Abstract
Background
Endoscopic mucosal resection is well-established for resecting flat or sessile benign colon polyps. The novel underwater endoscopic mucosal resection eschews submucosal injection prior to endoscopic mucosal resection. Reports about underwater endoscopic mucosal resection were limited to small series of single and/or tertiary-care referral centers, with single or supervised operators.
Objective
The purpose of this study was to determine feasibility and efficacy of underwater resection of polyps of any morphology (underwater polypectomy, here includes underwater endoscopic mucosal resection) in routine clinical practice.
Methods
This study involved a comparison of colonoscopy records of two community hospitals (January 2015–December 2016) for underwater polypectomy (n = 195) and gas insufflation polypectomy (n = 186).
Results
Comparable demographics, procedural data, overall distribution, morphology and size of resected lesions, number of en bloc and R0 resections (any polyp morphology and size); exception: overall, underwater polypectomy pedunculated polyps were significantly larger than those in the gas insufflation polypectomy group, p = 0.030. Underwater polypectomy (median, min) resection time was significantly shorter than gas insufflation polypectomy: sessile and flat polyps 6–9 mm, 0.8 vs 2.7 (p = 0.040); 10–19 mm, 2.0 vs 3.3 (p = 0.025), respectively; pedunculated polyps 6–19 mm, 0.8 vs 3.3 (p < 0.001). Underwater polypectomy resection of pedunculated polyps 6–19 mm showed significantly less immediate bleeding: 11.1% vs 1.5%, respectively (p = 0.031).
Conclusions
Underwater polypectomy can be efficaciously used in routine clinical practice for the complete resection of colon polyps, with several advantages over gas insufflation polypectomy.
Keywords: Colon polyp resection, endoscopic mucosal resection, post-polypectomy bleeding, underwater endoscopic mucosal resection
Key summary
Underwater endoscopic mucosal resection (UEMR) eschews submucosal injection of fluids prior to EMR. Reports about UEMR were limited to small series of single and/or tertiary-care referral centers, particularly focused on the resection of large, flat lesions.
In routine clinical practice at two community hospitals, compared with traditional gas insufflation polypectomy, the underwater resection of polyps (also with pedunculated morphology) achieved comparable proportions of en bloc and R0 resections, shortening resection time (all polyp morphologies with size of 6–19 mm), and with less immediate bleeding episodes for pedunculated polyps of 6–19 mm.
Introduction
Endoscopic mucosal resection (EMR) is a technique commonly used in the gas-insufflated colon for resecting flat or sessile benign polyps, particularly of large size.1 A fluid is injected in the submucosal space, lifting the lesion away from the muscularis propria2 to decrease the risk of iatrogenic damage.3
Underwater endoscopic mucosal resection (UEMR) is a novel technique: the bowel lumen is filled with water rather than gas eschewing submucosal injection,4 based on the endoscopic ultrasound (EUS) observation that in the water-filled lumen the mucosa and submucosa float away from the deeper layers.4,5
Reports attesting to the feasibility, efficacy and safety of UEMR were limited to small series of single and/or tertiary-care referral centres,5–11 with limited number of cases8,9 and with single5,7,11,12 or supervised operators,9,10 using caps fitted at the tip of the colonoscope,5–8,10,11 pediatric instruments7,11 or EUS evaluation of the polyps prior to resection.5,9,10 Minimal data exist from community-based practices where most colonoscopies are performed.12
We aimed to demonstrate that underwater polypectomy (UWP, in the current article comprising all underwater resections) can be efficaciously used to resect colorectal lesions irrespective of their size and morphology; and to determine if UWP has advantages over gas insufflation polypectomy (GIP).
Materials and methods
UEMR was adopted at our two community hospitals in Italy in late 2014, after reviewing a number of earlier published reports4,5,7 and associated videos.4,5
In January 2015, almost all flat or slightly elevated lesions were resected using EMR with just a few using the recently adopted UEMR. Gradually, UEMR became the technique of choice, replacing EMR. Within one year, both our hospitals transitioned from GIP (using either air or carbon dioxide (CO2), depending on the endoscopy room) to underwater resections, also for pedunculated polyps. Hence, in 2016 almost all lesions were resected underwater. The current observational retrospective study included two groups of patients that differ only in the endoscopic technique used: subjects >18 years old who underwent colonoscopy from January 2015–December 2016 with polypectomy carried out in a gaseous environment (mostly during 2015) or underwater (mostly during 2016). The treatment strategy and surveillance protocol were equally applied throughout the study period.
Our colonoscopy records reported polyp location, size, morphology (Paris classification)13 and associated pathology record; colon cleanliness (Boston Bowel Preparation Scale (BBPS)),14 and adverse events with their management. Resection time was recorded prospectively in cases enrolled in clinical trials unrelated to polyp resection technique and outcomes15,16 (Italian nationwide observational study about post-colonoscopy outcomes, protocol PG/2016/13127).
Before we adopted UWP, at our hospitals lesions of 6–9 mm were removed using either cold snare or hot snare with submucosal injection, depending on polyp location, endoscopist’s experience or preference. We included in the current analysis consecutive sessile, flat and pedunculated polyps judged ≥6 mm in diameter by the endoscopists, removed by hot snare either using GIP and submucosal injection or underwater.
Signed informed consent was obtained from each patient prior to polyp resection. The local Ethical Committee (Azienda Ospedaliera Universitaria di Cagliari) approved the study protocol (PG/2017/5571, 3 April 2017), which conformed to the Declaration of Helsinki, as part of a quality-improvement initiative. Patients received the standard of care and data was anonymized before analysis.
Colonoscopy
Four board-certified endoscopists with adequate levels of expertise in colonoscopy (2000–10,000 cases accrued) and EMR or polyp resection techniques performed the procedures. A split-dose bowel preparation was used to clean the colon. High-definition wide-angle adult video colonoscopes with auxiliary channel (Olympus HD 180–190 series, Olympus Corp, Hamburg, Germany) were used. CO2 was insufflated using the Olympus UCR unit.
Polyp location was noted; the polyp size was determined by comparison to the snare tip diameter and crosschecked with pathology reports.
Lesions were assessed by using both white-light and narrow-band imaging (NBI) to determine suitability for resection, defined as follows:17–19 (a) sessile and flat polyp with 0-Is, IIa or IIb morphology, respectively; (b) pedunculated polyps (0-Ip morphology); (c) benign lesion without Kudo pit pattern V20 or NICE type 3.21 For polyps meeting the suitability criteria, EUS was not used prior to resection.17,18
Standard polyfilament duckbill or oval snares of different measures (Heyinovo-Wilson, Shanghai, China; Endoflex GmbH, Voerde, Germany) were used for resection. At the discretion of the endoscopists, monofilament snares (Heyinovo-Wilson) were used to resect larger lesions.
Resections were carried out by using ERBE (ERBE Elektromedizin, Tübingen, Germany) electrosurgical units: VIO 200D or ICC200.
En bloc resection was always attempted, otherwise piecemeal resection was performed until resection borders appeared to be constituted only by normal mucosa. The mucosal defect and resection margins were carefully inspected with white light and NBI in order to evaluate the completeness of removal; any residual island of neoplasia was removed by snare or using biopsy forceps and stored in a separate jar. All resected material was retrieved for histologic examination.
At the discretion of the endoscopists, bleeding vessels at first were coagulated using snare-tip soft coagulation, then using argon plasma coagulation (APC) or obliterated using hemoclips.
EMR technique
Traditional EMR was performed as described,1–3 using the following electrosurgical unit settings: Endocut Q, effect 4, length 1, interval 1; Forced coagulation 25 W, effect 2.
Underwater EMR technique (Figures 1 and 2)
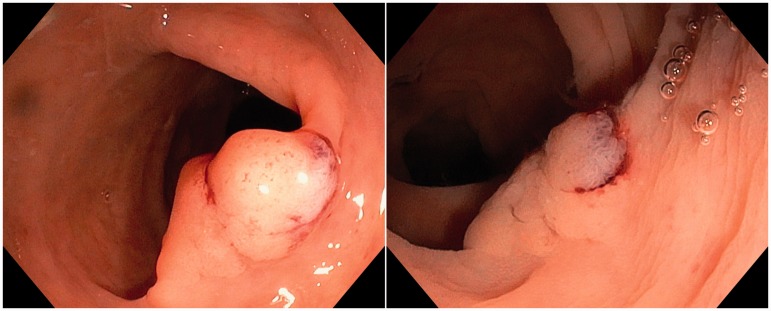
Figure 1.
Left: gas insufflation view of a type 0-Is-IIa (Paris classification)13 polyp. Right: the same lesion seen underwater.
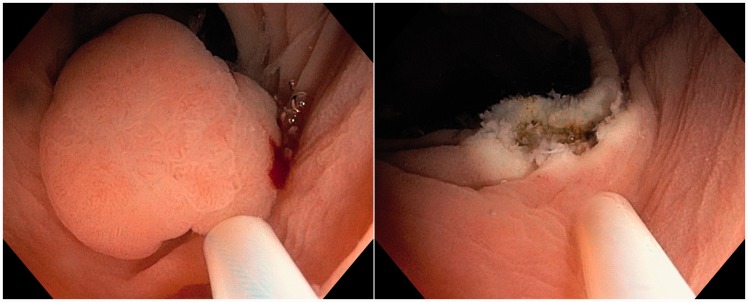
Figure 2.
Left: underwater view of the ensnared lesion. Right: underwater view of the resection site.
Before starting UEMR, any insufflated gas was replaced with warm-to-touch water to achieve complete filling of the lumen (usually between 100 ml and 300 ml) using a flushing pump (Olympus OFP2). At the discretion of the endoscopists, the margins of lesions >20 mm were marked within 3–5 mm of their borders using APC or the tip of a snare.
UEMR was performed as described,4 including normal mucosa outside the margins of the lesion or at the margins identified by the diathermic dots. The opened snare was pushed flush against the bowel wall and torqued to engage a pleat of tissue, maximizing tissue capture.4 The electrosurgical unit was set to Dry cut, effect 5, 60 W, Forced coagulation 60 W, effect 2 (VIO 200D) or Endocut effect 3, 35 W, Forced coagulation 35 W (ICC200).
Resection of pedunculated polyps
Resections were performed with the snare placed about halfway up the stalk, without prophylactic stalk clipping, ligature, or injection. Before starting underwater resection using the same setting as UEMR, any insufflated gas was substituted with water as described above. In the gas-distended colon, resection was carried out with the same settings as EMR, but with Forced coagulation set at 50 W.
Outcomes of interest
The outcomes of interest, presented as suggested by the European Society of Gastrointestinal Endoscopy (ESGE),19 were en bloc and R0 resection rates of sessile and flat lesions, resection outcomes of lesions of any morphology, and adverse events. R0 is defined as a complete en bloc resection of a lesion with tumor-free lateral and vertical margins.18 Our pathologists always report if resection margins are free of neoplastic tissue. Adverse events were identified from hospital files and from Regional Health Service informatics, categorized as immediate bleeding requiring endoscopy therapy after ineffective lavage and snare-tip soft coagulation; delayed bleeding within 30 days after the procedure resulting in an emergency department visit, hospitalization, or re-intervention (endoscopy, angiography, or surgery); post-polypectomy syndrome,1 and perforation (localized or diffuse release of gas or intestinal fluids into the peritoneum). Resection time is defined as the total time that included submucosal injection (EMR), marking around flat polyps, water infusion (underwater resections) and treatment of immediate complications (e.g. bleeding) with endoscopic therapy. We also evaluated BBPS score at the site of resection, and the use of hemoclips and/or of APC to treat bleeding.
Statistical analysis
Normally distributed variables are presented as means and standard deviations (SDs), and non-normally distributed variables are described with medians and interquartile ranges (IQRs). For categorical variables, p values were obtained using Chi Square test or Fisher’s exact test, as appropriate. For continuous variables, p values for comparison of the group means and medians were obtained using t-test and Mann-Whitney U test, respectively. A value of p < 0.05 was considered statistically significant.
Results
From January 2015–December 2016, 287 patients underwent either UWP (n = 146) or GIP (n = 141) (Table 1), for a total of 318 polypectomies (Table 2): 195 UWP and 186 GIP, respectively.
Table 1.
Patients and procedure characteristics.
| Underwater polypectomy | Gas insufflation polypectomy | ||
|---|---|---|---|
| Variable | n = 146 (50.9) | n = 141 (49.1) | p Value |
| Sex, n (%) | 0.278 | ||
| Female | 45 (30.8) | 52 (36.9) | |
| Male | 101 (69.2) | 89 (63.1) | |
| Age in years, mean (SD) | 64.7 (9.0) | 65.2 (10.7) | 0.717 |
| Indications, n (%) | 0.371 | ||
| Screening | 55 (37.7) | 46 (32.6) | |
| Symptoms | 91 (62.3) | 95 (67.4) | |
| Sedation, n (%) | 32 (21.9) | 45 (31.9) | 0.056 |
| Quality of colon preparation (Boston Bowel Preparation Scale score at polyp site), median (IQR) | 3.0 (2.0–3.0) n = 126 | 3.0 (2.0–3.0) n = 111 | 0.091 |
IQR: interquartile range; SD: standard deviation.
For categorical variables, p values were obtained using Chi Square test. For continuous variables, p values for comparison of the group means and medians were obtained using t-test and Mann-Whitney U test, respectively.
Table 2.
Characteristics of polyp resected.
| Underwater polypectomy | Gas insufflation polypectomy | ||
|---|---|---|---|
| Variable | n = 195 (51.2) | n = 186 (48.8) | p Value |
| Polyp distribution, n (%) | 0.685 | ||
| Right colon (cecum and ascending) | 38 (19.5) | 43 (23.1) | |
| Transverse colon | 29 (14.9) | 27 (14.5) | |
| Distal colon (descending to rectum) | 128 (65.6) | 116 (62.4) | |
| Polyp morphology, n (%) | |||
| Sessile and flat polyps | 108 (55.4) | 112 (60.2) | 0.340 |
| Pedunculated polyps | 87 (44.6) | 74 (39.8) | |
| Sessile and flat polyps, by size category (mm) | 0.551 | ||
| 6–9 | 27 (25.0) | 35 (31.3) | |
| 10–19 | 63 (58.3) | 58 (51.8) | |
| ≥20 | 18 (16.7) | 19 (16.9) | |
| Pedunculated polyps, by size category (mm) | 0.103 | ||
| 6–19 mm | 65 (74.7) | 63 (85.1) | |
| ≥20 mm | 22 (25.3) | 11 (14.9) | |
| Polyp size in mm, overall, median (IQR) | |||
| Sessile and flat polyps | 10 (9.25–15) n = 108 | 10 (8–15) n = 112 | 0.445 |
| Pedunculated polyps | 13 (10–20) n = 87 | 10.5 (8–15) n = 74 | 0.030 |
| Polyps ≥20 mm, overall size in mm, median (IQR) | |||
| Sessile and flat polyps | 22.5 (20.0–42.5) | 25.0 (20.0–32.0) | 0.855 |
| Pedunculated polyps | 20.0 (20.0–25.0) | 25.0 (20.0–25.0) | 0.721 |
IQR: interquartile range.
Polyp morphology assignment: flat, 0-IIa and 0-IIb; sessile, 0-Is; pedunculated, 0-Ip.13 For categorical variables, p values were obtained using Chi Square test. For continuous variables, p values for comparison of the group medians were obtained using Mann-Whitney U test.
Demographics, indications, use of sedation and colon cleanliness at the site of resection were comparable (Table 1).
Table 2 shows the comparable distribution and morphology (also by size category) of resected lesions. Overall, median sessile and flat polyp size was comparable, pedunculated polyps were significantly larger in the UWP than in the GIP group: 13 mm (IQR 10–20) vs 10.5 mm (IQR 8–15), respectively (p = 0.030).
Table 3 reports histology and resection outcomes. Most lesions were adenomas; there were nine carcinomas in situ in the UWP and three in the GIP group, respectively. Two lesions in the UWP group and one in the GIP group, containing infiltrating cancer were referred to surgery.
Table 3.
Histology and resection outcomes.
| Underwater polypectomy | Gas insufflation polypectomy | |||
|---|---|---|---|---|
| Variable | n = 195 (51.2) | n = 186 (48.8) | p Value | |
| Histology, n (%) | 0.195 | |||
| Adenoma | 171 (87.7) | 162 (87.1) | ||
| Hyperplastic | 11 (5.6) | 17 (9.1) | ||
| Carcinoma in situ | 9 (4.6) | 3 (1.6) | ||
| Invasive cancer | 2 (1.0) | 1 (0.5) | ||
| Other benign lesion | 2 (1.0) | 3 (1.6) | ||
| Resection time in minutes, median (IQR) | ||||
| Sessile and flat polyps, overall | 2.0 (0.8–5.0) n = 64 | 3.3 (2.5–6.0) n = 75 | 0.002 | |
| 6–9 mm | 0.8 (0.6–3.5) n = 17 | 2.7 (2.0–3.4) n = 25 | 0.040 | |
| 10–19 mm | 2.0 (0.8–4.5) n = 37 | 3.3 (2.5–5.0) n = 35 | 0.025 | |
| ≥20 mm | 7.0 (1.4–16.2) n = 10 | 10.0 (7.0–13.3) n = 15 | 0.579 | |
| Pedunculated polys, overall | 1.0 (0.7–3.3) n = 55 | 3.5 (2.5–5.0) n = 63 | <0.001 | |
| 6–19 mm | 0.8 (0.7–2.0) n = 43 | 3.3 (2.5–5.0) n = 54 | <0.001 | |
| ≥20 mm | 4.3 (1.4–7.8) n = 12 | 5.3 (4.2–8.5) n = 9 | 0.355 | |
| Resection outcomes, n (%) | ||||
| Sessile and flat polyps | ||||
| En bloc | Size category (mm) | |||
| 6–9 | 27 (100) | 35 (100) | NC | |
| 10–19 | 51 (81.0) | 46 (79.3) | 0.821 | |
| ≥20 | 7 (38.9) | 5 (26.3) | 0.414 | |
| Piecemeal | Size category (mm) | |||
| 6–9 | 0 (0) | 0 (0) | NC | |
| 10–19 | 12 (19.0) | 12 (20.7) | 0.821 | |
| ≥20 | 11 (61.1) | 14 (73.7) | 0.414 | |
| Pedunculated polyps | ||||
| En bloc | Size category (mm) | |||
| 6–19 | 64 (98.5) | 60 (95.2) | 0.361 | |
| ≥20 | 22 (100) | 11 (100) | NC | |
| Piecemeal | Size category (mm) | |||
| 6–19 | 1 (1.5) | 3 (4.8) | 0.361 | |
| ≥20 | 0 (0) | 0 (0) | NC | |
| R0a resections, n (%) | ||||
| Sessile and flat polyps, overall | 83 (97.6) | 86 (100) | 0.246 | |
| 6–9 mm | 27 (100) | 35 (100) | NC | |
| 10–19 mm | 49 (96.1) | 46 (100) | 0.496 | |
| ≥20 mm | 7 (100) | 5 (100) | NC | |
IQR: interquartile range; NC: not calculated.
Polyp morphology assignment: flat, 0-IIa and 0-IIb; sessile, 0-Is; pedunculated, 0-Ip.13 For categorical variables, p values were obtained using Chi Square test or Fisher’s exact test, as appropriate. For continuous variables, p values for comparison of the group medians were obtained using Mann-Whitney U test.
En block with uninvolved margins.
Resection time was available for 61% and 74.2% of UWP and GIP cases, respectively. Overall, median UWP resection time (sessile and flat polyps, 2.0 min, IQR 0.8–5.0; pedunculated polyps 1.0 min, IQR 0.7–3.3) was significantly shorter than GIP resection time (3.3 min, IQR 2.5–6.0; 3.5 min, IQR 2.5–5.0, respectively). In detail, median UWP resection time was significantly quicker than GIP for lesions of any morphology in the range of 6–19 mm (p from 0.040 to <0.001).
En bloc resections by polyp morphology and size, and the proportion of R0 resections were comparable.
Adverse events
These are reported in Table 4. No post-polypectomy syndrome or perforation cases occurred. UWP showed a lower, but comparable, proportion of adverse events than GIP (8.2% vs 12.3%, respectively; p = 0.369), mostly immediate bleeding episodes: 7.2% vs 11.8%. When stratifying by polyp morphology, immediate bleeding episodes for pedunculated lesions were significantly lower in the UWP than in the GIP group: 3.4% vs 13.5%, p = 0.019, due to lower occurrence in the cohort of lesions 6–19 mm in size (p = 0.031).
Table 4.
Adverse events.
| Adverse events, n (%) | Underwater polypectomies n = 195 | Gas insufflation polypectomies n = 186 | p Value |
|---|---|---|---|
| Overall | 0.369 | ||
| Immediate bleeding | 14 (7.2) | 22 (11.8) | |
| Delayed bleedinga | 2 (1.0) | 1 (0.5) | |
| Immediate bleeding | |||
| Sessile and flat polyps, overall, n (%) | 11 (10.2) n = 108 | 12 (10.7) n = 112 | 0.898 |
| 6–9 mm | 1 (3.7) n = 27 | 1 (2.9) n = 35 | >0.999 |
| 10–19 mm | 5 (7.9) n = 63 | 9 (15.5) n = 58 | 0.193 |
| ≥20 mm | 5 (27.8) n = 18 | 2 (10.5) n = 19 | 0.232 |
| Pedunculated polyps, overall, n (%) | 3 (3.4) n = 87 | 10 (13.5) n = 74 | 0.019 |
| 6–19 mm | 1 (1.5) n = 65 | 7 (11.1) n = 63 | 0.031 |
| ≥20 mm | 2 (9.1) n = 22 | 3 (27.3) n = 11 | 0.304 |
| Management | |||
| Hemoclip use, overall, n (%) | 14 (7.2) | 22 (11.8) | 0.121 |
| Sessile and flat polyps, overall, n (%) | 10 (9.3) | 12 (10.7) | 0.719 |
| Pedunculated polyps, n (%) | 4 (4.6) | 10 (13.5) | 0.045 |
| 6–19 mm | 2 (3.1) | 7 (11.1) | 0.093 |
| ≥20 mm | 2 (9.1) | 3 (27.3) | 0.304 |
| Use of argon plasma coagulation,b n (%) | 4 (21.1) | 1 (4.3) | 0.158 |
Polyp morphology assignment: flat, 0-IIa and 0-IIb; sessile, 0-Is; pedunculated, 0-Ip.13 For categorical variables, p values were obtained using Chi Square test or Fisher’s exact test, as appropriate.
Delayed bleeding occurred in two underwater polypectomy resections (one flat and one pedunculated polyp, respectively, each with a diameter of 15 mm) and in one gas insufflation polypectomy resection (pedunculated polyp with a diameter of 10 mm).
All sessile or flat polyps.
Overall, hemoclip use for sessile and flat lesions was comparable, also by polyp size. For pedunculated polyps, hemoclip use was significantly lower in the UWP than in the GIP group (4.6% vs 13.5%, respectively; p = 0.045), their use was less frequent for lesions 6–19 mm in size. Use of APC was comparable.
Follow-up surveillance colonoscopies
Forty patients with sessile or flat adenomas resected piecemeal (18 UEMR, 22 EMR) accepted to undergo short-term follow-up (median 14 months, IQR 3.5–21). Of them, 36 (90%; 16 UEMR, 20 EMR) returned to repeat the procedure. Biopsies were taken from post-polypectomy scars to rule out the presence of neoplastic tissue. Only three patients in the GIP group had a recurrent or residual neoplasia.
Discussion
In October 2014, after adopting UEMR, we further extended the technique to the underwater resection of all polyps. To the best of our knowledge, our is the first study comparing UWP and GIP with data recorded in routine clinical settings at two community hospitals, including pedunculated lesions and with procedures performed by endoscopists with different expertise. In the current study, notwithstanding a relatively short transition time from traditional GIP to UWP, the latter achieved comparable proportions of en bloc and R0 resections with the advantages of the avoidance of submucosal injection (flat lesions), a significantly shorter resection time (all polyp morphologies with size of 6–19 mm) and less immediate bleeding episodes for pedunculated polyps of 6–19 mm.
During conventional polypectomy, insufflated gas flattens polyps and thins the wall of the colon.4,5 This lead to the introduction of EMR to decrease the risk of iatrogenic damage to the colon wall.1 However, submucosal injection under flat lesions increases tissue tension and may paradoxically flatten the polyp relative to the surrounding tissue,4 making its ensnarement more difficult or impossible.4,22 Injection may displace the polyp into a less accessible location or constrict the lumen, hampering access to the lesion.4 Furthermore, there is the risk of dysplastic seeding into deeper wall layers;23 or the inadvertent injection outside the bowel wall may trigger local peritonitis and infection.4
UEMR is a safe and effective technique, relatively easy for experienced endoscopists,4,8–11 obviating the aforementioned drawbacks of submucosal injection, and the accidental capture of the circular muscular layer of the colon wall with the resecting snare is less probable.4 Compared with GIP, a heat-sink effect has been described for UEMR.24 Theoretically this should reduce the risk of a deeper transmural burn.
While transitioning from GIP to UWP, we found the latter easier to capture and resect lesions. We personally observed that infusing water to perform UWP floats the head of pedunculated polyps toward the center of the lumen, allowing for easier and precise snaring mid stalk, particularly helpful when the stalk is kinked. Change of patient position to put the polyp in the best position for ensnaring and resection is needed less frequently than when using GIP. Interestingly, apart from pedunculated polyps of 6–19 mm, polyp distribution, morphology, size, en bloc, and R0 resections were comparable between groups, and yet UWP resections of polyps of 6–19 mm (any morphology) were significantly quicker than in GIP (Table 3). This may have an impact in cases of multiple resections, where more insufflation is used, which can lead to more colon distention and associated discomfort or pain.25
Immediate bleeding following polyp resection is not considered an adverse event unless it results in hospitalization, transfusion, or surgery.5,9,26 For the purposes of our study we counted as adverse events all episodes of immediate bleeding lasting more than 30–60 s after washing the site of resection, and resistant to snare-tip coagulation. Immediate bleeding occurred less frequently in the UWP group, significantly so for pedunculated lesions of 6–19 mm, generally a preferred and easy target since their stalks are cut at some distance from the colon wall, minimizing the risk of perforation.1 Our observations suggest that UWP can offer a safer approach for the resection of pedunculated lesions within this size range. However, since UWP and GIP lesions were resected with different electrosurgical generator settings, as also previously reported,11 the observed differences might be related to the settings themselves, underwater resection, or both.
We speculate that immediate bleeding episodes were less frequent in the UWP group because of the latency between the application of the current and actual cutting. Anecdotally, when performing the procedure underwater a few seconds are required before the snare starts cutting into the ensnared tissue. It is conceivable that during UWP, delayed conduction and heat sink slightly prolongs the resection time, causing more coagulation along the cutting plane.
Within the timeframe of the current study, 108 submucosal injections were avoided to resect sessile and flat lesions using UWP, generating overall savings of about €2,394 (injection needles, €1,944; solution for submucosal injection, €450). The savings generated by UWP regarding only clinically significant lesions (≥10 mm, n = 81) added up to about €1,796 (injection needles, €1,458; colloidal solution, €338). Moreover, using UWP a cumulative time of 119 min was saved to resect all lesions (UWP = 514 min, GIP = 663 min, respectively).
In the UWP group, hemoclips were used less frequently than in the GIP group to treat bleeding originating from pedunculated lesions, especially those 6–19 mm in size. However, the savings generated by the less frequent use of hemoclips, which application varies widely in clinical practice at different hospitals, were marginal and partially offset by the use of APC. The issue of the economical impact of UWP deserves to be investigated in future studies.
The high proportions of R0 resections are likely due to the predominant number of lesions of small and intermediate size. The sample of polyps ≥20 mm is small, and outcomes relative to this subgroup analysis should be interpreted with caution.
We did all resections without using EUS, and yet only two cases (0.9%) of infiltrating cancer occurred in three lateral spreading tumors. This is consistent with the affirmation that EUS may not be routinely required before the resection of colorectal lesions that were adequately evaluated.17,18
Our follow-up comprised of a limited number of patients. The observation that the only three cases showing residual or recurrent neoplasia were in the GIP group is consistent with the reported lower proportion of recurrences of UEMR vs EMR, e.g. for the resection of large lesions.11
Our study has some strengths. Resection time, when available, was collected prospectively. The follow-up extended up to 22 months. Unlike previous reports of single tertiary-care centers with single endoscopists or performed by experts in this field,5–10 underwater resection of polyps was done in routine clinical settings at two community hospitals. This should provide reproducibility and generalizability of our results.
Our study also has some limitations. The analysis is retrospective. Resection time was available only for 67.5% of cases. Uncertainty resulting from selection and operator must be taken into account. However, their influence should have been limited, e.g. endoscopists judged UWP pedunculated lesions significantly bigger (Table 2), and yet adopted this technique. Nevertheless, the UWP technique might have changed, and adverse events thereby decreased over time, as the endoscopists gained experience, even if the study spanned a limited amount of time. Our patients’ records were accurately updated, and yet some adverse events occurring after discharge might have been missed.
In conclusion, most outcomes between the novel UWP and the time-tested GIP were comparable. Our data suggest that in everyday clinical practice transition to UWP can be implemented for the complete resection of colon lesions, irrespective of their size, with shorter resection time (lesions of 6–19 mm) and lower bleeding episodes (pedunculated polyps 6–19 mm). Based on this evidence, the use of UWP should be encouraged. Further randomized trials are needed to confirm our observations.
Declaration of conflicting interests
The authors have no competing interests to disclose.
Ethical approval
The local Ethical Committee (Azienda Ospedaliera Universitaria di Cagliari) approved the study protocol (PG/2017/5571, 3 April 2017).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Written informed consent was obtained from each patient at enrollment in clinical studies and before polyp resection.
References
- 1.Burgess NG, Bahin FF, Bourke MJ. Colonic polypectomy (with videos). Gastrointest Endosc 2015; 81: 813–835. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg 1955; 70: 120–122. [DOI] [PubMed] [Google Scholar]
- 3.Norton ID, Wang L, Levine SA, et al. Efficacy of colonic submucosal saline solution injection for the reduction of iatrogenic thermal injury. Gastrointest Endosc 2002; 56: 95–99. [DOI] [PubMed] [Google Scholar]
- 4.Binmoeller KF. Underwater endoscopic mucosal resection. J Interv Gastroenterol 2014; 4: 113–116. [Google Scholar]
- 5.Binmoeller KF, Weilert F, Shah J, et al. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc 2012; 75: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 6.Kim HG, Thosani N, Banerjee S, et al. Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc 2014; 80: 1094–1102. [DOI] [PubMed] [Google Scholar]
- 7.Wang AY, Flynn MM, Patrie JT, et al. Underwater endoscopic mucosal resection of colorectal neoplasia is easily learned, efficacious, and safe. Surg Endosc 2014; 28: 1348–1354. [DOI] [PubMed] [Google Scholar]
- 8.Uedo N, Nemeth A, Johansson GW, et al. Underwater endoscopic mucosal resection of large colorectal lesions. Endoscopy 2015; 47: 172–174. [DOI] [PubMed] [Google Scholar]
- 9.Curcio G, Granata A, Ligresti D, et al. Underwater colorectal EMR: remodeling endoscopic mucosal resection. Gastrointest Endosc 2015; 81: 1238–1242. [DOI] [PubMed] [Google Scholar]
- 10.Binmoeller KF, Hamerski CM, Shah JN, et al. Attempted underwater en bloc resection for large (2-4 cm) colorectal laterally spreading tumors (with video). Gastrointest Endosc 2015; 81: 713–718. [DOI] [PubMed] [Google Scholar]
- 11.Schenck RJ, Jahann DA, Patrie JT, et al. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc. Epub ahead of print 24 March 2017. DOI: 10.1007/s00464-017-5474-4. [DOI] [PubMed]
- 12.Amato A, Radaelli F, Spinzi G. Underwater endoscopic mucosal resection: The third way for en bloc resection of colonic lesions? United European Gastroenterol J 2016; 4: 595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endoscopic Classification Review Group. Update on the Paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005; 37: 570–578. [DOI] [PubMed] [Google Scholar]
- 14.Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009; 69: 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadoni S, Falt P, Gallittu P, et al. Impact of carbon dioxide insufflation and water exchange on post-colonoscopy outcomes in patients receiving on-demand sedation: a randomized controlled trial. Gastrointest Endosc 2017; 85: 210–218. [DOI] [PubMed] [Google Scholar]
- 16.Cadoni S, Falt P, Rondonotti E, et al. Water exchange for screening colonoscopy increases adenoma detection rate: a multicenter, double-blinded randomized controlled trial. Endoscopy 2017; 49: 456–467. [DOI] [PubMed] [Google Scholar]
- 17.Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011; 140: 1909–1918. [DOI] [PubMed] [Google Scholar]
- 18.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47: 829–854. [DOI] [PubMed] [Google Scholar]
- 19.Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2017; 49: 270–297. [DOI] [PubMed] [Google Scholar]
- 20.Kudo Se, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc 2008; 68: S3–S47. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013; 78: 625–632. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DB. Techniques for difficult polypectomy. Med Gen Med 2004; 6: 12–12. [PMC free article] [PubMed] [Google Scholar]
- 23.Zarchy T. Risk of submucosal saline injection for colonic polypectomy. Gastrointest Endosc 1997; 46: 89–90. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh YH, Binmoeller KF, Leung FW. Underwater polypectomy: Heat-sink effect in an experimental model. Gastrointest Endosc 2015; 83: AB385–AB385. [Google Scholar]
- 25.Fisher DA, Maple JT, Ben-Menachem T, et al. ASGE Standards of Practice Committee Complications of colonoscopy. Gastrointest Endosc 2011; 74: 745–752. [DOI] [PubMed] [Google Scholar]
- 26.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015; 81: 31–53. [DOI] [PubMed] [Google Scholar]