Abstract
Background
Portal hypertension is a major complication of liver cirrhosis. Transjugular intrahepatic portosystemic shunt is effective in treatment of portal hypertension. However, decreased parenchymal portal venous flow after transjugular intrahepatic portosystemic shunt insertion favours ischaemic liver injury which has been discussed to induce hepatocarcinogenesis causing hepatocellular cancer.
Aim
This study aimed to explore the association between transjugular intrahepatic portosystemic shunt placement and the development of hepatocellular cancer.
Methods
A total of 1338 consecutive liver cirrhosis patients were included in this retrospective study between January 2004–December 2015. Data were analysed with regard to development of hepatocellular cancer during follow-up. Binary logistic regression and Kaplan-Meier analyses were conducted for the assessment of risk factors for hepatocellular cancer development. In a second step, to rule out confounders of group heterogeneity, case-control matching was performed based on gender, age, model of end-stage liver disease score and underlying cause of cirrhosis (non-alcoholic steatohepatitis, alcoholic liver disease and viral hepatitis).
Results
Besides established risk factors such as older age, male gender and underlying viral hepatitis, statistical analysis revealed the absence of transjugular intrahepatic portosystemic shunt insertion as a risk factor for hepatocellular cancer development. Furthermore, matched-pair analysis of 432 patients showed a significant difference (p = 0.003) in the emergence of hepatocellular cancer regarding transjugular intrahepatic portosystemic shunt placement versus the non-transjugular intrahepatic portosystemic shunt cohort.
Conclusion
In patients with end-stage liver disease, transjugular intrahepatic portosystemic shunt insertion is significantly associated with reduced rates of hepatocellular cancer development.
Keywords: Ascites, variceal bleeding, liver cirrhosis, portal hypertension, transjugular intrahepatic portosystemic shunt, hepatocellular carcinoma, gut microbiota
Key summary
- Summarize the established knowledge on this subject:
- ^ TIPS placement is an established tool to treat portal hypertension in patients with liver cirrhosis.
- ^ The association between TIPS insertion and development of HCC is still unclear.
- ^ TIPS associated hypoxaemia might trigger hepatocarcinogenesis
- What are the significant and/or new findings of this study?
- ^ Alcoholic liver disease, NASH, viral hepatitis, patient age and sex are established risk factors for the development of HCC in cirrhotic patients.
- ^ TIPS insertion is significantly negatively correlated with the emergence of HCC in a large patient cohort calculated via Kaplan-Meier and multivariate analysis.
Introduction
The main complication of liver cirrhosis is portal hypertension which often causes variceal bleeding, therapy-refractory ascites and hepatorenal syndrome. Since the first description of transjugular intrahepatic portosystemic shunt (TIPS) insertion in dogs mentioned by Rösch et al.1 in 1969, the first successful TIPS insertion in patients was realised by Rössle et al. in Freiburg, Germany, in 1988.2 Nowadays, TIPS is regarded as an established procedure in the treatment of the above-mentioned consequences of liver cirrhosis resulting in a significantly reduced portal pressure. A more recent study from Garcia-Pagan et al.3 not only showed better prevention of re-bleeding rates but also a reduction of mortality in patients receiving TIPS versus endoscopic ligation therapy for secondary prophylaxis of variceal bleeding. Another feared complication of liver cirrhosis is the occurrence of hepatocellular carcinoma (HCC).4 Therefore, the surveillance of patients with liver cirrhosis is mandatory to improve prognosis by detecting HCC as early as possible in this high-risk population. According to the literature, additional risk factors for the development of HCC are male gender, older age, high CHILD-PUGH class (class B or C), the presence of viral hepatitis, and alcoholic aetiology of liver cirrhosis.5,6 A first description of a possible association between porto-systemic shunt and the development of HCC was published in a post-mortem histological study in the early 1980s.7 In this study, the authors found a higher prevalence of HCC in patients with surgical porto-systemic shunt. Recently, TIPS has become the most frequently used option of non-surgical porto-systemic shunting for the treatment of portal hypertensive complications.8 In a retrospective case-control study, Banares et al.9 suggested an association between the development of HCC and the placement of TIPS in patients who were mostly CHILD-PUGH C. Further studies from Libbrecht et al.10 and De Santis et al.,11 however, did not show such an association, and a similar incidence of primary liver cancer was detected in patients suffering from liver cirrhosis with and without TIPS. Further, a retrospective study from Borentain et al.12 did not find a clear association between TIPS insertion and development of HCC but the prevalence of liver cell dysplasia in patients with patent stent was increased. Specifically, small cell dysplasia, which is known to be a precancerous lesion,13 was detected to be similar between patients with and without TIPS, while large cell dysplasia was found to occur more frequently in cirrhotics with an indwelling stent. Some authors suggest that TIPS insertion may lead to reduced hepatic parenchymal oxygenation due to the diversion of portal venous blood flow into the systemic circulation resulting in an activation of hepatic stellate cells, an induction of neoangiogenesis and an increase in the secretion of various growth factors such as hepatocyte growth factor and vascular endothelial growth factor14 and, thus, possibly triggering hepatocarcinogenesis. By contrast, other studies have showed improved arterial blood flow in cirrhotic patients15–17 with adequate oxygenation of liver parenchyma after TIPS insertion. So, to date, just a few studies in small patient cohorts and with non-uniform results have explored the association of TIPS insertion and the development of HCC. Therefore, to tackle the above correlation more conclusively we, to the best of our knowledge, have conducted the largest study reported so far.
Patients and methods
We performed a retrospective study analysing patients with end-stage liver disease referred to Muenster University Hospital between January 2004–December 2015. The local Ethics Committee approved this study (reference number: 2016-046-f-S; approved 28 April 2016). A total of 2012 patient records were available to be analysed at our institution. The diagnosis of cirrhosis was made either via liver biopsy, ultrasound findings, and the presence of clinical and laboratory features compatible with cirrhosis or transient elastography. Inclusion criteria were the following: all patients underwent general physical examination and laboratory testing. Participants were eligible for the study if they were at least 18 years of age and suffering from end-stage liver disease. For further statistical analysis patients with evidence of HCC based on conventional ultrasound or other imaging studies at the time of the initial medical visit were excluded. Furthermore, patients with HCC development within six months after initial presentation or with a follow-up of less than six months were also excluded. Likewise, patients with permanent TIPS dysfunction/occlusion were excluded. Therefore, the remaining 1338 patients constituted the final cohort. Of these patients, 259 received TIPS placement (TIPS cohort) and 1079 did not (non-TIPS cohort).
In a second step, patients were compared with each other relating to TIPS insertion. Matching of age, gender, underlying causes of liver cirrhosis (non-alcoholic steatohepatitis (NASH), alcoholic liver disease, viral hepatitis) and severity of liver cirrhosis determined by the model for end-stage liver disease score (MELD score) was performed. A total of 432 patients were eligible for statistical analysis. Two hundred and sixteen patients underwent non-covered (n = 57) or covered (n = 159) TIPS insertion procedure. In all patients the objective MELD score was determined to judge the clinical status and the severity of chronic liver disease. MELD score was calculated using the following values: creatinine, bilirubin, clotting time in the formula according to the modified method of Wiesner et al.18
3.78(Ln serum bilirubin (mg/dl))+11.20 (Ln international normalized ratio)+9.57 (Ln serum creatinine (mg/dl))+6.43
TIPS procedure
TIPS procedures in our centre were conducted in close collaboration with an interventional radiologist and gastroenterologist using standard techniques.19 A transjugular venous approach was performed followed by catheterisation of the right hepatic vein. In the next step, puncture of an intrahepatic branch of the portal vein was conducted followed by measurement of portal pressure and blood pressure of the right atrium. Then dilation of the liver parenchyma followed. Optimal stent length was defined using a special catheter with opaque markers. Non-covered nitinol (E-Luminexx, Bard PV, Tempe, USA) or covered nitinol stents (Viatorr, Gore Medical, Newark, USA) were used for the insertion procedure. After stent placement the pressures of the portal vein and the right atrium were measured again using an Exadyn transducer set (Braun, Melsungen, Germany). Difference of the portal pressure minus the right atrium pressure resulted in the portal pressure gradient. Doppler ultrasonography was performed the next day after TIPS placement controlling stent patency. Every six months after insertion stents were checked by ultrasonography. A first interventional angiography follow-up of TIPS was performed after 12 months or earlier in case of sonographic evidence of stenosis or clinical features of recurrent portal hypertension. In case of re-stenosis or occlusion re-intervention was performed during the angiographic examination.
HCC detection
Diagnosis of HCC was made according to clinical practice guidelines.20,21 HCC detection in follow-up every six months was made using ultrasonography (even with contrast agent), laboratory values (alpha-fetoprotein (AFP)) and clinically. In patients suspected of HCC, computed tomography (CT) or magnetic resonance imaging (MRI) or even biopsy was performed as a matter of course. Localization and size of HCC was measured based on CT or MRI imaging.
Statistics
Results are expressed as medians with interquartile range (IQR) or ranges, means with standard deviation (SD) or numbers/percentages. Non-continuous parameters were analysed by chi-square test and continuous parameters were analysed by Mann–Whitney U-test as appropriate. A p-value below 0.05 was considered statistically significant.
The cumulative probability of developing HCC in the TIPS- and non-TIPS cohorts, respectively, of the entire study population was calculated via Kaplan-Meier analysis and curves were compared using the log-rank test. The cumulative incidence of the first HCC diagnosis at one, three and five years of follow-up was calculated.
Univariate analysis for identifying possible predictors of HCC development was performed. Only variables considered statistically significant by univariate analysis were used for multivariate analysis to identify independent predictive factors for HCC development.
In a second step, case-control matching on confounding variables to account for pre-existing differences was performed on a 1:1 basis. The primary endpoint was HCC diagnosis during follow-up.
Statistical analysis of factors influencing the development of HCC after TIPS insertion was performed using IBM SPSS Statistics 24 (IBM Corp., Armonk, New York, USA) evaluating whether, in our patient cohort, TIPS placement represents a statistically significant risk factor for the development of HCC in end-stage liver disease patients or not.
Results
A total of 1338 patients met the inclusion criteria and were considered in our statistical analysis. The causes of cirrhosis mainly encompassed alcoholic liver disease, NASH and viral hepatitis. The incidence per 100 person years of HCC in our study cohort was 0.14 which is in accordance with previous studies.22 Baseline characteristics of the entire study population are presented in Table 1. In binary logistic regression analyses older age, male gender and underlying viral hepatitis B or C were detected as significant risk factors for HCC development. Furthermore, higher MELD score, alcoholic liver cirrhosis and NASH were associated with a higher rate of HCC development (Table 2). Binary regression analysis also revealed TIPS implantation to be significantly negatively correlated with HCC occurrence. Prior episodes of hepatic encephalopathy or spontaneous bacterial peritonitis were not associated with higher risk of HCC development.
Table 1.
Baseline characteristics of the entire study cohort.
| Variable | (n) | (%) |
|---|---|---|
| Patients | 1338 | 100 |
| Age, years (IQR) | 56 (48–65) | |
| Sex, m/f | 819/519 | 61/39 |
| TIPS cohort | 259 | 19 |
| Covered TIPS | 177 | 68 |
| Non-covered TIPS | 82 | 32 |
| HCC | 118 | 9 |
| Underlying diseases in patients | ||
| Alcohol | 438 | 33 |
| NASH | 202 | 15 |
| HBV | 90 | 7 |
| HCV | 213 | 16 |
| Wilson disease | 8 | 1 |
| Haemochromatosis | 60 | 5 |
| Polycystic liver disease | 8 | 1 |
| Cirrhose cardiaque | 17 | 1 |
| Budd-Chiari | 13 | 0.9 |
| Portal or mesenterial vein thrombosis | 19 | 1 |
| Morbus Osler | 1 | 0.1 |
| PBC | 109 | 8 |
| SSC | 46 | 3 |
| AIH | 80 | 6 |
| PSC | 79 | 6 |
| Cryptogenic | 102 | 8 |
| CASH/DILI | 43 | 3 |
| HCC location | ||
| Left lobe | 21 | 18 |
| Right lobe | 73 | 62 |
| Bilobular | 23 | 20 |
| HCC size, cm ± SD | 5.6 ± 3.7 | n.a. |
| Laboratory parameters, median (IQR) | ||
| Serum bilirubin, mg/dl | 1.1 (0.6–2.2) | n.a. |
| INR | 1.17 (1.04–1.38) | n.a. |
| Creatinine, mg/dl | 0.9 (0.7–1.2) | n.a. |
| MELD | 11 (8–15) | n.a. |
AIH: autoimmune hepatitis; CASH: chemotherapy-associated steatohepatitis; DILI: drug induced liver injury; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; HE: hepatic encephalopathy; INR: international normalized ratio; IQR: interquartile range (25–75 percentile); MELD: model of liver end-stage disease; n.a.: not analysed; NASH: non-alcoholic steatohepatitis; PBC: primary biliary cholangitis; PSC: primary sclerosing cholangitis; SD: standard deviation; SSC: secondary sclerosing cholangitis; TIPS: transjugular portosystemic shunt.
Table 2.
Multivariate analysis for prediction of hepatocellular carcinoma (logistic binary regression) – entire study cohort.
| Predictive factors | Regression coefficient | OR | 95% CI | p Value | |
|---|---|---|---|---|---|
| TIPS | −1.052 | 0.35 | 0.163–0.746 | 0.001 | |
| Gender | 1.018 | 2.77 | 1.592–4.809 | <0.0001 | |
| Age | 0.047 | 1.05 | 1.028–1.069 | <0.0001 | |
| Alcohol | 1.173 | 3.23 | 1.562–6.689 | 0.002 | |
| NASH | 0.913 | 2.492 | 1.101–5.640 | 0.028 | |
| Viral hepatitis | 1.910 | 6.753 | 3.304–13.803 | <0.0001 | |
| Cholestatic liver disease | −0.334 | 0.72 | 0.289–1.772 | 0.470 | |
| Haemochromatosis | 0.823 | 2.28 | 0.905–5.731 | 0.081 | |
| MELD | −0.051 | 0.95 | 0.910–0.992 | 0.021 | |
| Constant | −6.73 |
CI: confidence interval; OR: odds ratio; MELD: model of liver end-stage disease; NASH: non-alcoholic steatohepatitis; TIPS: transjugular portosystemic shunt.
Cholestatic liver disease includes primary biliary cholangitis, primary sclerosing cholangitis and secondary sclerosing cholangitis; viral hepatitis includes chronic hepatitis C and chronic hepatitis B.
In our study cohort, the cumulative probability of developing HCC was calculated via Kaplan-Meier analysis and was found significantly greater in the non-TIPS group (log-rank test: p = 0.002) (Figure 1). The cumulative rate of HCC diagnosis at one, three and five years of follow-up was 7%, 11% and 20% for the non-TIPS cohort and 1%, 2% and 11% for the TIPS patients, respectively.
Figure 1.
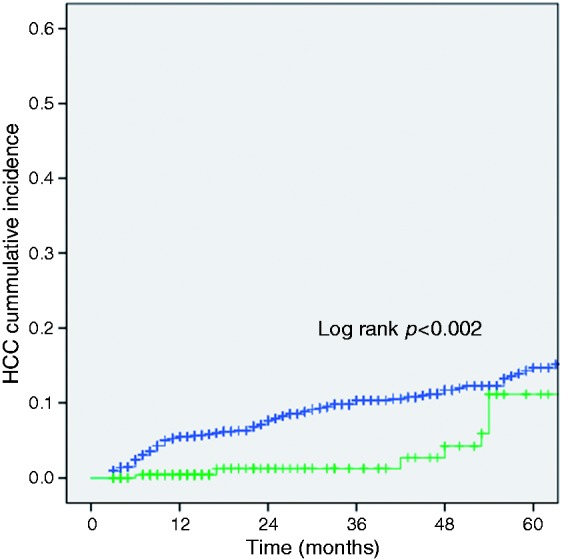
Kaplan-Meier curves showing cumulative probability of hepatocellular carcinoma development in patients with liver cirrhosis and implantation of transjugular portosystemic shunt (TIPS) (green line) compared to non-TIPS cirrhotic patients (blue line). The cumulative rate of hepatocellular carcinoma (HCC) diagnosis at one, three and five years was 7%, 11% and 20% for the non-TIPS cohort and 1%, 2% and 11% for the TIPS patients, respectively.
Case control matching based on the abovementioned parameters was performed to specify the influence of TIPS on HCC development and to rule out confounders of group heterogeneity. Of 216 patients per matched group, 141 were male (65%), 75 were female (35%). The median age was 58 years (51–65) in the TIPS group and 58 years (50–65) in the non-TIPS patients. The median MELD score was 12 (10–15) in the TIPS cohort and 11.7 (9.2–4.4) in the non-TIPS population (Table 3).
Table 3.
Baseline characteristics of the matched patient cohorts.
| Variable | TIPS | No TIPS | p Value |
|---|---|---|---|
| Number | 216 | 216 | |
| Age, years (IQR) | 58 (51–65) | 58 (50–65) | 0.760 |
| Sex, m/f | 141/75 | 141/75 | 1.000 |
| MELD (IQR) | 12 (10–14.8) | 11.7 (9.2–14.4) | 0.581 |
| f/u, months ± SD | 85.3 ± 266.7 | 42.9 ± 25.6 | <0.0001 |
| Covered/bare metal, n | 159/57 | ||
| Prior episode of HE | 24 (11%) | 14 (4.6%) | 0.069 |
| Underlying diseases in patients | |||
| Alcohol | 113 | 113 | 1.000 |
| NASH | 23 | 23 | 1.000 |
| Viral hepatitis | 1.000 | ||
| HBV | 7 | 10 | 0.452 |
| HCV | 24 | 22 | 0.768 |
| Cryptogenic | 21 | 11 | 0.069 |
| Wilson disease | 1 | 1 | 0.997 |
| Haemochromatosis | 13 | 10 | 0.528 |
| Budd-Chiari | 5 | 1 | 0.102 |
| Osler disease | 0 | 0 | 1.000 |
| PBC | 9 | 15 | 0.204 |
| PSC | 19 | 5 | 0.003 |
| SSC | 7 | 8 | 0.786 |
| Autoimmune hepatitis | 9 | 8 | 0.812 |
| Polycystic liver disease | 0 | 0 | 1.000 |
| Chronic portal vein thrombosis | 4 | 2 | 0.415 |
| CASH/DILI | 1 | 5 | 0.102 |
| HCC, n | 8 | 24 | 0.003 |
| HCC location, n (%) | 0.217 | ||
| Left lobe | 0 (0) | 5 (21) | |
| Right lobe | 7 (87,5) | 17 (71) | |
| Bilobular | 1 (12.5) | 2 (8) | |
| Extrahepatic spread, n (%) | 1 (12.5) | 6 (25) | 0.637 |
| Vascular infiltration, n (%) | 0 (0) | 1 (4) | 0.610 |
| HCC size, cm ± SD | 3.44 ± 1.76 | 5.63 ± 3.44 | 0.071 |
| HCC Nodules, n (IQR) | 1 (1–1.25) | 2 (1–3) | 0.066 |
| BCLC, n | 0.293 | ||
| Stage 0 | 0 | 2 | |
| Stage A | 5 | 11 | |
| Stage B | 0 | 3 | |
| Stage C | 1 | 8 | |
| Serum AFP, median (IQR) | 35 (4.4–1660) | 30.5 (7.5–496) | 0.767 |
| Laboratory parameters, median (IQR) | |||
| Serum bilirubin, mg/dl | 1.2 (0.8–1.9) | 1.5 (0.8–2.4) | 0.090 |
| INR | 1.3 (1.15–1.44) | 1.2 (1.03–1.4) | <0.0001 |
| Creatinine, mg/dl | 1 (0.8–1.3) | 1 (0.73–1.3) | 0.531 |
AFP: alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer Staging System; CASH: chemotherapy-associated steatohepatitis; DILI: drug-induced liver injury; f/u: follow-up; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; HE: hepatic encephalopathy; INR: international normalized ratio; IQR: interquartile range (25–75 percentile); MELD: model of liver end-stage disease; NASH: non-alcoholic steatohepatitis; PBC: primary biliary cholangitis; PSC: primary sclerosing cholangitis; SD: standard deviation; SSC: secondary sclerosing cholangitis.
The aetiology of liver disease was alcohol, NASH, hepatitis B, hepatitis C, autoimmune liver disease and cryptogenic liver disease in most cases (Table 3). Among those parameters, only primary sclerosing cholangitis (PSC) was statistically differently distributed in the two cohorts. Laboratory values of both groups including serum bilirubin, international normalized ratio (INR), and creatinine are also shown in Table 3. HCC was diagnosed in eight patients (3.7%) of the TIPS group and in 24 non-TIPS patients (11%; p = 0.003). HCC lesions were predominantly located in the right hepatic lobe, i.e. the site of TIPS insertion (Table 3).
Discussion and conclusion
Previous studies have analysed risk factors for HCC development in patients with liver cirrhosis.11 In binary regression analysis, we found established variables to be associated with HCC development (older age, male gender, severity of liver disease, underlying viral hepatitis B or C, alcoholic liver disease, NASH and hereditary liver disease). This is in accordance with previous findings that describe higher risk in patients with hepatitis infection23 in addition to older age, male gender, severity of liver disease and alcoholic liver disease.24,25
Former studies also suggest TIPS to be associated with an increased risk of HCC.9 However, study data are controversial concerning TIPS and its impact on HCC development. A study by De Santis et al.11 was not able to show a significant association of TIPS with HCC, although a trend towards higher HCC incidence in the TIPS cohort could be detected. Consistent with the data from De Santis et al.,11 in almost all cases of our study population, HCC occurred in lobule of TIPS insertion (right lobe) but this association missed the significance level. In contrast, in our investigation we could not detect TIPS to be related to a higher risk for HCC development. Both, Kaplan-Meier analyses and binary regression analysis of the entire study cohort as well as the matched case control evaluation showed the implantation of a TIPS shunt to be a protective factor with regard to the development of HCC.
As hypoxaemia is known to induce factors which regulate transcription of genes involved in cellular metabolism, inflammation, angiogenesis and proliferation,26 one might speculate that TIPS has an unfavourable effect on the hepatic blood supply. Some authors have suggested that TIPS insertion may lead to reduced hepatic parenchymal oxygenation due to diverting portal venous blood flow into the systemic circulation resulting in an activation of hepatic stellate cells, an induction of neoangiogenesis and an increased secretion of various growth factors such as hepatocyte growth factor and vascular endothelial growth factor.14 On the other hand, a study from Patel et al.16 showed an increased blood flow in the hepatic artery after TIPS insertion. Furthermore, a study from Stankovic et al.17 was also able to demonstrate changes in portal and splanchnic arterial haemodynamics in TIPS patients using four-dimensional flow MRI.17 Weidekamm et al.15 documented a statistically significant increase of the hepatic artery flow and of total hepatic perfusion after TIPS insertion using dynamic CT whereas no changes of the venous parenchymal perfusion could be observed. Taken together, improved arterial blood flow via the liver artery, potentially leading to better oxygenation in liver tissue and thus reducing the risk for the development of reactive oxygen species which are suggested to be involved in carcinogenesis,27 might contribute to a reduced risk of HCC.
Another aspect of the extenuated incidence of HCC in our TIPS cohort might be the impact of reduced portal hypertension on leaky gut. Patients with liver cirrhosis are at risk for the development of intestinal dysbiosis resulting in proinflammation.28 A review from Roderburg and Luedde28 discussed evidence suggesting intestinal microbiota are involved in the development of HCC due to the presence of pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharides (LPS). Zhang et al.29 reported dysbiosis to be associated with elevated portal LPS levels eventually resulting in hepatocarcinogenesis. TLR4 acts as a receptor for LPS and has been shown to be proinflammatory and to trigger profibrotic signalling pathways leading to liver fibrosis and is suggested to be directly connected with hepatocarcinogenesis.30 Furthermore, Tao et al.31 described a breakdown in intestinal barrier function to be involved into the development of HCC due to endotoxaemia caused by bacterial translocation. In patients with portal hypertension, gut permeability increases resulting in spontaneous bacterial peritonitis due to transmural migration of toxins and bacteria.32 Finally, damaged intestinal barrier function and bacterial overgrowth might favour the development of HCC.
A recent study by Meng and colleagues33 found reduced levels of LPS, as a surrogate parameter of bacterial translocation, in the portal vein after TIPS insertion. Furthermore, non-selective beta-blockers that are also used for lowering portal hypertension have been associated with a lower risk of HCC in a retrospective study of 291 patients with hepatitis C-related cirrhosis receiving either propranolol or not.34 A further meta-analysis recently published was also in favour of non-selective beta-blockers in regard to the development of HCC suggesting an important role of portal hypertension-related bacterial translocation promoted by increased intestinal permeability and shift in the gut microbiome that can be positively influenced by non-selective beta-blockers.35 This notion might be supported by the study from Ripoll et al.36 documenting a hepatic venous pressure gradient (HVPG) >10 mm Hg to be associated with a six-fold increase of HCC risk in patients with liver cirrhosis.
Therefore, we hypothesise that reduction of the portal hypertension by TIPS results in less gut permeability with less migration of microbial and toxic agents such as PAMPs and LPS, leading to less liver inflammation and thus levelling off the carcinogenic effects of TIPS placement such as hypoxia.
In conclusion, in the present study TIPS insertion could not be associated with higher incidence of HCC because of portal hypertension. Conversely, TIPS patients developed lower rates of HCC possibly due to increased hepatic arterial blood flow and reduction of gut permeability which may alleviate hepatocarcinogenic processes. Provocatively, our results might suggest that all patients with cirrhosis should go for TIPS installation. However, since this is a monocentric study such conclusions should not be drawn. Nevertheless, the findings of our study suggest a higher rate of HCC in non-TIPS patients that treating physicians might bear in mind in terms of surveillance of their patients.
Limitations
We do recognise that potential patient selection and information biases as results of the retrospective study design might have weakened the validity of the findings presented here. Also, the detection rate in the past might not have checked for all liver diseases, resulting in the diagnosis of cryptogenic liver disease, even possibly resulting in changes of group numbers, e.g. in the NASH group. Furthermore, as a tertiary referral centre the patient cohort with the percentage of underlying liver diseases might not reflect the situation observed at other hospitals. On the other hand, our study in a large number of subjects was solely performed at one medical institution with a uniform clinical, diagnostic and therapeutic work-up for patients with end-stage liver disease.
Furthermore, the duration of cirrhosis as a known risk factor for the development of HCC could not be assessed at the time of first presentation due to the retrospective character of our study. However, case-control matching as a quasi-experimental study design should have reduced the selection bias and improved the internal validity of our study thus evening out, at least in part, the abovementioned factors. In the end, the appropriate matching of the non-TIPS cohort with the TIPS population, as well as the use of Cox regression analysis as a standard statistical procedure, strongly indicates that TIPS insertion reduces the risk of HCC development in patients with end-stage liver disease.
Acknowledgements
Guarantor of the article: HS Heinzow is the submission's guarantor. Author contributions were as follows: A Hüsing-Kabar: study concept and design, analysis and interpretation of data, drafting of the manuscript, literature research; T Meister: statistical analysis, analysis and interpretation of data, technical support, critical revision of the manuscript; M Köhler: critical revision of the manuscript and literature research; W Domschke: drafting of the manuscript; interpretation of data, critical revision of the manuscript; I Kabar and C Wilms: technical support, critical revision of the manuscript; B Hild: technical support, critical revision of the manuscript. H Schmidt: critical revision of the manuscript; HS Heinzow: senior author, study concept and design, data acquisition, analysis and interpretation of data, drafting of the manuscript, literature research, study supervision. All authors approved the final version of the article.
Declaration of conflicting interests
All authors declare that they have nothing to disclose.
Funding
This work was supported by grants to A Hüsing-Kabar from the Dean’s Office of the Medical Faculty of the Westphalian Wilhelm University of Muenster.
Ethics approval
This study was performed in accordance with the Declaration of Helsinki 1975 and the International Conference on Harmonization Good Clinical Practice guidelines and was waived by the local Ethical Review Board of Muenster, Germany because of its retrospective study design (#2016-046-f-S; 28 April 2016).
Informed consent
Informed consent of patients prior to procedures was obtained in all cases.
References
- 1.Rosch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: An experimental study. Radiology 1969; 92: 1112–1114. [DOI] [PubMed] [Google Scholar]
- 2.Rossle M, Richter GM, Noldge G, et al. New non-operative treatment for variceal haemorrhage. Lancet 1989; 2: 153–153. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Pagan JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362: 2370–2379. [DOI] [PubMed] [Google Scholar]
- 4.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet 1999; 353: 1253–1257. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez RF, Rodriguez M, Navascues CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology 2003; 37: 520–527. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35: 421–430. [DOI] [PubMed] [Google Scholar]
- 7.Bjorneboe M, Andersen JR, Christensen U, et al. Does a portal-systemic shunt increase the risk of primary hepatic carcinoma in cirrhosis of the liver? Scand J Gastroenterol 1985; 20: 59–64. [DOI] [PubMed] [Google Scholar]
- 8.Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: Update 2009. Hepatology 2010; 51: 306–306. [DOI] [PubMed] [Google Scholar]
- 9.Banares R, Nunez O, Escudero M, et al. Patients with cirrhosis and bare-stent TIPS may have increased risk of hepatocellular carcinoma. Hepatology 2005; 41: 566–571. [DOI] [PubMed] [Google Scholar]
- 10.Libbrecht L, Maleux G, Verslype C, et al. Influence of TIPS on development of hepatocellular carcinoma in cirrhosis. Hepatology 2005; 42 236; author reply: 237. [DOI] [PubMed] [Google Scholar]
- 11.De Santis A, Iegri C, Kondili L, et al. Hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt: A retrospective case-control study. Dig Liver Dis 2014; 46: 726–730. [DOI] [PubMed] [Google Scholar]
- 12.Borentain P, Garcia S, Gregoire E, et al. Transjugular intrahepatic porto-systemic shunt is a risk factor for liver dysplasia but not hepatocellular carcinoma: A retrospective study of explanted livers. Dig Liver Dis 2015; 47: 57–61. [DOI] [PubMed] [Google Scholar]
- 13.Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int 2005; 25: 16–27. [DOI] [PubMed] [Google Scholar]
- 14.Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology 2000; 31: 141–148. [DOI] [PubMed] [Google Scholar]
- 15.Weidekamm C, Cejna M, Kramer L, et al. Effects of TIPS on liver perfusion measured by dynamic CT. AJR Am J Roentgenol 2005; 184: 505–510. [DOI] [PubMed] [Google Scholar]
- 16.Patel NH, Sasadeusz KJ, Seshadri R, et al. Increase in hepatic arterial blood flow after transjugular intrahepatic portosystemic shunt creation and its potential predictive value of postprocedural encephalopathy and mortality. J Vasc Interv Radiol 2001; 12: 1279–1284. [DOI] [PubMed] [Google Scholar]
- 17.Stankovic Z, Rossle M, Euringer W, et al. Effect of TIPS placement on portal and splanchnic arterial blood flow in 4-dimensional flow MRI. Eur Radiol 2015; 25: 2634–2640. [DOI] [PubMed] [Google Scholar]
- 18.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003; 124: 91–96. [DOI] [PubMed] [Google Scholar]
- 19.LaBerge JM, Ring EJ, Gordon RL, et al. Creation of transjugular intrahepatic portosystemic shunts with the wallstent endoprosthesis: Results in 100 patients. Radiology 1993; 187: 413–420. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–1236. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Ducreux M, Lencioni R, et al. European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 56: 908–943. [DOI] [PubMed]
- 22.Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004; 127: S35–S50. [DOI] [PubMed] [Google Scholar]
- 23.Ioannou GN, Splan MF, Weiss NS, et al. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2007; 5: 938–945. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–2576. [DOI] [PubMed] [Google Scholar]
- 25.Parikh S, Hyman D. Hepatocellular cancer: A guide for the internist. Am J Med 2007; 120: 194–202. [DOI] [PubMed] [Google Scholar]
- 26.Wilson GK, Tennant DA, McKeating JA. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: Current understanding and future directions. J Hepatol 2014; 61: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 27.Waris G, Ahsan H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J Carcinog 2006; 5: 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roderburg C and Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes 5: 441–445. [DOI] [PubMed]
- 29.Zhang HL, Yu LX, Yang W, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol 2012; 57: 803–812. [DOI] [PubMed] [Google Scholar]
- 30.Dapito DH, Mencin A, Gwak GY, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012; 21: 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao X, Wang N, Qin W. Gut microbiota and hepatocellular carcinoma. Gastrointest Tumors 2015; 2: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutz P, Nischalke HD, Strassburg CP, Spengler U. Spontaneous bacterial peritonitis: The clinical challenge of a leaky gut and a cirrhotic liver. World J Hepatol 2015; 7: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng J, Wang Q, Liu K, et al. Systemic and splanchnic lipopolysaccharide and endothelin-1 plasma levels in liver cirrhosis before and after transjugular intrahepatic portosystemic shunt. Gastroenterol Res Pract 2016: Article ID 8341030; 5 pages. [DOI] [PMC free article] [PubMed]
- 34.Nkontchou G, Aout M, Mahmoudi A, et al. Effect of long-term propranolol treatment on hepatocellular carcinoma incidence in patients with HCV-associated cirrhosis. Cancer Prev Res (Phila) 2012; 5: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 35.Thiele M, Albillos A, Abazi R, et al. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: A meta-analysis of randomized trials. Liver Int 2015; 35: 2009–2016. [DOI] [PubMed] [Google Scholar]
- 36.Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 2009; 50: 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]


