Abstract
Background
Accurate risk evaluation of choledocholithiasis prior to laparoscopic cholecystectomy is essential to determine optimal management strategy.
Objective
Our study aimed to evaluate the accuracy of separate predictors and Vilnius University Hospital Index (VUHI = A/30 + 0.4 × B; A = total bilirubin concentration (µmol/l), B = common bile duct (CBD) diameter (mm) measured by ultrasound) diagnosing choledocholithiasis and to assess different management strategies (cholecystectomy with intraoperative cholangiography and endoscopic retrograde cholangiopancreatography (ERCP)).
Methods
The retrospective study included 350 patients admitted to a tertiary care centre for laparoscopic cholecystectomy for cholecystolithiasis who were investigated for concomitant choledocholithiasis.
Results
Choledocholithiasis was diagnosed in 182 (76.2%) cases in the high-risk group (VUHI value ≥4.7) and 44 (39.6%) in the low, odds ratio is 4.86 (95% CI: 3.00–7.88). Its sensitivity was 80.5%, specificity 54.0%, accuracy 71.1%. Dilated CBD had the highest sensitivity (92.5%) of predictors.
ERCP showed better diagnostic performance than intraoperative cholangiography. Complications of ERCP were more frequent for patients without stones. There was no significant difference of outcomes between the two management strategies.
Conclusion
The prognostic index has good diagnostic accuracy but dividing patients into two risk groups is insufficient. The suggested model allows determining an intermediate-risk group, which requires additional investigation. Both management approaches are appropriate.
Keywords: Choledocholithiasis, choledocholithiasis risk, common bile duct stones, endoscopic retrograde cholangiopancreatography, intraoperative cholangiography, Vilnius University Hospital Index
Key summary
Common bile duct (CBD) obstruction by stones in case of cholecystolithiasis can lead to some serious outcomes;
use of endoscopic retrograde cholangiopancreatography (ERCP) for diagnostic means should be minimised as the procedure itself carries a certain risk of complications;
in the study centre an original prognostic index is being used for prediction of choledocholithiasis;
a value of the index indicating high risk (>50%) of choledocholithiasis showed 80.5% sensitivity, 54% specificity and 71.1% accuracy;
new thresholds for low, intermediate and high risk of choledocholithiasis are established;
additional non-invasive CBD investigation for the intermediate risk group is proposed;
no significant differences between two management strategies – ERCP before laparoscopic cholecystectomy and intraoperative cholangiography plus ERCP on demand – were observed.
Introduction
Choledocholithiasis is a common complication of cholecystolithiasis occurring for 10–18% of people undergoing cholecystectomy.1 Common bile duct (CBD) obstruction by stones can lead to acute biliary pancreatitis, mechanical jaundice, acute ascending cholangitis and even to fatal outcomes. Diagnosis of choledocholithiasis depends on a combination of biochemical tests and imaging studies’ findings. There is no clear consensus on the best therapeutic approach – surgical or endoscopic management of choledocholithiasis.2 Use of endoscopic retrograde cholangiopancreatography (ERCP) as a diagnostic tool should be minimised as it carries considerable risk (5–10%) of post-procedural complications: acute pancreatitis (1.3–6.7%), bleeding (0.7–2%), acute cholangitis (0.5–5%), duodenal perforation (0.3–1%).3,4 It is noticed that adverse events occur more often in patients with low risk of choledocholithiasis.4 Therefore the best possible patient selection for ERCP procedure is needed.
At the Centre of Abdominal Surgery of Vilnius University Hospital Santariskiu Klinikos, an original prognostic index (Vilnius University Hospital Index (VUH Index or VUHI)) has been used for evaluation of risk of choledocholithiasis since 1999.5 It is calculated by the formula VUHI = A/30 + 0.4 × B, where A = total bilirubin concentration (µmol/l), B = CBD diameter measured with ultrasound (US). When the value of the VUHI is equal to or higher than 4.7, the risk for choledocholithiasis is considered high and VUHI up to 4.7 is associated with low risk of choledocholithiasis. Considering diagnostic possibilities two decades ago, ERCP before laparoscopic cholecystectomy (LC) was the management of choice for high-risk patients and intraoperative cholangiography (IOC) was performed for the low-risk group.6,7 Currently non-invasive investigation methods (magnetic resonance cholangiography, endoscopic ultrasonography (EUS)) enable us to reduce unnecessary invasive examinations proportionally. So, revision of prognostic VUHI potential is essential in establishing the best diagnostic algorithm of choledocholithiasis.
The aims of our study were to evaluate the accuracy of separate predictors and the prognostic index, diagnosing choledocholithiasis for patients with gallbladder stones before LC, and to determine thresholds of VUHI values for intermediate choledocholithiasis risk, i.e. distinguish patients who would benefit from additional investigation. In addition, we aimed to assess safety and outcomes of different management strategies.
Patients and methods
The study consisted of patients admitted to the tertiary care centre Vilnius University Hospital Santariskiu Klinikos from January 2012 through December 2015 for LC for cholecystolithiasis for whom concomitant choledocholithiasis was suspected by clinical, radiological or biochemical findings. To identify study participants, we reviewed all the case records in our institution’s reporting database from this four-year period which included keywords ‘laparoscopic cholecystectomy’ in the operation protocol. Patients who underwent investigations for suspected choledocholithiasis (ERCP or IOC) were included. Exclusion criteria were: age under 18 years; surgically altered anatomy (Billroth II, Roux-en-y anastomosis, gastric bypass); a history of biliary surgery or stenting; suspected or known hepatopancreatobiliary malignancy; other known liver or biliary disease.
ERCP procedures were performed by experienced endoscopists (each had more than five years of experience in ERCP and more than 500 procedures completed) with Olympus side-viewing endoscopes TJF-160VR using standard technique.
All patients underwent a standard four-port LC. IOC was performed after distal clipping of the cystic duct that was incised and cannulated with a cholangiography catheter. A contrast material was injected gradually to visualise the lower end of the CBD and observe flow into the duodenum and then to outline the intrahepatic ducts. Cholangiograms were assessed by the operating surgeon and radiologist.
The following data were collected for each eligible participant: sex; age at the time of admission; duration from admission to intervention (IOC or ERCP) in days; total bilirubin concentration; CBD diameter and stones if seen on ultrasound (US), computed tomography (CT) or magnetic resonance cholangiopancreatography (MRCP); diagnosed acute cholecystitis, acute ascending cholangitis or acute biliary pancreatitis prior to ERCP; value of VUHI; physical status assessment according to the American Society of Anesthesiologists (ASA) classification; which investigation method of bile ducts was chosen first (ERCP or IOC), its results and outcome; type of cholecystectomy; adverse events of ERCP and their management; surgical complications (Clavien-Dindo classification); ERCP performed after cholecystectomy and its results; length of hospital stay. Jaundice was stated at a total bilirubin level of 34 µmol/l and higher.8 Diagnosis of acute biliary pancreatitis was acknowledged when stated in medical records or when lipase or amylase activity was at least three times higher than the upper limit of normal. Diagnosis of acute cholangitis was declared when stated in medical records. Diagnosis of acute cholecystitis was declared when suspected by clinical findings and confirmed histologically. CBD stone (CBDS) was considered detected when it was found and removed during ERCP, IOC or choledochotomy. CBDS at cholangiography (IOC or ERCP) was suspected when there was a filling defect seen on radiogram or delayed passage of contrast material into the duodenum was observed.
Statistical analysis was performed using SPSS version 17.0 (SPSS, Chicago, IL, USA) and statistical software R. Quantitative data were described as mean ± standard deviation (SD) and qualitative data as proportions. Independent-samples T-test was used to compare quantitative variables and chi-square test was used to compare categorical variables. Odds ratio (OR) was used to evaluate predictors. Two-sided hypotheses were checked and a p value <0.05 was considered statistically significant. For risk predictors and risk groups statistical measures of the performance of a binary classification test (sensitivity, specificity, positive predictive value, negative predictive value and accuracy) were assessed. A receiver operating characteristic (ROC) curve was evaluated to measure diagnostic accuracy. The relationship between VUHI and presence of S was selected using the analysis of variance (ANOVA) method and defined by logistic regression model.
This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Vilnius Regional Research Ethics Committee, certificate date 13 December 2016, number 158200-16-870-395. Written, informed consent for procedures was obtained from each patient included in the study.
Results
Patient characteristics and differences between patients with and without CBDS
During the study period 2313 patients had a cholecystectomy performed at our institution. Among them 350 patients (63.4% female, mean age 65.2 years, SD 17.89) underwent the aforementioned investigations for suspected choledocholithiasis and were eligible for the study.
CBDS were found in 226 cases (9.8% of all patients undergoing cholecystectomy); no stones were detected in 124 cases. Basic characteristics of the entire study population and differences between patients with and without CBDS are summarised in Table 1.
Table 1.
Characteristics of patients with and without CBDS.
| Variable | All patients n = 350 | CBDS (+) n = 226 | CBDS (–) n = 124 | Odds ratio (95% CI) | p value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (year), mean (SD) | 65.2 (17.9) | 66.3 (17.7) | 63.3 (18.1) | – | 0.130 |
| Female; n (%) | 222 (63.4) | 135 (59.73) | 87 (70.16) | 0.63 (0.4–1.0) | 0.053 |
| Clinical | |||||
| Jaundice, n (%) | 242 (69.1) | 165 (73.0) | 77 (62.1) | 1.65 (1.04–2.63) | 0.035 |
| Acute biliary pancreatitis, n (%) | 63 (18) | 30 (13.2) | 33 (26.6) | 0.42 (0.24–0.73) | 0.002 |
| Acute cholangitis, n (%) | 56 (16) | 45 (19.9) | 11(8.9) | 2.5 (1.24–5.04) | 0.002 |
| Acute cholecystitis, n (%) | 101 (28.9) | 60 (26.5) | 41 (33.1) | 0.73 (0.45–1.18) | 0.198 |
| Radiological | |||||
| Diameter of CBD (mm), mean (SD) | 10.17 (4.2) | 11.35 (4.11) | 7.94 (3.28) | – | <0.001 |
| CBDS seen on US, n (%) | 137 (39.1) | 112 (49.6) | 25 (20.2) | 5.81 (3.29–10.26) | <0.001 |
| Biochemical | |||||
| Total bilirubin (µmol/l), mean (SD) | 74.8 (63.3) | 82.1 (65.7) | 61.4 (56.4) | – | 0.002 |
CBDs: common bile duct stones; US: ultrasound; CI: confidence interval; SD: standard deviation. Statistically significant values are typed in bold.
Patients’ age and sex distribution did not differ significantly statistically. Patients in the stone-positive group had significantly higher total bilirubin concentration and CBD diameter, more cases of acute cholangitis (19.9% vs. 8.9%) but fewer cases of acute biliary pancreatitis (13.2% vs. 26.6%) as compared with the stone-negative group.
A total of 111 (31.71%) patients were classified as having a lower risk for choledocholithiasis (VUHI <4.7) and 239 (68.29%) patients were assigned to a higher-risk group (VUHI ≥4.7).
Predictors of choledocholithiasis
Performance characteristics of separate predictors: elevated bilirubin concentration, dilated CBD (diameter >6 mm) and CBDS seen or suspected by US were evaluated. In the stone-positive group, bilirubin was elevated above the upper limit of normal value in 189 cases (83.6% of patients with CBDS), dilated CBD was found in 209 cases (92.5%) and CBDS on US were seen in 112 cases (49.6%). In the stone-negative group, concentration of bilirubin was abnormal in 94 cases (75.8% of patients without CBDS), CBD was dilated in 84 cases (67.7%) and CBDS on US were seen or suspected in 18 cases (14.5% of US performed).
Evaluation of different criteria showed that dilated CBD and CBDS on US were stronger predictors than elevated total bilirubin (Table 2). Elevation of bilirubin above the upper limit of normal value (20 µmol/l) was not significantly different between the two groups but its increase above 30.78 µmol/l as a previously defined cut-off value for suspected choledocholithiasis2,9 was found to be a significant predictor. Dilated CBD had the highest sensitivity (92.5%), although its specificity was low (32.2%). CBDS found by US had low sensitivity (51.3%), despite high specificity (84.6%).
Table 2.
Prognostic values of different CBDS predictors and VUHI.
| Predictors | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) | Odds ratio (95% CI) | p value |
|---|---|---|---|---|---|---|---|
| Total bilirubin Cut-off >20 µmol/l | 83.6 | 24.2 | 66.8 | 44.8 | 62.6 | 1.63 (0.95–2.8) | 0.075 |
| Cut-off >30.78 µmol/l | 76.5 | 36.3 | 68.7 | 45.9 | 62.3 | 1.86 (1.15–3.0) | 0.011 |
| CBD > 6 mm | 92.5 | 32.2 | 71.3 | 70.2 | 71.1 | 5.85 (3.15–10.9) | <0.001 |
| CBDS on US | 51.3 | 84.6 | 86.2 | 48.3 | 63.0 | 5.81 (3.3–10.26) | <0.001 |
| VUHI ≥4.7 | 80.5 | 54.0 | 76.1 | 60.4 | 71.1 | 4.86 (3.00–7.88) | 0.000 |
CBDS: common bile duct stones; VUHI: Vilnius University Hospital Index; US: ultrasound; CI: confidence interval. Statistically significant values are typed in bold.
Evaluation of VUHI
The area under the ROC curve for VUHI was 0.742. VUHI ≥4.7 was found to be associated with more than a four-fold greater risk of having CBDS than VUHI <4.7 (OR 4.86) (Table 2). When counting CBDS on US as an additional factor for the higher-risk group (‘VUHI ≥4.7 or CBDS on US’) OR and performance rates improved, except specificity (OR 7.07). Additionally we included benign CBD strictures (n = 14) as a positive outcome presuming these patients would also benefit from ERCP. This modification raised the OR to 6.09 and overall accuracy to 74.0%.
In the higher-risk group ERCP was scheduled for 205 patients, and no pathology was detected (i.e. ERCP was performed unnecessarily) in 20 (9.76%) cases.
Distribution of detected CBDS according to VUHI value intervals is shown in Figure 1.
Figure 1.
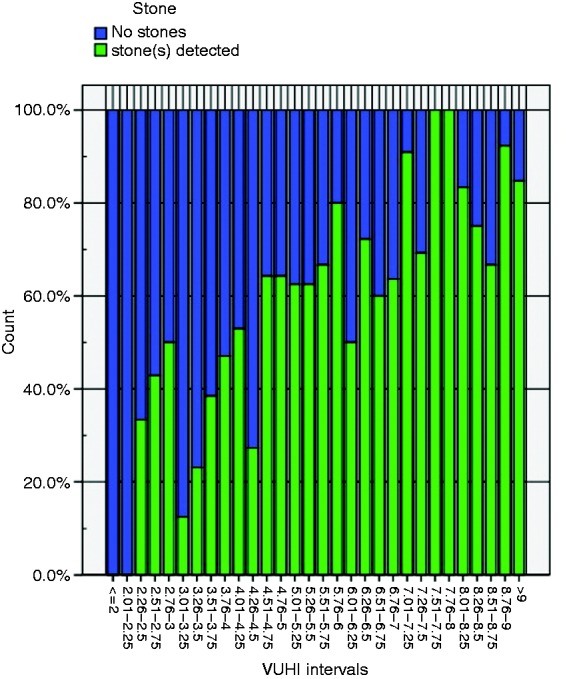
Proportion of CBD stones detected at different VUHI intervals. VUHI: Vilnius University Hospital Index.
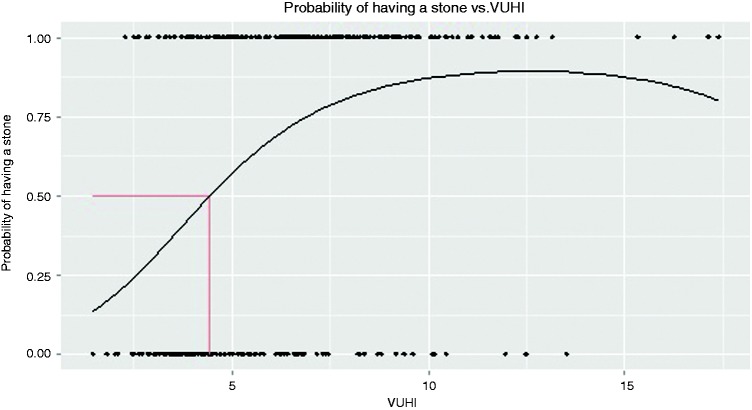
The relationship between VUHI and presence of S was defined by the logistic regression model
The predicted probability (πi) of finding an S while having a certain value of VUHI is obtained by the formula
Relation of predicted probability for CBDS and VUHI value is shown in Figure 2.
Figure 2.
Probability of CBD stones depending on VUHI. VUHI: Vilnius University Hospital Index.
Applying this equation, 50% probability of detecting stones would be at a VUHI value 4.42. The currently used value of 4.7 gives 53.6% probability.
Comparison of different management approaches
Two different choledocholithiasis management strategies were applied for these patients. A total of 118 patients first underwent the LC with intraoperative cholangiography (LC-IOC-first) and then ERCP ‘on demand’ in a single session (n = 18) or the next day (n = 10) depending on availability of the endoscopy unit. The other 232 patients had the two procedures in separate sessions: first, ERCP with sphincterotomy and necessary therapeutic interventions were performed and then cholecystectomy followed (ERCP-first). These two groups are compared in Table 3.
Table 3.
Comparison of different management approach groups.
| Variable | All patients n = 350 | LC-IOC-first n = 118 | ERCP-first n = 232 | p value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (year), mean (SD) | 65.2 (17.9) | 64.9 (17.4) | 65.4 (18.2) | 0.777 |
| Female; n (%) | 222 (63.4) | 76 (64.4) | 146 (62.9) | 0.786 |
| ASA grade | 0.429 | |||
| I–II | 165 (47.2) | 45 (38.5) | 12 (52.2) | |
| III–IV | 185 (52.8) | 73 (61.5) | 11 (47.8) | |
| Preoperative diagnosis | ||||
| Choledocholithiasis (CBDS on US/CT) | 133 (38) | 5 (4.5) | 128 (55.2) | 0.000 |
| Acute cholecystitis, n (%) | 101 (28.9) | 45 (38.1) | 56 (24.1) | 0.006 |
| Ascending cholangitis, n (%) | 56 (16) | 9 (7.7) | 47 (20.5) | 0.002 |
| Acute biliary pancreatitis, n (%) | 63 (18) | 25 (21.1) | 38 (16.4) | 0.268 |
| Predictors | ||||
| Preoperative bilirubin (µmol/l), mean (SD) | 74.8 (63.3) | 50.9 (47.9) | 86.9 (66.7) | 0.000 |
| Dilated CBDS on US >6 mm, n (%) | 293 (83.7) | 70 (59.3) | 223 (96.1) | 0.000 |
| CBD diameter (mm), mean (SD) | 10.17 (4.2) | 6.88 (2.3) | 11.8 (3.9) | 0.000 |
| VUHI ≥ 4.7, n (%) | 239 (68.3) | 34 (28.8) | 205 (88.4) | 0.000 |
| Outcomes | ||||
| CBDS detected, n (%) | 226 (64.6) | 35 (29.7) | 191 (82.3) | 0.000 |
| CBD pathology (stones + strictures), n (%) | 240 (68.6) | 35 (29.7) | 205 (88.4) | 0.000 |
| Duration from intervention to end of hospital treatment | 6.89 (8.62) | 4.67 (5.78) | 8.03 (9.57) | 0.000 |
LC-IOC: laparoscopic cholecystectomy with intraoperative cholangiography; ERCP: endoscopic retrograde cholangiopancreatography; ASA: American Society of Anesthesiologists; VUHI: Vilnius University Hospital Index; CBDS: common bile duct stones; US: ultrasound; CT: computed tomography; CI: confidence interval; SD: standard deviation. Statistically significant values are typed in bold.
For higher-risk patients (VUHI ≥4.7) the ERCP-first strategy was chosen in 205 cases and LC-IOC-first strategy in 34 cases. For patients with lower risk for choledocholithiasis (VUHI <4.7) LC-IOC as the first intervention was chosen in 84 cases and ERCP-first strategy in 27 cases, mostly when CBDS were seen on US/CT or other signs of possible choledocholithiasis were present (e.g. intrahepatic cholestasis).
Patients’ age, sex, physical status according to ASA grade and waiting time for the first intervention did not differ significantly between the different strategy groups. Duration from admission to the hospital to first intervention was less than two days (mean 1.34 days, 1.43 in the LC-IOC-first group and 1.29 in the ERCP-first group, p = 0.538). Values of separate predictors and VUHI were higher for ERCP-first patients. Acute cholecystitis was more frequent for the LC-IOC-first group, as a likely indication for urgent LC. Duration of hospital stay, both total and post-procedural, was longer in the ERCP-first group.
No significant differences were found for ERCP success rates and percentage of applied interventions between both groups. ERCP was successful at the first attempt for 93% of all patients (90.3% in LC-IOC-first, 93.3% in ERCP-first). Endoscopic treatment was unsuccessful for four (1.5%) patients; all of them belonged to the ERCP-first group. The complication rate was higher in the ERCP-first strategy group (14 vs. 1).
ERCP showed significantly better diagnostic performance than IOC, although diagnostic accuracy was very similar. If ERCP was evaluated just as a diagnostic procedure (cholangiography) it had 95.9% sensitivity, 78.8% specificity and 93.5% accuracy (eight false-negative and seven false-positive cases were found). All the ERCP patients had sphincterotomy and CBD revision performed as a standard procedure that allows detecting false-negative cases. This reduced missed CBDS count to one. Meanwhile, IOC had 90.6% sensitivity, 95.3% specificity and 94.1% accuracy but another intervention (ERCP) was needed to detect ‘falses’. There was no significant difference in conversion to open operation rate, CBD stenting or surgical complications (Clavien-Dindo classification) between the two groups (Table 4).
Table 4.
Results of diagnosing and managing CBDS and treatment outcomes in different strategy groups.
| LC-IOC-first n = 118 | ERCP-first n = 232 | p value | |
|---|---|---|---|
| Cholangiography positive for CBDS, n (%) | 33 (28.0%) | 198 (85.3%) | 0.000 |
| True positive for CBDS, n (%) | 29 (87.8%) | 191 (96.5%) | 0.032 |
| Success rates of ductal stone learance (all methods) | 28 (96.6%) | 189 (99.0%) | 0.298 |
| Missed CBDS | 3 (10.3%) | 1 (0.05%) | 0.000 |
| Incomplete stone clearance | 1 (3.45%)a | 0 | 0.01 |
| Conversion to open surgery | 2 (1.7) | 6 (2.6) | 0.597 |
| Choledochotomy | 1 (0.8) | 4 (1.7) | 0.513 |
| Biliary stent placement | 1 (0.8) | 10 (4.3) | 0.079 |
| Failure of CBD clearance | 0 | 2 (0.9) | 0.311 |
| Clavien-Dindo | |||
| 1–3 | 12 (10.2) | 44 (19.1) | 0.472 |
| 4–5 | 1 (0.8) | 8 (3.4) | |
| Mortalityb | 1 (0.8) | 3 (1.3) | 0.711 |
Endoscopic plastic stent insertion followed by postoperative ERCP after two days (n = 1).
Fatal outcomes were due to poor physical status, septic course of the disease and exacerbation of chronic illnesses. No deaths were caused by complications of surgical or endoscopic treatment.
CBDS: common bile duct stones; LC-IOC: laparoscopic cholecystectomy with intraoperative cholangiography; ERCP: endoscopic retrograde cholangiopancreatography. Statistically significant values are typed in bold.
Complications of interventions to CBD
ERCP-related complications occurred in 15 cases; the overall complication rate for 262 patients who underwent ERCP was 5.7%, being 4.5% (10 out of 221) in the stone-positive group and 12.2% (5 out of 41) in the stone-negative group, p = 0.052. The most common adverse event for all patients was post-ERCP pancreatitis (nine cases (4.1%), six in the stone-positive group, three in the stone-negative group) and this was followed by bleeding from the sphincterotomy site (three cases (1.4%)), perforation (two cases (0.9%)) and post-ERCP pancreatitis plus bleeding (one case (0.5%)). All complications were treated conservatively or endoscopically; no surgical treatment was necessary.
There were no complications of IOC reported.
Discussion
Risk assessment of choledocholithiasis for patients with symptomatic cholelithiasis tends to remain a topic of intensive debates recently. As a former gold standard for the diagnosis of CBDS, ERCP has a certain risk of complications, and it is important to distinguish which patients will benefit from ERCP as a therapeutic procedure as well, i.e. what features determine high risk for having concomitant CBDS.
We retrospectively evaluated the prognostic value of the choledocholithiasis index used in our institution. There were 76.2% of patients in the higher-risk category and 39.6% of patients in the lower-risk category who had CBDS, which gives a good sensitivity but unsatisfactory specificity. Analysis of separate constituents of VUHI (bilirubin concentration and US findings) showed them to be significant predictors of choledocholithiasis although elevation of bilirubin was significantly different for patients with and without CBDS just at a level of 30.78 µmol/l. Nevertheless, concentration of bilirubin makes up a distinctly lesser proportion in VUHI and the value of 20 µmol/l gives just 0.67 elevation of the index value. Our results are parallel with previous studies in which dilated CBD and/or hyperechoic shadows in it seen by US are the strongest predictors of choledocholithiasis.3,10–12 The diagnostic value of bilirubin is inconsistent in different studies: It has been found to be of highest specificity and accuracy among five biochemical parameters by Yang et al.,13 although other authors denied its significance.12,14
Clinical gallstone pancreatitis is ranked as a moderate predictor for choledocholithiasis.15 We found a negative association between biliary pancreatitis and risk for choledocholithiasis (OR = 0.42). Other authors also noticed that biliary pancreatitis is not associated with choledocholithiasis or can even be treated as a protective factor.2,16,17 This supports a hypothesis that biliary pancreatitis is associated with small CBDS size, and a spontaneous passage of such stones is more frequent.2
Although both non-invasive methods – MRCP and EUS – are available in our institution, we were not able to assess their effectiveness as MRCP was applied in just five cases and EUS was not performed at all for our cohort of patients.
As separate predictors seem to be insufficient to foresee CBDS, various prognostic systems are proposed for evaluation of this risk. A strategy to assign risk of choledocholithiasis in patients with symptomatic cholelithiasis proposed in 2010 by American Society for Gastrointestinal Endoscopy (ASGE guidelines) lately has been evaluated in different scenarios. We performed an analysis of seven different studies evaluating accuracy of the ASGE guidelines (Table 5).3,16–21 Altogether, 4613 patients were included in these studies; 2166 (46.95%) of them were classified as having a high risk for choledocholithiasis. Predictive values of high-risk criteria were evaluated: general sensitivity was found to be 52.4%, specificity 60.8%, positive predictive value 65.6%, negative predictive value 47.4%, accuracy 55.9%.
Table 5.
Diagnostic performance of ASGE guidelines.
| Authors, year of publication | Place, duration | Study type | Study aim | Participant characteristics | Results | Performance |
|---|---|---|---|---|---|---|
| Sethi et al., 20153 | One centre; Boston, MA, USA, 2011–2012 | Prospective observational cohort study | To assess accuracy of ASGE criteria | All patients referred for ERCP in unit with clinical suspicion for choledocholithiasis. 336 participants, 244 high-risk group, 92 intermediate | High risk: 185 (75.8%) had stones, intermediate: 45 (48.9%) | High-risk group: sens. 80.43%, spec. 44.34%, accur. 69.05% |
| Adams et al., 201518 | One centre; MI, USA, 2007–2012 | Retrospective cohort study | To test ASGE guidelines accuracy; impact of laboratory trends; predictors | Patients with suspected choledocholithiasis. 498 participants, 179 high-risk group; 319 intermediate or low | High-risk group: 99 (55.3%) with stones/sludge on MRC/EUS/ERCP; intermediate or low: 111 (34.8%) | High-risk group: sens. 47.7%, spec. 73%, accur. 62.1% |
| Magalhães et al, 201516 | One centre; Portugal, 2010–2013. | Retrospective study | To evaluate practical applicability of ASGE guidelines; different combination of predictors | Patients referred for ERCP for suspected bile duct lithiasis. 268 participants; 193 high risk, 73 intermediate, 2 low | High risk: 154 (79.8%) with stones; intermediate 25 (34.2%); low 0 | High-risk group (calculated): sens. 86.0%, spec. 56.1%, accur. 76.1% |
| Prachayakul et al., 201419 | One centre; Thailand, 2009–2012 | Retrospective study | To determine diagnostic yield and optimal timing of EUS in patients <…> requiring therapeutic ERCP | Patients with suspected choledocholithiasis who underwent EUS. 93 participants, 44 high-risk group, 49 intermediate | High risk: 17 (38.63%) had stones, intermediate 11 (22.44%) | High-risk group (calculated): sens. 60.7%, spec. 58.5%, accur. 59.1% |
| Suarez et al., 201620 | One centre; Charleston, SC, USA, 2009–2014 | Retrospective study | To evaluate performance characteristics of ASGE guidelines and to determine impact of laboratory trends | Patients with suspected choledocholithiasis. 173 participants, 71 high risk, 102 intermediate or low risk | High-risk group 39 (54.9%) had stones, intermediate or low risk 32 (31.4%) | High-risk group: sens. 54.9%, spec. 68.6%, accur. 63% |
| He et al., 201721 | One centre; Zhejiang, China, 2011–2013 | Retrospective study | To determine whether ASGE guidelines or other predictors can accurately identify patients with high risk of choledocholithiasis | Patients with suspected choledocholithiasis. 2724 participants, 1171 high-risk group, 1252 intermediate, 301 low | Calculated from performance: High-risk group 737 (62.9%) had stones, intermediate or low risk 316 (20.3%) | High-risk group: sens. 70%, spec.74%, accur. 72.5% |
| Rubin et al. 201317 | Two hospitals; Houston, TX, USA, 2007–2010 | Retrospective study | To assess validity and accuracy of current ASGE guidelines on choledocholithiasis | Patients who underwent ERCPs for suspected or confirmed choledocholithiasis 521 participants; 264 high risk, 249 intermediate, 8 low | High-risk group 189 (71.6%) had stones, intermediate 102 (41%), low 2 (25%) | High-risk group (calculated): sens. 64.5%, spec. 67.1%, accur. 65.6% |
ASGE: American Society for Gastrointestinal Endoscopy; ERCP: endoscopic retrograde cholangiopancreatography; sens.: sensitivity; spec.: specificity; accur.: accuracy; EUS: endoscopic ultrasonography; MRC: magnetic resonance cholangiography; USA: United States of America.
Our prognostic score shows comparable and, at some parameters, superior performance for predicting choledocholithiasis. The poorest measure here is specificity. This implies that the currently used threshold of VUHI is kind of a weak spot in this evaluation system. Our generated model for predicted probability of choledocholithiasis allows setting limits for an intermediate-risk group, i.e. determining which patients should undergo additional non-interventional investigation. The latest European Association for the Study of the Liver (EASL) guidelines state that patients with intermediate probability should undergo further evaluation with EUS or MRCP but do not define what this intermediate probability is.22
If we presume that the intermediate risk covers probability for CBDS from 25% to 75%, this corresponds to VUHI values of 2.54 to 6.88. In our study we had 202 such patients and CBDS were found for 105 (52.0%) of them. So, every other patient in this group would benefit from additional biliary imaging. In the potential low-risk group (VUHI <2.54) we had just nine patients, two (22.2%) of them had CBDS, both not larger than 5 mm. Respectively, in the presumed high-risk group (VUHI >6.88) we had 139 patients and 85.6% had choledocholithiasis.
Evaluating effectiveness of different management approaches, we found some advantages in both strategies: There were fewer missed stones and false-positive cholangiographies in the ERCP-first group, meanwhile the LC-IOC group had fewer ERCP-related complications. Mean length of hospital stay was approximately two days longer for the ERCP-first group, which in most cases reflected the waiting period for the LC. Meta-analyses of various different trials also show that there is no significant difference in the efficacy, mortality, morbidity, retained stones, and failure rates between single-stage and two-stage choledocholithiasis management.1,23,24 The main drawback of the preoperative ERCP strategy against various single-session approaches (intraoperative ERCP, LC with laparoscopic bile duct clearance, open bile duct clearance) is the time: Usually the waiting period between the two procedures prolongs duration of hospital stay and slightly increases the risk to develop recurrent biliary events and cholecystitis.22,25,26 Additionally, one of the biggest limitations to single-session ERCP and LC is difficult coordination of medical personnel, equipment and location of procedure.27,28 Despite these restraints a large survey of general surgeons in the United States showed that the majority of respondents preferred ERCP to laparoscopic CBD exploration for the management of choledocholithiasis.29
The most frequent adverse event of ERCP was post-ERCP pancreatitis. Its incidence is comparable to that observed in a systematic survey of 21 studies (3.47%).30 Among various established risk factors for this complication normal bilirubin level and non-dilated biliary ducts take an important place.31,32 This is compatible with our data that post-ERCP pancreatitis occurred more frequently in the low-risk group – for patients with less dilated CBD and lower bilirubin. Although complication count is too small to significantly confirm these findings, the tendency is seen.
There are some certain limitations of our study. The major limitation is its retrospective nature, which restricted evaluating clinical symptoms, as well as some other predictors, e.g. liver function tests other than bilirubin, because of lacking data. Also, timing from primary work-up to CBD exploration was not standardised as longer waiting duration can determine some spontaneously passed stones. We excluded patients with known or suspected hepatopancreatobiliary malignancy or other diseases; therefore, this model could be best applied before planned LC for relatively healthy patients.
In conclusion, the present study showed that our prognostic index has adequate diagnostic accuracy but dividing patients into two risk groups is insufficient in contemporary clinical practice. The suggested model allows determining an intermediate-risk group, which requires additional investigation. As no clinically significant differences between two management strategies were found, both approaches are suitable.
Declaration of conflicting interests
None declared.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval
This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Vilnius Regional Research Ethics Committee, certificate date 13 December 2016, number 158200-16-870-395.
Informed consent
Written, informed consent for procedures was obtained from each patient included in the study.
References
- 1.Dasari BV, Tan CJ, Gurusamy KS, et al. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev 2013, pp. CD003327–CD003327. [DOI] [PubMed] [Google Scholar]
- 2.Nárvaez Rivera RM, González González JA, Monreal Robles R, et al. Accuracy of ASGE criteria for the prediction of choledocholithiasis. Rev Esp Enferm Dig 2016; 108: 309–314. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S, Wang F, Korson AS, et al. Prospective assessment of consensus criteria for evaluation of patients with suspected choledocholithiasis. Dig Endosc 2015; 28: 75–82. [DOI] [PubMed] [Google Scholar]
- 4.Williams EJ, Green J, Beckingham I, et al. Guidelines on the management of common bile duct stones (CBDS). Gut 2008; 57: 1004–1021. [DOI] [PubMed] [Google Scholar]
- 5.Simutis G. Tulžies pūslės ir lydinčio tulžies latakų akmenligės gydymas minimaliai invaziniais chirurginiais būdais. Disertacija biomedicinos mokslų daktaro laipsniui įgyti. Vilnius: Vilniaus Universitetas, 1998, p.135.
- 6.Simutis G. Tulžies pūslės ir latakų chirurginės ligos. Vilnius: Vilniaus Universitetas, 2005, p.134.
- 7.Strupas K, Simutis G, Kontrimaviciute E, et al. Klinikinės chirurgijos diagnostikos ir gydymo vertinimo sistemos. Kaunas: Vitae Litera, 2008, p.207.
- 8.Merck manual. Professional version. Jaundice/Hepatic and biliary disorders, http://www.merckmanuals.com/professional/hepatic-and-biliary-disorders/approach-to-the-patient-with-liver-disease/jaundice (accessed 27 February 2017).
- 9.Barkun AN, Barkun JS, Fried GM, et al. Useful predictors of bile duct stones in patients undergoing laparoscopic cholecystectomy. McGill Gallstone Treatment Group. Ann Surg 1994; 220: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J, Paik K, Lee J, et al. The efficacy of clinical predictors for patients with intermediate risk of choledocholithiasis. Digestion 2016; 94: 100–105. [DOI] [PubMed] [Google Scholar]
- 11.Gurusamy KS, Giljaca V, Takwoingi Y, et al. Ultrasound versus liver function tests for diagnosis of common bile duct stones. Cochrane Database Syst Rev 2015; CD011548. [DOI] [PMC free article] [PubMed]
- 12.Jovanović P, Salkić NN, Zerem E, et al. Biochemical and ultrasound parameters may help predict the need for therapeutic endoscopic retrograde cholangiopancreatography (ERCP) in patients with a firm clinical and biochemical suspicion for choledocholithiasis. Eur J Intern Med 2011; 22: e110–e114. [DOI] [PubMed] [Google Scholar]
- 13.Yang MH, Chen TH, Wang SE, et al. Biochemical predictors for absence of common bile duct stones in patients undergoing laparoscopic cholecystectomy. Surg Endosc 2008; 22: 1620–1624. [DOI] [PubMed] [Google Scholar]
- 14.Isherwood J, Garcea G, Williams R, et al. Serology and ultrasound for diagnosis of choledocholithiasis. Ann R Coll Surg Engl 2014; 96: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maple JT, Ben-Menachem T, Anderson MA, et al. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 2010; 71: 1–9. [DOI] [PubMed] [Google Scholar]
- 16.Magalhães J, Rosa B, Cotter J. Endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis: From guidelines to clinical practice. World J Gastrointest Endosc 2015; 7: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin MIN, Thosani NC, Tanikella R, et al. Endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis: Testing the current guidelines. Dig Liver Dis 2013; 45: 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams MA, Hosmer AE, Wamsteker EJ, et al. Predicting the likelihood of a persistent bile duct stone in patients with suspected choledocholithiasis: Accuracy of existing guidelines and the impact of laboratory trends. Gastrointest Endosc 2015; 82: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prachayakul V, Aswakul P, Bhunthumkomol P, et al. Diagnostic yield of endoscopic ultrasonography in patients with intermediate or high likelihood of choledocholithiasis: A retrospective study from one university-based endoscopy center. BMC Gastroenterol 2014; 14: 165–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suarez AL, LaBarre NT, Cotton PB, et al. An assessment of existing risk stratification guidelines for the evaluation of patients with suspected choledocholithiasis. Surg Endosc 2016; 30: 4613–4618. [DOI] [PubMed] [Google Scholar]
- 21.He H, Tan C, Wu J, et al. Accuracy of ASGE high-risk criteria in evaluation of patients with suspected common bile duct stones. Gastrointest Endosc 2017; 86: 525–532. [DOI] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver (EASL). EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol 2016; 65: 146–181. [DOI] [PubMed] [Google Scholar]
- 23.Lu J. Two-stage vs single-stage management for concomitant gallstones and common bile duct stones. World J Gastroenterol 2012; 18: 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreras González JE, Torres Peña R, Ruiz Torres J, et al. Endoscopic versus laparoscopic treatment for choledocholithiasis: A prospective randomized controlled trial. Endosc Int Open 2016; 4: E1188–E1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kageoka M, Watanabe F, Maruyama Y, et al. Long-term prognosis of patients after endoscopic sphincterotomy for choledocholithiasis. Dig Endosc 2009; 21: 170–175. [DOI] [PubMed] [Google Scholar]
- 26.Byrne MF, McLoughlin MT, Mitchell RM, et al. The fate of patients who undergo “preoperative” ERCP to clear known or suspected bile duct stones. Surg Endosc 2009; 23: 74–79. [DOI] [PubMed] [Google Scholar]
- 27.ElGeidie AA. Single-session minimally invasive management of common bile duct stones. World J Gastroenterol 2014; 20: 15144–15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallick R, Rank K, Ronstrom C, et al. Single-session laparoscopic cholecystectomy and ERCP: A valid option for the management of choledocholithiasis. Gastrointest Endosc 2016; 84: 639–645. [DOI] [PubMed] [Google Scholar]
- 29.Baucom RB, Feurer ID, Shelton JS, et al. Surgeons, ERCP, and laparoscopic common bile duct exploration: Do we need a standard approach for common bile duct stones? Surg Endosc 2016; 30: 414–423. [DOI] [PubMed] [Google Scholar]
- 30.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: A systematic survey of prospective studies. Am J Gastroenterol 2007; 102: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 31.Rustagi T, Jamidar PA. Endoscopic retrograde cholangiopancreatography (ERCP)-related adverse events. Gastrointest Endosc Clin N Am 2015; 25: 107–121. [DOI] [PubMed] [Google Scholar]
- 32.Silviera M, Seamon M, Porshinsky B, et al. Complications related to endoscopic retrograde cholangiopancreatography: A comprehensive clinical review. J Gastrointest Liver Dis 2009; 18: 73–82. [PubMed] [Google Scholar]