Abstract
Background
There is limited evidence to support the belief that benzodiazepines increase cirrhosis patients’ risk of hepatic encephalopathy (HE).
Objective
We aimed to examine the association between benzodiazepine use and HE development in cirrhosis patients.
Methods
We used data on 865 cirrhosis patients with ascites from three trials to study the effect of benzodiazepine use on development of first-time HE. For each patient, we classified periods of benzodiazepine use by the number of days since initiation. We used Cox regression to compare the risk of HE in current benzodiazepine users vs. non-users adjusting for confounders.
Results
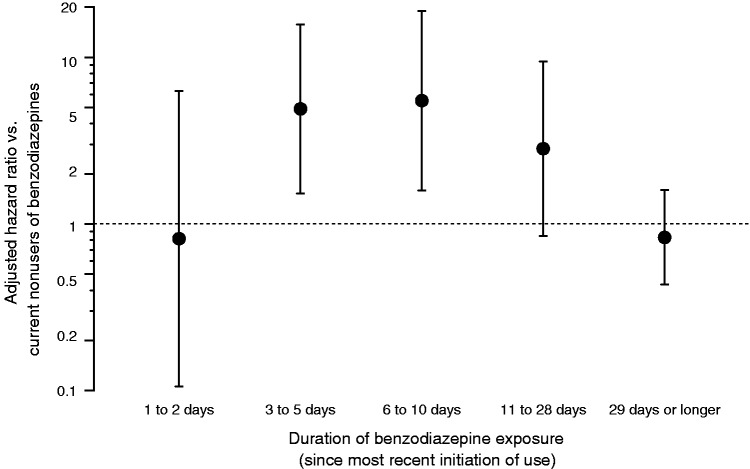
Cirrhosis patients were not at increased risk of HE for the first two days of benzodiazepine use, but then faced a five-fold increased risk of HE during days 3 to 10 of benzodiazepine use. The risk of HE was not increased for those who had been using benzodiazepines for more than 28 days.
Conclusion
Cirrhosis patients who had begun using benzodiazepines between 3 and 10 days previously had a markedly increased risk of developing first-time HE. Cirrhosis patients who had been using benzodiazepines for just one or two days or continued use for more than 28 days did not have such an excess risk.
Keywords: Cirrhosis, benzodiazepines, encephalopathy, risk, ascites
Key summary
Endogenous benzodiazepines have an inhibitory cerebral effect.
There is limited evidence to support the widespread belief that benzodiazepine use increases cirrhosis patients’ risk of hepatic encephalopathy.
Our study showed that cirrhosis patients who had begun using benzodiazepines between 3 and 10 days previously had a markedly increased risk of developing first-time hepatic encephalopathy.
Our study showed that cirrhosis patients who had been using benzodiazepines for just one or two days or continued use after four weeks did not have an excess risk of first-time hepatic encephalopathy.
Introduction
Hepatic encephalopathy (HE) is a frequent complication of cirrhosis1–3 and a strong predictor of mortality.4 It is widely believed that benzodiazepine use increases the risk of HE.5 This belief is supported by the “endogenous benzodiazepine” hypothesis proposing accumulation of endogenous benzodiazepines in the neuro-inhibitory gamma-aminobutyric-acid (GABA) system.6–9 However, the evidence that exogenous benzodiazepines can precipitate HE is mainly based on clinical experience, and only a few studies have described such an association.10–16 Patients with cirrhosis are often treated with benzodiazepines in relation to procedures and for anxiety or sleep disturbances, so it is clinically important to determine whether benzodiazepine use is a risk factor for HE development.
The aim of this study was to examine the association between benzodiazepine use and development of an episode of first-time HE in cirrhosis patients with ascites.
Materials and methods
Patients
This study used data from three large multinational trials conducted between July 2006 and December 2008. For each participating institution, the trial protocols, patient information, and consent forms were approved by human research committees and national ethics committees and registered on a public website (www.ClinicalTrials.gov, registration numbers NCT00358878, NCT00359437, and NCT 00366795). Written, informed consent to participation in the trials was obtained from all patients. The trials were conducted to evaluate the effect of satavaptan on ascites in patients with cirrhosis.17 Satavaptan is a vasopressin receptor antagonist that does not affect the risk for HE.18 The trials included 1198 patients with cirrhosis and ascites. For our study, out of the 1198 trial participants, 333 patients with a history of HE were excluded. Predictors of first-time HE and recurrent HE may differ,12,13,19 and prescription practice with respect to benzodiazepines may also differ for patients with and without a history of HE. Including all patients, regardless of history of HE, would therefore complicate interpretation of the study results.
Follow-up
The planned trial follow-up was 52 weeks with a safety follow-up visit one week after study completion or premature discontinuation of treatment.17 The patients were seen by a hepatologist at randomization and every four weeks during follow-up, and a systematic evaluation of symptoms and signs of HE was conducted, including HE episodes occurring between visits. HE episodes were categorized into grade 1–4 according to the West Haven criteria.2 Psychometric testing for minimal HE was not conducted. Precipitating factors for HE development were also registered when they were identified (e.g. bleeding, infection, medication, renal failure, constipation, dehydration, and electrolyte disorder). All medications and their indications were recorded, including benzodiazepines: Anatomical Therapeutic Chemical (ATC) codes N05BA (clonazepam, lorazepam, alprazolam, oxazepam, diazepam, prazepam, clorazepate); N05CD (midazolam, lormetazepam, nitrazepam); and the non-benzodiazepine class, N05CF (zopiclone, zolpidem), which also increase GABA transmission.
Statistical analyses
In this analysis of the trial data, follow-up began when patients were randomized and ended at a first-time episode of HE grade 1–4, at death, or on the date of the safety follow-up visit.
We examined the association between “time since initiation of benzodiazepines” and the hazard rate of first-time HE grade 1–4. First, we identified each patient’s periods of benzodiazepine use. Many patients did not use them at all, some used them continuously, some used them repeatedly, and some used them once. Second, we divided the periods according to the numbers of days since initiation using these categories: 1 to 2 days, 3 to 5 days, 6 to 10 days, 11 to 28 days, and 29 days or longer. For example, a patient who began using benzodiazepines May 1, 2007 was in the “1 to 2 days” category through May 2, 2007, then in the “3 to 5 days” category from May 3, 2007 through May 5, 2007. If benzodiazepine use was stopped, the patient was counted as unexposed from that day until he or she began using benzodiazepines again, at which time the duration of exposure was restarted from “1 to 2 days.” A first-time HE episode was considered benzodiazepine-associated if the patient was using benzodiazepines when HE developed, regardless of any concurrent precipitating factor.
We used Cox proportional hazards regression to examine the effect of benzodiazepine use on the hazard rate of first-time HE grade 1–4. The effect was expressed as the hazard ratio of developing a first-time episode of HE for a patient who on a particular date had currently been using benzodiazepines for a certain amount of time vs. a patient who on the same date was not using benzodiazepines. As described above, benzodiazepine use was a time-dependent variable in the Cox model. We adjusted the effect of benzodiazepines for confounding by gender, age, diabetes (yes or no), current model for end-stage liver disease (MELD) score, current plasma albumin, current plasma sodium, current platelet count, cirrhosis etiology (alcohol only (reference category), hepatitis B only, hepatitis C only, non-alcoholic steatohepatitis or cryptogenic cirrhosis only, or other etiology), current use of lactulose (yes or no), and current inpatient status (yes or no).
Results
We included 865 patients, and 189 first-time HE episodes (grade 1: 51%; grade 2: 27%; grade 3: 9%, and grade 4: 13%) developed during the total 509 person-years of follow-up. A total of 155 patients used benzodiazepines at some time during the study period, experiencing 199 spells of benzodiazepine use. Seventy-five patients used benzodiazepines at inclusion, and these patients were otherwise similar to benzodiazepine non-users at inclusion (Table 1). The stated indications for benzodiazepine use were generally nondescript, e.g. “anxiety” or “sedation.” Precipitating factors for HE development were found in 84 (44%) of 189 episodes of first-time HE grade 1–4 (infection: 22 episodes; renal failure: 15 episodes; dehydration: 14 episodes; electrolyte disorder: 12 episodes; constipation: eight episodes; medication: seven episodes; and bleeding: six episodes).
Table 1.
Characteristics of benzodiazepine non-users and benzodiazepine users at randomization.
| BZD non-users | BZD users | |
|---|---|---|
| Number | 790 | 75 |
| Men (%) | 542 (69) | 52 (69) |
| Median age (IQR) | 57 (50–64) | 60 (51–68) |
| Cirrhosis etiology | ||
| Alcohol (%) | 460 (58) | 55 (73) |
| Hepatitis B (%) | 42 (5) | 1 (1%) |
| Hepatitis C (%) | 105 (13) | 6 (8) |
| NASH or cryptogenic (%) | 65 (8) | 4 (5) |
| Other (%) | 117 (15) | 9 (12) |
| Child-Pugh class A/B/C | 11%/68%/21% | 7%/78%/15% |
| MELD score (median, IQR) | 11 (8–14) | 11 (8–14) |
| Creatinine, µmol/l (median, IQR) | 77 (63–95) | 86 (68–107) |
| Bilirubin, µmol/l (median, IQR) | 26 (15–43) | 27 (16–39) |
| INR (median, IQR) | 1.4 (1.2–1.6) | 1.4 (1.2–1.5) |
| Sodium, mmol/l (median, IQR) | 137 (134–140) | 137 (134–139) |
| Potassium, mmol/l (median, IQR) | 4.3 (4.0–4.7) | 4.3 (3.9–4.6) |
| Albumin, g/l (median, IQR) | 33 (29–38) | 35 (31–39) |
| Platelets, *109/l (median, IQR) | 132 (94–193) | 124 (91–195) |
| Diabetes (%) | 175 (22) | 19 (25) |
| Lactulose, any dose (%) | 166 (21) | 22 (29) |
BZD: benzodiazepine; IQR: interquartile range; MELD: model for end-stage liver disease; NASH: non-alcoholic steatohepatitis; INR: international normalized ratio.
The patients were five-fold times more likely to develop HE grade 1–4 after 3 to 10 days of benzodiazepine use than patients not using benzodiazepines (Figure 1 and Table 2). In contrast, benzodiazepine exposure for one or two days or for more than 28 days was not associated with HE grade 1–4. Confounding was substantial. Particularly “inpatient status” was a strong predictor both of benzodiazepine use and HE development. Other potential confounders were predictors of HE (Table 2) but not strongly associated with benzodiazepine use.
Figure 1.
Association between benzodiazepine exposure and development of a first-time episode of hepatic encephalopathy grade 1–4 in cirrhosis patients with ascites. The association is shown as confounder-adjusted hazard ratios on a logarithmic scale. The error bars show 95% confidence intervals.
Table 2.
Effects of benzodiazepine use and possible confounders on the hazard rate of first-time episode of hepatic encephalopathy grade 1–4 among cirrhosis patients.
| Adjusted HE hazard ratio (95% CI) | |
|---|---|
| BZD use vs. no BZD use | |
| BZD use for 1-2 days | 0.81 (0.11–6.29) |
| BZD use for 3-5 days | 4.90 (1.52–15.75) |
| BZD use for 6-10 days | 5.49 (1.59–19.01) |
| BZD use for 11-28 days | 2.83 (0.85–9.45) |
| BZD use for ≥ 29 days | 0.83 (0.43–1.60) |
| Men vs. women | 1.29 (0.91–1.81) |
| Age, per 10-year increment | 1.22 (1.05–1.43) |
| MELD score, per point increment | 1.10 (1.07–1.13) |
| Albumin, per 5 g/l increment | 0.73 (0.64–0.84) |
| Sodium, per 5 mmol increment | 0.67 (0.59–0.76) |
| Platelets, per 10*109/l increment | 1.01 (0.92–1.13) |
| Cirrhosis etiology | |
| Alcohol only (reference) | |
| Hepatitis B only | 1.42 (0.80–2.53) |
| Hepatitis C only | 1.55 (1.00–2.43) |
| NASH or cryptogenic | 1.67 (0.98–2.85) |
| Other etiology | 0.94 (0.60–1.47) |
| Diabetes vs. no diabetes | 1.56 (1.12–2.19) |
| Lactulose use vs. no lactulose use | 1.98 (1.45–2.69) |
| Hospitalization | 3.85 (2.62–5.66) |
BZD: benzodiazepine; CI: confidence interval; HE: hepatic encephalopathy; MELD: model for end-stage liver disease; NASH: non-alcoholic steatohepatitis.
Discussion
This study showed that cirrhosis patients who had begun using benzodiazepines between 3 and 10 days previously had a markedly increased risk of developing a first-time episode of HE grade 1–4. Cirrhosis patients who had been using benzodiazepines for just one or two days or for more than four weeks did not have such an excess HE risk.
The patients in this study were not randomized to receive benzodiazepines, and the pattern of use therefore reflects actual clinical practice around the world. Prescribed benzodiazepines were recorded by clinicians in the clinical record files; any misclassification of benzodiazepine use, e.g. due to noncompliance, would lead us to underestimate the true effect of benzodiazepines on HE development.20 It is a limitation of the study that we did not have the statistical power to stratify benzodiazepine use according to ATC code, specific benzodiazepine agent, indication, or dosage and half-life. Also, we did not have the information to subdivide benzodiazepine users into those with a first-time prescription and those with intermittent use, as data on medication were available only for the study period. Information on blood ammonia concentration was not available and anyway, such data are only indirectly related to the presence or grade of HE.19
Our study cohort included patients with alcoholic cirrhosis, and information on any active alcohol abuse in these patients was unavailable. However, patients were examined by experienced hepatologists, and we are confident that the differential diagnosis of alcohol intoxication was excluded in the HE diagnosing. Nevertheless, a relevant consideration is whether alcohol abuse might influence benzodiazepine metabolism, and data on alcohol abuse may have added information to our study.
The wide spectrum of data made it possible to adjust for a large number of potential confounders, and residual confounding is presumably minimal. The use of lactulose predicted a higher risk for HE, and this may be an example of confounding by indication, as lactulose was most likely given to patients considered being at high risk for HE development, i.e. those with the most severe liver disease. The definitive study with randomization to benzodiazepine or placebo is not feasible. Thus, the data in this study are probably the best possible because (1) they include a large cohort of patients followed for a relevant length of time, and (2) data were meticulously recorded according to a pre-specified protocol.
One explanation for our findings could be that benzodiazepines have an inhibitory cerebral effect via their activation of the neuro-inhibitory GABA system and thus might precipitate HE.7,8 Such an effect could manifest after some days’ accumulation of benzodiazepine, due to the reduced liver function,21–24 and that would likely lead to withdrawal of benzodiazepine. The lack of association with long-term use, then, could be explained by either development of tachyphylaxis against the neuro-inhibitory effect25,26 or selection bias of benzodiazepine users who tolerate the medication. An alternative explanation is that benzodiazepines have in fact no effect on HE development; they could be innocent bystanders, and the association we found would then be caused by the indication for giving the drugs. However, it speaks against this explanation that there was no excess risk with short-term use that was likely procedure related, e.g. in the case of variceal bleeding. The indication for several days of benzodiazepine use might be indications that mimic, or are part of HE, e.g. delirium or sleep disturbances. We have no good way of clarifying these issues.
The clinical interpretation of our findings is that treatment of cirrhosis patients with benzodiazepines does indeed increase the risk for HE grade 1–4. The risk for HE seems to be restricted to a critical period occurring after 3 to10 days of benzodiazepine use. Single-day use, such as that related to procedures, does not carry the same risk, nor does established benzodiazepine use after the critical period. These views seem to be in line with current clinical practice.
An older study suggested that exogenous benzodiazepines rather than endogenous benzodiazepines contributed to HE development in cirrhosis patients.14 A more recent Chinese study found that benzodiazepine use for >60 days was a risk factor for HE development among cirrhosis patients.10 However, the Chinese study differs from ours as they included recurrent HE, and they divided the spells of benzodiazepine use into time intervals of 30 days and thus may have missed the high-risk period of 3 to 10 days. Haq et al. examined cirrhosis patients undergoing upper endoscopy with midazolam sedation and found that subsequent HE development was much more likely in patients with Child-Pugh class C cirrhosis than in patients with Child-Pugh class A cirrhosis.11 That observation is consistent with our finding that a high MELD score predicts a high risk of HE development. Other studies examined the association between midazolam sedation for upper endoscopy and HE development in cirrhosis patients. They found that midazolam exacerbated subclinical HE confirmed by psychometric tests up to two hours after the procedure.15,16 However, no patients developed clinical symptoms of HE, and that is in line with our findings of no excess risk of HE grade 1–4 development in single day-use of benzodiazepines. Also, in agreement with our findings, two older studies found single-dose administration of benzodiazepines to be safe as regards to HE, except in patients with former HE.12,13
In conclusion, we found that cirrhosis patients who used benzodiazepines for more than two days were at much increased risk of HE grade 1–4 development for a short period after which the excess risk diminished and eventually disappeared. Single-day use of benzodiazepines was not associated with an excess HE risk.
Acknowledgment
The funding source had no involvement in the conduct of the research or preparation of this article.
Declaration of conflicting interests
Hugh Watson is an employee of Sanofi. Lisbet Grønbæk, Hendrik Vilstrup, and Peter Jepsen have no conflicts of interest to declare.
Funding
Lisbet Grønbæk has received funding from the Danish Foundation of 17.12.1981.
Informed consent
Written, informed consent to participation in the trials was obtained from all patients.
Ethics approval
The clinical trials were approved by human research committees and national ethical review boards and registered on a public website (www.ClinicalTrials.gov, registration numbers NCT00358878, NCT00359437, and NCT00366795). The trial protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
References
- 1.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology 2009; 50: 2014–2021. [DOI] [PubMed] [Google Scholar]
- 2.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: Final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35: 716–721. [DOI] [PubMed] [Google Scholar]
- 3.Romero-Gómez M, Boza F, García-Valdecasas MS, et al. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 2001; 96: 2718–2723. [DOI] [PubMed] [Google Scholar]
- 4.Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: A Danish population-based cohort study. Hepatology 2010; 51: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 5.Butterworth RF. Complications of cirrhosis III. Hepatic encephalopathy. J Hepatol 2000; 32(1 Suppl): 171–180. [DOI] [PubMed] [Google Scholar]
- 6.Avallone R, Zeneroli ML, Venturini I, et al. Endogenous benzodiazepine-like compounds and diazepam binding inhibitor in serum of patients with liver cirrhosis with and without overt encephalopathy. Gut 1998; 42: 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer DF, Jones EA. Hepatic encephalopathy and the gamma-aminobutyric-acid neurotransmitter system. Lancet 1982; 1: 18–20. [DOI] [PubMed] [Google Scholar]
- 8.Jones EA. Pathogenesis of hepatic encephalopathy. Clin Liver Dis 2000; 4: 467–485. [DOI] [PubMed] [Google Scholar]
- 9.Jones EA. Ammonia, the GABA neurotransmitter system, and hepatic encephalopathy. Metab Brain Dis 2002; 17: 275–281. [DOI] [PubMed] [Google Scholar]
- 10.Lee PC, Yang YY, Lin MW, et al. Benzodiazepine-associated hepatic encephalopathy significantly increased healthcare utilization and medical costs of Chinese cirrhotic patients: 7-year experience. Dig Dis Sci 2014; 59: 1603–1616. [DOI] [PubMed] [Google Scholar]
- 11.Haq MM, Faisal N, Khalil A, et al. Midazolam for sedation during diagnostic or therapeutic upper gastrointestinal endoscopy in cirrhotic patients. Eur J Gastroenterol Hepatol 2012; 24: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 12.Murray-Lyon IM, Young J, Parkes JD, et al. Clinical and electroencephalographic assessment of diazepam in liver disease. Br Med J 1971; 4: 265–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McConnell JB, Curry SH, Davis M, et al. Clinical effects and metabolism of diazepam in patients with chronic liver disease. Clin Sci (Lond) 1982; 63: 75–80. [DOI] [PubMed] [Google Scholar]
- 14.Perney P, Butterworth RF, Mousseau DD, et al. Plasma and CSF benzodiazepine receptor ligand concentrations in cirrhotic patients with hepatic encephalopathy: Relationship to severity of encephalopathy and to pharmaceutical benzodiazepine intake. Metab Brain Dis 1998; 13: 201–210. [DOI] [PubMed] [Google Scholar]
- 15.Assy N, Rosser BG, Grahame GR, et al. Risk of sedation for upper GI endoscopy exacerbating subclinical hepatic encephalopathy in patients with cirrhosis. Gastrointest Endosc 1999; 49: 690–694. [DOI] [PubMed] [Google Scholar]
- 16.Khamaysi I, William N, Olga A, et al. Sub-clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: A randomized controlled study. J Hepatol 2011; 54: 72–77. [DOI] [PubMed] [Google Scholar]
- 17.Wong F, Watson H, Gerbes A, et al. Satavaptan for the management of ascites in cirrhosis: Efficacy and safety across the spectrum of ascites severity. Gut 2012; 61: 108–116. [DOI] [PubMed] [Google Scholar]
- 18.Watson H, Jepsen P, Wong F, et al. Satavaptan treatment for ascites in patients with cirrhosis: A meta-analysis of effect on hepatic encephalopathy development. Metab Brain Dis 2013; 28: 301–305. [DOI] [PubMed] [Google Scholar]
- 19.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014; 60: 715–735. [DOI] [PubMed] [Google Scholar]
- 20.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet 2002; 359: 248–252. [DOI] [PubMed] [Google Scholar]
- 21.Peppers MP. Benzodiazepines for alcohol withdrawal in the elderly and in patients with liver disease. Pharmacotherapy 1996; 16: 49–57. [PubMed] [Google Scholar]
- 22.Albarmawi A, Czock D, Gauss A, et al. CYP3A activity in severe liver cirrhosis correlates with Child-Pugh and model for end-stage liver disease (MELD) scores. Br J Clin Pharmacol 2014; 77: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trouvin JH, Farinotti R, Haberer JP, et al. Pharmacokinetics of midazolam in anaesthetized cirrhotic patients. Br J Anaesth 1988; 60: 762–767. [DOI] [PubMed] [Google Scholar]
- 24.Chalasani N, Gorski JC, Patel NH, et al. Hepatic and intestinal cytochrome P450 3A activity in cirrhosis: Effects of transjugular intrahepatic portosystemic shunts. Hepatology 2001; 34: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 25.Grundström R, Holmberg G, Hansen T. Degree of sedation obtained with various doses of diazepam and nitrazepam. Acta Pharmacol Toxicol (Copenh) 1978; 43: 13–18. [DOI] [PubMed] [Google Scholar]
- 26.Shafer A. Complications of sedation with midazolam in the intensive care unit and a comparison with other sedative regimens. Crit Care Med 1998; 26: 947–956. [DOI] [PubMed] [Google Scholar]