter (11). A direct transmission model showed all pigs within a pen being infected with S. suis at a rate of 3.58 pigs/day following the introduction of a S. suis carrier (11). The indirect transmission model had infected pigs at a 1 m distance from non-infected pigs and after approximately 7 to 25 d the non-infected pigs in the neighboring pens became colonized with the bacteria (11). The rate and intensity of transmission between pigs with these models was attributed to the type of contact the pigs experienced, the virulence or serotype of S. suis, and the susceptibility of the pigs (11). If a pig is exposed to a pig that has died due to S. suis within a pen, the transmission of bacteria occurs at an even higher rate (12). To decrease within litter transmission of S. suis, it would likely be effective to quickly treat and isolate sick pigs when possible.
It is important to also consider that littermates experience similar risk factors such as having similar passive immunity, genetics, and pathogen exposure. Therefore, these pigs may share a similar set of risk factors that have led them to be more susceptible to disease than other litters, which is supported in the findings for this outbreak data set with respect to the large hazard ratio calculated for within litter mortality (12).
Although the risk factors at the pig-level are important for implementing prevention measures for mortality occurring during S. suis outbreaks, it is also important to explore sow-level factors on the survival of pigs within the nursery. This investigation revealed an interesting risk factor when comparing the mortality patterns within litters from the same sow over multiple parities. Only 15% of the sows in the study had multiple litters during the study period; however, within those sows there was a significant protective effect on the survival of pigs within a litter if pigs from a previous litter experienced mortality during the post-weaning stage. Based on this dataset, it is hypothesized that sows were able to provide greater immunological protection from infection with S. suis if they had a previous litter that experienced mortality due to S. suis. While this finding is not of a confirmatory nature, it indicates that interaction between the health of sows and their offspring perhaps needs to be tracked over time in multiple litters. This is an important area for further investigation as prevention measures at the sow-level can be both an economically viable and time-effective form of intervention (6).
Finally, season was identified as potentially a confounding observation based on the Cox’s regression model. During the colder months of January and December there were consistently higher mortality rates compared to October. The results surrounding seasonal effects are varied and month-wean was considered to be a confounding observation to include when analyzing the other identified risk factors.
There were a few limitations to consider when interpreting the results of this outbreak investigation. The major limitation was that not all mortalities included were confirmed cases of S. suis based on postmortem analysis and laboratory confirmation. Out of the 358 mortalities experienced during the 6-month duration of the trial, only 12 of those were submitted for postmortem analysis and confirmed to be due to S. suis infection. In our subsample, our confirmed serotype was serotype 2, a known virulent strain, but as there were only 12 subsamples, the all-cause mortality diagnosis provided more avenues for risk factor analysis. However, all the samples submitted were confirmed S. suis cases; therefore, it appears that clinical signs for acute meningitis used as a tool for diagnosis was effective. It would be beneficial in future studies to analyze the specific serotypes causing infection, as there are 35 serotypes of S. suis, all having the potential to cause various patterns of mortality, transmission routes, and potential for concurrent infections (1).
Another potential limitation was the lack of information on co-infection with other pathogens. Pneumonia and common viruses such as porcine reproductive and respiratory syndrome (PRRS) virus are known to influence the impact of S. suis (13). Additional research into this area may enhance our understanding of the transmission and mortality associated with S. suis.
There were missing data on pre-weaning mortality or parity for a proportion of the sows in the study (5% of sows, or 15 of 297 sows); therefore, these risk factors should be explored further as they still may be important to consider during outbreak scenarios.
In conclusion, the combination of sow- and pig-level factors had the potential to contribute in their own ways to mortality observed during this outbreak of S. suis disease. Although S. suis infection is complicated, requiring further research to understand its full impact in the nursery, we can use outbreak situations such as these to establish certain prevention measures aimed at minimizing the effects of S. suis. Further research on the influence that vaccinating sows could have on the immune status of piglets has the potential to create highly efficient prevention strategies. In addition, focusing efforts on treating and isolating sick pigs as quickly as possible to control bacterial transmission and decreasing the stress that pigs experience when entering the nursery through efficient husbandry practices could prove to be useful in the prevention and control of S. suis outbreaks in the nursery.
Acknowledgments
Funding for this research project was received from Ontario Pork and the University of Guelph — Ontario Ministry of Agriculture, Food, and Rural Affairs Research Partnership. Animal Health Laboratory, University of Guelph conducted the laboratory analysis. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Goyette-Desjardins G, Auger J-P, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent — An update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3:1–45. doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottschalk M. Streptococcosis. In: Zimmerman JJ, Karicker LA, Ramirez A, Schwartz KJ, Stevenson GW, editors. Diseases of Swine. 10th ed. Ames, Iowa: Wiley-Blackwell; 2012. pp. 841–855. [Google Scholar]
- 3.Windsor RS, Elliott SD. Streptococcal infection in young pigs and provisionally designated Streptococcus suis. J Hyg (Lond) 1975;75:69–78. doi: 10.1017/s0022172400047070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins R, Gottschalk M. An update on Streptococcus suis identification. J Vet Diagn Invest. 1990;2:249–252. doi: 10.1177/104063879000200324. [DOI] [PubMed] [Google Scholar]
- 5.Davis ME, Sears SC, Apple JK, Maxwell CV, Johnson ZB. Effect of weaning age and commingling after the nursery phase of pigs in a wean-to- finish facility on growth, and humoral and behavioral indicators of well-being. J Anim Sci. 2006;2:743–756. doi: 10.2527/2006.843743x. [DOI] [PubMed] [Google Scholar]
- 6.Baums CG, Kock C, Beineke A, et al. Streptococcus suis bacterin and subunit vaccine immunogenicities and protective efficacies against serotypes 2 and 9. Clin Vaccine Immunol. 2009;2:200–208. doi: 10.1128/CVI.00371-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rooke JA, Bland IM. The acquisition of passive immunity in the newborn piglet. Livest Prod Sci. 2002;78:13–23. [Google Scholar]
- 8.Baums CG, Valentin-Weigand P. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim Health Res Rev. 2009;10:65–83. doi: 10.1017/S146625230999003X. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk M, Higgins R, Boudreau M. Use of polyvalent coagglutination reagents for serotyping of Streptococcus suis. J Clin Microbiol. 1993;8:2192–2194. doi: 10.1128/jcm.31.8.2192-2194.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amass SF, Clark LK, Knox K, Wu CC, Hill MA. Streptococcus suis colonization of piglets during parturition. Swine Health Prod. 1996;4:4–7. [Google Scholar]
- 11.Dekker N, Bouma A, Daemen I, et al. Effect of spatial separation of pigs on spread of Streptococcus suis serotype 9. PLoS ONE. 2013;4:1–9. doi: 10.1371/journal.pone.0061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dee SA, Corey MM. The survival of Streptococcus suis on farm and veterinary equipment. Swine Health Prod. 1993;1:17–20. [Google Scholar]
- 13.Reams RY, Glickman LT, Harrington DD, Thacker HL, Bowersock TL. Streptococcus suis infection in swine: A retrospective study of 256 cases. Part II. Clinical signs, gross and microscopic lesions, and coexisting microorganisms. J Vet Diagn Invest. 1994;5:326–334. doi: 10.1177/104063879400600308. [DOI] [PubMed] [Google Scholar]