Abstract
Background
Impaired sleep quality is common, and can reduce work productivity in patients with functional dyspepsia (FD).
Objective
The objective of this article is to evaluate whether there is a direct association between the presence of FD and the severity of impaired sleep quality, and to calculate the economic loss due to the decreased work productivity associated with sleep quality.
Methods
In Study 1, using a web-based survey completed by workers with and without FD, we evaluated impaired sleep quality, work and daily productivity, and the severity of reflux and bowel symptoms. In Study 2, the association between the presence of FD and the severity of impaired sleep quality was validated in a hospital-based cohort.
Results
In both Study 1 and 2, although impaired sleep quality was more frequent in participants with FD than in those without FD, the independent association between the presence of FD and the severity of impaired sleep quality was not observed after adjustment for the severity of reflux and bowel symptoms. FD participants with impaired sleep quality reported additional economic loss of 53,500 Japanese yen/month.
Conclusion
Although the association between impaired sleep quality and FD was indirect, concomitant impaired sleep quality could worsen economic loss.
Keywords: Functional dyspepsia, economic loss, sleep disturbance, gastroesophageal reflux disease, irritable bowel syndrome
Key summary
- Summarize the established knowledge on this subject
- Abdominal symptoms due to functional gastrointestinal disorders (FGIDs) are frequently associated with impaired sleep quality.
- Although the frequent concomitance of impaired sleep quality has been documented in patients with functional dyspepsia (FD), a causal association between FD and impaired sleep quality has not been proven.
- What are the significant and/or new findings of this study?
- The severity of impaired sleep quality was not independently associated with the presence of FD, suggesting that impaired sleep quality in patients with FD is induced by concomitant reflux and/or bowel symptoms, but not by dyspepsia.
- The severity of impaired sleep quality was independently and more strongly associated with work productivity and daily activities than the severity of reflux or bowel symptoms in patients with FD.
- These results suggest that the improvement of sleep quality should be considered as a top priority to maintain the work productivity of patients with FD. To achieve this, controlling only dyspepsia is not enough, but controlling both reflux and bowel symptoms may be effective.
Introduction
Abdominal symptoms due to functional gastrointestinal disorders (FGIDs) are known to frequently impair sleep quality. Especially in patients with gastroesophageal reflux disease (GERD) or irritable bowel syndrome (IBS), the association between related abdominal symptoms and impaired sleep quality has been evident.1–3 The appropriate management of impaired sleep quality is urgently required in patients with FGIDs. Functional dyspepsia (FD) is a highly prevalent chronic disorder, characterized by one or more of the following: postprandial fullness, early satiation, epigastric pain, and/or epigastric burning that remains unexplained following esophagogastroduodenoscopy (EGD).4 Although FD is rarely a fatal disease, it is associated with reduced quality of life (QOL). A previous study reported that the FD-related reduction in QOL is similar to that seen in patients with mild heart failure.5
The frequent concomitance of impaired sleep quality is documented in patients with FD.6 Conceivably, dyspeptic symptoms themselves might directly disturb sleep quality, whereas another possibility is that overlapping gastroesophageal reflux symptoms or bowel symptoms could also have an effect.7 In Japan, 24%–35% of patients with FD are reported to have reflux symptoms and/or IBS.8,9
In this respect, we conducted a web-based survey to investigate the independent association between the presence of FD and the severity of impaired sleep quality, even after the adjustment of the severity of concomitant GI symptoms. The results were validated in a hospital-based cohort. In addition, we evaluated the loss of work productivity and activity impairment related to impaired sleep quality in participants with and without FD.
Methods
Study design and participants
In this study, we performed similar surveys in two independent populations. In Study 1, individuals aged 20 to 69 years who registered in a research program owned and managed by a survey company (MACROMILL Co., Tokyo, Japan), were sent an email invitation to participate in the screening survey between March 12, 2015 and March 16, 2015. After excluding non-workers, participants with FD or those without any dyspeptic symptoms were enrolled as either cases or study controls, respectively. FD was diagnosed based on the Rome III classification,10 since the present study was conducted prior to the publication of the Rome IV criteria.4 Among those with dyspepsia, participants with the following circumstances were excluded: those with no history of undergoing EGD within one year based on self-declaration; those with a history of neoplasms or other organic diseases that were likely to explain the upper GI symptoms; and/or those with history of upper GI surgery, disorder of the central nervous system, mental disorder, uncontrolled endocrine disorder, or pregnancy. A similar number of non-FD controls and FD cases were randomly selected without any matching, so that the controls represented the general population as closely as possible.
Participants selected as either FD cases or non-FD controls were sent the main survey. Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI).11 The loss of work productivity and the impairment of daily activities were assessed using the Work Productivity and Activity Impairment Questionnaire (WPAI).12 Additional details of the PSQI, WPAI, and the calculation of economic loss are provided in the supplementary materials. The survey also included questions about the presence/absence of IBS based on the Rome III classification,13 age, sex, height, weight, complications, medical history, past surgical history, smoking habits, alcohol habits, current medications, and the Gastroesophageal Reflux Disease Questionnaire (GerdQ).14,15
In Study 2, we prospectively recruited patients with dyspepsia and volunteers (students and staff members at Keio University) between April 2014 and July 2015. A complete medical history was obtained and a physical examination was performed to exclude individuals who had a history of abdominal surgery, Parkinson’s disease, or malignant diseases. All participants underwent EGD and the absence of organic diseases to explain dyspepsia was confirmed, except in the volunteers who were under 35 years of age. In addition, people who were taking a glucagon-like peptide-1 receptor agonist (e.g. exenatide), which might delay gastric emptying,16 were excluded from participation in this study. The presence of FD was diagnosed based on the Rome III criteria.10 We asked the study participants to answer the questionnaires, which consisted of the PSQI; questions about the presence/absence of IBS based on the Rome III classification; questions about participant age, sex, height, weight, complications, medical history, past surgical history, smoking habits, alcohol habits, and current medication use; the GerdQ; and the Hospital Anxiety and Depression Scale (HADS).17 The WPAI was not included in the questionnaires for the second cohort.
All participants in the study provided either electronic or written informed consent. The study was performed in accordance with the ethical principles for medical research of the World Medical Association’s Declaration of Helsinki, and was approved by the Ethics Committee of Keio University School of Medicine (No. 20140412 for Study 1 (10 March 2015) and No. 20120251 for Study 2 (24 September 2012)).
Statistical analysis
In both Study 1 and Study 2, impaired sleep quality and the other concomitant symptoms were expressed as four-point scales, as shown in Table 1. The severity of impaired sleep quality was categorized based on quantiles of the PSQI in Study 1. The severity of heartburn, regurgitation, and nausea was based on the original items of the GerdQ.14 The stool form was evaluated based on the Bristol Stool Form Scale.13 The severity of anxiety and depression was evaluated by the HADS with previously used cutoff scores.18 Further details of statistical analysis are provided in the supplementary materials.
Table 1.
Four-point scales to assess the severity of concomitant symptoms of functional dyspepsia.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Impaired sleep quality | ||||
| PSQI | <4 | 4, 5 | 6, 7 | 7< |
| Heartburn | ||||
| Frequency of symptom | 0 days/week | 1 day/week | 2–3 days/week | 4–7 days/week |
| Regurgitation | ||||
| Frequency of symptom | 0 days/week | 1 day/week | 2–3 days/week | 4–7 days/week |
| Nausea | ||||
| Frequency of symptom | 0 days/week | 1 day/week | 2–3 days/week | 4–7 days/week |
| Hard stool | ||||
| BSFS Type 1, 2 | <25% | 25–49% | 50–74% | ≥75% |
| Soft stool | ||||
| BSFS Type 6, 7 | <25% | 25–49% | 50–74% | ≥75% |
| Anxietya | ||||
| HADS-A | <8 | 8–10 | 11–14 | 14< |
| Depressiona | ||||
| HADS-D | <8 | 8–10 | 11–14 | 14< |
PSQI: Pittsburgh Sleep Quality Index; BSFS: Bristol Stool Form Scale; HADS: Hospital Anxiety and Depression Scale. aOnly in Study 2.
Results
Participant characteristics in Study 1
A total of 193,031 individuals were invited to participate in the study, and 40,000 individuals answered the screening survey (Figure S1). Excluding individuals with dyspepsia who failed to meet the inclusion criteria, 237 FD cases were invited to participate in the main survey. To obtain 250 answers for the controls, 420 non-FD participants were also invited to the same main survey, considering an expected response rate of 60%. Among the 657 participants, 125 participants did not answer the main survey, and 73 participants were excluded because their answers in the main survey were irrelevant. Finally, 202 participants with FD and 257 without FD were included in the analysis.
Table 2 shows participant characteristics. The average age was greater in the FD group than that in the non-FD group. Average monthly wages were higher in the FD group than those in the non-FD group. Loss of productivity was also compared between the FD group and the non-FD group (Table 2), with participants in the FD group reporting significantly greater loss of work productivity and activity impairment as compared with participants in the non-FD group. The economic loss was almost 32,000 Japanese yen (JPY)/month (≈258 Euro (EUR)/month) higher in the FD group than in the non-FD group. Alcohol consumption was also more frequent in the FD group than that in the non-FD group. Additionally, the proportion of proton pump inhibitor/prokinetic users was greater in the FD group than in the non-FD group, and the proportion of participants with reflux symptoms (GerdQ ≥ 8),14,15 IBS, and impaired sleep quality (PSQI ≥ 6)11 was higher in the FD group than in the non-FD group. The prevalence of impaired sleep quality was 16.5% higher in participants with FD than in participants without FD.
Table 2.
Participant characteristics (Study 1).
| non-FD (n = 257) | FD (n = 202) | p value | |
|---|---|---|---|
| Age (y) (mean ± S.D.) | 41.2 ± 10.5 | 43.9 ± 10.1 | 0.004a |
| Sex | 1.00b | ||
| Men | 137 (53.3%) | 108 (53.5%) | |
| Women | 120 (46.7%) | 94 (46.5%) | |
| BMI (kg m−2) (mean ± S.D.) | 22.3 ± 3.9 | 22.1 ± 3.9 | 0.59a |
| Monthly wage (.000 JPY) (mean ± S.D.) | 296 ± 84 | 313 ± 92 | 0.04a |
| Loss of work productivity (%) | 21.8 ± 25.7 | 32.7 ± 27.3 | <0.001a |
| Absenteeism (%) | 1.2 ± 9.2 | 1.8 ± 8.4 | |
| Presenteeism (%) | 21.6 ± 25.6 | 32.2 ± 26.9 | |
| Activity impairment (%) | 20.1 ± 23.4 | 34.0 ± 27.0 | <0.001a |
| Economic loss (.000 JPY/month) | 67.6 ± 88.4 | 99.6 ± 86.8 | <0.001a |
| Smoking habit | 0.051c | ||
| Current smokers | 44 (17.1%) | 44 (21.8%) | |
| Ex-smokers | 64 (24.9%) | 64 (31.7%) | |
| Non-smokers | 149 (58.0%) | 94 (46.5%) | |
| Alcohol drinking habit | 0.001c | ||
| Drinkers (≥once a week) | 82 (31.9%) | 96 (47.5%) | |
| Ex-drinkers | 6 (2.3%) | 7 (3.5%) | |
| Chance drinkers (<once a week) | 113 (44.0%) | 68 (33.7%) | |
| Non-drinkers | 56 (21.8%) | 31 (15.3%) | |
| Medication | |||
| PPI | 2 (0.8%) | 26 (12.9%) | <0.001b |
| Prokinetics | 1 (0.4%) | 14 (6.9%) | <0.001b |
| GerdQ ≥ 8 | 14 (5.4%) | 76 (37.6%) | <0.001b |
| IBS | 10 (3.9%) | 46 (22.8%) | <0.001b |
| IBS-C | 1 (0.4%) | 13 (6.4%) | |
| IBS-D | 3 (1.2%) | 19 (943%) | |
| IBS-M | 2 (0.8%) | 6 (3.0%) | |
| IBD-U | 4 (1.6%) | 8 (4.0%) | |
| PSQI ≥ 6 | 86 (33.5%) | 101 (50.0%) | <0.001b |
FD: functional dyspepsia; BMI: body mass index; PPI: proton pump inhibitors; GerdQ: gastroesophageal reflux disease questionnaire; IBS: irritable bowel syndrome; PSQI: Pittsburgh Sleep Quality Index; JPY: Japanese yen; y: years.
Student’s t-test. bFisher’s exact test. cPearson’s chi-square test.
Association between the presence of FD and the severity of concomitant symptoms
First, we evaluated whether the severity of impaired sleep quality was associated with the presence of FD using logistic regression analysis (Table 3). Since baseline characteristics such as age, sex, wage, alcohol consumption, and smoking status were different between participants with and without FD, these factors were statistically adjusted. The severity of concomitant symptoms, such as impaired sleep quality, heartburn, regurgitation, nausea, hard stool, and soft stool, was significantly associated with the presence of FD. However, multivariable analysis of these symptoms with a stepwise selection revealed that the severity of heartburn, regurgitation, hard stool, and soft stool was independently associated with the presence of FD, but that the severity of impaired sleep quality was not. This suggests that there is no direct causal association between impaired sleep quality and the presence of FD.
Table 3.
Association between the presence of FD and concomitant conditions (Study 1).
| Age-, sex-, wage-, alcohol consumption-, and
smoking status-adjusted analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Impaired sleep quality | 1.44 | 1.20–1.73 | ||
| Heartburn | 9.78 | 5.26–18.2 | 6.86 | 3.60–13.1 |
| Regurgitation | 4.11 | 2.84–5.94 | 2.52 | 1.72–3.70 |
| Nausea | 3.30 | 2.14–5.08 | ||
| Hard stool | 1.55 | 1.21–1.98 | 1.58 | 1.18–2.12 |
| Soft stool | 1.81 | 1.39–2.37 | 1.62 | 1.19–2.20 |
Multivariable analysis was further adjusted for factors selected by a stepwise method from marginally associated with the presence of FD (p < 0.1) in age-, sex-, wage-, alcohol consumption-, smoking status-adjusted analyses.
FD: functional dyspepsia; OR: odds ratio for one-point exaggeration of a symptom; CI: confidence interval. Bold values, p < 0.05.
Association between impaired sleep quality and loss of work productivity
To assess the association of the severity of impaired sleep quality with the loss of work productivity and the impairment of daily activities, linear regression analyses were performed (Table 4). According to the results of multivariable analyses, the severity of impaired sleep quality was independently and most strongly associated with work productivity and daily activities in participants with and without FD (β = 0.29 to 0.37). In participants without FD, the severity of nausea was also independently associated with work productivity and daily activities. In those with FD, the severity of soft stool was independently associated with work productivity and daily activities.
Table 4.
Association between work productivity or activity impairment and concomitant conditions in participants with and without FD.
| Non-FD |
FD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age- and sex-adjusted analysis |
Multivariable analysis |
Age- and sex-adjusted analysis |
Multivariable analysis |
|||||
| β | p value | β | p value | β | p value | β | p value | |
| Work productivity | ||||||||
| Impaired sleep quality | 0.33 | <0.001 | 0.32 | <0.001 | 0.33 | <0.001 | 0.34 | <0.001 |
| Heartburn | 0.04 | 0.565 | 0.15 | 0.028 | ||||
| Regurgitation | 0.19 | 0.002 | 0.19 | 0.008 | ||||
| Nausea | 0.24 | <0.001 | 0.23 | <0.001 | 0.23 | 0.001 | ||
| Hard stool | 0.07 | 0.299 | 0.05 | 0.542 | ||||
| Soft stool | 0.02 | 0.709 | 0.18 | 0.009 | 0.19 | 0.004 | ||
| Activity impairment | ||||||||
| Impaired sleep quality | 0.38 | <0.001 | 0.37 | <0.001 | 0.31 | <0.001 | 0.29 | <0.001 |
| Heartburn | 0.09 | 0.150 | 0.15 | 0.027 | ||||
| Regurgitation | 0.22 | <0.001 | 0.21 | 0.002 | ||||
| Nausea | 0.22 | <0.001 | 0.21 | <0.001 | 0.22 | 0.002 | 0.14 | 0.028 |
| Hard stool | 0.08 | 0.193 | −0.01 | 0.893 | ||||
| Soft stool | 0.05 | 0.388 | 0.25 | <0.001 | 0.24 | <0.001 | ||
Multivariable analysis was further adjusted for factors selected by a stepwise method from marginally associated with work productivity or activity impairment (p < 0.1) in the age- and sex-adjusted analyses.
FD: functional dyspepsia; β: standardized regression coefficient. Bold values, p < 0.05.
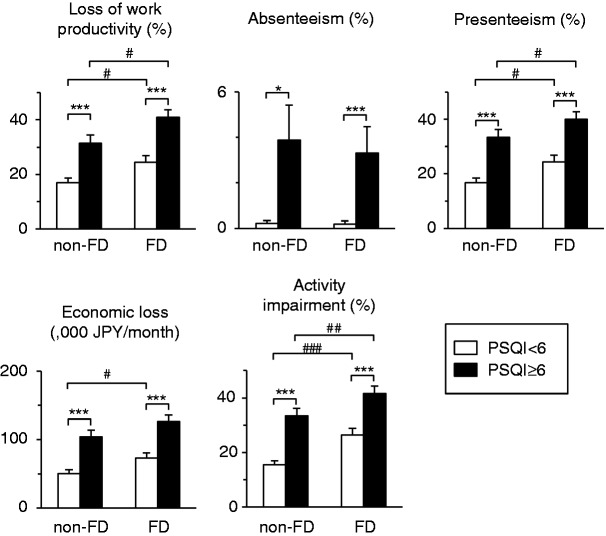
Impaired sleep quality also had a significant influence on the loss of work productivity and activity impairment, irrespective of the presence of FD (Figure 1). The difference in economic loss between those participants with the presence and absence of impaired sleep quality, respectively, was 48,700 JPY/month (≈393 EUR/month) in those without FD and 53,500 JPY/month (≈431 EUR/month) in those with FD. We further evaluated which components of impaired sleep quality were associated with work productivity. As shown in Figure 2, the scores of subjective sleep quality, sleep latency, sleep disturbance, use of sleep medication, and daytime dysfunction were associated with economic loss. However, neither sleep duration nor habitual sleep efficiency were associated with economic loss.
Figure 1.
Increased levels of loss of work productivity and activity impairment due to the presence of impaired sleep quality. Statistical differences between two groups were analyzed using Student’s t-test. p < 0.05 and ***p < 0.001 as compared between the presence and absence of impaired sleep quality. #p < 0.05, ##p < 0.01, and ## p < 0.001 as compared between the presence and absence of FD. Error bars represent one standard error of the mean.
FD: functional dyspepsia; PSQI: Pittsburgh Sleep Quality Index.
Figure 2.
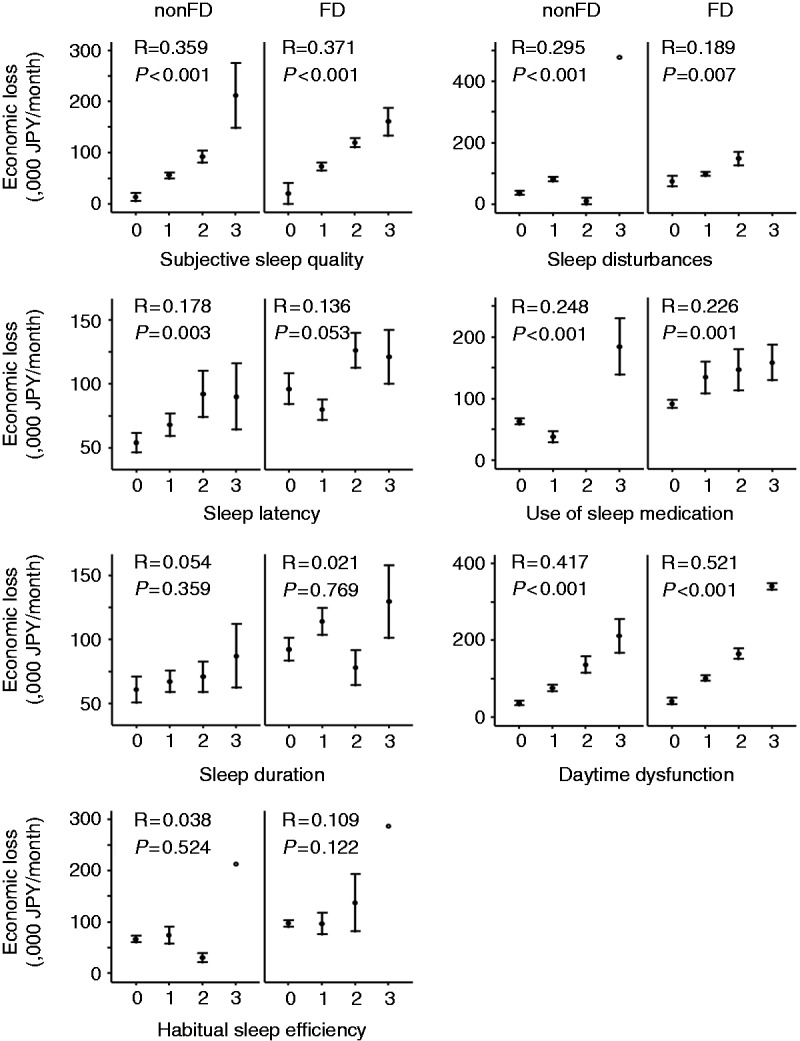
Alterations of the economic loss through the increased scores of each component of Pittsburgh Sleep Quality Index (PSQI). Error bars represent one standard error of the mean. R values indicate the correlation coefficient and p values indicate the statistical significance between the economic loss and the score of each component of PSQI calculated using Pearson’s correlation analysis.
FD: functional dyspepsia.
To summarize the results of Study 1, the presence of FD significantly affected work productivity and daily activities. Impaired sleep quality, rather than sleep quantity, was the strongest risk factor for poor work productivity and activity impairment, irrespective of the presence or absence of FD. On the other hand, an independent association between the presence of FD and the severity of impaired sleep quality was not observed, suggesting that poor sleep quality itself does not induce the symptoms of FD.
Validation of the association between the presence of FD and the severity of impaired sleep quality in Study 2
To confirm the results of Study 1, we validated the association between the presence of FD and the severity of impaired sleep quality in a hospital-based population. In Study 2, a total of 133 participants, which included 67 patients and 66 volunteers, were enrolled (Figure S2). After excluding 15 individuals, 118 participants were analyzed, including 78 participants without FD and 40 with FD. Although the WPAI was not performed, anxiety and depression symptoms were evaluated using the HADS in Study 2. The demographic characteristics of the study population are documented in Table S1. Age, sex, and body mass index (BMI) were significantly different between those with and without FD. The prevalence of impaired sleep quality was 25.6% higher in patients with FD than in participants without FD (non-FD, 26.9%; FD, 52.5%; p = 0.008).
Age-, sex-, and BMI-adjusted logistic regression analysis showed that the severity of impaired sleep quality was associated with the presence of FD (Table 5). The severity of heartburn, regurgitation, nausea, and anxiety was also associated with the presence of FD. However, multivariable logistic regression analysis using stepwise variable selection showed that the severity of regurgitation, but not impaired sleep quality, was independently associated with the presence of FD, which is congruent with the results of Study 1.
Table 5.
Association between the presence of FD and concomitant conditions (Study 2).
| Age-, sex-, and BMI-adjusted
analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Impaired sleep quality | 1.56 | 1.01–2.42 | ||
| Heartburn | 1.86 | 1.03–1.08 | ||
| Regurgitation | 3.13 | 1.68–5.86 | 3.13 | 1.68–5.86 |
| Nausea | 2.11 | 1.14–3.92 | ||
| Hard stool | 1.94 | 0.85–4.46 | ||
| Soft stool | 1.22 | 0.63–2.36 | ||
| Anxiety | 1.87 | 1.01–3.48 | ||
| Depression | 1.01 | 0.45–2.44 | ||
Multivariable analysis was adjusted for age, sex, BMI, and factors selected by a stepwise method from marginally associated with the presence of FD (p < 0.1) in the age-, sex-, and BMI-adjusted analyses.
FD: functional dyspepsia; BMI: body mass index; OR: odds ratio for one-point exaggeration of a symptom; CI: confidence interval. Bold values, p < 0.05.
Discussion
In concordance with previous studies, a significant association was validated between the presence of FD and impaired sleep quality. However, following a statistical adjustment for reflux, bowel, and anxiety symptoms, an independent association between the presence of FD and the severity of impaired sleep quality was not observed. This suggests that FD is not a direct cause of impaired sleep quality, and vice versa. Vakil et al. demonstrated that impaired sleep quality due to the presence of reflux symptoms is common in patients with FD who do not have GERD.1 Futagami et al. also reported that impaired sleep quality is related to GERD symptoms in FD patients, and furthermore, that these individuals showed a significant improvement of sleep quality when a histamine (H2)-receptor antagonist was administered for four weeks, in comparison with the administration of a placebo.19 These results suggest that the treatment of concomitant symptoms, such as reflux symptoms, could be effective in assisting in the improvement of sleep quality, as compared with the provision of treatment for dyspeptic symptoms themselves.
We also evaluated the loss of work productivity and activity impairment due to impaired sleep quality in this study, and calculated the economic loss associated with the loss of work productivity in participants with FD in Study 1. As baseline data, in the present study, the loss of overall work productivity and activity impairment were 32.7% and 34.0% in the FD group, and 23.0% and 21.9% in the non-FD group, respectively. According to a previous study that evaluated the loss of work productivity in 565 Japanese individuals with atrial fibrillation with and without dyspepsia by using the same method (WPAI:GH),20 the loss of overall work productivity and activity impairment were 32.2% and 30.8% in the dyspepsia group, and 17.6% and 23.8% in the non-dyspepsia group, respectively. Such similar results suggest that we could obtain a representative population for our analysis. Multivariable linear regression analysis showed that the severity of impaired sleep quality was independently and most strongly associated with a loss of work productivity and activity impairment among participants with FD. Impaired sleep quality has also been reported to be the greatest mediator of work productivity in some other chronic disorders, such as GERD3,21 and psoriasis.22 Since the prevalence of impaired sleep quality was greater in those with FD than in those without FD, the total loss of work productivity and activity impairment was also greater in those with FD, even when the levels of the loss of work productivity (FD, 16.5%; non-FD, 14.4%) and activity impairment (FD, 15.2%; non-FD, 16.1%) due to impaired sleep quality were not significantly different between the FD and the non-FD groups. Among those with FD, the presence of impaired sleep quality resulted in 53,500 JPY (≈431 EUR) of economic loss per person per month. Since, according to the Annual Report on the Labour Force Survey 2016 published by the Ministry of Internal Affairs and Communications in Japan, more than 60 million people were working at the time, and the prevalence of FD was reported to be approximately 10% in Japan,9 if we assume that our participants represent the general population, the estimated economic loss due to impaired sleep quality concomitant with FD would be more than 1.9 trillion JPY (≈15 billion EUR) each year. In addition, our results suggest that the use of interventions for impaired sleep quality could have a large impact on the improvement of work productivity in patients with FD.
The present study has several limitations. In Study 1, the prevalence of FD was quite low in the survey responders. To verify the presence/absence of organic diseases that explain dyspepsia, we excluded workers who did not undergo EGD within one year of the survey from the FD group. However, since the annual double-contrast barium radiograph method is currently used in mass screening for gastric cancer in Japan, most Japanese workers did not undergo EGD in the time period that we specified for inclusion in this study. Therefore, a limited number of participants met the criteria in our web-survey cohort, which could have led to selection bias. In addition, it was not guaranteed that the answers of participants were totally correct, since we could not refer to actual medical information, such as comorbidities, past medical histories, and/or the findings of the EGD. This might also lead to the misdiagnosis of FD in some proportion of participants. In Study 2, we conducted the survey involving patients in a tertiary-care hospital and healthy participants, which also could have led to selection bias. However, the fact that we arrived at the same result, despite studying two different cohorts, suggests that our findings are highly reliable. Finally, we did not adjust the prevalence of non-GI diseases (e. g. osteoarhrosis, asthma) that might have an impact on patients’ QOL. Further investigations are needed to evaluate how much non-GI diseases would additionally affect sleep quality and work productivity in patients with FD.
In conclusion, the present study showed that although impaired sleep quality was a significantly frequent and concomitant symptom of FD, the association between impaired sleep quality and FD depended on the presence of other concomitant symptoms, such as reflux symptoms. This suggests that FD does not directly impair sleep quality. Therefore, the treatment of concomitant symptoms of FD might be an effective method for improving sleep quality. In addition, also showed that impaired sleep quality was the greatest factor that reduced work productivity and impaired daily activities. Thus, maintaining healthy sleep quality is the most important factor to consider for the optimization of QOL in patients with FD.
Supplementary Material
Ethics approval
The study was approved by the Ethics Committee of Keio University School of Medicine (No. 20140412 for Study 1 (10 March 2015) and No. 20120251 for Study 2 (24 September 2012)).
Informed consent
All participants in the study provided electronic informed consent in Study 1 and written informed consent in Study 2.
Declaration of conflicting interests
During the last two years, H.S. received scholarship funds for the research from AstraZeneca K.K., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Tsumura Co., and received service honoraria from Astellas Pharma Inc, AstraZeneca K.K., Otsuka Pharmaceutical Co. Ltd., Daiichi-Sankyo Co., Takeda Pharmaceutical Co. Ltd., Mylan EPD Co., and Zeria Pharmaceutical Co. Ltd. T.K. received scholarship funds for the research from Astellas Pharm Inc, Astra- Zeneca K.K., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Eisai Pharmaceutical Co. Ltd., Zeria Pharmaceutical Co. Ltd., Tanabe Mitsubishi Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Kyorin Pharmaceutical Co. Ltd., and received service honoraria from Astellas Pharm Inc, Eisai Pharmaceutical Co. Ltd., JIMRO Co. Ltd., Tanabe Mitsubishi Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Miyarisan Pharmaceutical Co. Ltd., and Zeria Pharmaceutical Co. Ltd. The other authors have nothing to declare.
Funding
This work was supported by a Grant-in-Aid for Scientific Research B (16H05291, to H.S.), from the Japan Society for the Promotion of Science (JSPS), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411003, to H.S.), the Princess Takamatsu Cancer Research grants (to H.S.), Keio Gijuku Academic Development Funds (to H.S.), and a research grant from AstraZeneca K.K. (to J.M.). The sponsors had no roles in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the article for publication.
References
- 1.Vakil N, Wernersson B, Wissmar J, et al. Sleep disturbance due to heartburn and regurgitation is common in patients with functional dyspepsia. United European Gastroenterol J 2016; 4: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fass R, Fullerton S, Tung S, et al. Sleep disturbances in clinic patients with functional bowel disorders. Am J Gastroenterol 2000; 95: 1195–2000. [DOI] [PubMed] [Google Scholar]
- 3.Mody R, Bolge SC, Kannan H, et al. Effects of gastroesophageal reflux disease on sleep and outcomes. Clin Gastroenterol Hepatol 2009; 7: 953–959. [DOI] [PubMed] [Google Scholar]
- 4.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology 2016; 150: 1380–1392. [DOI] [PubMed] [Google Scholar]
- 5.Moayyedi P, Mason J. Clinical and economic consequences of dyspepsia in the community. Gut 2002; 50(Suppl 4): iv10–iv12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacy BE, Everhart K, Crowell MD. Functional dyspepsia is associated with sleep disorders. Clin Gastroenterol Hepatol 2011; 9: 410–414. [DOI] [PubMed] [Google Scholar]
- 7.Yao X, Yang YS, Cui LH, et al. The overlap of upper functional gastrointestinal disorders with irritable bowel syndrome in Chinese outpatients: A multicenter study. J Gastroenterol Hepatol 2016; 31: 1584–1593. [DOI] [PubMed] [Google Scholar]
- 8.Kaji M, Fujiwara Y, Shiba M, et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol 2010; 25: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzaki J, Suzuki H, Fukushima Y, et al. High frequency of overlap between functional dyspepsia and overactive bladder. Neurogastroenterol Motil 2012; 24: 821–827. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130: 1466–1479. [DOI] [PubMed] [Google Scholar]
- 11.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 12.Tang K. Estimating productivity costs in health economic evaluations: A review of instruments and psychometric evidence. Pharmacoeconomics 2015; 33: 31–48. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 14.Jones R, Junghard O, Dent J, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther 2009; 30: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, Matsuzaki J, Okada S, et al. Validation of the GerdQ questionnaire for the management of gastro-oesophageal reflux disease in Japan. United European Gastroenterol J 2013; 1: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acosta A, Camilleri M, Burton D, et al. Exenatide in obesity with accelerated gastric emptying: A randomized, pharmacodynamics study. Physiol Rep 2015; 3: e12610–e12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 18.Langley RG, Feldman SR, Han C, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol 2010; 63: 457–465. [DOI] [PubMed] [Google Scholar]
- 19.Futagami S, Yamawaki H, Izumi N, et al. Impact of sleep disorders in Japanese patients with functional dyspepsia (FD): Nizatidine improves clinical symptoms, gastric emptying and sleep disorders in FD patients. J Gastroenterol Hepatol 2013; 28: 1314–1320. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita Y, Dibonaventura M, Rossi B, et al. Burden of comorbidities among Japanese patients with atrial fibrillation: A case study of dyspepsia. Clin Exp Gastroenterol 2013; 6: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahlqvist P, Reilly MC, Barkun A. Systematic review: The impact of gastro-oesophageal reflux disease on work productivity. Aliment Pharmacol Ther 2006; 24: 259–272. [DOI] [PubMed] [Google Scholar]
- 22.Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: Sleep problems are a mediating factor. J Drugs Dermatol 2016; 15: 183–188. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.